Abstract
Kainate receptors are a family of ionotropic glutamate receptors whose physiological roles differ from those of other subtypes of glutamate receptors in that they predominantly serve as modulators, rather than mediators, of synaptic transmission. Neuronal kainate receptors exhibit unusually slow kinetic properties that have been difficult to reconcile with the behaviour of recombinant kainate receptors. Recently, however, the neuropilin and tolloid-like 1 (NETO1) and NETO2 proteins were identified as auxiliary kainate receptor subunits that shape both the biophysical properties and synaptic localization of these receptors.
Kainate-type glutamate receptors are expressed throughout the mammalian CNS and affect neural circuit activity through the modulation of excitatory and inhibitory tone, neuronal excitability, synaptic development and various other aspects of brain function1. Kainate receptors have been implicated in human diseases ranging from epilepsy to neuropathic pain and migraine1, and they are attractive pharmacological targets for therapeutic intervention because of their modulatory effects on neural circuit activity. The bewildering array of kainate receptor effects has made it challenging to develop a consensus view of their impact on neural systems. Simple, yet crucially important, aspects of kainate receptor function remain poorly understood despite more than a decade of research into these receptors since their initial description at hippocampal synapses2–4. For example, many currents mediated by postsynaptic kainate receptors are over an order of magnitude slower than those elicited from their recombinant counterparts. In addition, the cellular mechanisms that control the heterogeneous use and polarized localization of kainate receptors in different populations of neurons are unknown, as is the mechanism by which the ‘metabotropic’ function of these ion channels is transduced. The limited availability of selective pharmacological and biochemical tools for kainate receptors have made these issues difficult to resolve.
Recently, at least some of the uncertainties regarding the molecular basis of kainate receptor activity in the mammalian brain were resolved in a series of studies that described a new set of kainate receptor-interacting proteins, which are known as neuropilin and tolloid-like (NETO) proteins5–9. NETO proteins profoundly alter kainate receptor function and probably account for many of the disparities between recombinant and neuronal kainate receptor gating. The evolving picture of NETO proteins and their impact on kainate receptors has many parallels to the earlier discovery of obligatory partners for AMPA-type glutamate receptors, namely stargazin and other transmembrane AMPA receptor regulatory proteins (TARPs)10. TARPs were found to be distinct from the myriads of AMPA receptor-interacting proteins in that the stability and obligatory nature of their association with these receptors met criteria previously developed for classification of bona fide auxiliary proteins of voltage-gated ion channels11, 12 (BOX 1). Although the NETO proteins are structurally distinct from mammalian AMPA receptor auxiliary proteins, their discovery and characterization could prove to be no less enlightening with regards to kainate receptor function. In this Review, we will briefly summarize kainate receptor actions in the CNS and recapitulate the evidence that the actions of NETO proteins as auxiliary proteins are important for those functions.
Box 1 | Auxiliary subunits versus interacting proteins.
Many proteins associate with glutamate receptors and regulate key aspects of their biogenesis, localization and function10, 104, 105. Why are some, like stargazin and other transmembrane AMPA receptor regulatory proteins, referred to as auxiliary (or accessory) proteins, whereas others (such as postsynaptic density 95 and other membrane-associated guanylate kinases) fall short of this connotation despite making crucial contributions to ionotropic glutamate receptor localization? As has been noted in recent reviews, a set of principles previously established for auxiliary subunits of voltage-gated ion channels11, 106 provides a useful standard to apply to ligand-gated ion channels generally, and AMPA and kainate receptors most recently10, 79. This definition for classification of an interacting protein as an auxiliary subunit has four principal criteria. First, the candidate auxiliary protein should not constitute an integral component of the transduction pathway. Channel pores of AMPA, kainate and NMDA receptors are formed exclusively from membrane domains contributed by their respective subunit proteins, which exhibit primary sequence homology and a conserved tertiary architecture17. Second, an auxiliary protein remains stably associated with its partner receptor for the majority, if not the entirety, of its lifetime following assembly in the endoplasmic reticulum. Third, an auxiliary protein should affect multiple aspects of receptor pharmacology, function and subcellular trafficking or targeting. Fourth, co-assembly with auxiliary subunits should be required for proper neuronal functionality of the receptor. Consequently, gene-targeting or other effective mechanisms of silencing of the auxiliary subunit should, at a minimum, profoundly alter receptor function in the CNS. An auxiliary subunit is therefore an extension of the receptor, modifying biophysical features inherent to the individual subunits themselves. We know from the field of voltage-gated ion channels that auxiliary subunits that meet these various criteria can be either cytoplasmic or integral membrane proteins (such as the β and γ auxiliary subunits of voltage-gated calcium channels, respectively107). Interacting proteins, however, display transient and often dynamic interactions with receptors and generally influence singular aspects of receptor function, usually involving their biogenesis, trafficking or synaptic localization104.
Kainate receptor properties
Recombinant kainate receptors
Kainate receptors are tetrameric ionotropic glutamate receptors (iGluRs) that are composed of diverse combinations of five subunit proteins: GluK1, GluK2, GluK3, GluK4 and GluK5 (previously known as GluR5, GluR6, GluR7, KA1 and KA2, respectively). Kainate receptors were the second family of iGluRs to be cloned in the early 1990s13, and their sensitivity to agonists that included the natural excitotoxins kainic and domoic acid clearly aligned them with the pharmacologically defined kainate receptors that were identified first definitively in neurons of the dorsal root ganglia14, 15 and later in cultured hippocampal neurons16. The five subunits are divided into two subfamilies on the basis of their primary sequence homology and functional distinctions. GluK1, GluK2 and GluK3 subunits can form homo- and heteromeric receptors, whereas the ‘high-affinity’ subunits GluK4 and GluK5 form obligate heteromers with GluK1, GluK2 or GluK3.
Initial investigations focused on the physiological characterization of recombinant receptors in heterologous systems, in which kainate receptors exhibited biophysical properties qualitatively similar to those of AMPA receptors; that is, kainate receptors could mediate glutamate-evoked currents that were rapidly activated with sub-millisecond kinetics and decayed in less than 10 ms, which was consistent with a putative role in transducing rapid synaptic depolarizations17. However, in subsequent years, intriguing differences between kainate and AMPA receptors emerged that set the former apart functionally from the latter. Heteromeric receptor combinations exhibited distinct pharmacological properties from those of homomeric kainate receptors, leading to the seemingly reasonable expectation that specific receptor populations could be selectively targeted in the brain17, 18 (an approach that has only seen limited success, unfortunately). Furthermore, kainate receptors were slower (on average, by an order of magnitude) to recover from desensitization than AMPA receptors19–21. This would, in principle, impose temporal constraints on their contribution to phasic, high-frequency postsynaptic depolarizations. At the molecular level, kainate receptors composed of the principal GluK2 subunit were found to require extracellular ions for receptor gating22–26, a requirement that is unique among ligand-gated ion channels. Sodium and chloride ions are coordinated at the extracellular surface of the receptor25, 26, stabilizing its ligand-binding domain in an open conformation. This has led to the notion that neuronal kainate receptors (unlike AMPA receptors) have the ability to ‘sense’ extracellular ion concentrations to regulate local excitability27. These studies in recombinant systems have been critical for developing an understanding of the mechanisms of iGluR function and have proven complementary to investigations of native receptors.
