Abstract
Mammalian spermatogenesis is a complex developmental program in which a diploid progenitor germ cell transforms into highly specialized spermatozoa. One intriguing aspect of sperm production is the dynamic change in membrane lipid composition that occurs throughout spermatogenesis. Cholesterol content, as well as its intermediates, differs vastly between the male reproductive system and nongonadal tissues. Accumulation of cholesterol precursors such as testis meiosis-activating sterol and desmosterol is observed in testes and spermatozoa from several mammalian species. Moreover, cholesterogenic genes, especially meiosis-activating sterol-producing enzyme cytochrome P450 lanosterol 14α-demethylase, display stage-specific expression patterns during spermatogenesis. Discrepancies in gene expression patterns suggest a complex temporal and cell-type specific regulation of sterol compounds during spermatogenesis, which also involves dynamic interactions between germ and Sertoli cells. The functional importance of sterol compounds in sperm production is further supported by the modulation of sterol composition in spermatozoal membranes during epididymal transit and in the female reproductive tract, which is a prerequisite for successful fertilization. However, the exact role of sterols in male reproduction is unknown. This review discusses sterol dynamics in sperm maturation and describes recent methodological advances that will help to illuminate the complexity of sperm formation and function.
Keywords: cholesterol, cytochrome P450, male germ cells, neutral sterols, sperm development, sterols in reproduction, sterol intermediates, testis-specific gene expression
DISCOVERY OF STEROLS THAT AFFECT MEIOSIS IN VITRO
The cholesterol biosynthesis pathway is a sequence of enzymatic reactions involving various intermediates. Early intermediates of cholesterol synthesis have long been known to function as precursors for the synthesis of essential compounds such as dolichol, heme A, and coenzyme Q10 (1). The first steroidal intermediate, lanosterol, was found to be formed from squalene in one of the most complex single enzymatic reactions ever identified (2). Late sterol intermediates, alternatively known as neutral sterols (Fig. 1), were long thought to be dedicated exclusively to the synthesis of the end product, cholesterol; however, a potential functional role of neutral sterols in mammals was first proposed in 1995 (3). Screens for naturally occurring compounds with meiosis-activating potency have led to the identification of cholesterol synthesis intermediates, termed meiosis-activating sterols (MAS). Experiments demonstrated the ability of MAS to trigger the resumption of meiosis in cultured mouse oocytes in vitro. Two structurally related sterols with similar biological effects were initially primarily identified in human preovulatory ovarian follicular fluid (FF-MAS) and testis of adult bull (T-MAS) (3). The chemical names are 4,4-dimethyl-5α-cholest-8,14,24-triene-3β-ol for FF-MAS and 4,4-dimethyl-5α-cholest-8,24-diene-3β-ol for T-MAS. Chemically synthesized sterols MAS-414 and MAS-412, which closely resemble FF-MAS and T-MAS, were able to trigger the resumption of oocyte meiosis in vitro, similar to their naturally occurring counterparts. These were the first examples implicating sterol intermediates in direct biological roles apart from being transitory intermediates in the chain of reactions leading up to the synthesis of a functional end product, cholesterol. MAS were found to accumulate primarily in the gonadal tissue of both sexes and were shown by in vitro assays to stimulate meiosis in sex- and species-nonspecific manners. On the basis of these initial biochemical studies, a potentially important in vivo role of MAS in reproduction was proposed (3, 4).
