Abstract
Rapamycin is an immunosuppressive agent routinely used in organ transplantation, but also paradoxically, exerts antiviral and anti-tumor activities. Pathogen-specific memory CD8+ T cell (TCD8) responses were recently found to be augmented by rapamycin. However, whether rapamycin influences the magnitude and quality of anticancer TCD8 responses is unknown. Importantly, how rapamycin may regulate simultaneous virus/tumor-specific and alloreactive TCD8 in the same host remains unexplored. To answer these questions, we primed wild-type mice with allogeneic cells concomitantly expressing simian virus 40 large tumor antigen (T Ag), a viral oncoprotein with well-defined epitopes. Rapamycin selectively enhanced the cross-priming of TCD8 specific for T Ag’s most immunodominant epitope called site IV but not TCD8 alloreactivity. Rapamycin-treated mice also had a high percentage of splenic CD127highKLRG1low TCD8 as well as an increased frequency of site IV-specific T cells long after the peak of their primary response. When site IV was presented as a cytosolic minigene encoded by a recombinant vaccinia virus, rapamycin failed to boost the site IV-specific response. Therefore, the nature and presentation mode of antigen determine the susceptibility to the adjuvant effect of rapamycin. Our findings reveal the unexpected benefit of rapamycin treatment in recipients of allografts co-expressing tumor/viral Ags.
Keywords: Rapamycin, mTOR, CD8+ T cells, alloreactivity, anti-tumor response, memory
Introduction
Allograft rejection by immunological mechanisms constitutes a formidable obstacle to life-saving organ transplantation. Initially discovered as an anti-fungal macrolide produced by Streptomyces hygroscopicus, rapamycin is an immunosuppressive agent commonly used in the clinic to hamper alloaggressive T cells in renal graft recipients (1). It potently and specifically inhibits the mammalian target of rapamycin (mTOR), an intracellular serine/threonine protein kinase that controls cellular metabolism, growth and survival. Various aspects of innate and adaptive immune responses are modulated by mTOR (2). The inhibition of the mTOR signaling pathway by rapamycin leads to altered T cell trafficking (3), attenuated effector T cell proliferation and enhanced regulatory T cell function (4,5), all of which likely contribute to rapamycin-induced immunosuppression.
Recent studies have revealed that rapamycin surprisingly improves, rather than weakens, memory CD8+ T cell (TCD8) responses to lymphocytic choriomeningitis virus (LCMV) in mice (6) and vaccinia virus (VV) in rhesus macaques (6,7). However, several important questions remain regarding TCD8 immunomodulation by rapamycin. First, it is not clear whether TCD8 specific for tumor antigens (Ags) are controlled by mTOR. This is particularly important given the increased risk of malignancy in allograft recipients. Second, to what degree rapamycin treatment influences TCD8 cross-priming is unknown. Cross-priming is spearheaded by professional Ag-presenting cells (pAPCs), particularly dendritic cells (DCs), which acquire antigenic materials from client cells (e.g., an allogeneic graft cell) that are incapable of activating naïve TCD8 on their own (8). Cross-priming is a robust pathway for inducing TCD8 responses to tumors of non-hematopoietic origin and to viruses that paralyze the MHC class I pathway in infected host cells. Although Ags displayed by allografted tissues may trigger TCD8 cross-priming, TCD8 alloreactivity may also result from direct priming. Third, whether rapamycin affects the epitope breadth of TCD8 responses is not understood. Of thousands of potentially immunogenic peptides harbored by complex Ags, only a handful elicit detectable TCD8 responses of varying magnitude, thus creating an immunodominance hierarchy among Ag-specific TCD8 clones (9). Immunodominance may influence the effectiveness of TCD8 responses to tumors, pathogens and transplants. Last, but certainly not least, it is not known how rapamycin may regulate concurrent TCD8 responses mounted towards allografts and other Ags in the same host at the same time. This is a relevant question in light of the clinical facts that: 1) current organ deficit may justify the usage of allografts prepared from “high-risk” kidneys containing tumor masses for recipients with limited life expectancy (10), which could introduce tumor Ags to the recipients’ immune system; 2) donor-derived infections with a variety of microbes (e.g., cytomegalovirus and BK polyoma virus) still occur with significant frequency (11); 3) community-acquired and nosocomial infections or reactivation of endogenous latent viruses are common in immunocompromised allograft recipients.
