Abstract
The objective of this study was to establish the role of apoA-IV, ABCA1, and LCAT in the biogenesis of apoA-IV-containing HDL (HDL-A-IV) using different mouse models. Adenovirus-mediated gene transfer of apoA-IV in apoA-I−/− mice did not change plasma lipid levels. ApoA-IV floated in the HDL2/HDL3 region, promoted the formation of spherical HDL particles as determined by electron microscopy, and generated mostly α- and a few pre-β-like HDL subpopulations. Gene transfer of apoA-IV in apoA-I−/− × apoE−/− mice increased plasma cholesterol and triglyceride levels, and 80% of the protein was distributed in the VLDL/IDL/LDL region. This treatment likewise generated α- and pre-β-like HDL subpopulations. Spherical and α-migrating HDL particles were not detectable following gene transfer of apoA-IV in ABCA1−/− or LCAT−/− mice. Coexpression of apoA-IV and LCAT in apoA-I−/− mice restored the formation of HDL-A-IV. Lipid-free apoA-IV and reconstituted HDL-A-IV promoted ABCA1 and scavenger receptor BI (SR-BI)-mediated cholesterol efflux, respectively, as efficiently as apoA-I and apoE. Our findings are consistent with a novel function of apoA-IV in the biogenesis of discrete HDL-A-IV particles with the participation of ABCA1 and LCAT, and may explain previously reported anti-inflammatory and atheroprotective properties of apoA-IV.
Keywords: apolipoprotein A-IV, lipoproteins, genetically altered mice, lecithin:cholesterol acyltransferase, ATP binding cassette transporter A1
ApoA-IV (Mr = 46 kDa) is a major component of HDL and chylomicrons in rats (1). Similar to apoA-I and apoE, apoA-IV contains repeated units mainly of 22 residues long that are organized in amphipathic α-helices (2, 3) and have been implicated in lipid binding. In humans and the majority of animal species, apoA-IV is synthesized primarily by the intestine and, to a lesser extent, by the liver, and is found in plasma, the lymph chylomicrons, and the cerebrospinal fluid (3–5). An exception is the rabbit, where both the liver and the intestine are major sites of apoA-IV mRNA synthesis (6). Following synthesis in the intestine, apoA-IV is incorporated into chylomicrons, secreted into the lymph, and reaches the plasma (4). Hydrolysis of the triglycerides of chylomicrons by lipoprotein lipase in plasma causes dissociation of apoA-IV and its redistribution in either in HDL or the d>1.21 g/ml fraction (4). ApoA-IV mRNA and protein synthesis in mammals is controlled by hormonal (7) and nutritional factors (8). Plasma apoA-IV levels increase following a fat meal (4, 9) and under conditions of hypertriglyceridemia (10). In rats under fasting conditions, 50% of plasma apoA-IV is produced by the intestine (11). In humans, apoA-IV has two common alleles, designated apoA-IV-1 and apoA-IV-2, that result from a Q360H substitution, and a few rare alleles that follow Mendelian inheritance and may affect plasma lipid levels (12).
The in vitro and in vivo properties of apoA-IV have been investigated extensively, and various potential physiological functions have been suggested. These include a role in lipid absorption, secretion, metabolism (4), and food uptake (13–15), and protective functions against inflammatory diseases (16, 17) and atherosclerosis (17–19). ApoA-IV has structural (2, 3) and several functional similarities with apoA-I and apoE. Thus lipid-free apoA-IV promotes cholesterol efflux from cells (20–22), and rHDL-A-IV particles activate LCAT (23). ApoA-IV was also shown to bind saturably to cell surface sites (21, 24), as well as to hepatic cell membranes (25), to potentiate the apoCII-mediated activation of lipoprotein lipase (26) and the activity of cholesteryl ester transfer protein (27). Furthermore, apoA-IV was reported to have anti-oxidant (28) and anti-inflammatory (16, 29) properties, and similarly to apoA-I (30), and apoE (31), may also play some role in the development of Alzheimer's disease (32). A difference between apoA-IV and apoA-I or apoE exists on the contribution of the C-terminal domain of these proteins to the solubilization of dimyristoyl-l-α-phosphatidyl-choline (DMPC) phospholipids (33, 34). In the case of apoA-I and apoE, deletion of the C-terminal domain drastically reduced the ability of the truncated forms to solubilize DMPC phospholipids and to associate with preformed HDL (35, 36). In the case of apoA-IV, deletion of the 44 C-terminal residues increased its ability to solubilize DMPC phospholipids (34). Subsequent studies showed that deletion of the C-terminal residues 333–343 strongly increased the rate of association of truncated apoA-IV with DMPC phospholipids, and this enhancement required residues 11–20 of the truncated apoA-IV (37). The reduced capacity of the full-length apoA-IV to associate with phospholipids was attributed to intramolecular interactions of C- and N-terminal regions that contain residues F334 and F335, and W12 and F15, respectively (33). In cell culture studies, lipid secretion and the size of secreted lipoprotein particles increased dramatically with the deletion of the 344–354 region that contains three EQQQ motifs and one EQVQ motif in human apoA-IV (38). Increased lipid secretion was also observed in newborn swine, where apoA-IV lacks the EQQQ sequences, suggesting that these sequences modulate chylomicron packaging and secretion (38).
Studies with transgenic mice showed that overexpression of apoA-IV in the intestine did not affect the intestinal absorption of cholesterol and triglycerides and fat-soluble vitamins or the clearance of chylomicrons. It also did not cause weight gain and did not alter feeding behavior in transgenic mice as compared with control mice (15). Similar conclusions regarding lipid absorption and weight gain were reached by the study of apoA-IV-deficient mice (14). Previous studies had implicated apoA-IV as a satiety factor (13). The transgenic mice expressing the mouse apoA-IV gene mostly in the intestine had reduced levels of atherosclerotic lesions in response to atherogenic diets (19). The lipid profiles of these mice were similar, but not identical to those of the control wild-type (WT) mice (15). Plasma isolated from the mouse apoA-IV-transgenic mice had increased endogenous cholesterol esterification rates, and their HDL, isolated following fat feeding, promoted more efficiently cholesterol efflux from cholesterol-loaded human monocytes, as compared with HDL obtained from WT mice (19). Reduced atherosclerotic lesions were also observed in transgenic mice expressing human apoA-IV mainly in the intestine in an apoE-deficient background. Injection of lipopolysaccharide into these human apoA-IV-transgenic mice in an apoE-deficient background resulted in fewer atherosclerotic lesions than in apoE-deficient mice. The protective effect of apoA-IV in this case was attributed to its antioxidant properties (17) and the stronger Th1 response of the lymphocytes in the presence of apoA-IV. Lymphocytes isolated from human apoA-IV × apoE−/−−transgenic mice produced lower levels of proinflammatory cytokines as compared with apoE−/− mice (29). The anti-inflammatory properties of apoA-IV were also manifested by intraperitoneal injection of the recombinant protein in WT and apoA-IV-deficient mice. This treatment delayed the onset and reduced the severity of the inflammation associated with experimentally induced colitis in rats (16). Reduced atherosclerosis was also observed in transgenic mice overexpressing the apoA-IV gene in the liver of either normal or apoE-deficient mice under the control of the hepatic control region of the apoE/apoC-I gene cluster (18).