Postsynaptic kainate receptors in the CNS
As summarized above, many research groups found that kainate receptors expressed robustly in various heterologous expression systems seemed to have biophysical properties that would enable them to have a postsynaptic role that would be similar to that of AMPA receptors (albeit with less sensitivity to higher frequencies of synaptic input)1, 17. Nevertheless, neuronal kainate receptor currents proved elusive prey in the mammalian CNS in part because they could not be differentiated conclusively from AMPA receptor currents with the antagonists that were available in the early 1990s. Furthermore, selective kainate receptor activation in the hippocampal CA1 region produced effects that were more consistent with a potential presynaptic locus of action2, raising the possibility that postsynaptic kainate receptors simply did not exist in the CNS. This idea, however, was put to rest when the first postsynaptic kainate receptor-mediated excitatory postsynaptic currents (EPSCsKA) were detected at mossy fibre–CA3 synapses in the hippocampus3, 4 (FIG. 1a), an achievement that was made possible only by eliminating the much larger amplitude synaptic AMPA receptor currents with a newly developed, highly selective non-competitive antagonist16, 28.
Figure 1. Distinctive features of synaptic kainate receptors.
a | Synaptic kainate receptors mediate smaller amplitude currents with much slower kinetics than AMPA receptors. Traces from recordings of synaptic currents at mossy fibre–CA3 synapses in the hippocampus show a mixed AMPA and kainate receptor-mediated excitatory postsynaptic current (EPSCAMPA–KA; black), which is predominantly mediated by AMPA receptors, the low-amplitude pharmacologically isolated EPSCKA (grey), and the peak-scaled EPSCKA (red), which illustrates the relatively slow kinetics of kainate receptors. b | Repetitive activation of pharmacologically isolated mossy fibre kainate receptors (at 20 Hz in the example) results in EPSCKA summation and increases the total charge transferred during the synaptic currents. c | Excitatory postsynaptic potentials (EPSPs) recorded during a train of mossy fibre stimulation exhibit a tonic depolarization ‘envelope’ that is contributed to by the activation of kainate receptors. This is evident in the example traces as the difference between EPSPAMPA–KA (black) and EPSPAMPA (blue) recorded in the presence of the selective postsynaptic kainate receptor antagonist UBP310 (REF. 29). d | Incorporation of the GluK4 and GluK5 subunits shape postsynaptic kainate receptor responses. The left panel shows example traces of EPSCsKA recorded from CA3 pyramidal cells following stimulation of mossy fibre projections from wild-type (black) and GluK4−/− (blue) mice (A. Contractor, unpublished results). The right panel shows that under the same recording conditions as in the previous experiment, the EPSCKA at this synapse exhibits faster kinetics in GluK5−/− mice (green) than in wild-type mice (black)46. Parts a and d (right panel) are modified, with permission, from REF. 46 © (2003) Society for Neuroscience. Part b is modified, with permission, from REF. 126 © (2007) Society for Neuroscience. Part c is modified, with permission, from REF. 29 © (2012) Oxford University Press.
The direct recording of EPSCsKA confirmed that these receptors have a postsynaptic role in excitatory signal transduction and led to the identification of the first of several conserved principles of kainate receptor signalling. In stark contrast to recombinant kainate receptors, EPSCsKA exhibited exceptionally slow decay kinetics (FIG. 1a) and summated effectively during high-frequency trains of activation (FIG. 1b); when voltage is measured, this property of kainate receptor gating produces a tonic level of depolarization during which phasic signals drive neurons closer to the threshold for action potential firing29 (FIG. 1c). The slow time course of the postsynaptic current decay was proposed to impart an associative property to synaptic communication during bursts of excitatory synaptic input30. With few exceptions, the hallmark slow kinetics of EPSCsKA were observed throughout the nervous system, from mossy fibre–CA3 pyramidal cell3, 4 or Schaffer collateral–CA1 interneuron synapses31, 32 in the hippocampus to developing thalamocortical synapses33 and in the periphery at primary sensory inputs to dorsal horn neurons of the spinal cord34. These unusual properties were just the first of a number of puzzling aspects of kainate receptor function.
The slow kinetics of EPSCsKA defied easy explanation; they were not a result of perisynaptic distribution of the kainate receptors underlying the synaptic currents3, 35–38 and, with one possible exception, could not generally be reproduced by the expression of recombinant receptors in heterologous systems20, 39–43. Of all the diverse receptor stoichiometries examined, only the heteromeric GluK2–GluK5 receptors exhibited relatively slow kinetics in response to brief pulses of physiological concentrations of glutamate17, 44, but this was not observed at higher, saturating levels of glutamate7, 8. GluK2 and GluK5 were identified as crucial components of postsynaptic kainate receptors in mossy fibre synapses through comparative analysis of EPSCsKA in gene-targeted mice lacking one or more receptor subunits. Knockout of the GluK2 subunit completely eliminated the EPSCKA, demonstrating an obligatory role for this subunit in forming functional receptors at this synapse45. Ablation of the ‘high-affinity’ GluK4 and GluK5 subunits had a bidirectional effect on synaptic kinetics: mice lacking GluK4 exhibited far slower kinetics, whereas loss of GluK5 resulted in a more rapid current decay46, 47 (FIG. 1d). Moreover, elimination of both GluK4 and GluK5 subunits in double knockout mice precluded localization of kainate receptors to mossy fibre postsynaptic sites but did not wholly prevent the expression of functional receptors in extrasynaptic domains47. Together with the conclusions from work on recombinant receptors, the in vivo studies suggested that the unique kinetics observed at synapses could not be solely explained by the biophysical properties of the receptor subunits themselves; additional mechanisms were at play.
Compartmentalized localization
A second feature of kainate receptors that was presaged by the isolation of the hippocampal mossy fibre EPSCsKA was the highfidelity and selective nature of kainate receptor localization in neurons. On CA3 pyramidal neurons, kainate receptor-mediated synaptic currents were only detected at mossy fibre inputs, which are the most proximal synapses along the apical dendrites of the neurons, and were absent from the abundant associational–commissural synapses3, 4. Conversely, many other neurons were shown to use kainate receptors specifically as presynaptic mediators of neurotransmitter release or axonal excitability and completely exclude them from postsynaptic densities (PSDs). Hippocampal granule cells are one such cell type; presynaptic kainate receptors facilitate glutamate release from mossy fibre terminals and contribute to the marked frequency-dependent short-term potentiation of glutamate release at this synapse48–50. CA1 interneurons, by contrast, express both postsynaptic and presynaptic kainate receptors31, 32, 51 (reviewed in REF. 1). Selective distribution of kainate receptors also occurs outside the hippocampus; cerebellar granule cells use the receptors exclusively as presynaptic modulators of glutamate release at parallel fibre synapses onto stellate or Purkinje neurons52, whereas kainate receptors in Purkinje cells themselves are only found in postsynaptic domains apposed to climbing fibre terminals53. Thus, neurons target kainate receptors to functional domains in a variegated pattern that seems to be different for each type of neuron or synaptic input examined. Remarkably, the cellular control pathways and chaperone proteins that presumably dictate these patterns of expression remain a mystery.
An ionotropic receptor operating in metabotropic mode
A third unusual aspect of kainate receptors in the CNS is that some aspects of their signalling do not appear to be dependent on channel gating but rather rely on G protein-mediated, or metabotropic, signalling pathways. This surprising hypothesis was first formulated when kainate receptor modulation of GABA release from CA1 interneurons was found to be independent of ion channel permeation, to require protein kinase C activity and to be sensitive to occlusion by pertussis toxin54. An analogous metabotropic pathway underlies kainate receptor-mediated inhibition of a calcium-activated potassium conductance (the slow after-hyperpolarizing current), which increases the intrinsic excitability of CA1 and CA3 pyramidal neurons55–57. The GluK5 subunit has been proposed to play a unique part in initiating metabotropic signalling58 (although this result was not reproduced in a subsequent study47). In large part, however, the molecular mechanisms that couple conformational changes induced by glutamate binding to kainate receptors with G protein-dependent activation of downstream effectors, such as protein kinase C, remain unknown.