To address all the above questions in a non-transgenic, physiologically relevant setting, we primed wild-type mice with allogeneic kidney epithelial cells expressing a clinically relevant tumor Ag, the simian virus 40 (SV40) large tumor Ag (T Ag), which is in fact homologous to the BK virus T Ag detected in human kidneys. We demonstrate that rapamycin selectively improves the TCD8 response elicited by cross-priming against the most immunodominant epitope of T Ag (site IV) while not affecting or slightly attenuating alloreactive TCD8 present in the same host. In addition, rapamycin failed to boost the TCD8 response to site IV in mice infected with a recombinant vaccinia virus (rVV) expressing site IV. Therefore, the mode of TCD8 priming and the immunodominance status of targeted epitopes determine susceptibility to the immunostimulatory effect of rapamycin on TCD8. Our findings have clear clinical implications in allotransplantation and in therapeutic vaccine design.
Materials and Methods
Mice
Adult female C57BL/6 (B6)(H-2b) mice were purchased from Charles River Canada Inc. (St. Constant, QC), housed at the University of Western Ontario animal care facility under specific pathogen-free conditions, and cared for in accordance with institutional and national guidelines.
Cell lines
The SV40-transformed cell lines KD2SV (H-2d) and C57SV (H-2b) were grown in Dulbecco modified Eagle medium supplemented with 5% fetal bovine serum (FBS). The mouse mastocytoma cell line P815 (H-2d) was maintained in complete RPMI 1640 medium containing 10% FBS, nonessential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate and 50 μM 2-mercaptoethanol.
Immunization and rapamycin treatment
Age- and gender-matched mice received daily intraperitoneal (i.p.) injections of freshly prepared rapamycin (LC Laboratories, Woburn, MA) at 1.5 μg/dose in PBS or of vehicle (PBS containing Phosal 50 PG and Tween 80). This regimen provides a blood rapamycin concentration of ~5–20 ng/ml (6), which is consistent with its clinical dosing in humans. Treatment with rapamycin or vehicle started one day prior to i.p. immunization with 2×107 allogeneic KD2SV cells or 5×106 plaque-forming units of a rVV expressing the T Ag’s immunodominant peptide site IV (rVV-IV) and ended one day before the animals were euthanized.
Intracellular cytokine staining (ICS) and cytofluorimetric analyses
Unless otherwise indicated, mice were euthanized 9 or 7 days after immunization with KD2SV cells or rVV-IV, respectively, time points at which corresponding primary TCD8 responses reach their peak (12). Erythrocyte-depleted splonocytes and peritoneal exudate cells were then stimulated ex vivo, as appropriate, with C57SV cells, KD2SV cells, P815 cells or the following synthetic peptides corresponding to T Ag- and VV-derived epitopes (12,13): T Ag peptides: SAINNYAQKL (site I), CKGVNKEYL (site II/III), VVYDFLKC (site IV), QGINNLDNL (site V); VV peptides: TSYKFESV (B8R20), AAFEFINSL (A47L138), YSLPNAGDVI (K3L6), YAPVSPIVI (A42R88), VSLDYINTM (A19L47). An H-2b-restricted peptide derived from HSV-1, gB498 (SSIEFARL), served as irrelevant control. All the above peptides were >95% pure and generously provided by Drs. Jonathan Yewdell and Jack Bennink (National Institutes of Health). All peptides were used at a 500 nM final concentration. After 2-hour incubation at 37°C, brefeldin A was added at 10 μg/ml and cultures were continued for an additional 3 hours. Cells were then washed, stained for surface CD8α, CD127 (IL-7Rα), killer cell lectin-like receptor G1 (KLRG1), fixed and permeablized to enable staining for intracellular interferon (IFN)-γ and Bcl-2. All fluorochrome-labeled Abs were from eBioscience except for anti-KLRG1-FITC (Southern Biotech, Birmingham, AL) and anti-Bcl-2-FITC (BD Pharmingen). Isotype controls were purchased from eBioscience or BD Pharmingen. MHC class I tetramers were prepared and used as we previously described (14). A BD FACSCanto II flow cytometer and FlowJo software (Tree Star, Ashland, OR) were used for data acquisition and analysis. The percentage of IFN-γ+ or tetramer+ cells was determined after live gating on CD8+ events and Ag-specific TCD8 were enumerated accordingly.
