Abstract
Fluid monolayers of 1-palmitoyl-2-oleoyl-phosphatidylcholine collapse from an air/water interface to form a three-dimensional bulk phase at the equilibrium spreading pressure (πe) of ~47 mN/m. This phase transition limits access to higher surface pressures under equilibrium conditions or during slow continuous compressions. We have shown previously that these films avoid collapse and become metastable when compressed on a captive bubble to surface pressures above 60 mN/m and that the metastability persists during expansion at least to πe. Here, we first documented the extent of this persistent metastability. Rates of isobaric collapse during expansion of the metastable films were up to 3 orders of magnitude slower than those during the initial compression to high surface pressures. Recovery of the ability to collapse depended on the surface pressure to which the films were expanded and how long they were held there. Films reverted after brief exposure to 20 mN/m and after 1 h at 35 mN/m. At πe, films remained capable of reaching high surface pressures during slow compressions after 65 h, although an increase in compressibility above 55 mN/m suggested somewhat increased rates of collapse. We also determined if the films remained metastable when they acquired sufficient free area to allow reinsertion of collapsed material. Faster isobaric expansion in the presence of more collapsed material and with further deviation below πe supported the existence of reinsertion. The persistence of metastability to πe shows that films with sufficient free area to allow reinsertion remain resistant to collapse. Observations that suggest heterogeneous reinsertion, however, argue that free area may be distributed heterogeneously and leave open the possibility that metastability persists because significant regions retain a restricted free area.
Introduction
Fluid monolayers at an air/water interface that are compressed fast enough to reach high surface pressures can form structures that are metastable far from equilibrium1,2 (Figure 1). During slow compressions under quasi-equilibrium conditions, the surface pressure that fluid films can achieve is limited by a two-to-three-dimensional phase transition in which constituents collapse from the interface to form a bulk phase.3 The equilibrium coexistence of the monolayer and the collapsed phase defines an equilibrium spreading pressure (πe), and the monolayer exists at higher surface pressures only as a nonequilibrium structure. If compressed on a captive bubble, however, fast enough to outrun collapse, phosphatidylcholine films in the fluid liquid-expanded (LE) phase can reach surface pressures well above πe.1,2 At those pressures, rates of collapse slow, and films that return to πe from lower surface pressures in seconds can persist with little or no further compression for hours.1,2 At the high surface pressures, the fluid monolayers are metastable, analogous in several respects to three-dimensional liquids supercooled toward a glass transition.2
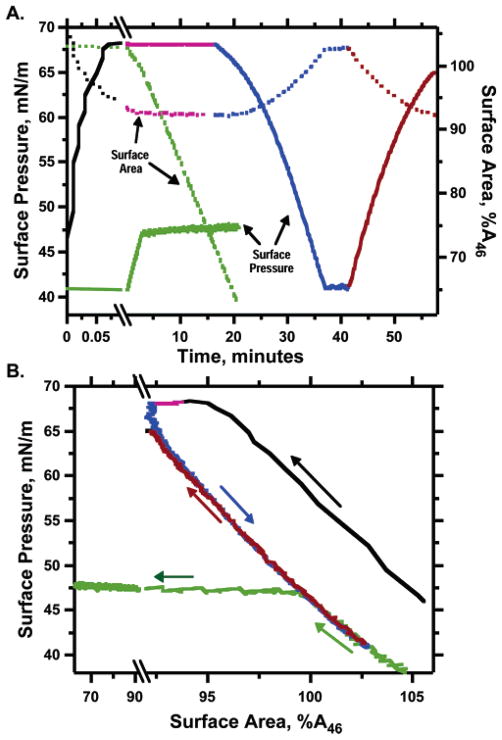
Figure 1.
Slow and fast compression of POPC monolayers. Films of POPC, spread on a captive bubble to <40 mN/m, were heated to 26 °C and then compressed either continuously at 0.9 h−1 (green) or from 46 mN/m at 15 min−1 (black). For the more rapidly compressed films, bubbles were first manipulated to hold surface pressure constant for 15 min (magenta), then expanded at 1.0 h−1 to 41 mN/m (blue), and finally recompressed at the same rate (red). Surface areas are expressed relative to the value at 46 mN/m (A46), using the area during slow expansion for the films compressed to high surface pressure. (A) Surface pressure (solid lines, left axis) and surface area (dotted lines, right axis) expressed as functions of time. The split time scale distinguishes the initial rapid compression from the subsequent slower manipulations. (B) The same data, with the sequence of colors preserved, expressed as surface pressure vs area. Arrows indicate the temporal progression of measurements. Comparable data have been published previously.2
A second characteristic of the films at high surface pressures is that they become not only metastable but also trapped in their transformed state.1,2 The resistance to collapse observed at high surface pressures persists during slow expansion of the films at least to πe (Figure 1). During compression at the same temperature and slow rates from the same surface pressure, films exhibit very different behavior before and after exposure to high surface pressure. After the rapid compression, films that originally collapsed at πe instead increase surface pressure along a steeply rising isotherm. The different behaviors at the same thermodynamic conditions indicate that the films become trapped in the metastable state, unable to relax over the course of standard experiments to their initial structures. The persistence of the metastable state upon return to conditions of equilibrium coexistence, although possible,4 is not a general property of supercooled liquids. This aspect of the supercompressed fluid monolayers is therefore particularly interesting.