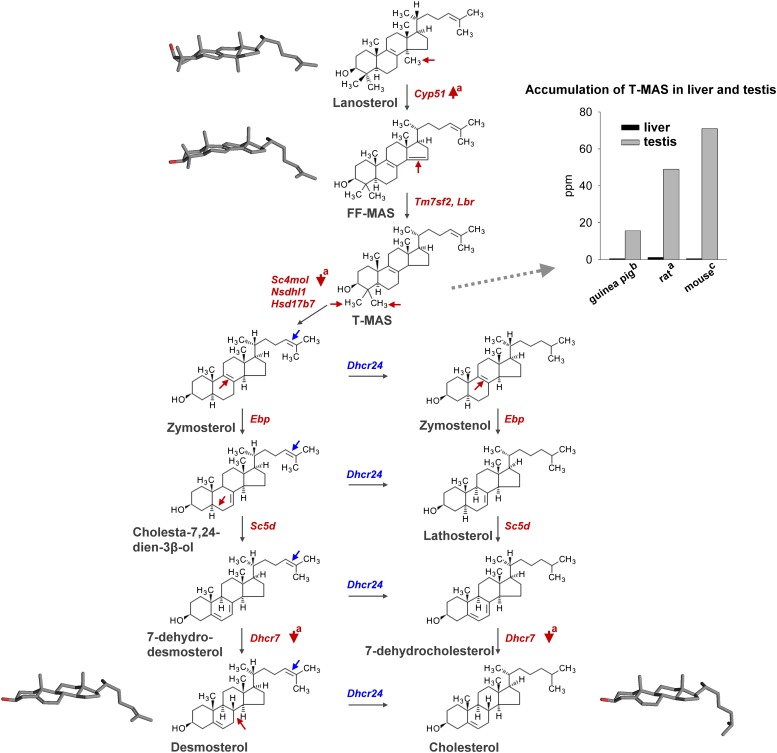
Fig. 1.
Postlanosterol intermediates of cholesterol synthesis. Mouse genes encoding enzymes that catalyze individual enzymatic steps are displayed in red or blue. Sites of enzymatic changes in individual sterol intermediates are displayed with arrows. Note the up-regulation of Cyp51 and down-regulation of postMAS genes Sc4mol and Dhcr7 reported during development of the rat testis (a) (9). Discordant regulation of cholesterogenic enzymes is a possible reason for the accumulation of T-MAS in the testis of rat (a), guinea pig (b) (12), and mouse (c) (13), compared with the quantity of T-MAS in the liver. Three-dimensional structures of lanosterol, FF-MAS, desmosterol, and cholesterol obtained with a Pc3D molecule viewer display the spatial conformation of individual intermediates, which might affect sperm membrane properties.
The identification of the meiosis-activating potency of MAS was soon followed by numerous in vitro studies examining the potential role of MAS in oocyte maturation. One early hypothesis suggested that MAS produced by cumulus cells in response to a gonadotropin surge during ovulation serve as paracrine factors to stimulate the resumption of oocyte meiosis (4). MAS were initially considered to be important in the treatment of infertility and in the development of novel contraceptive drugs that would not be based on sex steroids or sex steroid-like compounds (4); however, contradictory data indicated that MAS are not essential for the resumption of meiosis mediated by gonadotropin hormones (5). Subsequently, eight endogenous MAS molecules with similar structures and equipotent meiosis-activating activities were identified (6) and demonstrated that FF-MAS and related MAS analogs possess an obvious dose-dependent meiosis-activating effect in vitro. In addition, MAS compounds were demonstrated to have a positive influence on cytoplasmic maturation, an important process occurring during the final steps of oocyte development that enables completion of nuclear maturation, fertilization, and proper early embryo development. In contrast to the initially proposed role of MAS in the resumption of meiosis, it was suggested that MAS might have another role in meiotic nuclear processes and cytoplasmic maturation (6); however, the exact mechanism of MAS in female reproduction remains controversial.
ACCUMULATION OF MEIOSIS-ACTIVATING STEROLS IN THE TESTIS AND SPERM
Although MAS function in oocytes has been examined extensively, studies investigating its function in spermatogenesis are scarce. Early in vitro experiments demonstrated that a diffusible component present in adult mouse testes triggers nondifferentiated fetal male germ cells to enter meiosis (7, 8). After its identification in 1995, the role of MAS in male germ cell development was often suggested, but its causal relationship was never demonstrated conclusively in vivo or in vitro. Most studies aimed to quantify MAS in the male reproductive tract or the expression levels of MAS-producing enzyme cytochrome P450 14α-demethylase (CYP51) in testes from different mammalian species and to correlate these parameters with reproductive function. First reports of MAS in adult testes of bull, mouse, and horse demonstrated that MAS accumulate at concentrations above 30 μg/g (parts per million [ppm]) (3, 4); however, lower concentrations of MAS were detected in isolated rat seminiferous tubules (15 ppm) (4). Nevertheless, T-MAS was found to be the predominant sterol intermediate in the rat testis, with a 40-fold higher concentration than in the liver (Fig. 1). During sexual maturation (i.e., 19–70 days of age), the concentration of T-MAS in the rat testis also increased 8-fold (i.e., 6.1–49 ppm) (9). Another group demonstrated a 4-fold increase of T-MAS in the rat testis during puberty (10). No other sterol precursor has shown comparable changes in testis during puberty, implying the important role of T-MAS in spermatogenesis.