Statistical analysis
Statistical comparisons were performed using Student’s t-test with the aid of GraphPad Prism software. *, ** and *** denote p<0.05, p<0.01 and p<0.001, respectively.
Results and Discussion
mTOR regulation of concomitant tumor-specific and alloreactive TCD8 responses
It was recently demonstrated that anti-pathogen TCD8 can be augmented by rapamycin treatment (6,7,15). An important and elegant study by Ferrer et al. found that rapamycin enhances the responsiveness of adoptively transferred ovalbumin-specific TCD8 in mice infected with recombinant Listeria monocytogenes encoding ovalbumin but not in recipients of an ovalbumin-expressing skin allograft (15). The above study examined transgenic TCD8 responses in parallel, not in the same host. Therefore, we set to explore the effect of rapamycin on simultaneously ongoing TCD8 responses against tumor/viral Ags and alloantigens within the same wild-type animal. To do so, we injected B6 mice (H-2b) with KD2SV cells (H-2d) that are transformed with SV40 and, as such, express T Ag, a viral oncoprotein with well characterized TCD8 epitopes (14). TCD8 responses in this model mimic the “real life” situation because they are elicited against two types of clinically relevant Ags (i.e., alloantigens and T Ag) expressed by kidney epithelial cells (a known target of T cells in renal allograft recipients) in wild-type animals harboring a natural T cell repertoire.
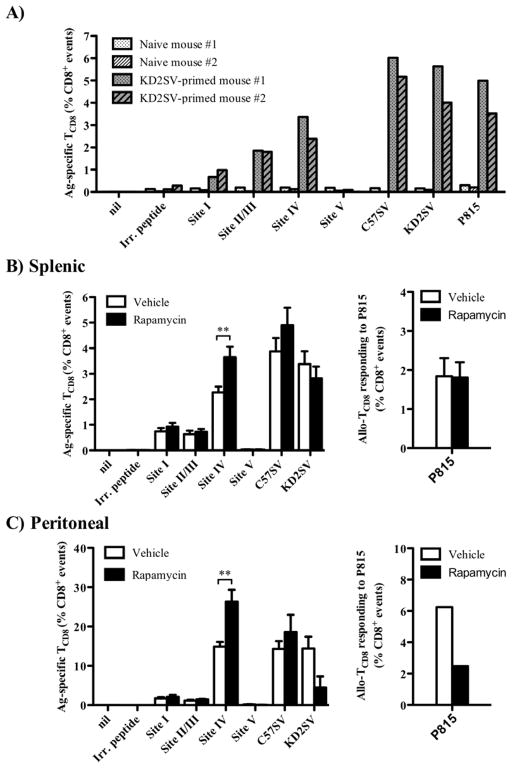
We first confirmed that in our model, T Ag-specific and alloreactive TCD8 responses require in vivo priming with KD2SV cells and are not detectable in naïve animals (Fig. 1A). Treatment with rapamycin increased the frequency of both the splenic and peritoneal TCD8 specific for site IV, the most immunodominant epitope of T Ag (14), as judged by intracellular staining for IFN-γ (Fig. 1B, 1C). Peritoneal and splenic TCD8 represent local and systemic responders to site IV, respectively (8,12). There was also a trend for an enhanced TCD8 response to C57SV cells, T Ag+ fibroblastic cells of B6 origin, when they were used in lieu of T Ag-derived peptides for ex vivo TCD8 restimulation. We