The origin and the metabolic fate and the physiological significance of apoA-IV that resides on the HDL particle are not fully understood. Here we show that apoA-IV participates in the biogenesis of apoA-IV-containing HDL (HDL-A-IV) particles using the same pathway that is utilized by apoA-I and apoE. The HDL-A-IV particles formed may explain, at least partially, the previously reported anti-inflammatory and atheroprotective functions of apoA-IV.
EXPERIMENTAL PROCEDURES
Materials
Materials not mentioned in the experimental procedures have been obtained from sources described previously (39).
Methods
Generation of an adenovirus expressing the human apoA-IV.
The apoA-IV cDNA was generated by RT-PCR of human mRNA using 5′ and 3′ primers contained restriction sites for Bgl-II and EcoRV, respectively. The apoA-IV cDNA was digested with Bgl-II and EcoRV and cloned into the corresponding sites of the pAdTrack-CMV vector. The recombinant adenoviruses were constructed and purified using the Ad-Easy-1 system where the adenovirus construct is generated in bacteria BJ-5183 (Agilent Technologies; Santa Clara, CA) as described (39). Correct clones were propagated in RecA DH5α cells (Invitrogen; Carlsbad, CA). The recombinant adenoviral vectors were linearized with PacI and used to transfect 911 cells. Following large-scale infection of HEK293 cell cultures with virus-containing cell lysates, the recombinant adenoviruses were purified by two consecutive Caesium chloride ultracentrifugation steps, dialyzed, and titrated (39).
Cholesterol efflux measurements.
ATP-binding cassette transporter (ABC) A1-mediated cholesterol efflux measurements by lipid-free apoA-IV using HEK293-EBNA cells was performed as described (39). Net efflux was calculated by subtracting the efflux obtained in the untransfected cells from that of the ABCA1-transfected cells (40). Scavenger receptor BI (SR-BI)-mediated cholesterol efflux by reconstituted HDL-A-IV (rHDL-A-IV) using CHO ldlA[mSR-BI] cells was performed as described (39, 41, 42). Net efflux was calculated by subtracting the efflux obtained in the parent IdlA CHO cells from that of ldlA[mSR-BI] CHO cells.
Animal studies, plasma lipids, fractionation of plasma, two-dimensional gel electrophoresis, electron microscopy, and apoA-IV mRNA analyses.
ApoA-I−/− (ApoA1tm1Unc) C57BL/6J mice (43) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice deficient for apoA-I and apoE were a gift of Dr. Fayanne Thorngate and Dr. David Williams (44). Mice deficient in ABCA1 (45) (purchased from Jackson Laboratories) were provided by Dr. Mike Filtzerald. Mice deficient for LCAT were a gift of Dr. Santa-Marina Fojo (46). The mice were maintained on a 12 h light/dark cycle and standard rodent chow. All procedures performed on the mice were in accordance with National Institutes of Health guidelines and following an approved IACUC protocol. Mice, 6–8 weeks of age, were injected via the tail vein with 0.5 to 1.5 × 109 pfu of recombinant adenovirus per animal. Four days postinjection, following a 4 h fast, blood was drawn and the livers were collected for further analyses.
The fractionation of plasma by fast-protein liquid chromatography (FPLC) and density gradient ultracentrifugation, the two-dimensional gel electrophoresis of plasma, the cholesterol and triglyceride measurements, the electron microscopy (EM) of the HDL fractions, and the apoA-IV mRNA quantification were performed as described (47). For details, please see the Supplementary Methods.
Statistics
Statistical analyses were performed by two-tailed Student's-t test with equal variance.
RESULTS
In vitro properties of apoA-IV
We have generated a recombinant adenovirus expressing apoA-IV and used it to study its in vivo and in vitro properties.
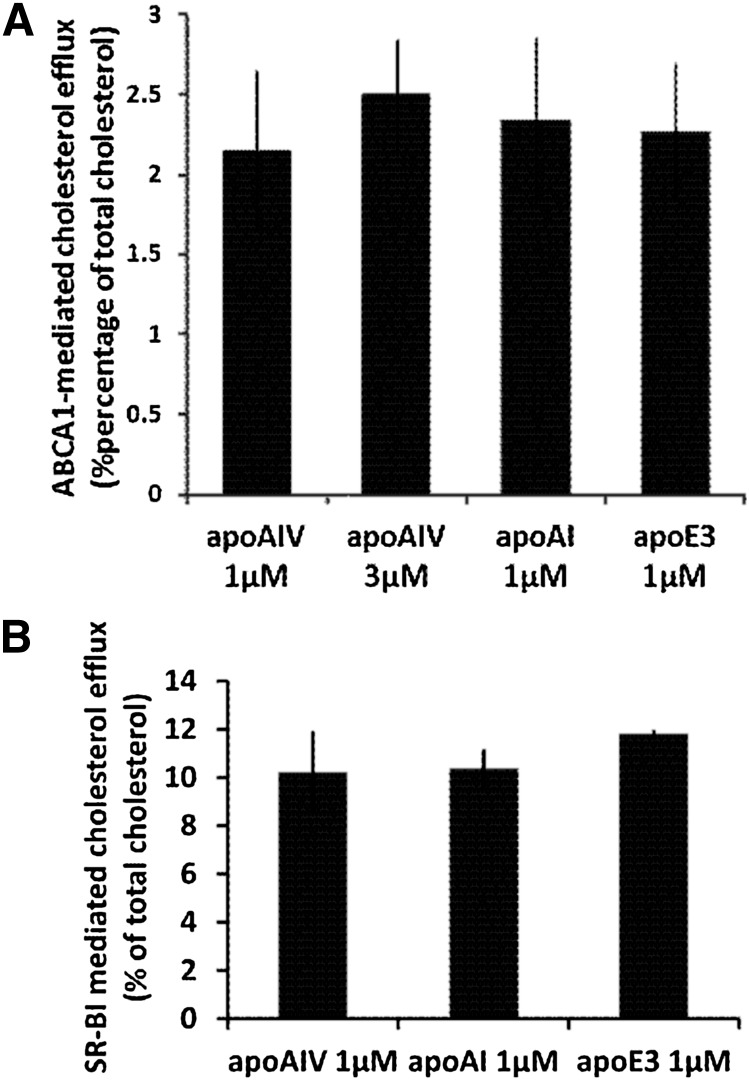
ApoA-IV secreted in the culture medium of adenovirus-infected HTB-13 grown on a large scale was purified and used to study its cholesterol efflux potential and its physicochemical properties. As shown in Fig. 1A, the ABCA1-mediated cholesterol efflux to lipid-free apoA-IV, which represents the first step in the biogenesis of HDL, was comparable to that of lipid-free apoA-I and apoE. Similarly the SR-BI-mediated cholesterol efflux of rHDL-A-IV was comparable to those of rHDL, containing apoA-I or apoE (Fig. 1B).
