Abstract
Purpose
To compare anterior segment and ocular biometric parameters in unaffectedfellow eyes of patients with a previous attack of acute angle closure (AAC), primary angle closure suspect (PACS) eyes, and normal eyes; and to identify eyes at high risk of AAC among primary angle closure suspects.
Methods
In this case-control study, 16 unaffected fellow eyes of patients with aprevious attack of AAC (group I), 20 PACS eyes (group II) and 18 normal eyes (group III) underwent Pentacam and A-scan echography.
Results
Mean anterior chamber volume was 72±18, 77±18 and 176±44 µl in groupsI, II, and III, respectively (P<0.001). Corresponding values for anterior chamber angle in the same order were 24.8±4.6, 22.6±6.3 and 35.8±6.9 degrees (P<0.001), and that for anterior chamber depth measured from the corneal endothelium were 1.80±0.26, 1.93±0.23 and 3.13±0.59 mm, respectively (P<0.001). Using receiver operating characteristic (ROC) curves, anterior chamber volume ≤100 µl was associated with a high risk of AAC with sensitivity of 93.3% and specificity of 100%. Corresponding values for anterior chamber depth ≤2.1 mm were 86.7% and 100%, and that for anterior chamber angle ≤26° were 73.3% and 88.2%, respectively. Age, sex, central corneal thickness, and lens thickness were comparable among the study groups (all P values >0.05).
Conclusion
Eyes with anterior chamber volume ≤100 µl, depth ≤2.1 mm and angle≤26° may be considered at high risk for developing AAC. These criteria could be helpful for making decisions regarding prophylactic laser peripheral iridotomy.
Keywords: Acute Angle Closure, Glaucoma, Scheimpflug Imaging
INTRODUCTION
Glaucoma is the second cause of blindness worldwide.1,2 The prevalence of different types of glaucoma vary across the world; primary angle closure glaucoma (PACG) is the most common type in eastern Asia and India and is now believed to be more prevalent than considered before.1,3 Several anatomic characteristics including small corneal diameter and radius of curvature, shallow central anterior chamber depth (ACD), anterior lens position, excessive lens thickness, and short axial length(AL) are considered as predisposing factors for development of angle closure glaucoma.4-8
Recently recommended classifications for angle closure (glaucoma) based on gonioscopy and clinical examination consist of: primary angle closure suspect (PACS) characterized by iridotrabecular contact exceeding 180˚,9,10or 270˚,11but otherwise normal findings; primaryangle closure (PAC) is defined in the presence of PACS characteristics plus peripheral anterior synechiae (PAS) formation, high intraocular pressure (IOP), or iris/lens changes suggestive of a previous attack of angle closure in the absence of signs of optic disc damage or visual field defects; primary angle closure glaucoma (PACG) is defined as the above mentioned characteristics together with evidence of end organ damage such as glaucomatous optic disc changes and/or visual field defects.11
Another classification is based on angle appearance and presentation of the disease which is categorized as acute, subacute or chronic angle closure glaucoma.12 While the acute, symptomatic phase is dramatic, it occurs only in a minority of patients with PACG and the chronic, asymptomatic form of the disease predominates.
It has been shown that without treatment, 22% of PACS eyes progress to PAC over a period of 5 years.9 Additionally, the 5-year incidence for progression from PAC to PACG was shown to be 28.5%.10 As damage by acute angle closure (AAC) is irreversible, prophylactic laser peripheral iridotomy (LPI) of PACS eyes with high risk characteristics for developing AAC is crucial. No biometric parameter was found to predict progression within the angle closure spectrum in either of the above mentioned studies, and there are no well established criteria for performing LPI in PACS eyes. It has also been shown that for patients with a history of AAC attack, without treatment, the risk for developing AAC in the fellow eye is 40-80% over 5-10 years.12 For this reason, development of AAC in one eye is probably the strongest indication for performing prophylactic LPI in fellow PACS eyes.