The analogy to three-dimensional materials suggests that the metastability of the film is likely to reflect changes in viscosity. For a supercooled liquid, crystallization slows because of the “super-Arrhenius” increase in viscosity that culminates in the glass transition.5 Fluid monolayers containing phosphatidylcholines collapse by the flow of the continuous lamella into a stacked smectic liquid crystal,6 suggesting that the rate of collapse should depend directly on the film’s viscosity. Free area, defined as the area not excluded by interfacial molecules, is an important determinant of a film’s viscosity,7,8 just as free volume of a liquid in large part determines its viscosity.9 The metastability of the highly compressed films might therefore reflect a restriction of free area. This model would not explain the slow relaxation after return to equilibrium conditions. It would, however, predict that, as long as the film remains transformed to structures that resist collapse, free area should remain restricted.
Quantitative assessment of free area is difficult. The rapid compression invariably concludes at high surface pressures with some final isobaric change in area (Figure 1), suggesting that the decreased area of the metastable films reflects a loss of constituents as well as a change in molecular area. The unknown number of molecules in the film after the fast compression prevents accurate estimation of molecular area, and therefore of free area. In the studies reported here, we have instead taken a qualitative approach. Material that has collapsed from the interface can reinsert into the monolayer only if sufficient free area is available to accommodate the additional constituents. During reintroduction of free area by expansion, we addressed where the film regained its ability to collapse and when reinsertion became possible. We then determined if the conditions for the two processes coincide.
Materials and Methods
Materials
1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) was obtained from Avanti Polar Lipids (Alabaster, AL) and used without further purification or characterization. High purity chloroform and methanol (Honeywell Burdick & Jackson, Muskegon, MI) were used as spreading solvents. Water was distilled and then filtered through Macropure, Ultrapure DI, and Organic Free cartridges from Barnstead/Thermolyne (Dubuque, IA). The following reagents were purchased commercially and used without further purification or analysis: N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) (GibcoBRL brand, Life Technologies, Grand Island, NY); CaCl2·2H2O (J. T. Baker, Inc., Phillipsburg, NJ); and NaCl (Mallinckrodt Specialty Chemicals Co., Paris, KY). Captive bubbles were suspended in 10 mM HEPES (pH 7.0), 150 mM NaCl, and 1.5 mM CaCl2 (HSC) that was filtered through 0.45-μm micropore filters (Millipore, Bedford, MA) to remove particulate contaminants.
Methods
These studies measured the characteristics of films spread at an air/water interface using a captive bubble10,11 as the “surface balance”.12 Bubbles offer several advantages over standard surface balances for the studies reported here. Rates of compression are possible that are much faster than on a Langmuir trough, and the bubble’s continuous air/water interface eliminates the need for confining barriers that produce artifacts from escape of the confined film.13 Temperature regulation and environmental isolation are better than with open troughs, making reliable long-term experiments possible.
Films are spread at the continuous air/water interface of a bubble, which is suspended in HSC below an agarose dome in a small chamber, and compressed by infusion of buffer from a computer-controlled syringe pump to shrink the bubble’s volume and surface area.14 The instrument uses a video camera to capture the profile of an axisymmetric bubble through a framegrabber to a computer, which analyzes the captured profile and measures the height and the diameter of the bubble. Surface tension, volume, and surface area are calculated through the use of polynomial equations in terms of the height and the diameter of the bubble.15,16 Surface pressure is calculated from the known surface tension of water interpolated from published values according to the measured temperature. The chamber was warmed to 26 °C using a temperature controller (Cole-Palmer, Mt. Vernon, NJ) and heating pads (Minco, Minneapolis, MN) applied along the sides of the sample chamber. Surface pressure–area (π–A) isotherms obtained at the same temperature and with the same rate of compression exactly replicate curves measured on Langmuir troughs for dipalmitoyl phosphatidylcholine14 and for palmitic acid (data not shown).
For standard experiments, bubbles ranged from 100 to 120 μL before compression. Films were spread in small volumes (~0.10 μL) of chloroform/methanol (1:1, v/v) to initial surface pressures of 25–40 mN/m at ambient temperatures. After removal of the spreading solvent by exhaustive exchange of the subphase,14 these bubbles were heated at atmospheric pressure to the desired temperature and compressed slowly to 46 mN/m. The bubbles were then compressed at ~120% V0/s to ~50% of their initial volume (V0). The maximum extent of compression was limited by the pressure tolerance of the system at ~3.5 atm. Because compression changed volume at a constant rate, the variation of area was nonlinear. Rates of compression, expressed as the fractional rate of change in area or the rate of strain, (dA/A)/dt = d(ln A)/dt, changed during the infusions. The reported values were obtained during the initial change in area when rates were roughly constant. For comparison to rates commonly reported for Langmuir troughs, 3 Å2/(molecule·min) at 100 Å2/molecule represents a fractional rate of 1.8 h−1.
Rates of collapse were determined during isobaric compression. Using simple feedback, the computer manipulated the syringe pump to maintain constant surface pressure. If relaxations within the monolayer can be ignored,2 then the molecular area (Ā) when surface pressure is held constant should be invariant with time. Consequently,
The rate of collapse, defined as 1/n dn/dt, the fractional rate at which constituents leave the interface, can therefore be measured from the rate of fractional isobaric compression. The curved isobars obtained for POPC indicate that collapse varied with time. The results reported here compare initial rates of collapse measured during an interval specified for each experiment directly after reaching the target surface pressure.