Fig. 1.
A: ABCA1-mediated cholesterol efflux from HEK293 EBNA-T cells transfected with an ABCA1-expressing plasmid using human apoA-I, apoE, and apoA-IV as cholesterol acceptors. Cholesterol efflux was determined as described in Experimental Procedures. The concentration of the acceptor apoA-IV in the medium was 1 μM or 3 μM and the concentration of apoA-I and apoE was 1 μM as indicated. The net efflux was calculated by subtracting the efflux obtained in the untransfected HEK293 EBNA-T cells from that of ABCA1-transfected cells. The difference in the net efflux promoted by apoA-IV, apoA-I, or apoE3 was not statistically significant. B: SR-BI-mediated cholesterol efflux from IdlA[mSR-BI] CHO cell line expressing the murine SR-BI (42), using rHDL-containing human apoA-I, apoE3, and apoA-IV as cholesterol acceptors. The concentration of each acceptor apolipoprotein bound to rHDL in the medium was 1 μM. The net efflux was calculated by subtracting the efflux obtained in the untransfected IdlA CHO cells from that of IdlA[mSR-BI] CHO cells. Values are the means ± SE from three experiments performed in duplicate. The difference in the net efflux promoted by rHDL-A-IV, rHDL-A-I, and rHDL-E3 was not statistically significant.
Recombinant ApoA-IV had structural and thermodynamic properties that were reminiscent of apoA-I and apoE. Circular dichroism measurements revealed a significant helical content of 41.4%, albeit reduced compared with apoA-I and apoE (48, 49). Upon mixing with egg yolk phosphatidyl-choline, recombinant apoA-IV readily formed HDL-like particles with increased helical content of 46.7% (supplementary Fig. I A,B and Table I). Thermal denaturation of apoA-IV revealed a single limited-cooperativity transition with a Tm of 45.6°C (supplementary Fig. II A). The thermal denaturation of apoA-IV was largely reversible, inasmuch as the protein recovered more than 95% of its secondary structure after cooling (supplementary Fig. II A,B). rHDL-A-IV particles were significantly more stable versus thermal denaturation (Tm = 61.4°C) and exhibited a limited-cooperativity nonreversible transition (supplementary Table I and Fig. II A,B). Chemical denaturation of apoA-IV revealed single-step transition with limited cooperativity that lacked the intermediate described for the thermal denaturation of apoE (49). Chemical denaturation of rHDL-A-IV showed a highly noncooperative transition (supplementary Fig. II C,D). Overall, biophysical analysis of recombinant apoA-IV suggests extensive conformational changes upon lipid binding similar to those described for other apolipoproteins. Furthermore, this analysis suggests that although apoA-IV has structural and thermodynamic properties similar to those of apoA-I and apoE, it still retains a unique structural and thermodynamic profile that may be consistent with distinct functional roles.
Effect of apoA-IV on lipid and lipoprotein profiles and the generation of HDL-A-IV
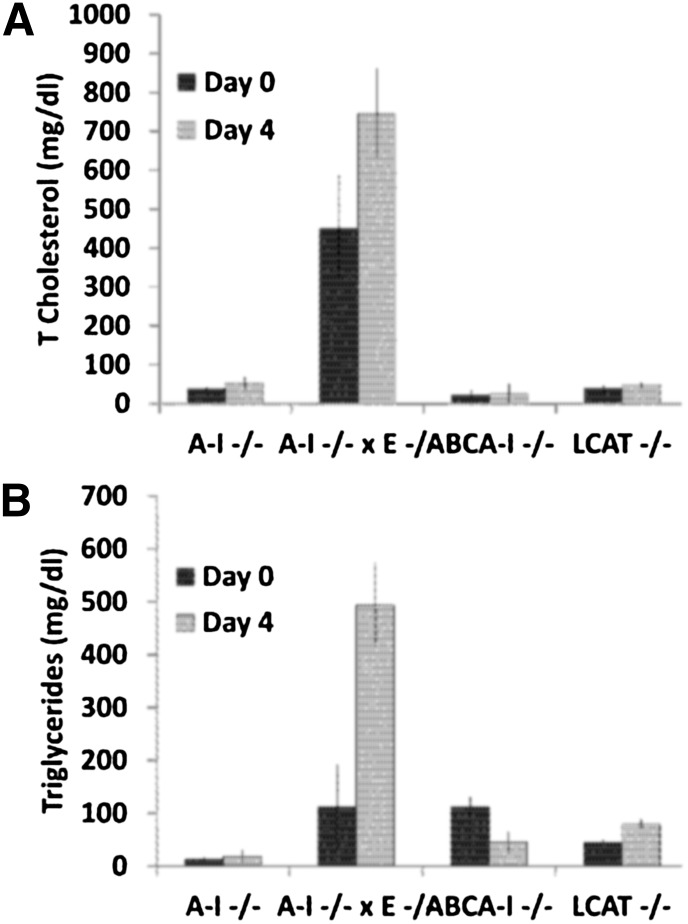
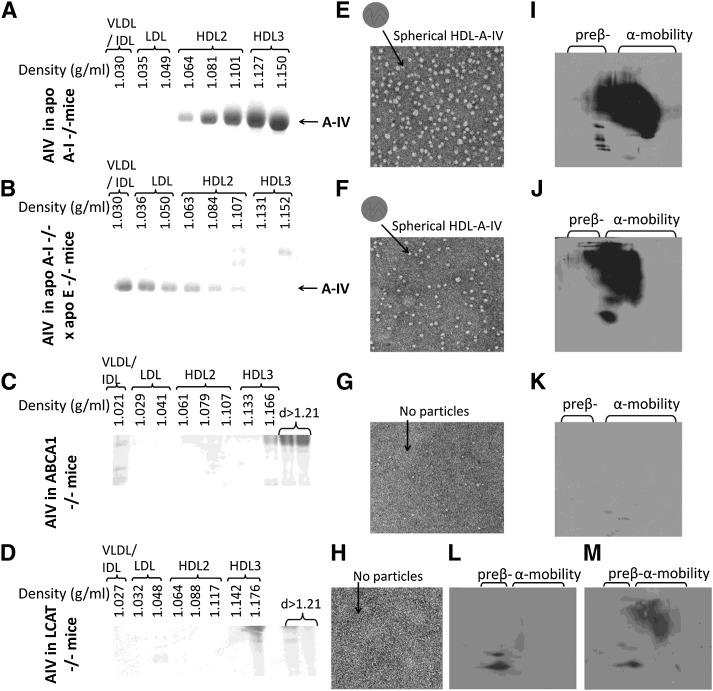
The changes in the lipid and lipoprotein profiles as a result of hepatic expression of apoA-IV were studied in different mouse models by adenovirus-mediated gene transfer 4 days postinfection. Gene transfer of apoA-IV in apoA-I−/− mice did not significantly alter total plasma lipid levels or the cholesterol and triglyceride FPLC profiles (Fig. 2A, B; Fig. 3A, B,, and supplementary Table II). The distribution of apoA-IV to different lipoprotein fractions was determined by density gradient ultracentrifugation of plasma followed by SDS-PAGE of the resulting fractions. This analysis showed that apoA-IV was distributed predominantly to HDL3 and, to a lesser extent, to the HDL2 fraction (Fig. 4A). EM of the HDL fractions showed that hepatic expression of apoA-IV promoted the formation of spherical particles (Fig. 4E). Two-dimensional gel electrophoresis of plasma showed that apoA-IV generated predominantly α-HDL particles with smaller amount of pre-β-like particles (Fig. 4I).