Interacting proteins and kainate receptor signalling
Just as with other types of iGluRs, kainate receptors have no shortage of associated proteins that have important roles in subcellular trafficking and postsynaptic receptor stability59. These proteins affect kainate receptors predominantly through the intracellular C-terminal domain of the receptor subunits, in which a number of protein–protein interaction motifs have been identified59. Scaffolding molecules such as PSD95 and other membrane-associated guanylate kinases60, 61, glutamate receptor-interacting protein (GRIP) and protein interacting with C kinase 1 (PICK1)62, as well as the SNARE complex protein SNAP25 (REF. 63), constitutively or through activity-dependent mechanisms regulate the amplitude of EPSCsKA at the mossy fibre–CA3 pyramidal synapse. However, these and other transiently interacting proteins controlling distinct aspects of kainate receptor signalling (such as catenins and cadherin64, COPI complex proteins65 and actinfilin66) do not affect receptor function to an appreciable degree (with the possible exception of PSD95 (REFS 60,61)) and do not appear to contribute to either metabotropic signalling or the highly polarized localization of neuronal kainate receptors. The characteristics of their association with kainate receptors make it clear that none meets the criteria for receptor auxiliary proteins, which is not surprising given the diverse roles of these proteins in many aspects of subcellular trafficking, postsynaptic scaffolding or control of integral membrane protein stability. Additionally, most are not uniquely associated with kainate receptors; indeed, many are crucial for the synaptic maintenance of other iGluRs67.
A cytosolic protein that was isolated recently, the BTB/kelch domain protein KRIP6 (kainate receptor-interacting protein for GluR6; also known as KLHL24), is not as easy to categorize. It was found to interact with the GluK2 subunit, and although KRIP6 did not affect the plasma membrane expression of GluK2 receptors, co-expression of these proteins did decrease peak current amplitudes while increasing the steady-state currents68, which is suggestive of a fundamental alteration in receptor biophysical properties (although current decay rates were not affected). Thus, KRIP6 certainly alters receptor functional properties, albeit in ways that are not well understood. Given the generally accepted criteria for auxiliary subunits (BOX 1), however, KRIP6 is considered an interacting protein rather than an auxiliary subunit because it has not yet been shown to be essential for some aspect of kainate receptor function in the CNS.
In summary, despite the variety, sheer number and recognized importance of these proteins to kainate receptor signalling, none could adequately account for any of the strikingly unusual aspects of kainate receptor function in the CNS. Into this void of satisfactory explanations came the NETO proteins to settle at least one of the major puzzles associated with these receptors.
NETO proteins are auxiliary subunits
Discovery and domain structure
NETO2 is a single-pass integral membrane protein that was identified in 2009 through a proteomic approach combining immunoprecipitation and mass spectrometry as an interacting partner for neuronal kainate receptors5. Concurrently, the structurally related NETO1 protein was proposed to act as an interacting protein for NMDA-type glutamate receptors69; this provocative observation has not yet been elaborated upon and thus we will confine our discussion to the role of NETO proteins in kainate receptor signalling. Complementary DNAs for NETO1 and NETO2 had been previously cloned in genetic screens for transcripts expressed in the brain and retina, but the functional relevance of the NETO proteins was unknown70–72. It was hypothesized that these proteins had a role in nervous system development owing to their two extracellular CUB (complement C1r/C1s, Uegf and Bmp1) domains, which are well-characterized protein–protein interaction domains initially found in members of the complement family. The CUB domains in NETO proteins had highest sequence homology with analogous domains in neuropilins, which mediate associations with semaphorin ligands and are therefore crucial for axonal pathfinding and appropriate wiring of the nervous system73–75. In NETO1 and NETO2, the CUB domains are followed by an extracellular juxtamembrane low-density lipoprotein class A domain, which can form a complex with calcium and adopt a rigid secondary structure that restricts conformational freedom. The intracellular C-terminal domains of NETO proteins possess an AP2 adaptor protein endocytic motif and, in the case of NETO1, a type I PDZ ligand at the extreme C terminus. Other proteins built on a similar template act as auxiliary proteins for iGluRs and nicotinic acetylcholine receptors in the invertebrate Caenorhabditis elegans76–78, and thus CUB domains appear to be a conserved structural feature for a family of auxiliary proteins for diverse ligand-gated ion channels (BOX 2).
Box 2 | CUB domain proteins as auxiliary subunits.
Neuropilin and tolloid-like (NETO) proteins contain two discrete CUB (complement C1r/C1s, Uegf and Bmp1) domains, which are structurally conserved, ~110 amino acid protein interaction domains that were initially characterized in signalling molecules that are crucial for development73. The identified roles of CUB domain proteins have diversified considerably to include acting as auxiliary subunits for ligand-gated ion channels. NETO1 and NETO2 are the latest members of this family and the first acting in this capacity that have been identified in mammals (see the figure). Genetic screens in the nematode Caenorhabditis elegans identified an auxiliary subunit of the invertebrate homologue of mammalian glutamate receptors, GLR-1 (REFS 77,78). The protein, SOL-1, has a single transmembrane domain similar to the NETO proteins but contains four extracellular CUB domains and lacks the juxtamembrane low-density lipoprotein class A (LDLa) domain that is present in NETO proteins. SOL-1 is required for GLR-1 gating but not for localization to synapses; in the absence of the auxiliary subunit, the open state of GLR-1 receptors is destabilized resulting in non-functional receptors108. Co-assembly with SOL-1 slows receptor desensitization and speeds recovery from desensitization, similar to the effect of NETO2 on kainate receptors5, 6, 8. Two other CUB domain C. elegans proteins, LEV-9 and LEV-10, are important for targeting levamisole-sensitive acetylcholine receptors (l-AChRs) to postsynaptic sites of the neuromuscular junction (NMJ)76, 103. In contrast to the effects of SOL-1, loss of the integral membrane protein LEV-10 does not affect surface expression or functional properties of l-AChRs, but these receptors are completely absent from synapses. A truncated, soluble form of LEV-10 is able to cluster the l-AChRs at NMJs and restore synaptic currents. Similarly, loss of LEV-9, which is a secreted protein that only contains CUB domains, causes similar deficits in synaptic clustering, suggesting that interactions with the CUB domains are necessary and sufficient to cluster receptors at sites of synaptic contact. The addition of the NETO proteins to this family further underscores the role of CUB domain-containing proteins in shaping neurotransmitter signalling as auxiliary subunits. CKAMP44, cystine-knot AMPAR modulating protein of 44 kDa; CNIH, cornichon homologue; TARP, transmembrane AMPA receptor regulatory protein.
NETO proteins and recombinant kainate receptors
Following the initial identification of NETO2 as a protein associating with the GluK2 subunit, both NETO1 and NETO2 were shown to interact with other recombinant kainate receptors but not with AMPA receptors5–7, 9. This interaction occurs primarily through the second extracellular CUB domain and is absent when both CUB domains are deleted9. NETO1 and NETO2 seem to affect kainate receptors in many of the same ways that TARPs alter AMPA receptor function and distribution; that is, NETO co-assembly influences nearly all aspects of kainate receptor signalling, including changes in receptor pharmacology, channel gating properties and receptor trafficking in heterologous as well as neuronal systems. The changes in receptor function are dependent on both the receptor subtype and NETO isoform6, 7.