found a similar increase in both the absolute number and mean fluorescence intensity (MFI) of site IV-specific IFN-γ+ TCD8 in the spleens of rapamycin-treated animals (Fig. 2A, 2B). Contrary to T Ag-specific TCD8, the frequency of alloreactive TCD8, defined by their ex vivo responsiveness to KD2SV cells, was unaltered or decreased to varying degrees upon rapamycin treatment (Fig. 1). This decrease was most pronounced within the peritoneal cavity (Fig. 1C). This response is of allogeneic nature and independent of T Ag expression by KD2SV cells because these cells express H-2d allomorphs and cannot be directly recognized by T Ag-specific TCD8 that are H-2b-restricted. The above notion is supported by our observation that rapamycin treatment of KD2SV-primed mice failed to increase the frequency of alloreactive cells restimulated with P815 cells, a T Ag− H-2d+ cell line (Fig. 1B, 1C).
Figure 1.
Treatment with rapamycin increases the frequency of functional TCD8 specific for the T Ag immunodominant epitope (site IV), but not that of alloreactive TCD8. To confirm the requirement for in vivo priming in the generation of T Ag-specific and alloreactive TCD8 responses, splenocytes from naïve B6 mice or B6 mice immunized with allogeneic (H-2d) T Ag+ KD2SV cells were restimulated ex vivo with T Ag-derived peptides (sites I, II/III, IV or V), syngeneic (H-2b) T Ag+ C57SV cells, allogeneic KD2SV cells, or allogeneic P815 cells used at 2 × 105 cells/well. The frequency of cognate TCD8 was calculated as the percentage of IFN-γ+ cells after live gating on CD8+ events. Individual mouse data are shown (A). To assess the effect of rapamycin, splenocytes (B) and peritoneal exudate cells (C) from vehicle- and rapamycin-treated B6 mice that were immunized with KD2SV cells were restimulated ex vivo with T Ag-derived peptides, C57SV, KD2SV or P815 cells. The frequency of cognate TCD8 was determined as described above. Data are shown as mean ± SEM obtained from 19 mice/group pooled from 5 independent experiments except in the case of the TCD8 response to P815 cells that was assessed in two experiments.
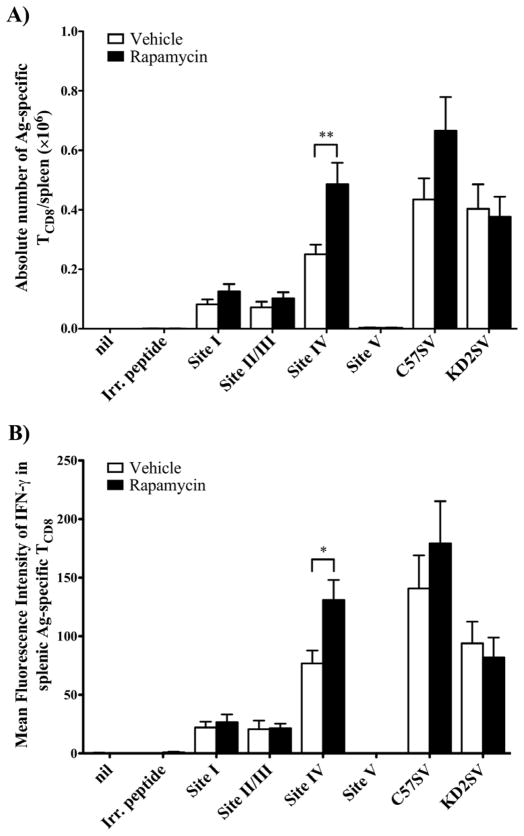
Figure 2.