Recent advances in anterior segment imaging have improved the ability to evaluate and measure anterior segment parameters in an objective and repeatable way, and have resulted in a better understanding of the pathophysiology of primary angle closure disease and diagnosis of secondary types of angle closure glaucoma.13 Pentacam imaging is a noninvasive noncontact method which uses a single rotating Scheimpflug camera for anterior segment imaging in a quantitative and reproducible way.13,14 Anterior segment imaging modalities such as Pentacam may help define and detect high risk eyes. Considering the fact that unaffected fellow eyes of subjects with a previous attack of AAC have the highest risk for developing AAC, this study was conducted to evaluate anterior segment parameters in these eyes and compare them with those in PACS and normal control eyes; the objective was to find eyes at high risk of developing AAC attacks. We also sought to determine predictive criteria for establishing an indication for prophylactic LPI. For primary outcome measures, we employed the Pentacam rotating Scheimpflug camera (PTC, Oculus Inc., Wetzlar, Germany).
methods
This case-control study included 16 unaffected fellow eyes of patients with a previous attack of AAC (group I) who presented to the emergency room from March 2008 to February 2009, 20 PACS eyes of 20 subjects (group II), and 18 normal eyes of 18 subjects (group III). The study was approved by the Ethics Committee (equivalent to Institutional Review Board) of the Ophthalmic Research Center. Written informed consent was obtained from all patients prior to enrollment after providing adequate explanation about the study goals. The study protocol adhered to the tenets of the Declaration of Helsinki.
All patients underwent a complete ophthalmologic examination including slit lamp biomicroscopy, tonometry, gonioscopy and funduscopy. Gonioscopy was performed in a dimly lit room using a 4-mirror goniolens (Sussmann model, Ocular Instruments, Bellevue, WA, USA), without indentation, in primary position and with a slit beam of 2 millimeters.
Enrollment criteria for group I included the presence of ocular pain, nausea and/or vomiting, IOP over 30 mmHg (as measured by Goldmann applanation tonometry), ciliary injection, corneal epithelial edema, fixed mid-dilated pupil, shallow anterior chamber and an occluded angle on gonioscopy in the AAC eye and presence of PACS in the unaffected fellow eye. Group II met the criteria for PACS, namely the posterior trabecular meshwork was not visible in at least 270 degrees of the angle circumference by gonioscopy in dim-lit conditions without indentation in primary position. Group III were selected from spouses or non-related subjects accompanying other patients. Inclusion criteria for these individuals consisted of no history of ocular disease or surgery, normal ocular examination and an open drainage angle in the entire angle circumference. Only one eye of each subject was randomly considered for the study in groups II and III by simple randomization. Subjects with history of ocular trauma or intraocular disease and/or surgery were excluded from the study. None of the eyes had been treated with miotics.
In patients with AAC, after performing LPI in the involved eye and controlling the acute attack, the unaffected fellow eye was considered for the study before receiving any medication or undergoing LPI.
All eligible eyes underwent anterior segment imaging using Pentacam (PTC, Oculus Inc., Wetzlar, Germany) and A-scan echography (Echoscan US-800; Nidek Co, Tokyo, Japan). Anterior segment parameters including anterior chamber volume (ACV), anterior chamber angle (ACA), anterior chamber depth (ACD) from the endothelium, central corneal thickness (CCT) and keratometry (KR) were measured by Pentacam. For each patient, Pentacam was performed twice within a 5-minute interval and the mean values were considered for analysis.
Lens thickness (LT), vitreous length (VL) and axial length (AL) were measured using A-scan echography. Ratios of ACD/AL and LT/ AL were also calculated. Although measurement of lens thickness and densitometry are possible with Pentacam, these were not obtained because they require pupil dilatation.
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA).
Mean, standard deviation (SD), 95% confidence interval (CI), frequency and percentage values were used to describe the data. Quantitative and qualitative variables were compared by analysis of variance and Chi-square tests, respectively. To adjust for multiple comparisons in two by two group evaluations, we employed the Bonferroni method.
Since there is no gold standard to definitely predict the chance of developing AAC, we chose fellow eyes of AAC patients because they have the highest risk of developing the condition. Accordingly a receiver operating characteristic (ROC) curve was constructed for each parameter, and the area under the ROC curve (AUC) was calculated for each parameter within each of the study groups. P-values less than 0.05 were considered as statistically significant.