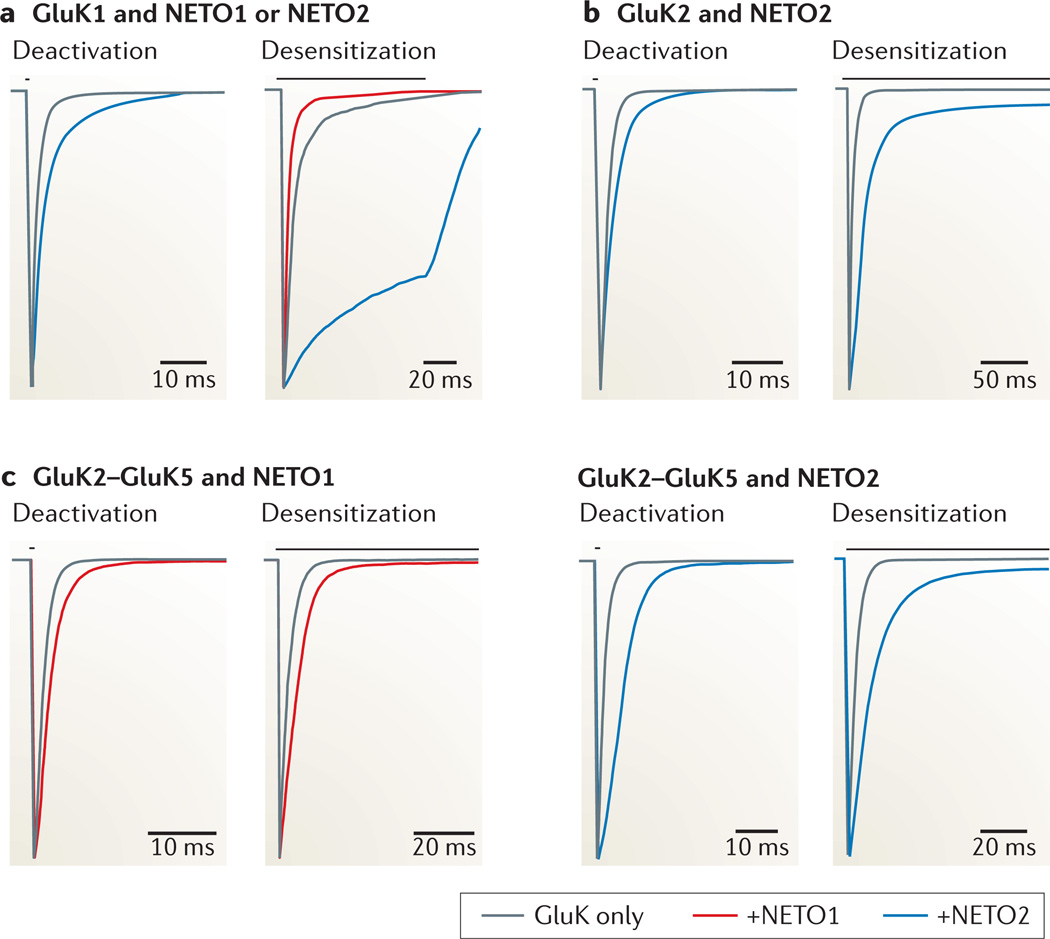
NETO2 was first shown to slow the deactivation and desensitization of glutamate-gated currents from homomeric GluK2 receptors, and this observation was subsequently extended to many other homomeric and heteromeric subunit combinations5–8 (FIG. 2). NETO1 has a bidirectional impact on receptor kinetics, slowing the deactivation and desensitization of GluK2–GluK5 heteromeric receptors but hastening entry into the desensitized state for GluK1 receptors6, 7 (FIG. 2). The degree to which NETO2 slows deactivation rates is relatively consistent at approximately twofold5, 8, whereas increases in desensitization rates can be as large as an order of magnitude, as was observed with homomeric GluK1 receptors6. Both NETO1 and NETO2 generally increase the rate of kainate receptor recovery from desensitization. Furthermore, NETO2 increases the open probability and open time of channels without affecting single-channel conductance5 (BOX 3). Agonist efficacy and potency are also increased when kainate receptors incorporate NETO proteins; for example, co-expression of NETO1 with recombinant GluK2–GluK5 receptors enhances affinity for kainic acid7. NETO2 is able to increase the efficacy of the partial agonist kainate, relative to the full agonist glutamate, for both recombinant GluK2 receptors in Xenopus laevis oocytes and native GluK2 receptors in cultured hippocampal neurons5. Our understanding of the extent to which NETO proteins modulate receptor pharmacology is still limited, however, and further studies are required to test the hypothesis that all ligands exhibit increases in potency or efficacy and, more importantly, what effect the auxiliary subunits have on antagonist sensitivity and selectivity (BOX 3).
Figure 2. Neuropilin and tolloid-like protein co-assembly alters the biophysical properties of kainate receptors.
a | GluK1 receptors are bidirectionally modulated by neuropilin and tolloid-like (NETO) co-assembly. NETO1 (red) hastens GluK1 entry into a desensitized state, whereas NETO2 (blue) slows both deactivation and desensitization rates6, 8. The grey horizontal bars illustrate the duration of glutamate application (10 mM). To determine deactivation rates, the application time was 1 ms; desensitization was elicited with 100 ms or longer applications. b | NETO2 (blue) slows both the deactivation and desensitization rates of GluK2 receptors5. c | Both deactivation and desensitization of heteromeric receptors composed of GluK2 and GluK5 subunits are slowed by both NETO1 (red) and NETO2 (blue)7, 8. Traces were originally derived from whole-cell and outside-out patch recordings as described in the respective reports and were adapted for the purposes of the illustration. The left panel of part a and right panel of part c are modified, with permission, from REF. 8 © (2011) Society for Neuroscience. The right panel of part a is modified, with permission, from REF. 6 © (2011) Society for Neuroscience. Part b is modified, with permission, from REF. 5 © (2009) Elsevier. The left panel of part c is modified, with permission, from REF. 7 © (2011) Macmillan Publishers Limited. All rights reserved.
Box 3 | Modulation of ionotropic glutamate receptor function by auxiliary subunits.
With the identification of neuropilin and tolloid-like (NETO) proteins as modulators of kainate receptor function, it appears that a growing number of proteins meet the criteria as auxiliary subunits for ionotropic glutamate receptors (iGluRs). The first glutamate receptor auxiliary subunit to be identified, stargazin, led to the discovery of a large family of transmembrane-associated regulatory proteins (TARPs)10. Subsequently, three additional families of proteins have been proposed to act as auxiliary subunits — the cornichon homologues (CNIH2 and CNIH3), cystine-knot AMPAR modulating protein of 44 kDa (CKAMP44)109, 110 and a recent addition, the distant TARP relative germ cell-specific gene 1-like protein (GSG1L)111, 112 (see the table). Elucidation of auxiliary subunit effects on AMPA receptor function has provided satisfying molecular explanations to anomalous aspects of their physiological behaviour and pharmacological sensitivity in brain10, and in many ways acts as an instructive template for current and future investigations into NETO protein effects on kainate receptor function. As such, we briefly highlight here a few features modified by the prototypical auxiliary subunit family, TARPs; this subject has also been reviewed recently in significantly more detail10.
TARPs modulate nearly every aspect of AMPA receptor behaviour, from receptor pharmacology to gating and trafficking. TARP co-assembly increases glutamate affinity113–116 in addition to increasing the efficacy of partial agonists such as kainite115, 116. The classically defined competitive antagonist CNQX instead acts as a partial agonist on AMPA receptors associated with the prototypical TARP stargazin; this effect has been attributed to a reduction in the energetic barrier between ligand binding-related conformational changes and channel gating117. TARP co-assembly can affect channel gating directly by altering AMPA receptor single-channel conductance and open probability115, 118–120. Channel kinetics are perhaps the most extensively characterized aspects of AMPA receptor function that are affected by co-assembly with TARPs; for example, both deactivation and desensitization are slowed in a receptor subunit- and TARP-specific manner84, 114–116, 118. Lastly, the trafficking of AMPA receptors is critically dependent on TARPs; their association occurs early during receptor biogenesis and promotes both forward trafficking115, 116, 121, 122 and synaptic localization through interactions with postsynaptic density 95 (REFS 123,124). Gene-targeting of mosaics of TARP isoforms conclusively showed that these auxiliary proteins are obligatory constituents of AMPA receptor complexes targeted for synapses96, 125.