Fig. 2.
Changes in the plasma cholesterol (A) and triglyceride (B) levels caused by expression of human apoA-IV in different mouse models (apoA-I−/−, apoA-I−/− × apoE−/−, ABCA1−/−, and LCAT−/− mice).
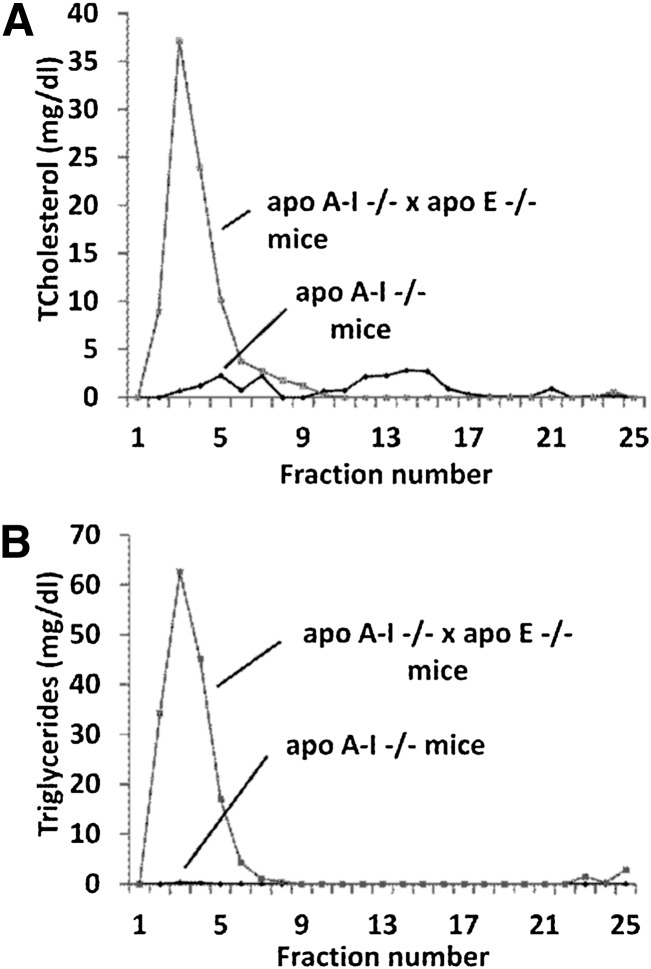
Fig. 3.
FPLC profiles of total cholesterol (A) and triglycerides (B) of apoA-I−/− and apoA-I−/− × apoE−/− mice 4 days post-infection with adenoviruses expressing the human apoA-IV as indicated.
Fig. 4.
Analyses of plasma of apoA-I−/−, apoA-I−/− × apoE−/−, ABCA1−/−, and LCAT−/− mice infected with the adenovirus expressing the human apoA-IV by density gradient ultracentrifugation and SDS-PAGE, EM, and two-dimensional gel electrophoresis. A–D: SDS-PAGE analysis of density gradient ultracentrifugation fractions. E–H: EM pictures of HDL fractions 6–7 obtained from mice expressing human apoA-IV following density gradient ultracentrifugation of plasma, as indicated. The photomicrographs were taken at 75,000× magnification and enlarged three times. I–M: Analysis of plasma obtained from mice expressing the human apoA-IV following two-dimensional gel electrophoresis and Western blotting. A, E, I: Analyses of apoA-I−/− mice. B, F, J: Analyses of apoA-I−/− × apoE−/− mice. C, G, K: Analyses of ABCA1−/− mice. D, H, L, M: Analyses of LCAT−/− mice.
A different picture was obtained by adenovirus mediated gene transfer of apoA-IV in apoA-I−/− × apoE−/− double-deficient mice. Hepatic apoA-IV expression in these mice increased plasma cholesterol to levels greater than those of the uninfected controls and induced hypertriglyceridemia (Fig. 2A, B). FPLC analysis showed that all the cholesterol and triglycerides were found in the VLDL/IDL region (Fig. 3A, B). SDS-PAGE analyses of the lipoprotein fractions separated by density gradient ultracentrifugation of plasma, showed that the observed dyslipidemia was associated with distribution of the majority (80%) of apoA-IV in the VLDL/IDL/LDL region and to a lesser extend to the HDL2/HDL3 region (Fig. 4B). The apoA-IV fractions that float in the VLDL/IDL/LDL region also contain large amounts of apoB-48 (data not shown). EM showed formation of spherical HDL (Fig. 4F) and two-dimensional gel electrophoresis of plasma showed predominantly the formation of α-HDL and a small amount of pre-β-like HDL particles (Fig. 4J). The findings shown in Fig. 4A, B, E, F, I, and J suggest strongly that apoA-IV participates in the generation of HDL-A-IV particles. The findings shown in Fig. 3A, B and Fig. 4A, B show for the first time that in the absence of both apoE and apoA-I, apoA-IV has increased affinity for triglyceride-rich lipoproteins and that this increased affinity is associated with the induction of hypertriglyceridemia.
ABCA1 and LCAT are required for the biogenesis of HDL-A-IV
The next task was to determine the role of ABCA1 and LCAT in the biogenesis of HDL-A-IV. Adenovirus-mediated gene transfer of apoA-IV in ABCA1−/− mice failed to form HDL particles. The density gradient ultracentrifugation did not show the presence of apoA-IV in the HDL region (Fig. 4C), and the EM analysis of the HDL fractions, combined with the two-dimensional gel electrophoresis of plasma, failed to demonstrate formation of HDL particles (Fig. 4G, K).
A similar picture emerged from adenovirus-mediated gene transfer of apoA-IV in LCAT−/− mice. Following gene transfer, apoA-IV was not present in the HDL fractions (Fig. 4D). HDL particles were not detected by EM (Fig. 4H), and the two-dimensional gel electrophoresis of the plasma showed the formation of two types of particles with pre-β-like mobility (Fig. 4L). The relationship of these particles with α-HDL particles formed in apoA-I−/− mice expressing apoA-IV was established by mixing experiments (Fig. 4M).