Results
We have previously reported qualitative evidence that monolayers of POPC can be transformed.2 Freshly spread films that collapse at 47 mN/m become metastable if compressed fast enough to reach high surface pressures (Figure 1). A rapid compression alone is insufficient to transform the films if they do not reach high surface pressures. When compressed at the same rates through the same difference in surface pressures that produced transformation, but to final surface pressures below πe, the films retained their susceptibility to collapse.2 The metastability achieved at high surface pressures is not restricted to those conditions but persists after return to the surface pressures just above πe at which the film originally collapsed (Figure 1). In addition to qualitative evidence for transformation obtained from the film’s compressibility during slow maneuvers, measurements of the rates at which the films collapsed also provided more quantitative information (Figure 2). After reaching the different target surface pressures, films were compressed isobarically. If rearrangements within the monolayer at each surface pressure can be ignored,2 then the fractional rates of compression and collapse under isobaric conditions must be equal (see the Methods section). The slope of the isobar therefore provided the rate of collapse. Experiments here extended previous measurements during the initial compression2 to compare rates of collapse before and after reaching 68 mN/m (Figure 2).
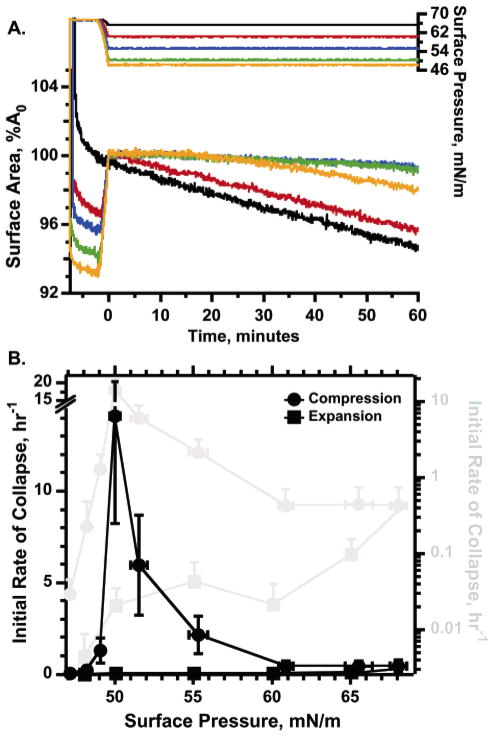
Figure 2.
Rates of collapse during compression and expansion. Films were compressed from 46.0 mN/m at 20 ± 3 min−1 and 26 °C either directly to the target surface pressures (“compression”) or to 68 mN/m, at which the films were maintained isobarically for 5 min prior to expansion at 1.4 ± 0.7 h−1 to the target surface pressure (“expansion”). At each target surface pressure, films were manipulated to maintain surface pressure constant. The initial rate of collapse was then obtained from the average initial slope of the isobar in semilogarithmic plots (see the Methods section). For target surface pressures achieved during the initial compression (“compression”), the average slope was determined between 20 and 60 s after beginning isobaric compression. During expansion, because the slower collapse produced minimal changes in area during that interval, collapse was determined from the slope during the initial 15 min of isobaric compression. (A) Variation of surface area (left axis) and surface pressure (right axis) with time for collapse measured during expansion. (Comparable data obtained during compression have been published previously.2) The time scale is set to 0 at the beginning of the isobaric manipulations, and area is expressed relative to the value at that time (A0). Curves of the same color for surface area and surface pressure indicate data from the same experiment. Curves are representative of four experiments at each surface pressure. (B) Rates of collapse at different surface pressures reached during the initial compression or during expansion from 68 mN/m. Results are presented relative to both the left linear axis (black) and the right logarithmic scale (gray). Symbols give mean values, with error bars indicating SD, for both target surface pressure and rate of collapse.
The isobars during the initial compression from πe and during expansion from 68 mN/m differed in two respects. First, the temporal progression of collapse showed opposite trends. At surface pressures reached during the initial compression, collapse slowed.2 The slope of the compression isobar became less negative with time. After compression to high surface pressure followed by expansion, however, collapse instead accelerated (Figure 2A). The second difference was that collapse during expansion was slower at all surface pressures than during the initial compression. Rather than replicating the variation with surface pressure observed during compression, where collapse passed through a sharply defined maximum,2 the rates during expansion decreased as surface pressures returned toward equilibrium values (Figure 2B). Consequently, at 50 mN/m, where collapse reached its maximum during compression, rates of collapse fell from 14.1 ± 5.9 h−1 during the initial compression to 0.021 ± 0.014 h−1 after reaching 68 mN/m.
Experiments that expanded the film further to surface pressures below πe demonstrated that the transformation was reversible. When expanded to 1–2 mN/m and then recompressed at 0.6 h−1, the film collapsed at 47 mN/m (Figure 3), the same surface pressure at which they collapsed during the initial compression from beyond lift-off. If expanded sufficiently, the original behavior of the film prior to the rapid compression was restored.
Figure 3.
Full expansion of a rapidly compressed monolayer. Films of POPC compressed at 26 °C from 46 mN/m at 59.0 ± 3.1 min−1 and held for 7 min at the highest surface pressure achieved were expanded at 0.6 ± 0.1 h−1 to 1.5 mN/m and then recompressed at the same rate (black curves). For comparison, freshly spread POPC was compressed at 0.9 h−1 from lift-off (gray curve). Areas are expressed relative to the final value at 46 mN/m (A46). Each curve is representative of three experiments.