This abbreviated survey of the large body of work on a single family of AMPA receptor auxiliary subunits, the TARPs, makes it clear that they modify nearly every aspect of AMPA receptor gating, pharmacology and neuronal localization, probably in concert with members of other auxiliary subunit families. It remains unclear whether NETO proteins control signalling through neuronal kainate receptors to an analogous degree, but even in these early days, it is clear that many parallels exist in their impact on receptor gating.
| Effect on glutamate receptors |
Kainate receptor auxiliary subunits |
AMPA receptor auxiliary subunits | |||
|---|---|---|---|---|---|
| NETO1 | NETO2 | TARPs | CNIH2, CNIH3 | CKAMP44 | |
| Pharmacology | |||||
| Agonist affinity | + | + | + | ? | + |
| Agonist efficacy | ? | + | + | No effect | + |
| Polyamine block | ? | ? | −− | −− | ? |
| Gating | |||||
| Popen | ? | ++ | Variable | No effect | ? |
| Open time | ? | ++ | + | ? | ? |
| Conductance | ? | No effect | + | + | ? |
| Peak amplitude | + | ++ | + | + | − |
| Deactivation rate | − | − | + | + | + |
| Desensitization rate | +/− | −− | + | + | − |
| Rate of recovery from desensitization | −− | − | +/− | No effect | + |
| Trafficking* | |||||
| Forward trafficking | ? | + | + | + | No effect |
| Synaptic targeting | − | ++ | ++ | Variable | + |
| EPSC decay rate | −− | − | − | Variable | ? |
+ indicates increases; ++ indicates strongly increases; − indicates decreases; −− indicates strongly decreases; +/− indicates that the effect varies depending on the receptor subunit or auxiliary protein; ? indicates unknown. Note that many effects of auxiliary subunits are receptor- and auxiliary subunit-dependent, which is not reflected by the qualitative assessment in the table. EPSC, excitatory postsynaptic current; Popen, channel open probability. NETO1, TARPs and CKAMP44 but not NETO2, CNIH2 and CNIH3 contain a PDZ ligand.
Whether NETO proteins promote plasma membrane expression of kainate receptors seems to be a contentious issue79. In Drosophila, a NETO orthologue was shown to be absolutely required for both neuromuscular synaptic development and iGluR clustering at that synapse80. Studies with mammalian recombinant receptors were less definitive; initially, studies in the X. laevis oocyte expression system suggested that neither NETO1 nor NETO2 influenced the membrane expression of GluK2a (an alternatively spliced variant of GluK2) subunitcontaining kainate receptors5, 7. However, the GluK2a subunit contains a strong forward trafficking signal that efficiently promotes membrane expression of homomeric assemblies of these kainate receptors81, 82. This makes it unclear whether expression of GluK2a proteins on the plasma membrane could be enhanced beyond the already-high levels driven by its C terminus trafficking signal. By contrast, NETO2 doubled the number of recombinant GluK1 receptors, which lack this forward trafficking motif, on the surface of transfected mammalian cells while increasing the peak current amplitudes sevenfold6. Thus, it is likely that both trafficking and channel opening probability were enhanced, and indeed the latter effect was observed in single-channel recordings from GluK2–NETO2 versus GluK2 receptors5. An even more profound effect on GluK1 membrane targeting was observed when the receptors were co-expressed with NETO2 in cultured hippocampal neurons: in addition to promoting localization to the plasma membrane, NETO2 redistributed GluK1-containing receptors to dendritic spines and sites of synaptic contact6. This effect was surprising, given that NETO1 but not NETO2 contains a PDZ ligand that could potentially bind to scaffolding proteins within the PSD. Whatever the underlying mechanism, the evidence suggests that the effects of NETO proteins on trafficking, like their modulation of receptor kinetics, can vary depending on kainate receptor subunit composition and incorporation of specific NETO protein isoforms.
NETO subunits shape synaptic currents
If NETO proteins are indeed auxiliary subunits for kainate receptors, then their presence should be required for normal kainate receptor-mediated signalling in the CNS. Both NETO1 and NETO2 are expressed in the CA3 pyramidal cell layer, although NETO1 expression predominates7, 9 (FIG. 3a). Thus, both isoforms might have been expected to contribute to synaptic kainate receptor function or localization. Two separate groups recently provided crucial confirmation of this notion by examining EPSCsKA at mossy fibre–CA3 pyramidal neuron synapses in Neto1 and Neto2 knockout mice (Neto1−/− mice and Neto2−/− mice, respectively)7, 9. Both groups found that constitutive Neto1−/− mice exhibit much faster mossy fibre EPSCKA decay kinetics; in the absence of NETO1, the EPSCKA decayed with a time course of 17–20 ms, whereas in wild-type mice this decay occurred over 50–70 ms7, 9 (FIG. 3b). Neto2−/− mice, by contrast, did not exhibit any alterations in amplitude or decay kinetics at this synapse, and ablation of both NETO isoforms did not have any effect on EPSCsKA beyond that observed in the Neto1−/− mice. What are the functional implications of these alterations in synaptic kainate receptor output? As mentioned above, the slow decay kinetics of mossy fibre EPSCsKA are thought to enhance synaptic integration and the likelihood of action potential initiation during repetitive bursts of input from the granule cells. Consequent to their faster decay rates, EPSCsKA in the absence of NETO1 show reduced temporal summation and charge transfer7, 9 during repetitive stimulation, resulting in attenuation in spiking fidelity (FIG. 3c) and an increase in spike jitter in the CA3 pyramidal neurons7. These kinetic data therefore provided longsought insight to the molecular mechanisms underlying the slow kinetics of EPSCsKA. The same cannot be said with respect to presynaptic kainate receptor function, however, because gene-targeting of Neto1 and Neto2 did not alter measures of release probability or synaptic plasticity7, 9 at mossy fibre synapses despite the well-characterized contributions by kainate receptors to short- and long-term plasticity48–50 (FIG. 3d). Thus, NETO1 is required to confer the biophysical properties thought to underlie the principle role of postsynaptic mossy fibre kainate receptors.
Figure 3. Gene-targeting of Neto1 alters synaptic kainate receptor function.
a | In situ hybridization demonstrates that neuropilin and tolloid-like 1 (NETO1) and NETO2 are expressed in the hippocampal region of adult mice. Neto1 mRNA is much more prominent in the pyramidal cell layers, especially in the CA3 region. NETO2 is present in scattered neurons in all regions. b | Idealized kainate receptor-mediated excitatory postsynaptic currents (EPSCsKA) from wild-type (black) and Neto1−/− mice demonstrate the much faster decay of the latter. c | Spike probability during a brief 3 Hz train of mossy fibre stimuli shows that CA3 pyramidal neurons are more likely to fire an action potential when kainate receptor currents are slowly decaying (wild-type; black) than in the faster gating Neto1−/− mice (red). d | Facilitation of mixed AMPA–kainate EPSCs by low frequencies of stimulation is similar in wild-type and Neto1−/− mice, which is consistent with the interpretation that presynaptic kainate receptor function is intact in the Neto1−/− mice. Part a is modified from the Allen Mouse Brain Atlas © (2012) Allen Institute for Brain Science. Parts b, c and d were modified, with permission, from REF. 7 © (2011) Macmillan Publishers Limited. All rights reserved. DG, dentate gyrus.