Results
A total of 54 eyes of 54 subjects including 16 eyes in group I, 20 eyes in group II and 18 eyes in group III were studied. Mean± SD age of study participants was 59±10.8 (median, 58; range, 37 to 80) years among whom 28 (51.9%) subjects were female. The age range in groups I, II, and III were 39 to 73, 39 to 80, and 37 to 73 years, respectively. There were no statistically significant differences among the study groups in terms of age (P=0.2), and gender (P=0.39).
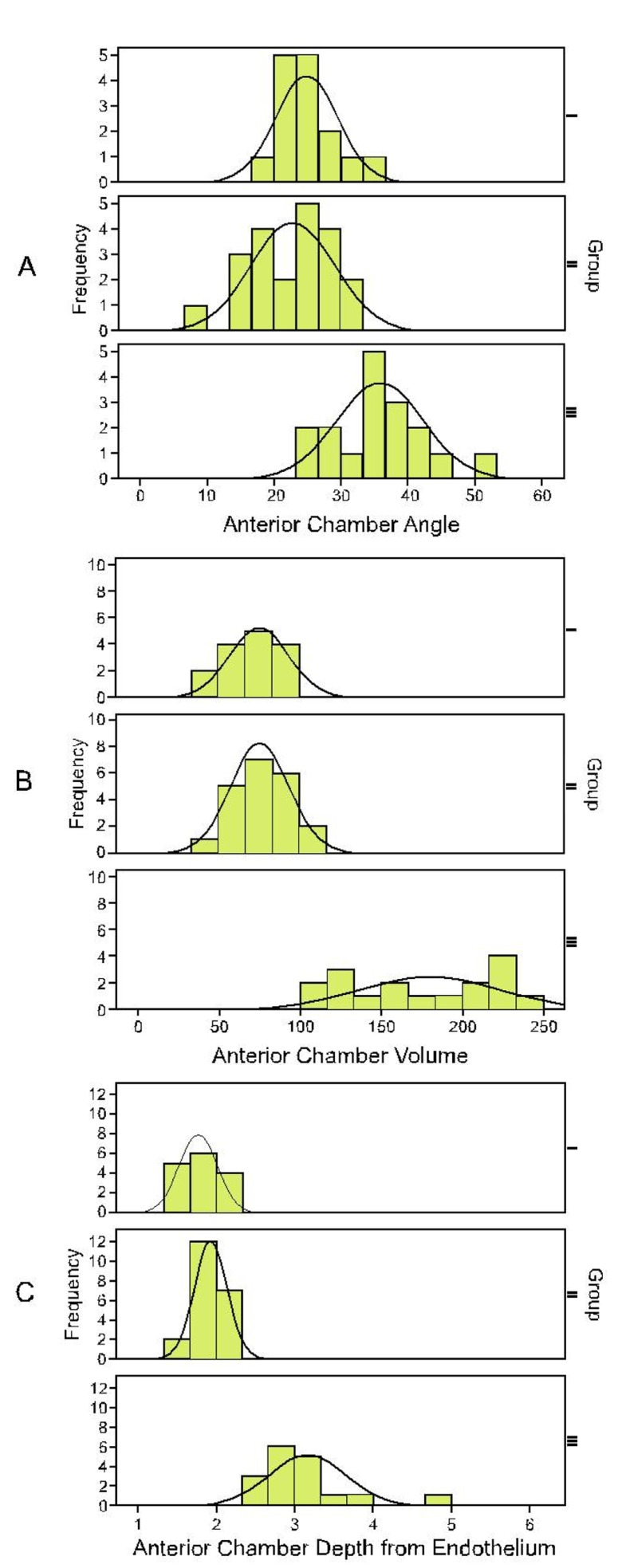
Mean ACV was 72±18 (range, 46 to 100) µl in group I, 77±18 (range, 42.5 to 106) µl in group II, and 176±44 (range, 110 to 246) µl in group III, (P<0.001). Corresponding values for ACA were 24.8±4.6 (range, 18.8 to 36.4) degrees, 22.6±6.3 (range, 9.1 to 33) degrees, and 35.8±6.9 (range, 24.5 to 50.5) degrees, (P<0.001) in the study groups respectively. Central ACD values were 1.80±0.26 (range, 0.08 to 0.13) mm in group I, 1.93±0.23 (range, 0.09 to 0.13) mm in group II, and 3.13±0.59 (range, 0.09 to 0.15) mm in group III (P<0.001, Fig. 1). No significant difference was observed between groups I and II in any of the parameters and ratios (all P values >0.3). Mean ACV, ACA, and ACD were significantly different in groups I and II as compared to group III (all P values <0.001). Mean AL was also significantly different between groups I and III (P<0.001), and also between groups II and III (P=0.017).