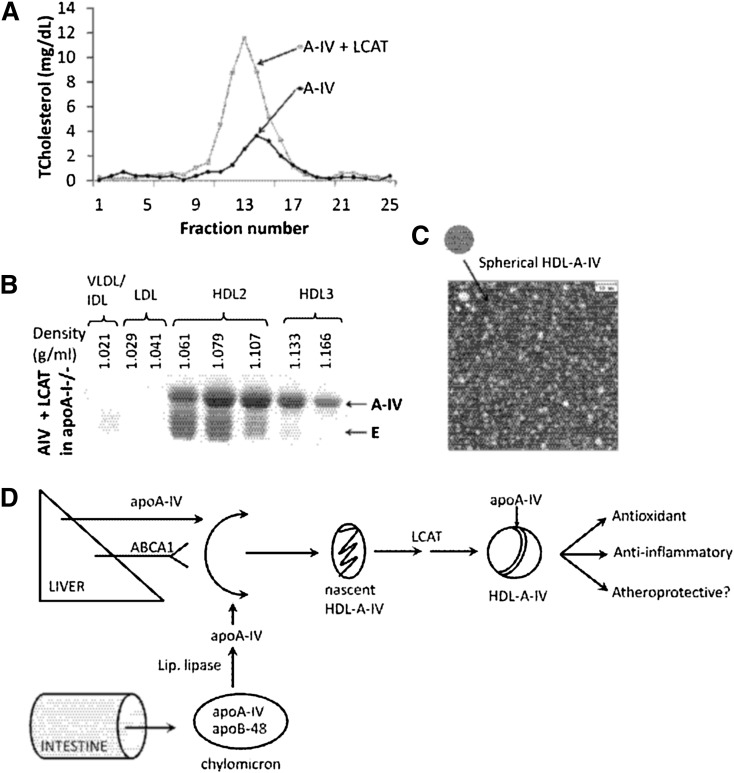
The role of LCAT in the biogenesis of apoA-IV-containing HDL was also explored by coexpression of apoA-IV and LCAT in apoA-I−/− mice. This treatment increased the plasma HDL cholesterol levels as determined by FPLC (Fig. 5A). It also promoted the flotation of apoA-IV in the HDL2 and HDL3 region (Fig. 5B) and generated spherical HDL-A-IV particles (Fig. 5C). The LCAT treatment also increased the concentration of the mouse apoE in the HDL2 fraction (Fig. 5B).
Fig. 5.
Analysis of plasma from apoA-I−/− mice coinfected with 109 pfu adenovirus expressing human apoA-IV and 5 × 108 pfu adenovirus expressing human LCAT. A: FPLC profiles. B: SDS-PAGE of the fractions isolated by density gradient gel electrophoresis. C: EM analysis of the HDL2 fractions shown in B. D: Schematic representation of the pathway of biogenesis and the putative beneficial functions of HDL-A-IV.
The overall pathway of the biogenesis and the potential functions of HDL-A-IV are depicted in Fig. 5D.
DISCUSSION
Role of apoA-IV, ABCA1, and LCAT in the biogenesis of HDL-A-IV
Although the functions of the intestinally delivered apoA-IV have been extensively studied during the past 35 years, there is limited information on the physiological significance and the functions of apoA-IV synthesized by the liver. Earlier studies showed that when ApoA-IV is purified from plasma by immunoprecipitation, immunoaffinity, gel filtration, or nondenaturing gradient gel electrophoresis, it is found on the HDL density fraction (50–52), but it dissociates from lipoproteins following ultracentrifugation of plasma (53). This raises the question whether apoA-IV-containing HDL particles originate from the transfer of apoA-IV that is displaced from chylomicrons to the surface of a preformed HDL molecule that contains apoA-I and in some instances other apolipoproteins. An alternative possibility is that HDL-A-IV particles are synthesized de novo by the liver.
Clues pertinent to this question were obtained from studies of transgenic mice expressing the apoA-IV gene under the control of its natural promoter or a heterologous hepatic promoter (15, 17, 19). Transgenic mice carrying the apoA-IV gene under the control of the common apoA-I/apoCIII/apoA-IV promoter and enhancer (54) express apoA-IV predominantly in the intestine and to a lesser extend in the liver (15). When the plasma of these transgenic mice was fractionated by gel filtration, the majority of apoA-IV was distributed in the same HDL fractions where apoA-I was also found (15). Such localization of apoA-IV reinforces the concept that lipid-free apoA-IV originating from chylomicrons or secreted by the liver may contribute in the de novo synthesis of HDL-A-IV particles.
We have shown previously that de novo synthesis of HDL particles containing apoA-I or apoE is initiated by interactions of the lipid-poor apolipoproteins with the ABCA1 lipid transporter. These functional interactions catalyze the transfer of phospholipids and subsequently cholesterol from intracellular membrane pools to lipid-free apoA-I or apoE leading to the formation of minimally lipidated particles which are gradually converted to discoidal particles (39, 47, 55, 56). Subsequent esterification of the cholesterol of the nascent pre-β and discoidal particles by LCAT generates the spherical HDL particles present in the plasma that can be visualized by EM (55, 56). In the present study the ability of apoA-IV to promote de novo formation of HDL-A-IV particles was established by adenovirus mediated gene transfer in four different mouse models. To ensure that pro-inflammatory conditions resulting from adenovirus over expression were not reached, we monitored the plasma transamimase levels during the experiments. Gene transfer of apoA-IV in apoA-I−/− mice showed that apoA-IV expressed in the liver was distributed in the HDL fraction of plasma. EM showed the presence of spherical particles and two-dimensional gel electrophoresis showed α-migrating HDL particles and pre-β-like HDL particles. To exclude the possibility that the spherical HDL particles observed in these experiments did not originate from apoE, we performed gene transfer experiments in apoA-I and apoE double-deficient mice. These studies also showed the formation of spherical HDL particles and pre-β-like and α-migrating HDL particles. These findings are consistent with in vivo interactions of lipid-free apoA-IV with ABCA1. As shown in Fig. 1A and documented in previous studies (20), lipid free apoA-IV promotes ABCA1 mediated cholesterol efflux to the same extend as lipid free apoA-I and apoE. The functional interactions of lipid-free apoA-IV with ABCA1 in vivo are expected to lipidate apoA-IV and lead to the generation of nascent HDL-A-IV particles. These particles may subsequently mature to spherical HDL-A-IV that can interact functionally with SR-BI. As shown in Fig. 1B, rHDL-A-IV promotes SR-BI mediated cholesterol efflux to similar extend as rHDL-A-I or rHDL-E (41, 57).
The requirement of ABCA1 and LCAT for the formation of HDL-A-IV was established by adenovirus-mediated gene transfer of apoA-IV in ABCA1- and LCAT-deficient mice, respectively. In these experiments, as expected, deficiency in ABCA1 prevented the formation of nascent or mature HDL-A-IV particles. The absence of LCAT also appears to prevent the formation of nascent or mature HDL-A-IV particles. It is possible that in the absence of LCAT, nascent HDL-A-IV particles formed by initial interactions of lipid-free apoA-IV with ABCA1 are susceptible to fast catabolism. This interpretation is supported by coexpression of apoA-IV and LCAT in LCAT−/− mice. This treatment increased the HDL cholesterol peak and the plasma apoA-IV levels, promoted the formation of spherical HDL-A-IV particles and resulted in the distribution of apoA-IV in the HDL2 and HDL3 regions. Fast catabolism of pre-β-apoA-I-containing HDL particles by the kidney has been described previously (58).