There was not perfect concordance between the two studies, however, on the potential role of NETO1 in determining the plasma membrane expression or synaptic content of kainate receptors. On the one hand, one group found that surface expression of GluK2 and GluK3 subunits or synaptic levels of GluK2, GluK3 and GluK5 were unchanged in the Neto knockout mice7. On the other hand, the other group noted a marked decrease in levels of GluK2, GluK3 and GluK5 within PSD fractions from Neto1−/− mice9 without any changes in the levels of AMPA or NMDA receptor proteins, suggesting that synaptic targeting of kainate receptors was selectively affected. Both studies noted a similar reduction in the amplitude of the EPSCKA relative to EPSCsAMPA 9 or EPSCsNMDA 7, which could be accounted for either by a reduction in receptor number or by a decrease in the probability of opening in response to synaptic release of glutamate (as there was no apparent alteration in presynaptic function). It remains difficult to directly reconcile the biochemical and physiological findings because at least two other likely components of postsynaptic mossy fibre kainate receptors are not detectable with the current set of antibodies (for example, GluK4 (REF. 47) and GluK2b83).
In summary, these knockout studies provided strong evidence that NETO1 contributes to postsynaptic kainate receptors at hippocampal mossy fibre synapses. Although these data provide validation of NETO1 as an auxiliary subunit, the data also raise yet more questions: most obviously, what role does NETO2 protein have in neuronal kainate receptor function and/or localization? This isoform is much more widely distributed in the CNS than NETO1 and is clearly present even in the termination zone for hippocampal mossy fibre afferents, the stratum lucidum9. Of course there are other aspects of kainate receptor function that were not examined and that could require NETO2: for example, metabotropic signalling or presynaptic modulation of GABA release. These questions remain to be explored.
Future directions: everything old is new
The discovery of the NETO proteins has initiated an exciting new era in kainate receptor research, which is analogous to the wealth of studies re-examining AMPA receptor function following the discovery of their auxiliary subunits. These range from structure–function studies to provide a mechanistic basis for functional modulation of kainate receptors by NETO proteins, to studies exploring how the auxiliary subunits differentially target kainate receptor signalling complexes to synapses and shape their diverse responses throughout the nervous system.
Structure–function studies: kainate receptor modulation
Understanding how co-assembly with each NETO protein modulates the kinetic properties and pharmacological profiles of kainate receptors made up of distinct combinations of subunits is an important initial goal. Is the bidirectional modulation of homomeric GluK1 desensitization by NETO1 and NETO2 the sole exception to the general rule that NETO proteins tend to slow channel kinetics? In addition, what is the stoichiometry of NETO protein co-assembly with kainate receptors? Is the ratio fixed or permissive to incorporation of a variable number of NETO subunits to potentially tune receptor properties in a graded manner, analogous to effects seen in AMPA receptors84, 85?
Co-assembly with auxiliary subunits typically has a profound impact on the pharmacological properties of their associated receptors10, 86. NETO1 and NETO2 have been shown to modify aspects of kainate receptor pharmacology, but the spectrum of compounds examined is quite limited (glutamate and kainate). How might NETO proteins affect agonist potencies and efficacies and antagonist activity? TARP association with AMPA receptors converts the classical competitive antagonist CNQX into a weak partial agonist — is this also the case for kainate receptor–NETO complexes? Might compounds exist that selectively target kainate receptors co-assembled with their auxiliary subunits? These would become very useful tools in dissecting out particular features of individual receptor populations, which have largely been unavailable18.
From a mechanistic point of view, it will be particularly interesting to determine how NETO incorporation differentially alters kainate receptor kinetics. The impact of NETO on receptor gating clearly depends on both the NETO isoform and the particular kainate receptor subunit composition. What domains and discrete elements in the NETO proteins or receptor subunits confer this selectivity in effect? For that matter, why do NETO proteins selectively associate with kainate receptors and not AMPA receptors? It seems reasonable to postulate that NETO modulation occurs through interactions with the ligand binding domain, perhaps by altering stability of the dimer interface that is known to be key to receptor desensitization87, 88, but other regions that couple ligand binding to receptor gating could be candidate sites of contact as well89, 90. Resolution of these mechanistic questions will probably come through the effective combination of structural and mutagenic approaches that have provided such a wealth of insight into the molecular basis for biophysical function of iGluRs.
Presynaptic function
Kainate receptors are localized to presynaptic terminals at many sites in the CNS, in which they function as autoreceptors to regulate levels of neurotransmitter release (reviewed in REFS 1,91,92). To what degree might NETO proteins contribute to this function? At mossy fibre synapses, the answer appears to be ‘not much’, given that knockout mice lacking one or both NETO proteins had normal frequency-dependent facilitation of presynaptic release7, 9. Although not formally tested, these results suggest that kainate receptor contributions to facilitation were intact in these mice, and therefore that NETO proteins did not seem to be necessary for either presynaptic localization or function. This observation was somewhat surprising, at least for NETO1, given the prominent expression of this auxiliary subunit isoform in the dentate gyrus. Presynaptic autoreceptor function is, however, challenging to assess directly, and the site and mechanism of action of axonal and terminal kainate receptors have been difficult to pin down unambiguously38, 46, 48, 50, 93–95. Furthermore, multiple distinct kainate receptor populations probably exist in the axonal and presynaptic domains50 and confer a subtlety to presynaptic kainate receptor function that was not clearly sampled in the recent studies of NETO knockout mice. A more exhaustive examination at this and other synapses will be required to rigorously explore their contributions to presynaptic function.
Auxiliary subunits and receptor trafficking
NETO2 targets GluK1 receptors to synapses in cultured hippocampal neurons, which suggests that determinants within the NETO2 protein are able to mediate this effect, as the short C terminus of the GluK1–2a splice variant lacks any known trafficking or protein interaction domains6. The intracellular domains of both NETO proteins are highly divergent and therefore could use distinct cellular mechanisms for their localization. Identification of NETO-interacting proteins may provide clues as to how their function is regulated on a subcellular level. In addition to exploring the role of NETO-interacting proteins, it will be of interest to explore whether and how NETO proteins are regulated by post-translational modifications, which might be crucial for activity-dependent modulation of channel function or localization. How post-translational modification participates in the trafficking, synaptic targeting and regulated expression of other members of the glutamate receptor family is much better understood and has contributed significantly towards explaining many aspects of receptor signalling and behaviour. Studies of AMPA receptor auxiliary subunits have pointed to their role in not just synaptic targeting but also in dynamic regulation of their delivery and localization96–98. It remains an important question to determine whether kainate receptor auxiliary subunits might have similar roles.
Receptor compartmentalization
Kainate receptor distribution displays cell-type-specific expression of individual subunits, and assembled signalling complexes exhibit highly compartmentalized distributions. In CA3 pyramidal cells, for example, kainate receptors are exclusively localized to proximal dendrites receiving input from mossy fibres and excluded from distal synapses on both apical and basal dendrites, an observation not seen with other glutamate receptors. How kainate receptors achieve this selective localization is currently unknown and a major question in the field. Might the co-assembly of NETO proteins, which are both strongly localized to the CA3 region9, 71, specifically target receptors to mossy fibre synapses? This question was not examined in recent studies. Compartmentalization of kainate receptors is also observed in interneurons within the CA1 region of the hippocampus, in which they are found postsynaptically at excitatory synapses from Schaffer collateral inputs originating from CA3 pyramidal cells32, 99. Synapses in these interneurons can contain only kainate receptors, only AMPA receptors or a mixture of both types of receptors99. This selective distribution is not restricted to interneurons within the hippocampus. Off bipolar cells within the retina possess the ability to segregate AMPA and kainate receptors to distinct sites of contact from cone photoreceptor cells, in which they take advantage of the unique features of both receptor families to filter and process incoming visual information100–102. Postsynaptic kainate receptor-mediated responses in both cell types are not explained by unique subunit combinations, suggesting that NETO co-assembly may modulate both the unique functional properties and compartmentalization observed in these neurons. Curiously, a soluble isoform of NETO1 is expressed exclusively in the retina70, and it would be interesting to test whether this affects aspects of kainate receptor signalling in ways analogous to soluble CUB domain-containing proteins in invertebrates103.
Are NETOs alone?