Figure 1.
Frequency distribution of anterior chamber angle (A), volume (B) and depth (C) from the endothelium in each group. Group I, unaffected eyes of patients with a previous attack of acute angle closure; Group II, primary angle closure suspects; Group III, normal eyes.
CCT, KR, LT, VL and LT/AL ratio were not statistically different among the groups (all P values >0.1), but ACD/AL ratio was significantly smaller in groups I and II as compared to group III (P<0.001, Table 1).
Table 1.
Biometric parameters and ratios within each study group and two by two comparisons
| Parameters | Groups | P* | Diff II-I† | Diff III-I† | Diff III-II† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | Mean (95% CI) | P | Mean (95% CI) | P | Mean (95% CI) | P | |||
| ACV (µl) | 72±18 | 77±18 | 176±44 | <0.001 | 4(-20,29) | >0.999 | 104(78,129) | <0.001 | 99(76,123) | <0.001 | |
| ACD (mm) | 1.8±0.26 | 1.93±0.23 | 3.13±0.59 | <0.001 | 0.14(-0.19,0.46) | >0.999 | 1.33(0.99,1.67) | <0.001 | 1.2(0.88,1.51) | <0.001 | |
| ACA (°) | 24.8±4.6 | 22.6±6.3 | 35.8±6.9 | <0.001 | -2.2(-7.3,2.9) | >0.999 | 11(5.6,16.3) | <0.001 | 13.2(8.3,18.1) | <0.001 | |
| CCT (µm) | 551±68 | 542±31 | 525±31 | 0.249 | -9(-46,29) | >0.999 | -26 (-65,13) | <0.001 | -17(-54,19) | 0.719 | |
| Lens thickness(mm) | 4.41±0.88 | 4.43±0.88 | 4.14±0.41 | 0.589 | 0.02(-0.72,0.76) | >0.999 | -0.27 (-1.04,0.5) | >0.999 | -0.28 (-1.04,0.47) | >0.999 | |
| Vitreous length(mm) | 15.8±1.4 | 15.5±2.4 | 16.7±1.1 | 0.207 | -0.3(-2,1.5) | >0.999 | 1(-0.8,2.7) | >0.999 | 1.2(-0.5,3) | 0.269 | |
| Axial length(mm) | 22.5±0.7 | 22.3±2 | 23.9±1 | 0.012 | -0.1(-1.4,1.2) | >0.999 | 1.4(0,2.8) | <0.001 | 1.6(0.2,2.9) | 0.017 | |
| Keratometry(Diopter) | 43.9±2.1 | 44.9±1.7 | 43.7±1.6 | 0.088 | 1(-0.5,2.6) | <0.001 | -0.2(-1.8,1.4) | >0.999 | -1.3(-2.7,0.2) | 0.123 | |
| ACD/AL ratio | 0.08±0.01 | 0.09±0.01 | 0.13±0.01 | <0.001 | 0.01(0,0.02) | 0.301 | 0.04(0.03,0.06) | <0.001 | 0.04(0.03,0.05) | <0.001 | |
| LT/AL ratio | 0.2±0.04 | 0.2±0.04 | 0.17±0.02 | 0.173 | 0(-0.03,0.04) | >0.999 | -0.02 (-0.06,0.01) | <0.001 | -0.03 (-0.06,0.01) | 0.266 | |
Group I, unaffected eyes of patients with previous attack of acute angle closure; Group II, primary angle closure suspects; Group III, normal eyes; Diff, difference; CI, confidence interval; ACV, anterior chamber volume; ACD, anterior chamber depth from the endothelium; ACA, anterior chamber angle; CCT, central corneal thickness; AL, axial length; LT, lens thickness.
Based on analysis of variance.
Adjusted for multiple comparison based on Bonferroni method.