Effect of apoA-IV on lipid and lipoprotein profiles in different mouse models
The experiments described above also showed that following gene transfer in apoA-I−/− mice, apoA-IV was distributed in the HDL2 and HDL3 regions and the mice had normal triglycerides. In contrast following gene transfer of apoA-IV in the apoA-I−/− × apoE−/− mice, 80% of apoA-IV was distributed in the VLDL/IDL region where apoB is also found and the mice developed hypertriglyceridemia. This implies that deficiency for both apoA-I and apoE increased the affinity of apoA-IV for apoB-containing lipoprotein particles and this might have triggered the hypertriglyceridemia.
Is there a role for HDL-A-IV in atheroprotection?
Numerous previous studies have indicated that the conventional apoA-I-containing HDL particles promote cholesterol efflux (42, 59), prevent oxidation of LDL (60), and inhibit expression of proinflammatory cytokines by macrophages (61), as well as expression of adhesion molecules by endothelial cells (62). HDL inhibits cell apoptosis (63) and promotes endothelial cell proliferation and migration (64). HDL stimulates release of NO from endothelial cells, thus promoting vasodilation (65). Other studies have also indicated that several beneficial effects of HDL on the arterial wall cells are mediated through signaling mechanisms mediated by SR-BI or other cell surface proteins (65–67). Owing to these properties, the conventional apoA-I-containing HDL particles are thought to protect the endothelium and inhibit several steps in the cascade of events that lead to the pathogenesis of atherosclerosis and various other human diseases.
The ability of apoA-IV to form discrete populations of HDL-A-IV particles reported in this study provides the basis for exploring further the previously reported atheroprotective functions of apoA-IV. Such functions were demonstrated in mouse models expressing apoA-IV in the intestine or the liver (15, 17, 19) as well as of apoA-IV knock-out mice (14).
Overall, the present study establishes that apoA-IV has the capacity to promote the de novo biogenesis of discrete HDL-A-IV particles. The formation of these particles requires the functions of ABCA1 and LCAT. Further work is required to establish whether the generation of HDL-A-IV by the liver is responsible, at least partially, for the previously reported anti-inflammatory and atheroprotective functions of apoA-IV (16–19, 29).
Supplementary Material
Acknowledgments
A. Kateifides and P. Fotakis are students in the graduate program “The Molecular Basis of Human Disease” of the University of Crete Medical School. The authors thank Gayle Forbes for technical assistance.
Footnotes
Abbreviations:
- DMPC
- dimyristoyl-l-α-phosphatidyl-choline
- EM
- electron microscopy
- FPLC
- fast-protein liquid chromatography
- SR-BI
- scavenger receptor BI
- WT
- wild type
This work was supported by Grant HL-48739 from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies. P. Fotakis has been supported by pre-doctoral training Fellowship HERAKLEITOS II of the Greek Ministry of National Education. D. Georgiadou was supported by the graduate fellowship program of the National Center for Scientific Research “Demokritos”.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures, two tables, and supplemanary Methods.
REFERENCES
- 1.Swaney J. B., Reese H., Eder H. A. 1974. Polypeptide composition of rat high density lipoprotein: characterization by SDS-gel electrophoresis. Biochem. Biophys. Res. Commun. 59: 513–519 [DOI] [PubMed] [Google Scholar]
- 2.Li W. H., Tanimura M., Luo C. C., Datta S., Chan L. 1988. The apolipoprotein multigene family: biosynthesis, structure, structure-function relationships, and evolution. J. Lipid Res. 29: 245–271 [PubMed] [Google Scholar]
- 3.Karathanasis S. K., Yunis I., Zannis V. I. 1986. Structure, evolution, and tissue-specific synthesis of human apolipoprotein AIV. Biochemistry. 25: 3962–3970 [DOI] [PubMed] [Google Scholar]
- 4.Green P. H. R., Glickman R. M., Riley J. W., Quinet E. 1980. Human apolipoprotein-A-IV. Intestinal origin and distribution in plasma. J. Clin. Invest. 65: 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utermann G., Beisiegel U. 1979. Apolipoprotein-A-IV: protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur. J. Biochem. 99: 333–343 [DOI] [PubMed] [Google Scholar]
- 6.Lenich C., Brecher P., Makrides S., Chobanian A., Zannis V. I. 1988. Apolipoprotein gene expression in the rabbit: abundance, size, and distribution of apolipoprotein mRNA species in different tissues. J. Lipid Res. 29: 755–764 [PubMed] [Google Scholar]
- 7.Apostolopoulos J. J., La Scala M. J., Howlett G. J. 1988. The effect of triiodothyronine on rat apolipoprotein A-I and A-IV gene transcription. Biochem. Biophys. Res. Commun. 154: 997–1002 [DOI] [PubMed] [Google Scholar]
- 8.Weinberg R. B., Dantzker C., Patton C. S. 1990. Sensitivity of serum apolipoprotein A-IV levels to changes in dietary-fat content. Gastroenterology. 98: 17–24 [DOI] [PubMed] [Google Scholar]
- 9.Go M. F., Schonfeld G., Pfleger B., Cole T. G., Sussman N. L., Alpers D. H. 1988. Regulation of intestinal and hepatic apoprotein synthesis after chronic fat and cholesterol feeding. J. Clin. Invest. 81: 1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verges B., Rader D., Schaefer J., Zech L., Kindt M., Fairwell T., Gambert P., Brewer H. B., Jr 1994. In vivo metabolism of apolipoprotein A-IV in severe hypertriglyceridemia: a combined radiotracer and stable isotope kinetic study. J. Lipid Res. 35: 2280–2291 [PubMed] [Google Scholar]
- 11.Windmueller H. G., Wu A. L. 1981. Biosynthesis of plasma apolipoproteins by rat small intestine without dietary or biliary fat. J. Biol. Chem. 256: 3012–3016 [PubMed] [Google Scholar]
- 12.Lohse P., Brewer H. B. J. 1991. Genetic polymorphism of apolipoprotein A-IV. Curr. Opin. Lipidol. 2: 90–95 [Google Scholar]
- 13.Fujimoto K., Fukagawa K., Sakata T., Tso P. 1993. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J. Clin. Invest. 91: 1830–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstock P. H., Bisgaier C. L., Hayek T., Aalto-Setala K., Sehayek E., Wu L., Sheiffele P., Merkel M., Essenburg A. D., Breslow J. L. 1997. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J. Lipid Res. 38: 1782–1794 [PubMed] [Google Scholar]