To determine where between 47 and 1–2 mN/m the film regained its ability to collapse, rapidly compressed films were expanded cyclically to progressively lower surface pressures. Beginning with 35 mN/m, each cycle lowered surface pressure by an additional 3 mN/m, with a recompression to 65–66 mN/m at the end of each expansion to test the film’s continued ability to reach high surface pressures. In four experiments, transformed POPC regained the ability to collapse after expansion to 20 ± 3 mN/m (Figure 4). Collapse occurred, however, at 49.5 ± 0.2 mN/m rather than at 47 mN/m, the value observed for the freshly spread film. Even at 27 mN/m below the point at which the initial film collapsed, return to the original behavior was incomplete.
Figure 4.
Restoration of collapse during sequential expansions to progressively lower surface pressures. POPC monolayers, compressed at 26 °C and 29 ± 2 min−1 to >65 mN/m, were expanded at 1.2 ± 0.1 h−1 and then recompressed at the same rate to test for collapse at surface pressures up to 65 mN/m. Subsequent cycles of expansion and recompression, represented by different colors, lowered surface pressure further by 3 mN/m on each successive cycle. Area is expressed relative to the value at 46 mN/m achieved during the first expansion (A46). The data shown are representative of four replicates.
The cyclic expansions provided information only on the immediate effects of lower surface pressures. Subsequent experiments also tested whether films would regain the ability to collapse if held at 35 mN/m for longer durations (Figure 5). Films compressed above 65 mN/m were expanded to 35 mN/m and then held at constant surface pressure. Recompression above 52 mN/m tested resistance to collapse. Initially, the recompressions were cyclic, repeated every 40 min. By this criterion, films regained the ability to collapse between 3.7 and 4.3 h. The collapse plateau again occurred at a surface pressure (50.1 ± 2.0 mN/m) above the value observed for freshly spread films (47 mN/m), suggesting incomplete return to the original state (Figure 5). The change in behavior with time at constant surface pressure did fit with return from the transformed state by a kinetic process, although one that was slow.
Figure 5.
Duration of metastability at 35 mN/m tested by cyclic recompression. POPC monolayers were compressed at 14.1 ± 0.8 min−1 from 46 mN/m at 26 °C to 67 mN/m, expanded at 1.19 ± 0.01 h−1 to 35 mN/m, and then held at that surface pressure by manipulating surface area. At 40-min intervals, the films were recompressed at the same slow rate to 52 mN/m as a test of continued ability to sustain surface pressure above πe. Area is expressed relative to the value at 46 mN/m during the initial expansion (A46). (A) Surface pressure (dashed curves, left axis) and surface area (solid curves, right axis) represented as functions of time. (B) The same data, with the color sequence preserved for the different cycles, expressed as surface pressure vs surface area. The data shown are representative of four replicates.
Close examination of the isobars showed that, during each of the 40-min isobaric intervals, the films expanded (Figure 5). Each recompression returned the area at 35 mN/m approximately to its value prior to the expansion. To determine if the cyclic recompressions affected the kinetics of reversion, the films were instead held isobarically for different intervals and then recompressed only once (Figure 6). With this approach, the films recovered the ability to collapse between 40 and 60 min. The faster return to the initial behavior demonstrated that recompression, even to surface pressures as low as 52 mN/m, at least partially restored metastability.
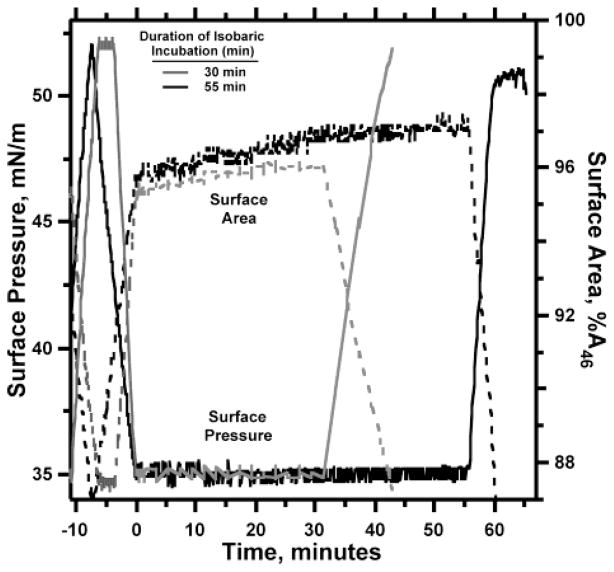
Figure 6.
Duration of metastability at 35 mN/m tested by a single compression. POPC monolayers, compressed at 42.3 ± 1.3 min−1 and 26 °C from 46 mN/m to >65 mN/m, were expanded at 1.04 ± 0.01 h−1 to 35 mN/m, recompressed at the same slow rate to 55 mN/m to confirm the metastability above 46 mN/m, and then held isobarically at 35 mN/m for 30 or 55 min. The metastability of the film was then tested by compression at 1.0 h−1 to surface pressures above 52 mN/m. Time is expressed relative to the beginning of the isobaric incubation, and area, relative to the value at 46 mN/m during the first reexpansion (A46). The experiment shown is representative of three replicates for 40-min incubations and four replicates for 60-min incubations.