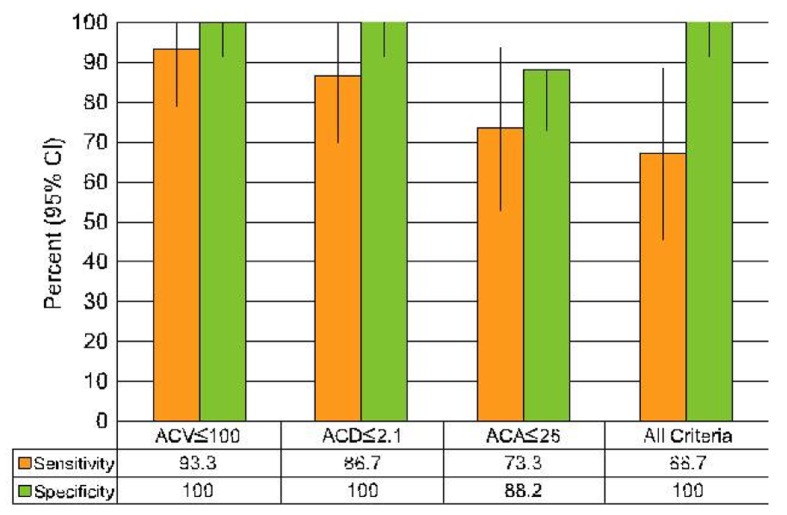
ROC curves were constructed for each parameter to distinguish group I eyes from those in group III. ACV ≤100 µl predicted a high risk of AAC according to the study presumption with sensitivity of 93.3% (95% CI: 70 to 99) and specificity of 100% (95% CI: 82 to 100). Corresponding values for ACD ≤2.1 mm were 86.7% (95% CI: 62 to 96) sensitivity and 100% (95% CI: 82 to 100) specificity (Fig. 2). Eyes with ACA ≤26° were also at high risk with sensitivity of 73.3% (95% CI: 48 to 89) and specificity of 88.2% (95% CI: 66 to 97). If an eye meets all of the three aforementioned criteria(ACV ≤100 µl, ACA ≤26° and ACD ≤2.1 mm), it can be considered at high risk for an attack of AAC with sensitivity of 66.7% (95% CI: 42 to 85) and specificity of 100% (95% CI: 82 to 100).
Figure 2.
Figure 2. Mean (95% confidence interval) sensitivity and specificity for each criterion alone and all criteria together for detecting eyes at high risk of developing acute angle closure. ACA, anterior chamber angle; ACD, anterior chamber depth from the endothelium; ACV, anterior chamber volume.
Discussion
In this study we evaluated and compared anterior segment and ocular biometric variables among unaffected fellow eyes of patients with a previous attack of AAC, PACS eyes and normal eyes using Pentacam and A-scan echography.
We looked for predictive parameters to identify eyes at high risk for developing AAC. The large AUCs found for ACV, ACA and ACD indicated that all three parameters are probably powerful indicators for determining the risk of AAC with cutoff values of ACV ≤100 µl, ACA≤26°, and ACD ≤2.1mm.
Although less common than chronic angle closure glaucoma, AAC is a dramatic condition resulting in irreversible damage. An attack of AAC may result in peripheral field loss from ischemic damage to the optic nerve head, some loss of visual acuity even in subjects receiving early treatment, and endothelial cell loss with attacks of more than 3 days’ duration.15 For these reasons, AAC attacks must be prevented if at all possible. These eyes show an anatomical predisposition as evident by a crowded anterior segment which is usually bilateral, and any case with unilateral AAC and normal fellow eye should be diagnosed as secondary angle closure glaucoma. The unaffected fellow eye is at high risk of developing AAC.12 Without treatment, this may occur simultaneously or at any time after the initial attack, most commonly in the first month.15 In a population based study it has been shown that over a period of 5 years, 22% of eyes categorized as PACS may progress to PAC, however no predictive parameter was recognized.9 Prophylactic administration of miotics such as pilocarpine, although frequently used until LPI can be performed, are ineffective for long term prevention of the acute attack.16 Laser peripheral iridotomy (LPI) is effective and the major preventive measure to decrease such risk in the fellow eye. Although LPI is easily performed, it may be accompanied by complications among which progression of cataracts and bullous keratopathy are most important.17,18 Thus LPI has not been recommended for all patients with anatomically narrow angles.19 A more practical approach would be to distinguish eyes at high risk of developing AAC which has been a subject of great interest.