- 15.Aalto-Setala K., Bisgaier C. L., Ho A., Kieft K. A., Traber M. G., Kayden H. J., Ramakrishnan R., Walsh A., Essenburg A. D., Breslow J. L. 1994. Intestinal expression of human apolipoprotein A-IV in transgenic mice fails to influence dietary lipid absorption or feeding behavior. J. Clin. Invest. 93: 1776–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vowinkel T., Mori M., Krieglstein C. F., Russell J., Saijo F., Bharwani S., Turnage R. H., Davidson W. S., Tso P., Granger D. N., et al. 2004. Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Invest. 114: 260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostos M. A., Conconi M., Vergnes L., Baroukh N., Ribalta J., Girona J., Caillaud J. M., Ochoa A., Zakin M. M. 2001. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 1023–1028 [DOI] [PubMed] [Google Scholar]
- 18.Duverger N., Tremp G., Caillaud J. M., Emmanuel F., Castro G., Fruchart J. C., Steinmetz A., Denefle P. 1996. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 273: 966–968 [DOI] [PubMed] [Google Scholar]
- 19.Cohen R. D., Castellani L. W., Qiao J. H., Van Lenten B. J., Lusis A. J., Reue K. 1997. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J. Clin. Invest. 99: 1906–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remaley A. T., Stonik J. A., Demosky S. J., Neufeld E. B., Bocharov A. V., Vishnyakova T. G., Eggerman T. L., Patterson A. P., Duverger N. J., Santamarina-Fojo S., et al. 2001. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem. Biophys. Res. Commun. 280: 818–823 [DOI] [PubMed] [Google Scholar]
- 21.Steinmetz A., Barbaras R., Ghalim N., Clavey V., Fruchart J. C., Ailhaud G. 1990. Human apolipoprotein-A-IV binds to apolipoprotein-A-I/apolipoprotein-A-II receptor-sites and promotes cholesterol efflux from adipose-cells. J. Biol. Chem. 265: 7859–7863 [PubMed] [Google Scholar]
- 22.Gomaraschi M., Putt W. E., Pozzi S., Iametti S., Barbiroli A., Bonomi F., Favari E., Bernini F., Franceschini G., Talmud P. J., et al. 2010. Structure and function of the apoA-IV T347S and Q360H common variants. Biochem. Biophys. Res. Commun. 393: 126–130 [DOI] [PubMed] [Google Scholar]
- 23.Steinmetz A., Utermann G. 1985. Activation of lecithin: cholesterol acyltransferase by human apolipoprotein A-IV. J. Biol. Chem. 260: 2258–2264 [PubMed] [Google Scholar]
- 24.Savion N., Gamliel A. 1988. Binding of apolipoprotein A-I and apolipoprotein A-IV to cultured bovine aortic endothelial cells. Arteriosclerosis. 8: 178–186 [DOI] [PubMed] [Google Scholar]
- 25.Weinberg R. B., Patton C. S. 1990. Binding of human apolipoprotein-A-IV to human hepatocellular plasma membranes. Biochim. Biophys. Acta. 1044: 255–261 [DOI] [PubMed] [Google Scholar]
- 26.Goldberg I. J., Scheraldi C. A., Yacoub L. K., Saxena U., Bisgaier C. L. 1990. Lipoprotein ApoC-II activation of lipoprotein-lipase. Modulation by apolipoprotein-A-IV. J. Biol. Chem. 265: 4266–4272 [PubMed] [Google Scholar]
- 27.Main L. A., Ohnishi T., Yokoyama S. 1996. Activation of human plasma cholesteryl ester transfer protein by human apolipoprotein A-IV. Biochim. Biophys. Acta. 1300: 17–24 [DOI] [PubMed] [Google Scholar]
- 28.Qin X. F., Swertfeger D. K., Zheng S. Q., Hui D. Y., Tso P. 1998. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am. J. Physiol. 274: H1836–H1840 [DOI] [PubMed] [Google Scholar]
- 29.Recalde D., Ostos M. A., Badell E., Garcia-Otin A. L., Pidoux J., Castro G., Zakin M. M., Scott-Algara D. 2004. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 24: 756–761 [DOI] [PubMed] [Google Scholar]
- 30.Lewis T. L., Cao D., Lu H., Mans R. A., Su Y. R., Jungbauer L., Linton M. F., Fazio S., LaDu M. J., Li L. 2010. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J. Biol. Chem. 285: 36958–36968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtzman D. M., Bales K. R., Tenkova T., Fagan A. M., Parsadanian M., Sartorius L. J., Mackey B., Olney J., McKeel D., Wozniak D., et al. 2000. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 97: 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y., Huang M., He Y., Zhang S., Luo Y. 2011. Genetic ablation of apolipoprotein A-IV accelerates Alzheimer's disease pathogenesis in a mouse model. Am. J. Pathol. 178: 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tubb M. R., Silva R. A., Pearson K. J., Tso P., Liu M., Davidson W. S. 2007. Modulation of apolipoprotein A-IV lipid binding by an interaction between the N and C termini. J. Biol. Chem. 282: 28385–28394 [DOI] [PubMed] [Google Scholar]
- 34.Pearson K., Saito H., Woods S. C., Lund-Katz S., Tso P., Phillips M. C., Davidson W. S. 2004. Structure of human apolipoprotein A-IV: a distinct domain architecture among exchangeable apolipoproteins with potential functional implications. Biochemistry. 43: 10719–10729 [DOI] [PubMed] [Google Scholar]
- 35.Laccotripe M., Makrides S. C., Jonas A., Zannis V. I. 1997. The carboxyl-terminal hydrophobic residues of apolipoprotein A-I affect its rate of phospholipid binding and its association with high density lipoprotein. J. Biol. Chem. 272: 17511–17522 [DOI] [PubMed] [Google Scholar]
- 36.Li X., Kypreos K., Zanni E. E., Zannis V. 2003. Domains of apoE required for binding to apoE receptor 2 and to phospholipids: implications for the functions of apoE in the brain. Biochemistry. 42: 10406–10417 [DOI] [PubMed] [Google Scholar]
- 37.Pearson K., Tubb M. R., Tanaka M., Zhang X. Q., Tso P., Weinberg R. B., Davidson W. S. 2005. Specific sequences in the N and C termini of apolipoprotein A-IV modulate its conformation and lipid association. J. Biol. Chem. 280: 38576–38582 [DOI] [PubMed] [Google Scholar]
- 38.Lu S., Yao Y., Cheng X. Y., Mitchell S., Leng S. Y., Meng S. M., Gallagher J. W., Shelness G. S., Morris G. S., Mahan J., et al. 2006. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 281: 3473–3483 [DOI] [PubMed] [Google Scholar]
- 39.Chroni A., Liu T., Gorshkova I., Kan H. Y., Uehara Y., von Eckardstein A., Zannis V. I. 2003. The central helices of apoA-I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux. Amino acid residues 220–231 of the wild-type apoA-I are required for lipid efflux in vitro and high density lipoprotein formation in vivo. J. Biol. Chem. 278: 6719–6730 [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald M. L., Mendez A. J., Moore K. J., Andersson L. P., Panjeton H. A., Freeman M. W. 2001. ATP-binding cassette transporter A1 contains an NH2-terminal signal anchor sequence that translocates the protein's first hydrophilic domain to the exoplasmic space. J. Biol. Chem. 276: 15137–15145 [DOI] [PubMed] [Google Scholar]