Treatment with rapamycin increases the absolute number and mean IFN-γ production (on a per cell basis) of splenic site IV-specific TCD8, but not those of alloreactive TCD8. Splenocytes from vehicle- and rapamycin-treated B6 mice that were immunized with KD2SV cells were restimulated ex vivo with peptides corresponding to T Ag epitopes (sites I, II/III, IV or V), syngeneic T Ag+ C57SV cells, or allogeneic KD2SV cells. The frequency of cognate IFN-γ+ TCD8 was used to enumerate the absolute number of these cells within each spleen (A), and their MFI for IFN-γ was also determined using FlowJo software (B). Data are shown as mean ± SEM obtained from 19 mice/group pooled from 5 independent experiments.
Our finding that site IV-specific TCD8 in rapamycin-treated mice produce more IFN-γ on a per cell basis – hence their higher MFI – indicates that rapamycin improves the functional fitness of these TCD8. This is consistent with our unpublished observation that treatment with rapamycin also amplifies cytotoxic responses of cross-primed, T Ag-specific TCD8 (data not shown).
Previous studies have documented the positive effect of rapamycin on memory but not primary TCD8 responses. Rapamycin treatment reportedly failed to increase LCMV-specific TCD8 numbers at the peak of their primary response (6). In contrast, the primary TCD8 response to VV in rhesus macaques and that to a heat shock protein-based vaccine in mice were boosted by the inhibition of mTOR (7,16). These discrepancies may have stemmed from different readouts used in these studies. The former study enumerated LCMV-specific TCD8 by tetramer staining, whereas the latter two studies used functional assays similar to ours. In fact, when we detected site IV-specific TCD8 by tetramer staining in a head-to-head comparison with ICS, we did not find any difference between mice receiving rapamycin or vehicle by tetramer staining (supplemental Fig. 1).
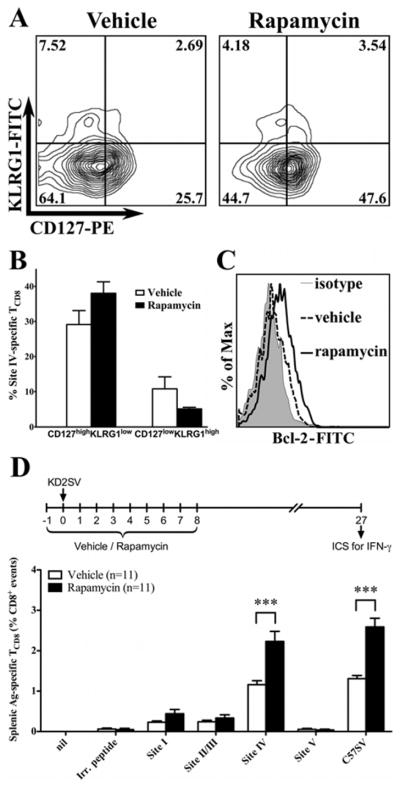
It was of interest to determine whether rapamycin affects the quality of primary T Ag-specific TCD8 and their progression to a memory state. We found that the site IV-specific TCD8 pool in rapamycin-treated animals had a higher proportion of CD127highKLRG1low cells and a lower proportion of CD127lowKLRG1high cells, which are considered memory TCD8 precursors and short-lived effectors, respectively (6)(Fig. 3A, 3B). Rapamycin treatment also increased the expression of the pro-survival protein Bcl-2 in site IV-specific TCD8 at the peak of their primary response (Fig. 3C). Importantly, treatment with rapamycin during the initial priming phase (i.e., during the first 9 days) led to a higher frequency of site IV-specific TCD8 detected at a later time point (day 27)(Fig. 3D). In a different setting that simulates clinical conditions requiring continuous treatment, daily administration of rapamycin up until day 27 resulted in a higher proportion of T Ag-specific (but not alloreactive) TCD8 (supplemental Fig. 2). These results collectively show that rapamycin ameliorates the functional fitness of primary anti-tumor TCD8 and raises both their primary and long-term frequencies.