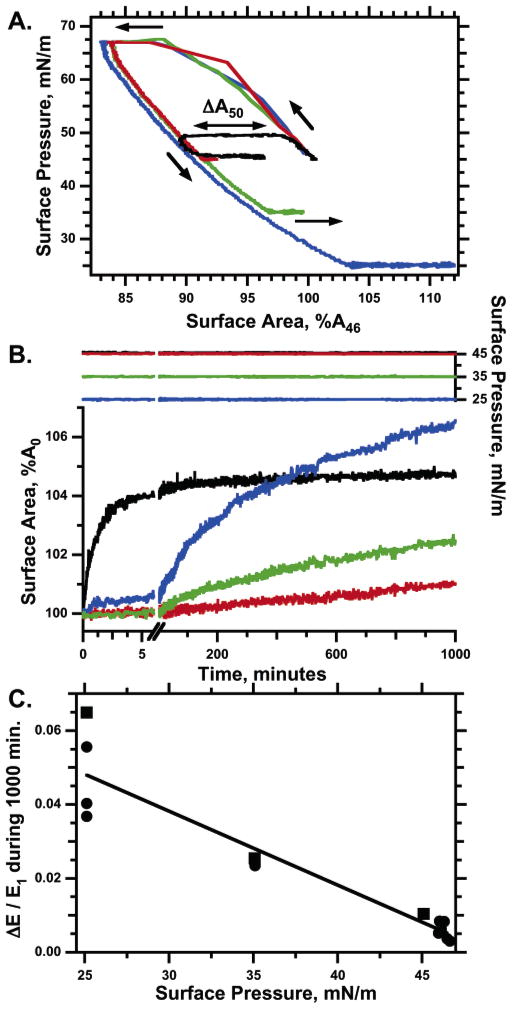
A key issue for these studies was the extent to which the reverted film contained sufficient free area to allow material that collapsed during compression at high surface pressures to reinsert into the interface. To establish whether reinserted material contributed to the film’s expansion, we determined the effect of overcompression at high surface pressure on the rate of expansion at 35 mN/m. Films were compressed to 68 mN/m and then maintained isobarically at that level through variable changes in area (Figure 7). After lowering surface pressure to 35 mN/m, the extent of isobaric expansion was measured during the subsequent 1000 min. Rates of expansion were faster for greater overcompression (Figure 7C), consistent with reexpansion at least partially by reinsertion of collapsed material.
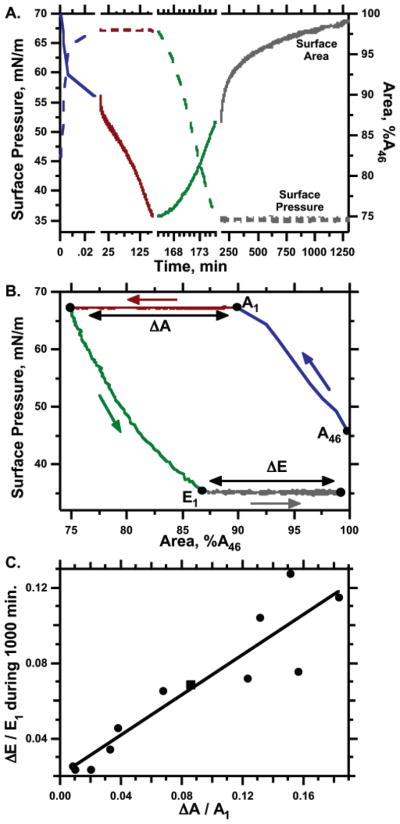
Figure 7.
Dependence of the isobaric expansion rate at 35 mN/m on the extent of collapse at 68 mN/m. Spread films were compressed from an initial area (A46) at 46 mN/m to a smaller area (A1) at 68 mN/m and then held isobarically at that high surface pressure for different intervals to produce a range of changes in area (ΔA). After expanding the area at <1.2 h−1 to the area (E1) at 35 mN/m, the films were allowed to expand isobarically for 1000 min through a change in area of ΔE. Areas are expressed relative to A46. (A) Surface pressure (dashed lines, left axis) and surface area (solid lines, right axis) as functions of time for an individual experiment. The split time scale distinguishes the different manipulations of the film. (B) The same data expressed as surface pressure vs area. The color scheme from part A is preserved to indicate the temporal sequence. (C) Dependence of fractional expansion on the extent of collapse for 12 experiments. The square symbol indicates the experiment displayed in parts A and B. The solid line gives the least-squares fit to a straight line through the data (r2 = 0.86).
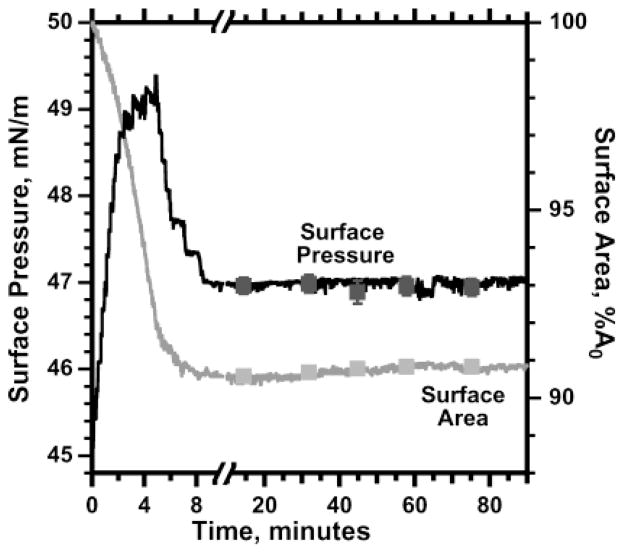
We then tested the extent to which the expansion rate for the metastable films depended on the deviation of surface pressure below πe. These experiments required accurate values of πe, which represented a major landmark for our studies. Collapse would be restricted to surface pressures above πe, and reinsertion would only occur at lower pressures. To determine πe, we compressed films until they began to collapse and then held them at constant surface area while surface pressure fell to constant values (Figure 8). These experiments estimated πe to be 46.8 ± 0.2 mN/m.