Accumulation of T-MAS was further demonstrated in sexually maturing stallions. A study on 16 stallions aged 1–12 years demonstrated a positive correlation between testicular T-MAS concentration and testicular weight as well as a negative association between testicular T-MAS concentration and cryptorchidism after puberty. Testis of mature studs contained a 7-fold higher concentration of T-MAS than that of immature males (19.3 vs. 2.8 ppm) (11). The concentration of T-MAS in isolated spermatozoa has been only measured in humans, where it was 4-fold higher (2 ppm) than in the ejaculate (< 0.5 ppm), illustrating accumulation of MAS in sperm cells and not in seminal plasma (4). However, measurements of MAS and several other sterol intermediates in guinea pig testes revealed that, by quantity, T-MAS ranked third (15.6 ppm) after lathosterol (23.5 ppm) and desmosterol (21.4 ppm). The concentration of T-MAS in the testis was at least 15-fold higher than in the liver (Fig. 1) (12). In the adult mouse testis (10–12 weeks), T-MAS was shown to be a predominant sterol (71 ppm), followed by desmosterol (15 ppm). The concentration of T-MAS in testis was at least 70-fold higher than in the liver (Fig. 1) (13). These results obtained by HPLC are not completely in line with recent gas chromatography-mass spectrometry-based sterol profiling studies that used mice testes of the same age because desmosterol was found to be higher than T-MAS (52 vs. 40 ppm) (Keber et al., unpublished data). Nevertheless, T-MAS has been reported to be present in sexually mature testes and sperm from various mammalian species, providing an excellent argument for the potentially important role of MAS in sperm development.
UNIQUE TESTICULAR EXPRESSION PATTERN OF CYP51 ENCODING THE MAS-PRODUCING ENZYME
Substantial support for the role of MAS in spermatogenesis came from studies on lanosterol 14α-demethylase, which encodes the CYP51 enzyme that is known to catalyze the conversion of lanosterol to FF-MAS. CYP51 is a unique member of the cytochrome P450 (CYP) superfamily. Of the more than 10,000 cytochrome P450s in existence, it is the most widely distributed and the only one present in all biological kingdoms (http://drnelson.uthsc.edu/P450.statsfile.html). From an evolutionary standpoint, Cyp51 is the most conserved gene within the P450 superfamily (14). Mammalian CYP51 from different species displays over 90% sequence identity at the protein level (15). Moreover, CYP51 from distant eukaryotic organisms has been found to be strikingly similar at the structural level (16–19). These data indicate that CYP51 function has remained highly conserved throughout evolution, suggesting an essential role of this gene in organisms of various taxa. In mammals, CYP51 catalyzes an essential late step in cholesterol biosynthesis, the demethylation of lanosterol and 24,25-dihydrolanosterol into the intermediate FF-MAS, which is further converted into T-MAS by one of the enzymes with sterol-Δ14-reductase activity, transmembrane 7 superfamily member 2, or lamin B receptor. At least seven additional enzymatic steps are required to synthesize cholesterol (Fig. 1). The essential role of Cyp51 in de novo cholesterol synthesis and embryo development in vivo has recently been demonstrated by our group (20). In addition to its role in cholesterol synthesis, Cyp51 is thought to have an essential role in reproduction as an enzyme producing the intermediate FF-MAS. This function was first suggested when MAS was found to have meiosis-activating potency (3). To investigate the proposed role of Cyp51 in reproduction, the expression pattern of Cyp51 in testes from several mammalian species was examined. Northern analyses using various human tissues revealed high levels of Cyp51 transcripts in the testis primarily due to the synthesis of additional shorter testis-specific transcript (21). Accumulation of this testis-specific Cyp51 transcript was later confirmed in sexually mature rats, in contrast to prepubertal animals (22). Interestingly, in situ hybridization and northern analysis using testis cross-sections and different testicular cell fractions, respectively, revealed stage-specific expression of Cyp51 in germ cells. Cyp51 expression was lowest in pachytene spermatocytes but increased, reaching its highest level in elongating spermatids; however, Cyp51 mRNA transcripts were not detected in most elongated spermatids that line the luminal edge of the seminiferous epithelium. With increasing accumulation of the shorter testis-specific transcript, the expression of the longer somatic transcripts declined (22). Moreover, a similar stage-specific expression pattern of Cyp51 was later confirmed in mouse (23) and human testes (9). Only background levels of Cyp51 mRNA were detected in steroidogenic Leydig cells. Studies showing high evolutionary conservation of the Cyp51 gene and a unique mRNA expression profile in testis suggested an important role of Cyp51 (and its product MAS) in reproduction.