Figure 3.
Rapamycin treatment improves the quality of primary T Ag-specific TCD8 and promotes their progression to memory cells. Site IV-specific TCD8 identified by ICS for IFN-γ were gated upon and assessed for their expression of CD127 and KLRG1 (A,B) as well as intracellular Bcl-2 (C). Representative FACS plots for these markers are shown. In addition, the frequencies of CD127highKLRG1low (memory TCD8 precursors) and CD127lowKLRG1high (short-lived effectors) are shown for 6 mice/group (B). Statistical comparisons revealed that rapamycin-treated mice had a higher proportion of CD127highKLRG1low cells compared with vehicle-treated animals (38 ± 3.3 vs. 29.1 ± 4, p=0.11) and a lower proportion of CD127lowKLRG1high cells (5.1 ± 0.4 vs. 10.8 ± 3.4, p=0.15). In separate experiments, B6 mice were immunized with KD2SV cells and treated with rapamycin or vehicle during the initial priming phase as illustrated (D). Mice were left untreated until day 27, at which point the frequency of T Ag-specific TCD8 was determined by ICS for IFN-γ. Data are shown as mean ± SEM obtained from 11 mice/group pooled from 3 independent experiments.
Inhibition of mTOR affects TCD8 cross-priming and immunodominance
The T Ag-specific response in our model occurs exclusively through cross-priming (8,12). This is because: 1) KD2SV cells are of kidney epithelial origin, not pAPCs, and lack B7 costimulatory molecules, a prerequisite for naïve TCD8 activation; 2) they are allogeneic to B6 mice and unable to directly prime TCD8 in this strain according to the rule of MHC restriction; 3) they are transformed with subgenomic fragments of SV40 and fail to produce SV40 virions, thus eliminating any possibility that the ensuing TCD8 responses are due to the infection of host pAPCs (8). Therefore, our finding that the TCD8 response to T Ag in this model is improved by rapamycin constitutes the initial report describing the effect of this agent on cross-priming. This is important for allotransplantation because TCD8 responses to microbial and tumor Ags of donor origin, which are believed to occur at least partially through cross-priming, are likely to be heightened by rapamycin. We have recently found that anti-influenza TCD8 responses can also be augmented by rapamycin in a cross-priming model (8)(unpublished data).
We found that rapamycin strengthens the TCD8 response to site IV, but typically not those targeting subdominant epitopes (sites I, II/III and V). This indicates that even among TCD8 clones recognizing the same Ag, some are more prone than others to the immunostimulatory effect of rapamycin. We previously reported that site IV-specific TCD8 are sufficient for eradication of T Ag-induced choroid plexus brain tumors in irradiated mice (17). However, it is noteworthy that TCD8 clones specific for immunodominant epitopes are not always necessarily the most protective TCD8 against all forms of cancer and infectious diseases. Therefore, the selective adjuvanticity of rapamycin for some but not all TCD8 clones need to be taken into consideration in therapeutic vaccine design.