The existence of auxiliary subunits for ligand- and voltage-gated ion channels has explained a variety of anomalous points of comparison between recombinant and native biophysical properties and pharmacological sensitivities. The NETO proteins are perhaps the newest members of this exclusive club based on their ability to profoundly modulate function and localization of kainate receptors. The highly structured interaction domains in the NETO proteins suggest that optimism is warranted with respect to resolution of a clearer physical model for NETO modulation of kainate receptor function. Our understanding of the spectrum of NETO contributions to kainate receptor function in the CNS is still in its infancy, consisting entirely of recordings from a single synapse in the hippocampus. Will they turn out to be all-important partners to kainate receptors, as TARPs are to AMPA receptors, for example? How do NETO proteins alter the unexplained metabotropic function of kainate receptors? The answers await further analysis of the complex and subtle rules of engagement of these newly discovered dancing partners.
Acknowledgements
We thank A. Contractor (Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA) for contributing original data to this article and C. Mulle (University of Bordeaux II, Bordeaux, France) for sharing data in advance of its publication. This work was supported by grants R01 NS44322 and R01 NS071952 from the US National Institute of Neurological Disorders and Stroke to G.T.S.
Glossary
- Recombinant receptors
Receptors produced subsequent to the introduction into cells of complementary DNA-encoding component receptor subunits.
- Desensitization
A decrease in a biological response in the continued presence of or due to repeated exposure of a receptor to an agonist.
- Excitatory postsynaptic currents
(EPSCs). Membrane currents arising from activation of excitatory (depolarizing) postsynaptic ligand-gated ion channels by a physiological stimulus, typically measured when membrane potential is held constant in voltage clamp experiments.
- Hippocampal mossy fibre
Axons arising from dentate gyrus granule cells that form bouton-type synapses on proximal dendrites of CA3 pyramidal neurons and filopodial synapses on interneurons in the stratum lucidum.
- Short-term potentiation
A predominantly presynaptic form of plasticity in which synaptic transmission is transiently enhanced, principally due to the engagement of mechanisms that enhance vesicle release probability.
- Chaperone proteins
Proteins that facilitate or are necessary for trafficking of cargo proteins through the secretory pathway in cells, which can include targeting receptors to their pre- or postsynaptic sites of action.
- Pertussis toxin
The causative agent of whooping cough, which causes the persistent activation of Gi proteins by catalysing ADP-ribosylation of the Gαi subunit.
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor.
- Deactivation
A reduction in a receptor-mediated biological response subsequent to the removal of agonist.
- Open probability
The probability that an ion channel protein is in the open state under a given experimental condition.
- Open time
The average time an ion channel dwells in a conducting state during a single activation event.
- Single-channel conductance
An expression of the rate of ion flow through an ion channel during a single activation event; it is derived from Ohm’s Law as a proportionality constant equal to the inverse of resistance.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Allen Mouse Brain Atlas: http://mouse.brain-map.org
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chittajallu R, et al. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- 3.Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- 4. Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. This study, with reference 3, first described postsynaptic currents mediated by kainate receptors, which were found at hippocampal mossy fibre–CA3 pyramidal cell synapses. The observed currents exhibited remarkably slow kinetics that were incongruous with the gating behaviour of recombinant kainate receptors.
- 5. Zhang W, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. This study was the first to identify the NETO proteins as potential auxiliary subunits that had a profound impact on kainate receptor functional properties.
- 6. Copits BA, Robbins JS, Frausto S, Swanson GT. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (Neto) proteins. J. Neurosci. 2011;31:7334–7340. doi: 10.1523/JNEUROSCI.0100-11.2011. This study was the first to report a role for NETO proteins in the trafficking and synaptic targeting of kainate receptors. Additionally, NETO1 and NETO2 co-assembly was found to have a bidirectional effect on GluK1 receptor function.
- 7.Straub C, et al. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nature Neurosci. 2011;14:866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub C, Zhang W, Howe JR. Neto2 modulation of kainate receptors with different subunit compositions. J. Neurosci. 2011;31:8078–8082. doi: 10.1523/JNEUROSCI.0024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang M, et al. Neto1 Is an auxiliary subunit of native synaptic kainate receptors. J. Neurosci. 2011;31:10009–10018. doi: 10.1523/JNEUROSCI.6617-10.2011. This report and the report in reference 7 demonstrated that NETO1 confers slow gating kinetics on postsynaptic kainate receptors at the hippocampal mossy fibre synapse, thereby providing key evidence that it is an auxiliary protein for kainate receptors.
- 10.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol. Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straub C, Tomita S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr. Opin. Neurobiol. 2012;22:488–495. doi: 10.1016/j.conb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br. J. Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 16. Lerma J, Paternain AV, Naranjo JR, Mellstrom B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc. Natl Acad. Sci. USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. These authors, for the first time, recorded pharmacologically isolated kainate receptor-mediated currents from neurons derived from the CNS using a newly developed non-competitive AMPA receptor antagonist, thereby setting the stage for subsequent detection of postsynaptic kainate receptors.
- 17.Traynelis SF, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2008;56:90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys. J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J. Physiol. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson GT, Heinemann SF. Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J. Physiol. 1998;513:639–646. doi: 10.1111/j.1469-7793.1998.639ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowie D, Lange GD. Functional stoichiometry of glutamate receptor desensitization. J. Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paternain AV, Cohen A, Stern-Bach Y, Lerma J. A role for extracellular Na+ in the channel gating of native and recombinant kainate receptors. J. Neurosci. 2003;23:8641–8648. doi: 10.1523/JNEUROSCI.23-25-08641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong AY, Fay AM, Bowie D. External ions are coactivators of kainate receptors. J. Neurosci. 2006;26:5750–5755. doi: 10.1523/JNEUROSCI.0301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron. 2007;53:829–841. doi: 10.1016/j.neuron.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Plested AJ, Vijayan R, Biggin PC, Mayer ML. Molecular basis of kainate receptor modulation by sodium. Neuron. 2008;58:720–735. doi: 10.1016/j.neuron.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowie D. Ion-dependent gating of kainate receptors. J. Physiol. 2010;588:67–81. doi: 10.1113/jphysiol.2009.178863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro PS, et al. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex. 2012 Feb;17 doi: 10.1093/cercor/bhs022. [DOI] [PubMed] [Google Scholar]
- 30. Frerking M, Ohliger-Frerking P. AMPA receptors and kainate receptors encode different features of afferent activity. J. Neurosci. 2002;22:7434–7443. doi: 10.1523/JNEUROSCI.22-17-07434.2002. This report laid experimental and theoretical groundwork for the now prevalent concept that postsynaptic AMPA and kainate receptors have distinct roles in synaptic integration as a result of their divergent gating kinetics.
- 31.Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nature Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- 32.Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nature Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- 33.Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- 34.Li P, et al. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 35.Petralia RS, Wang YX, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J. Comp. Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- 36.Min MY, Rusakov DA, Kullmann DM. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: role of glutamate diffusion. Neuron. 1998;21:561–570. doi: 10.1016/s0896-6273(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 37.Kidd FL, Isaac JT. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J. Neurophysiol. 2001;86:1139–1148. doi: 10.1152/jn.2001.86.3.1139. [DOI] [PubMed] [Google Scholar]
- 38.Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J. Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herb A, et al. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- 40.Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 41.Swanson GT, Gereau RW, Green T, Heinemann SF. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron. 1997;19:913–926. doi: 10.1016/s0896-6273(00)80972-1. [DOI] [PubMed] [Google Scholar]
- 42.Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J. Neurosci. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J. Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barberis A, Sachidhanandam S, Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J. Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mulle C, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. This was the first study to use gene-targeting to investigate neuronal kainate receptor function. The authors found that the GluK2 (GluR6) subunit was an essential constituent of postsynaptic kainate receptors in the CA3 region of hippocampus.