Figure 8.
Estimate of πe. Films of POPC were spread to <40 mN/m, compressed continuously from 40 mN/m at 0.6 ± 0.2 h−1 until the steep rise in surface pressure slowed at ~48 mN/m, suggesting the onset of collapse. The bubble’s volume was then held constant while surface pressure relaxed to a relatively constant value, after which surface area was held constant for >2 h. Area is expressed relative to the initial value (A0). The curves are representative of four experiments. The symbols give the mean ±SD at specific times.
Metastable films expanded during isobaric incubation below πe at rates that depended on the surface pressures at which they were held. During the 1000 min directly after expansion to a final surface pressure, the change in area grew linearly with decreasing surface pressure (Figure 9). Close to πe, the isobaric expansion approached zero. The expansion rate therefore demonstrated the same general dependence on deviation of surface pressure below πe expected for the reinsertion of collapsed material into a normal LE film.
Figure 9.
Dependence of expansion on surface pressure. Films of POPC were spread to <40 mN/m and compressed at 26 °C and 0.6 h−1 to 46 mN/m and then at 22 ± 5 min−1 to ~68 mN/m (colored lines). After ~1–2 min at that surface pressure, films were expanded at <1.2 h−1 to different target surface pressures and then allowed to expand isobarically. Control experiments measured isobaric expansion at 45.5 mN/m after collapse at <50 mN/m (black curve). (A) Surface pressure expressed relative to surface area. The arrows indicate the temporal progression of the full maneuvers. The curves are representative of at least four experiments for isobaric expansion at each surface pressure. (B) Surface pressure and surface area expressed relative to time after onset of the isobaric expansion. The curves of the same color in parts A and B indicate the same data. (C) Rate of expansion at different surface pressures. Expansion is expressed as the fractional change in area during a 1000-min interval. ΔE and E1 remain as defined in Figure 7. Square symbols indicate the particular experiments presented in parts A and B. The straight line gives the least-squares linear fit to the individual measurements (r2 ± 0.89).
These rates for the transformed films were considerably slower at all surface pressures than the measured rates for LE films. In control experiments, POPC films were compressed through a collapse plateau at 50 mN/m to produce the same relative change in area experienced by the transformed films (Figure 9). After return to 45 mN/m, area expanded by 3.7 ± 0.5% during the first 10 min of isobaric expansion. For the films transformed at high surface pressures, the expansion was much slower (Figure 9). Even at 25 mN/m, expansion during the first 10 min was only 0.4 ± 0.1%. Our experiments left open the possibility that the different rates of expansion following collapse at high and low surface pressures reflected differences in the collapsed material, the structure of which is unknown. The different rates, however, were also consistent with the hypothesis that the transformed and LE films, which behave differently in other respects, also differ in their ability to accommodate reinserted material.
The presence of reinserted material below πe suggested two mechanisms by which the films might revert to their original behavior. The transformed monolayer might return to its original equilibrium structure, or it might remain unchanged but allow the reinsertion of sufficient untransformed material that the entire film would regain its ability to collapse. To distinguish between these two possibilities, we tested the extent to which restoration of the original behavior depended on the presence of reinserted material. Following rapid compression to high surface pressures, films were expanded to surface pressures close to or below πe. The films were then incubated isobarically at the lower surface pressure for variable durations to produce a range of changes in area (Figure 10A). Within 1 mN/m of πe, area changed <1% during intervals as long as 77 h (Figure 10B). The variation of area at different surface pressures again fit with reinsertion that was greater at surface pressures further below πe and that stopped at πe.
Figure 10.
Effects of change in area during isobaric incubation close to or below πe on resistance to collapse. Freshly spread films of POPC were compressed from 46 mN/m at 26 °C and 22 ± 5 min−1 to >65 mN/m. After ~1–2 min at the highest surface pressures, films were expanded at <1.2 h−1 to different surface pressures and maintained isobarically for intervals lasting up to 77 h in an attempt to produce a range of expansions. Films were then recompressed at <1.2 h−1. A subset of three films that reached high surface pressures was then subjected to a further cycle of slow (<1.2 h−1) expansion and recompression. (A) Surface pressure vs area for representative curves showing two characteristic behaviors for films incubated within 0.5 mN/m of πe. Area is expressed relative to the initial value at 46 mN/m (A46). Arrows indicate the temporal progression of the different maneuvers. (B) Highest surface pressure reached during recompression (square symbols) for films that experienced different changes in area during the isobaric incubation. Negative changes in area indicate collapse during the incubation. ΔE and E1 remain as defined in Figure 7. Triangular symbols indicate the surface pressures at which the films were incubated. Open symbols indicate the subset of 10 films for which the isobaric incubation lasted between 66 and 77 h.