Mode of Ag presentation determines the susceptibility of TCD8 responses to rapamycin adjuvanticity
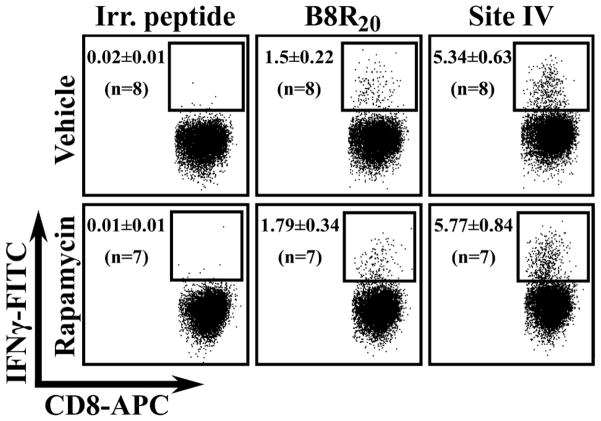
Next, we asked whether rapamycin affects TCD8 responses to antigenic peptides encoded by a viral vector. We infected vehicle- and rapamycin-treated mice with a rVV that expresses site IV as a cytosolic minigene (12). Direct priming is presumed to be the predominant pathway in activating naïve TCD8 recognizing peptides encoded by such minigenes. This experiment also enabled us to examine the effect of rapamycin on mouse TCD8 responses to VV epitopes that we previously characterized (13). TCD8 responses to both site IV and the VV-derived epitopes B8R20, A47L138, K3L6, A42R88 and A19L47 remained unaltered upon rapamycin treatment (Fig. 4 and data not shown). Therefore, we conclude that: 1) regardless of whether site IV-specific TCD8 activation following rVV-IV infection can be dubbed as direct priming, the mode of Ag presentation can clearly dictate the susceptibility of TCD8 responses to rapamycin; 2) the adjuvanticity of rapamycin cannot be generalized to all pathogen-specific TCD8 responses, and even to TCD8 responses against the same pathogen in different host species. This is because mouse TCD8 responses to VV epitopes are resistant to rapamycin treatment, whereas the bulk VV-specific TCD8 response is reportedly augmented in rapamycin-treated rhesus macaques (7). It will be important to explore the susceptibility of VV-specific TCD8 to rapamycin in humans since rVVs are pursued as suitable vectors in therapeutic vaccination.
Figure 4.
VV- and site IV-specific TCD8 induced through infection with rVV-IV are not affected by rapamycin. Splenocytes from rVV-IV-infected mice that received daily treatment of rapamycin or vehicle were restimulated ex vivo with VV-derived peptides (including B8R20) or the T Ag site IV peptide. The frequencies of epitope-specific TCD8 are shown as mean ± SEM obtained from 7–8 mice/group pooled from 2 independent experiments that yielded almost identical results. Representative dot plots are also shown.
How rapamycin modulates TCD8 responses is unclear. Using RNA interference to knock down mTOR, raptor [an important component of the mTOR complex 1(mTORC1)] or FKBP12 (a binding partner of rapamycin) exclusively in LCMV-specific transgenic TCD8, a previous study found that rapamycin operates in a T cell-intrinsic fashion to accelerate memory TCD8 differentiation (6). Whether this is true also for wild-type TCD8 is currently unknown. The role of mTORC2, whose activity may be reduced in some cell types after prolonged exposure to rapamycin (18), remains to be elucidated. mTOR is known to modulate autophagy in DCs, and rapamycin-induced autophagy in these cells enhances their ability to prime T cells in vitro (19). We favor the possibility that APCs may participate in modulation of TCD8 by rapamycin. This is because: 1) tumor-specific and alloreactive TCD8 primed in the same host behave differently in response to rapamycin; 2) TCD8 clones recognizing various epitopes of the same Ag show variation in response to the immunostimulatory effect of rapamycin (site IV versus other epitopes); 3) the TCD8 priming route for the same epitope determines the response to rapamycin (site IV expressed by allogeneic non-APCs as opposed to site IV encoded by a rVV), potentially implicating various APC subsets in the observed effect; 4) TCD8 found in different environments exhibit varying degrees of susceptibility to rapamycin (splenic versus peritoneal alloresponses). The activity of mTORC1 and/or mTORC2 and the specialized functions of distinct APC subsets (e.g., autophagy) may be subject to differential rapamycin regulation. Infection with replicating viral vectors (e.g., rVV) expressing tumor Ags may yield a high Ag load and simultaneously trigger viral pattern recognition receptors within APCs. This would be absent in responses to cell-associated Ags.