- 41.Chroni A., Nieland T. J., Kypreos K. E., Krieger M., Zannis V. I. 2005. SR-BI mediates cholesterol efflux via its interactions with lipid-bound ApoE. Structural mutations in SR-BI diminish cholesterol efflux. Biochemistry. 44: 13132–13143 [DOI] [PubMed] [Google Scholar]
- 42.Gu X., Kozarsky K., Krieger M. 2000. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J. Biol. Chem. 275: 29993–30001 [DOI] [PubMed] [Google Scholar]
- 43.Williamson R., Lee D., Hagaman J., Maeda N. 1992. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc. Natl. Acad. Sci. USA. 89: 7134–7138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorngate F. E., Yancey P. G., Kellner-Weibel G., Rudel L. L., Rothblat G. H., Williams D. L. 2003. Testing the role of apoA-I, HDL, and cholesterol efflux in the atheroprotective action of low-level apoE expression. J. Lipid Res. 44: 2331–2338 [DOI] [PubMed] [Google Scholar]
- 45.McNeish J., Aiello R. J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K. L., Roach M. L., Royer L. J., de Wet J., et al. 2000. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97: 4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai N., Vaisman B. L., Koch C. A., Hoyt R. F., Jr, Meyn S. M., Talley G. D., Paiz J. A., Brewer H. B., Jr, Santamarina-Fojo S. 1997. Targeted disruption of the mouse lecithin:cholesterol acyltransferase (LCAT) gene. Generation of a new animal model for human LCAT deficiency. J. Biol. Chem. 272: 7506–7510 [DOI] [PubMed] [Google Scholar]
- 47.Vezeridis A. M., Chroni A., Zannis V. I. 2011. Domains of apoE4 required for the biogenesis of apoE-containing HDL. Ann. Med. 43: 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorshkova I. N., Liadaki K., Gursky O., Atkinson D., Zannis V. I. 2000. Probing the lipid-free structure and stability of apolipoprotein A-I by mutation. Biochemistry. 39: 15910–15919 [DOI] [PubMed] [Google Scholar]
- 49.Chroni A., Pyrpassopoulos S., Thanassoulas A., Nounesis G., Zannis V. I., Stratikos E. 2008. Biophysical analysis of progressive C-terminal truncations of human apolipoprotein E4: insights into secondary structure and unfolding properties. Biochemistry. 47: 9071–9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisgaier C. L., Sachdev O. P., Megna L., Glickman R. M. 1985. Distribution of apolipoprotein-A-IV in human plasma. J. Lipid Res. 26: 11–25 [PubMed] [Google Scholar]
- 51.Lagrost L., Gambert P., Boquillon M., Lallemant C. 1989. Evidence for high-density lipoproteins as the major apolipoprotein A-IV-containing fraction in normal human-serum. J. Lipid Res. 30: 1525–1534 [PubMed] [Google Scholar]
- 52.Duverger N., Ghalim N., Ailhaud G., Steinmetz A., Fruchart J. C., Castro G. 1993. Characterization of apoA-IV-containing lipoprotein particles isolated from human plasma and interstitial fluid. Arterioscler. Thromb. 13: 126–132 [DOI] [PubMed] [Google Scholar]
- 53.Weinberg R. B., Spector M. S. 1985. Structural properties and lipid-binding of human apolipoprotein A-IV. J. Biol. Chem. 260: 4914–4921 [PubMed] [Google Scholar]
- 54.Zannis V. I., Kan H. Y., Kritis A., Zanni E. E., Kardassis D. 2001. Transcriptional regulatory mechanisms of the human apolipoprotein genes in vitro and in vivo. Curr. Opin. Lipidol. 12: 181–207 [DOI] [PubMed] [Google Scholar]
- 55.Kypreos K. E., Zannis V. I. 2007. Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem. J. 403: 359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zannis V. I., Chroni A., Krieger M. 2006. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 84: 276–294 [DOI] [PubMed] [Google Scholar]
- 57.Liadaki K. N., Liu T., Xu S., Ishida B. Y., Duchateaux P. N., Krieger J. P., Kane J., Krieger M., Zannis V. I. 2000. Binding of high density lipoprotein (HDL) and discoidal reconstituted HDL to the HDL receptor scavenger receptor class B type I. Effect of lipid association and APOA-I mutations on receptor binding. J. Biol. Chem. 275: 21262–21271 [DOI] [PubMed] [Google Scholar]
- 58.Miettinen H. E., Gylling H., Miettinen T. A., Viikari J., Paulin L., Kontula K. 1997. Apolipoprotein A-IFin. Dominantly inherited hypoalphalipoproteinemia due to a single base substitution in the apolipoprotein A-I gene. Arterioscler. Thromb. Vasc. Biol. 17: 83–90 [DOI] [PubMed] [Google Scholar]
- 59.Nakamura K., Kennedy M. A., Baldan A., Bojanic D. D., Lyons K., Edwards P. A. 2004. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J. Biol. Chem. 279: 45980–45989 [DOI] [PubMed] [Google Scholar]
- 60.Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., Fogelman A. M. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41: 1495–1508 [PubMed] [Google Scholar]
- 61.Okura H., Yamashita S., Ohama T., Saga A., Yamamoto-Kakuta A., Hamada Y., Sougawa N., Ohyama R., Sawa Y., Matsuyama A. 2010. HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. J. Atheroscler. Thromb. 17: 568–577 [DOI] [PubMed] [Google Scholar]
- 62.Cockerill G. W., Rye K. A., Gamble J. R., Vadas M. A., Barter P. J. 1995. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 15: 1987–1994 [DOI] [PubMed] [Google Scholar]
- 63.Nofer J. R., Levkau B., Wolinska I., Junker R., Fobker M., von Eckardstein A., Seedorf U., Assmann G. 2001. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J. Biol. Chem. 276: 34480–34485 [DOI] [PubMed] [Google Scholar]
- 64.Seetharam D., Mineo C., Gormley A. K., Gibson L. L., Vongpatanasin W., Chambliss K. L., Hahner L. D., Cummings M. L., Kitchens R. L., Marcel Y. L., et al. 2006. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ. Res. 98: 63–72 [DOI] [PubMed] [Google Scholar]
- 65.Mineo C., Yuhanna I. S., Quon M. J., Shaul P. W. 2003. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 278: 9142–9149 [DOI] [PubMed] [Google Scholar]
- 66.Terasaka N., Westerterp M., Koetsveld J., Fernandez-Hernando C., Yvan-Charvet L., Wang N., Sessa W. C., Tall A. R. 2010. ATP-binding cassette transporter G1 and high-density lipoprotein promote endothelial NO synthesis through a decrease in the interaction of caveolin-1 and endothelial NO synthase. Arterioscler. Thromb. Vasc. Biol. 30: 2219–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okajima F., Sato K., Kimura T. 2009. Anti-atherogenic actions of high-density lipoprotein through sphingosine 1-phosphate receptors and scavenger receptor class B type I. Endocr. J. 56: 317–334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.