During slow recompression, all films that expanded >1.0% collapsed and failed to reach high surface pressures (Figure 10B). For expansions between 0.2 and 1.0%, results varied. Within that range, two films collapsed at ≤55 mN/m and eight films persisted to high surface pressures. For expansion <0.2%, all films remained capable of reaching 65 mN/m. The extent of reinsertion, interpreted from the extent of reexpansion, therefore predicted the behavior during recompression.
For the films with minimal or no expansion, the ability to reach high surface pressures persisted for remarkable durations. Films held at πe ± 0.5 mN/m for more than 65 h still reached 65 mN/m during slow recompressions. Their behavior, however, did change subtly during the incubation. The isotherms during recompression developed a discrete break between 50 and 60 mN/m with a lower slope at higher surface pressures (Figure 10A). Compressibility, defined as −d(ln A)/dπ, during recompression between 60 and 65 mN/m increased from 4.2 ± 0.1 m/N immediately after the rapid compression to 6.5 ± 1.1 m/N following the isobaric incubation. For the three films subjected to a further cycle of slow expansion and recompression, the final isotherm again showed a single linear slope (Figure 10A), consistent with restoration of the original behavior by exposure to high surface pressure. The increased compressibility immediately following the isobaric incubation showed that although films with no change in area, and therefore no reinserted material, failed to revert completely to their original behavior, they did all show a trend in that direction.
Discussion
When compressed to high surface pressures, monomolecular films of POPC become not only metastable2 but also trapped in their transformed state. Return to the initial conditions just above πe at which collapse progressed rapidly before exposure to high surface pressures fails in standard experiments to restore their original behavior. Rates of collapse show extensive hysteresis, with differences before and after reaching high surface pressures approaching 3 orders of magnitude (Figure 2). The studies here address the extent to which the persistent metastability of the supercompressed films reflects a persistent restriction of free area. Because collapse at high surface pressures prevents accurate tracking of molecular area, we have taken a qualitative approach to the determination of free area. Our experiments establish whether the supercompressed films during expansion remain resistant to collapse when free area is sufficient to allow the reinsertion of collapsed material. Our studies require that we demonstrate reinsertion, define when it begins, and establish whether at that point the films remain metastable.
Two sets of experiments provide evidence for reinsertion. Both detect the reinserted constituents from an isobaric expansion of the film. The analysis requires a distinction between an increase in molecular area of the transformed monolayer itself and an increase in the number of constituents at the interface, both of which would increase the film’s area. The distinction relies on the different responses of the two processes, greater molecular area and reinsertion, to specific manipulations of the system.
Experiments that vary the extent of collapsed material provide the best evidence for reinsertion. The rate of reexpansion at 35 mN/m depends directly on the extent to which the film is overcompressed along an isobaric plateau above 65 mN/m. The plateau at high surface pressure presumably reflects collapse of the film, during which the structure of the monolayer itself remains essentially unchanged. A longer plateau should increase the pool of material available for reinsertion, which would increase the rate at which reinsertion occurs. In contrast, for the alternative explanation that reexpansion reflects an increasing molecular area, a direct relationship between the extent of collapse at high surface pressures and the rate of reexpansion would be unexpected. The dependence on the extent of overcompression therefore suggests that reinsertion accounts for at least a significant portion of isobaric reexpansion at 35 mN/m.
The variation of isobaric expansion at different surface pressures strengthens this conclusion. The rate of reexpansion increases linearly with decreasing surface pressure. This observation alone does not distinguish between expansion caused by reinsertion or by an increase in molecular area of the transformed monolayer. The rates of both processes should increase with greater deviation from the surface pressure at which the two structures, the transformed monolayer and the collapsed phase, can form, and thus, lower surface pressure should accelerate either mechanism. The surface pressures at which the two structures arise, however, are distinct. Collapse occurs at an πe of 47 mN/m. The metastable films, indicated by the slow rates of collapse, only form above 55–60 mN/m. For the transformed monolayer, a reduction in surface pressure to πe represents a significant deviation from the conditions of transformation, and the driving force for expansion of the molecular area should be significant. The observed rates of expansion, which are undetectable at πe and increase linearly at progressively lower surface pressures, agree well with the behavior expected if reinsertion of collapsed material accounts for most of the increased area.
Our results also suggest that reinsertion of untransformed material represents the major mechanism by which films recover the ability to collapse. At πe, where the constant area during isobaric incubation indicates the absence of reinsertion, the films remain resistant to collapse for days. At 35 mN/m, however, where the films do expand, suggesting that reinsertion does occur, the films revert substantially to their original behavior within 1 h. The major implication of these experiments is that the prolonged duration of the transformed state may not terminate at πe. If reinsertion could be eliminated, the metastable structure might well persist for long periods not only at πe but also at lower surface pressures.
The behavior of the transformed monolayer itself, unaffected by reinserted material, can still be investigated if expansion stops at or short of πe. Under conditions at which reinsertion does not occur, the metastable films do show evidence of return toward their original behavior. The films still reach high surface pressures during slow recompression, but at these high pressures, compressibility increases. The slope of the compression isotherm above πe reflects, in addition to elasticity, the relative rates of compression and relaxation, both by rearrangements within the interface and by collapse from the monolayer. The effect of rearrangements should be small. Measurements on freshly spread monolayers below πe, where collapse cannot occur, suggest that rearrangements in these films contribute only minimally to changes in their area.2 If the elasticity of the monolayer itself remains unchanged, then the greater compressibility indicates an increased rate of collapse. Reversion from the transformed state, however, is quite slow. Even at 15 mN/m below the surface pressures at which the films become metastable, their return to original behavior after 67 h is limited.