In summary, we show for the first time that rapamycin augments the vigor, fitness and quality of TCD8 responses induced by cross-priming against a clinically relevant viral oncoprotein but not the alloreactive TCD8 response occurring in the same host. The ultimate question is whether human TCD8 are prone to the adjuvant effect of rapamycin. Rapamycin is not only used in allograft recipients but is also an approved therapeutic agent for advanced renal cell carcinoma. How TCD8 in allograft recipients and cancer patients under rapamycin therapy respond to viruses and tumor Ags warrants further investigation.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR grant MOP 86601), The Cancer Research Society Inc. and the Multi-Organ Transplant Program, London Health Sciences Centre to SMMH, and by grant CA025000 from the National Cancer Institute/National Institutes of Health to TDS. SMMH holds a Canada Research Chair in Viral Immunity & Pathogenesis. SMV was a recipient of a CIHR Training Grant in Cancer Research & Technology Transfer, and MR was a recipient of a Canada Graduate Scholarship from Natural Sciences and Engineering Research Council of Canada. The authors wish to thank Dr. Rafi Ahmed and Dr. Koichi Araki (Emory Vaccine Center) and Dr. Joaqúin Madrenas (McGill University) for their critical review of this work.
Abbreviations
- B6
C57BL/6
- FBS
fetal bovine serum
- ICS
intracellular cytokine staining
- IFN
interferon
- KLRG1
killer cell lectin-like receptor G1
- LCMV
lymphocytic choriomeningitis virus
- MFI
mean fluorescence intensity
- mTOR
mammalian target of rapamycin
- mTORC
mTOR complex
- TCD8
CD8+ T cell
- rVV
recombinant vaccinia virus
- SV40
simian virus 40
- T Ag
large tumor antigen
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional supporting information may be found in the online version of this article.
Supplemental Figure 1 depicts a head-to-head comparison of ICS for IFN-γ and tetramer staining for detection of site I- and site IV-specific TCD8 at the peak of their primary response.
Supplemental Figure 2 demonstrates the effect of continuous rapamycin treatment on long-term frequencies of T Ag-specific and alloreactive TCD8.
References
- 1.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner AP, V, Shaffer O, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus enhances the magnitude and quality of viral-specific CD8(+) T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant. 2011;11:613. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Masterman KA, Basta S, Haeryfar SM, Dimopoulos N, Knowles B, Bennink JR, Yewdell JW. Cross-priming of CD8+ T cells by viral and tumor antigens is a robust phenomenon. Eur J Immunol. 2004;34:194. doi: 10.1002/eji.200324257. [DOI] [PubMed] [Google Scholar]
- 9.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Sener A, Uberoi V, Bartlett ST, Kramer AC, Phelan MW. Living-donor renal transplantation of grafts with incidental renal masses after ex-vivo partial nephrectomy. BJU Int. 2009;104:1655. doi: 10.1111/j.1464-410X.2009.08681.x. [DOI] [PubMed] [Google Scholar]
- 11.Grossi PA, Fishman JA. Donor-derived infections in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S19–S26. doi: 10.1111/j.1600-6143.2009.02889.x. [DOI] [PubMed] [Google Scholar]
- 12.Haeryfar SM, DiPaolo RJ, Tscharke DC, Bennink JR, Yewdell JW. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol. 2005;174:3344. doi: 10.4049/jimmunol.174.6.3344. [DOI] [PubMed] [Google Scholar]
- 13.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mylin LM, Schell TD, Roberts D, Epler M, Boesteanu A, Collins EJ, Frelinger JA, Joyce S, Tevethia SS. Quantitation of CD8(+) T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J Virol. 2000;74:6922. doi: 10.1128/jvi.74.15.6922-6934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer. 2011;104:643. doi: 10.1038/bjc.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatum AM, Mylin LM, Bender SJ, Fischer MA, Vigliotti BA, Tevethia MJ, Tevethia SS, Schell TD. CD8+ T cells targeting a single immunodominant epitope are sufficient for elimination of established SV40 T antigen-induced brain tumors. J Immunol. 2008;181:4406. doi: 10.4049/jimmunol.181.6.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.