- 46.Contractor A, et al. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J. Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes HB, et al. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 49.Contractor A, Swanson GT, Heinemann SF. Kainate receptors are involved in short and long term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 50.Pinheiro PS, et al. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc. Natl Acad. Sci. USA. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cossart R, et al. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron. 2001;29:497–508. doi: 10.1016/s0896-6273(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 52.Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36:475–482. doi: 10.1016/s0896-6273(02)01008-5. [DOI] [PubMed] [Google Scholar]
- 53.Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE. Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J. Neurosci. 2004;24:103–111. doi: 10.1523/JNEUROSCI.4473-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodríguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. This study introduces the concept of metabotropic signalling by kainate receptors acting as heterosynaptic modulators of GABA release in the CA1 region.
- 55. Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. These authors demonstrated that kainate receptors regulate intrinsic conductances in CA1 pyramidal neurons through G protein-mediated signalling pathways.
- 56.Melyan Z, Lancaster B, Wheal HV. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J. Neurosci. 2004;24:4530–4534. doi: 10.1523/JNEUROSCI.5356-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisahn A, Heinemann SF, McBain CJ. The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurones. J. Physiol. 2005;562:199–203. doi: 10.1113/jphysiol.2004.077412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J. Neurosci. 2005;25:11710–11718. doi: 10.1523/JNEUROSCI.4041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coussen F. Molecular determinants of kainate receptor trafficking. Neuroscience. 2009;158:25–35. doi: 10.1016/j.neuroscience.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 60.Garcia EP, et al. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 61.Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. Allosteric regulation and spatial distribution of kainate receptors bound to ancillary proteins. J. Physiol. 2003;547:373–385. doi: 10.1113/jphysiol.2002.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirbec H, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selak S, et al. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron. 2009;63:357–371. doi: 10.1016/j.neuron.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Coussen F, et al. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J. Neurosci. 2002;22:6426–6436. doi: 10.1523/JNEUROSCI.22-15-06426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vivithanaporn P, Yan S, Swanson GT. Intracellular trafficking of KA2 kainate receptors mediated by interactions with coatomer protein complex I (COPI) and 14-3-3 chaperone systems. J. Biol. Chem. 2006;281:15475–15484. doi: 10.1074/jbc.M512098200. [DOI] [PubMed] [Google Scholar]
- 66.Salinas GD, et al. Actinfilin is a CUL3 substrate adaptor, linking GluR6 kainate receptor subunits to the ubiquitin-proteasome pathway. J. Biol. Chem. 2006;281:40164–40173. doi: 10.1074/jbc.M608194200. [DOI] [PubMed] [Google Scholar]
- 67.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laezza F, et al. KRIP6: a novel BTB/kelch protein regulating function of kainate receptors. Mol. Cell. Neurosci. 2007;34:539–550. doi: 10.1016/j.mcn.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng D, et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stöhr H, Berger C, Fröhlich S, Weber BH. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene. 2002;286:223–231. doi: 10.1016/s0378-1119(02)00438-9. [DOI] [PubMed] [Google Scholar]
- 71.Michishita M, et al. A novel gene Btcl1, encoding CUB and LDLa domains is expressed in restricted areas of mouse brain. Biochem. Biophys. Res. Commun. 2003;306:680–686. doi: 10.1016/s0006-291x(03)01035-0. [DOI] [PubMed] [Google Scholar]
- 72.Michishita M, et al. Expression of Btcl2, a novel member of Btcl gene family, during development of the central nervous system. Dev. Brain. Res. 2004;153:135–142. doi: 10.1016/j.devbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Bork P, Beckmann G. The CUB domain: a widespread module in developmentally regulated proteins. J. Mol. Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- 74.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 75.Kolodkin AL, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 76.Gally C, Eimer S, Richmond JE, Bessereau JL. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature. 2004;431:578–582. doi: 10.1038/nature02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. This study reports the first CUB domain-containing auxiliary subunit for iGluRs, SOL-1, using a forward genetic screen in C. elegans.
- 78.Zheng Y, et al. SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2006;103:1100–1105. doi: 10.1073/pnas.0504612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan D, Tomita S. Defined criteria for auxiliary subunits of glutamate receptors. J. Physiol. 2012;590:21–31. doi: 10.1113/jphysiol.2011.213868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim YJ, Bao H, Bonanno L, Zhang B, Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev. 2012;26:974–987. doi: 10.1101/gad.185165.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaskolski F, et al. Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J. Neurosci. 2004;24:2506–2515. doi: 10.1523/JNEUROSCI.5116-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan S, et al. A C-terminal determinant of GluR6 kainate receptor trafficking. J. Neurosci. 2004;24:679–691. doi: 10.1523/JNEUROSCI.4985-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coussen F, et al. Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron. 2005;47:555–566. doi: 10.1016/j.neuron.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 84.Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gill MB, et al. Cornichon-2 modulates AMPA receptor–transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology. J. Neurosci. 2011;31:6928–6938. doi: 10.1523/JNEUROSCI.6271-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010;33:241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 88.Sun Y, et al. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 89.Yelshansky MV, Sobolevsky AI, Jatzke C, Wollmuth LP. Block of AMPA receptor desensitization by a point mutation outside the ligand-binding domain. J. Neurosci. 2004;24:4728–4736. doi: 10.1523/JNEUROSCI.0757-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vivithanaporn P, Lash LL, Marszalec B, Swanson GT. Critical roles for the M3–S2 transduction linker domain in kainate receptor assembly and postassembly trafficking. J. Neurosci. 2007;27:10423–10433. doi: 10.1523/JNEUROSCI.2674-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lerma J. Kainate receptor physiology. Curr. Opin. Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nature Rev. Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 93.Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J. Physiol. 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lauri SE, et al. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- 95.Kwon HB, Castillo PE. Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron. 2008;60:1082–1094. doi: 10.1016/j.neuron.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rouach N, et al. TARP γ-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nature Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 97.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 98.Opazo P, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Cossart R, et al. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 100.DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- 101.DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 102.DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 103.Gendrel M, Rapti G, Richmond JE, Bessereau JL. A secreted complement-control-related protein ensures acetylcholine receptor clustering. Nature. 2009;461:992–996. doi: 10.1038/nature08430. [DOI] [PubMed] [Google Scholar]
- 104.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Ann. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 105.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 107.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 108.Walker CS, et al. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc. Natl Acad. Sci. USA. 2006;103:10787–10792. doi: 10.1073/pnas.0604520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwenk J, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 110.von Engelhardt J, et al. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- 111.Schwenk J, et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 112.Shanks NF, et al. Differences in AMPA and kainate receptor interactomes faciliate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep. 2012;1:590–598. doi: 10.1016/j.celrep.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamazaki M, et al. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci. Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 114.Priel A, et al. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J. Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomita S, et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 116.Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J. Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- 118.Cho C-H, St-Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 119.Soto D, et al. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, γ-5. Nature Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jackson AC, et al. Probing TARP modulation of AMPA receptor conductance with polyamine toxins. J. Neurosci. 2011;31:7511–7520. doi: 10.1523/JNEUROSCI.6688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomita S, et al. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bedoukian MA, Weeks AM, Partin KM. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J. Biol. Chem. 2006;281:23908–23921. doi: 10.1074/jbc.M600679200. [DOI] [PubMed] [Google Scholar]
- 123.Chen L, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 124.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl Acad. Sci. USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Menuz K, O’Brien JL, Karmizadegan S, Bredt DS, Nicoll RA. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. 2008;28:8740–8746. doi: 10.1523/JNEUROSCI.1319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rebola N, Sachidhanandam S, Perrais D, Cunha RA, Mulle C. Short-term plasticity of kainate receptor-mediated EPSCs induced by NMDA receptors at hippocampal mossy fiber synapses. J. Neurosci. 2007;27:3987–3993. doi: 10.1523/JNEUROSCI.5182-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]