The Van Herrick grading system is a noninvasive and simple method based on peripheral ACD for evaluating eyes with PACS, but its major shortcoming is being imprecise and subjective.20 The same holds true for gonioscopy, a subjective method with high intra- and inter-observer variability which is also affected by pressure exerted on the eye during the examination.
Advances in anterior segment imaging have allowed quantitative and reproducible measurement of anterior segment parameters. The Pentacam is a noninvasive noncontact method which uses a single rotating Scheimpflug camera for anterior segment imaging in a quantitative and reproducible way. It takes up to 50 slit images of the anterior segment in 2 seconds with 500 true elevation points in each image. A 3-dimensional model of the anterior segment is then constructed using the obtained data, and corneal thickness, corneal topographic parameters, central ACD, ACA, ACV and some other parameters can be measured.13,14 Using Pentacam, the anterior chamber angle is calculated by projecting the posterior corneal surface and the iris contour; this ignores the convex iris configuration in eyes with narrow angles thus resulting in overestimation of the anterior chamber angle. Therefore angle measurement by the Pentacam may not be accurate14; this may be the cause for our observations of unexpectedly smaller ACA in PACS eyes as compared to fellow eyes of AAC patients. Furthermore this may justify why ACA had lower sensitivity and specificity for discriminating fellow eyes of AAC patients from normal individuals as compared to ACV and ACD.
Different imaging techniques have been used to determine high risk features for development of AAC. Ocular biometry has revealed significantly shallower ACD, thicker lens, shorter AL and higher LT/AL ratio in AAC and fellow eyes than eyes with chronic angle closure glaucoma.7 In the current study we also observed shallower ACD and shorter AL in unaffected fellow eyes in group I. In another study by George et al, no significant difference in biometric values was found between angle closure glaucoma and occludable angles, however they were significantly different from normal eyes; this observation is also in line with our findings.8 Using ultrasound biomicroscopy (UBM), Sawada et al showed that fellow eyes with AAC have different topologic features and a higher incidence of appositional angle closure as compared to normotensive narrow angled eyes.21 In another UBM study on fellow eyes of patients with AAC, Mérula et al showed that these eyes have a more crowded anterior segment and shallower ACD than narrow angled eyes.22 Aung et al evaluated the relationship between ACD and the risk of PAS and PAC and found a threshold value of 2.4 mm for ACD in Mongolian subjects, a cutoff value below which the rate of PAS increased rapidly.23 Kurita et al compared Pentacam and UBM and reported the former to be safe and useful in screening for PACS and PAC using parameters such as ACD and ACV, but not ACA.24 Our findings are in agreement with the mentioned study in that we found ACD and ACV to be more powerful predictors of AAC than ACA.
One of the limitations of our study is the relatively small number of cases in each group, nevertheless the cutoff values we found can be considered as a guideline for LPI in a larger prospective randomized study.