The behavior of the films during recompression suggests that the reinserted material may remain distinct from constituents in the transformed monolayer. For films held at 35 mN/m, recompression to 52 mN/m significantly prolongs their metastability (Figures 5 and 6). Exposure to that surface pressure seems unlikely to affect the monolayer itself by reversing the relaxation of tight packing. Although 52 mN/m is past the point at which rates of collapse reach their maximum during an initial compression, that surface pressure is well short of the level at which collapse slows dramatically (Figure 2A2). Standard untransformed LE sections of the film, however, would collapse at that surface pressure. These observations raise the possibility that when expanded, the transformed film becomes heterogeneous, breaking into compact regions that retain the characteristics of the film at high surface pressures and regions with relatively low surface concentrations that allow reinsertion. In the extreme version of this model, compact regions retain the restricted molecular area of the transformed film at high surface pressures and the monolayer resembles an ice pack breaking into individual floes separated by gaps of open water.
Our results then lead to somewhat equivocal conclusions concerning the role of free area in transformation of the supercompressed films. The simplest interpretation of our data is that a restricted free area is unlikely to explain the altered behavior. Expansion to πe does introduce sufficient free area to allow collapsed material to reinsert, but when they first reach that surface pressure, the films remain resistant to collapse. During incubation for 3 days, the increased compressibility at high surface pressures indicates a return of the films toward their original behavior, but the change occurs without any increase in area. Some factor other than restricted free area seems likely. Our conclusions, however, are subject to the major reservation that expansion could be heterogeneous. Sections of the film might then retain the tightly packed configuration achieved at high surface pressures and so remain resistant to collapse, while intervening regions acquire the low surface concentrations necessary to allow reinsertion.
In summary, our results show the remarkable extent to which fluid films of POPC, made metastable by compression to high surface pressures, can persist in the transformed state despite return to the initial conditions at which they originally showed rapid collapse. Restoration of the original behavior occurs most readily by reinsertion of collapsed material at surface pressures below πe. Perpetuation of transformation by compression to surface pressures just above πe suggests that reinserted material may remain distinct from components of the transformed monolayer and that the metastable film may expand heterogeneously. At surface pressures just above πe, where reinsertion cannot occur, the metastable films do show signs of returning to their original behavior, but only over the course of several days.
Acknowledgments
The authors gratefully acknowledge the assistance of Drs. Jon Goerke and Günther Putz in initiating studies with captive bubbles and helpful discussions with Drs. David Grainger, Charles Knobler, and Shankar Rananavare. This work was supported by grants from the American Lung Association of Oregon, the National Institutes of Health (HL 03502 and 60914), and the Whitaker Foundation.
References
- 1.Crane JM, Hall SB. Biophys J. 2001;80:1863–1872. doi: 10.1016/S0006-3495(01)76156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EC, Crane JM, Laderas TG, Hall SB. Biophys J. 2003;85:3048–3057. doi: 10.1016/S0006-3495(03)74723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaines GL., Jr . Insoluble monolayers at liquid–gas interfaces. Interscience Publishers; New York: 1966. [Google Scholar]
- 4.Courtney TH. Mechanical Behavior of Materials. McGraw-Hill; New York: 1990. p. 330. [Google Scholar]
- 5.Debenedetti PG. Metastable Liquids: Concepts and Principles. Princeton University Press; Princeton, NJ: 1996. [Google Scholar]
- 6.Schief WR, Antia M, Discher BM, Hall SB, Vogel V. Biophys J. 2003;84:3792–3806. doi: 10.1016/S0006-3495(03)75107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters R, Beck K. Proc Natl Acad Sci USA. 1983;80:7183–7187. doi: 10.1073/pnas.80.23.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galla HJ, Hartmann W, Theilen U, Sackmann E. J Membr Biol. 1979;48:215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- 9.Macedo PB, Litovitz TA. J Chem Phys. 1965;42:245–256. [Google Scholar]
- 10.Schürch S, Bachofen H, Goerke J, Possmayer F. J Appl Physiol. 1989;67:2389–2396. doi: 10.1152/jappl.1989.67.6.2389. [DOI] [PubMed] [Google Scholar]
- 11.Putz G, Goerke J, Schürch S, Clements JA. J Appl Physiol. 1994;76:1417–1424. doi: 10.1152/jappl.1994.76.4.1417. [DOI] [PubMed] [Google Scholar]
- 12.Kwok DW, Tadros B, Deol H, Vollhardt D, Miller R, Cabrerizo-Vilchez MA, Neumann AW. Langmuir. 1996;12:1851–1859. [Google Scholar]
- 13.Goerke J, Gonzales J. J Appl Physiol. 1981;51:1108–1114. doi: 10.1152/jappl.1981.51.5.1108. [DOI] [PubMed] [Google Scholar]
- 14.Crane JM, Putz G, Hall SB. Biophys J. 1999;77:3134–3143. doi: 10.1016/S0006-3495(99)77143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malcolm JD, Elliott CD. Can J Chem Eng. 1980;58:151–153. [Google Scholar]
- 16.Schoel WM, Schürch S, Goerke J. Biochim Biophys Acta. 1994;1200:281–290. doi: 10.1016/0304-4165(94)90169-4. [DOI] [PubMed] [Google Scholar]