In summary ACV, ACA, and ACD from the endothelium as measured by Pentacam were found to be reliable parameters to predict eyes at high risk of developing AAC. These criteria can be helpful when making a decision to proceed for prophylactic laser PI in borderline cases. Our findings revealed that eyes with ACV ≤100 µl can be considered at high risk with sensitivity of 93.3% and specificity of 100%. ACD ≤2.1 mm was another considerable risk factor for development of AAC with sensitivity of 86.7% and specificity of 100%. Corresponding values for ACA ≤26° were 73.3% and 88.2% respectively. Additionally, any eye that meets all of these three criteria (ACV ≤100 µl, ACA ≤26° and ACD ≤2.1 mm) could also be considered at high risk with sensitivity of 66.7% and specificity of 100%.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World HealthOrgan. 1995;73:115–121. [PMC free article] [PubMed] [Google Scholar]
- 3.Yip JL, Foster PJ. Ethnic differences in primary angle-closure glaucoma. Curr Opin Ophthalmol. 2006;17:175–180. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- 4.Alsbirk PH. Primary angle-closure glaucoma. Oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol Suppl. 1976;127:5–31. [PubMed] [Google Scholar]
- 5.Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle closure glaucoma. Br J Ophthalmol. 1970;54:161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sihota R, Lakshimaiah NC, Agrawal HC, Pandey RM, Titiyal JS. Ocular parameters in the subgroups of angle closure glaucoma. Clin ExperimentOphthalmol. 2000;28:253–258. doi: 10.1046/j.1442-9071.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 7.Lan YW, Hsieh JW, Hung PT. Ocular biometry in acute and chronic angle-closure glaucoma. Ophthalmologica. 2007;221:388–394. doi: 10.1159/000107498. [DOI] [PubMed] [Google Scholar]
- 8.George R, Paul PG, Baskaran M, Ramesh SV, Raju P, Arvind H, et al. Ocular biometry in occludable angles and angle closure glaucoma: a population based survey. Br J Ophthalmol. 2003;87:399–402. doi: 10.1136/bjo.87.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2003;87:450–454. doi: 10.1136/bjo.87.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas R, Parikh R, Muliyil J, Kumar RS. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: a population-based study. Acta Ophthalmol Scand. 2003;81:480–485. doi: 10.1034/j.1600-0420.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 11.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaucoma, Section 10, Basic and Clinical Science Course. American Academy of Ophthalmology; San Francisco: 2008-2009. Angle-Closure Glaucoma. pp. 128–131. [Google Scholar]
- 13.Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: a new era for ophthalmic diagnosis? Br JOphthalmol. 2007;91:551–557. doi: 10.1136/bjo.2006.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J Cataract Refract Surg. 2006;32:456–459. doi: 10.1016/j.jcrs.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 15.Edwards RS. Behaviour of the fellow eye in acute angle-closure glaucoma. Br J Ophthalmol. 1982;66:578–579. doi: 10.1136/bjo.66.9.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamed Q, Fahey DK, Manners RM. Angle closure in fellow eye with prophylactic pilocarpine treatment. Br J Ophthalmol. 2001;85:1263. doi: 10.1136/bjo.85.10.1260b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quigley HA. Long-term follow-up of laser iridotomy. Ophthalmology. 1981;88:218–224. doi: 10.1016/s0161-6420(81)35038-6. [DOI] [PubMed] [Google Scholar]
- 18.Ang LP, Higashihara H, Sotozono C, Shanmuganathan VA, Dua H, Tan DT, et al. Argon laser iridotomy-induced bullous keratopathy-a growing problem in Japan. Br J Ophthalmol. 2007;91:1613–1615. doi: 10.1136/bjo.2007.120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilensky JT, Ritch R, Kolker AE. Should patients with anatomically narrow angles have prophylactic iridectomy? Surv Ophthalmol. 1996;41:31–36. doi: 10.1016/s0039-6257(97)81991-1. [DOI] [PubMed] [Google Scholar]
- 20.Van Herick, Shaffer RN, Schwartz A. Estimation of width of angle of anterior chamber. Incidence and significance of the narrow angle. Am J Ophthalmol. 1969;68:626–629. doi: 10.1016/0002-9394(69)91241-0. [DOI] [PubMed] [Google Scholar]
- 21.Sawada A, Sakuma T, Yamamoto T, Kitazawa Y. Appositional angle closure in eyes with narrow angles: comparison between the fellow eyes of acute angle closure glaucoma and normotensive cases. JGlaucoma. 1997;6:288–292. [PubMed] [Google Scholar]
- 22.Mérula RV, Cronemberger S, Diniz Filho, Calixto N. New comparative ultrasound biomicroscopic findings between fellow eyes of acute angle closure and glaucomatous eyes with narrow angle. Arq BrasOftalmol. 2008;71:793–798. doi: 10.1590/s0004-27492008000600005. [DOI] [PubMed] [Google Scholar]
- 23.Aung T, Nolan WP, Machin D, Seah SK, Baasanhu J, Khaw PT, et al. Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol. 2005;123:527–532. doi: 10.1001/archopht.123.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Kurita N, Mayama C, Tomidokoro A, Aihara M, Araie M. Potential of the pentacam in screening for primary angle closure and primary angle closure suspect. J Glaucoma. 2009;18:506–512. doi: 10.1097/IJG.0b013e318193c141. [DOI] [PubMed] [Google Scholar]