An immunolocalization study revealed CYP51 to be highest in Leydig cells and round and elongated spermatids in the rat testis (Fig. 2) (24). When these findings are interpreted together with the aforementioned expression results, we can propose that translation of Cyp51 mRNA most likely occurs without delay and continues in later stages of the epithelial cycle that lack mRNA synthesis. Translation of the testis-specific Cyp51 mRNA might be less efficient to supply a template for its protein synthesis during the final stages of spermatogenesis. On the other hand, CYP51 translation in Leydig cells where low expression resulted in an abundance of protein appears to be highly efficient, in contrast to germ cells. CYP51 protein was not restricted to the cytoplasm of the developing germ cells where the endoplasmic reticulum resides but was also seen in the acrosomal regions of round and elongated spermatids and in residual bodies (25). The presence of CYP51 protein in residual bodies has led to the hypothesis that MAS synthesis in haploid germ cells might be important for the initiation of meiosis in premeiotic stages. If the synthesis of MAS in postmeiotic germ cells is important for the regulation of meiosis during early spermatogenesis, MAS would need to be reversely transported to premeiotic germ cells. One possible transport route would be via residual bodies that are formed during spermiogenesis and that contain excess cytoplasm and organelles. Residual bodies are phagocytosed by surrounding Sertoli cells and transported toward the basal membrane (26, 27). Transport of compounds contained in residual bodies from the adluminal to the basal cell compartment has been reported previously (28). In addition to CYP51, the expression of cytochrome P450 oxidoreductase (POR), which serves as an obligate CYP51 electron donor, was examined in the testis. POR protein was present in Leydig cells, residual bodies, and late-stage elongated spermatids (Fig. 2). The entire lanosterol 14α-demethylase complex (CYP51 and POR) has also been detected in the acrosomal membranes of round and elongated spermatids in the mouse (25). In addition, conversion of lanosterol to FF-MAS was observed in isolated acrosomal membranes from ejaculated bull sperm, suggesting that spermatozoa might produce FF-MAS in situ (25). It was therefore speculated that spermatid-produced MAS sterols might trigger the resumption of meiosis in quiescent oocytes upon fertilization. This hypothesis was also supported by the fact that the highest concentration of MAS was not found in the ovary but in the testis and spermatozoa (4).
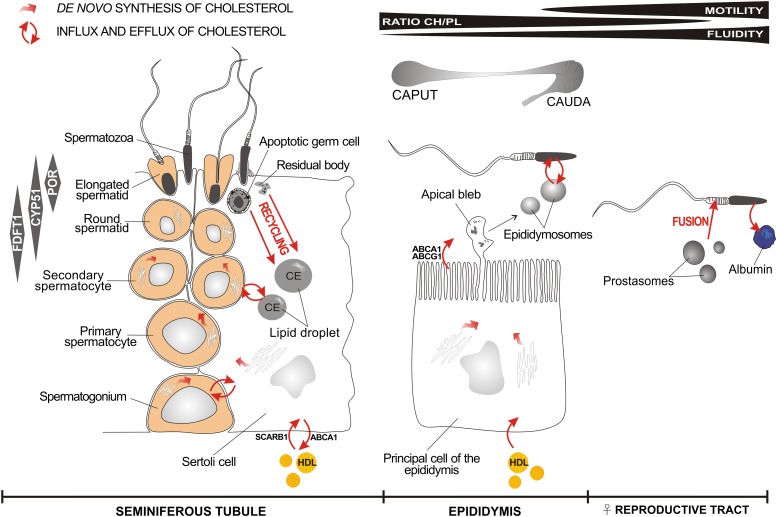
Fig. 2.
Schematic representation of major cholesterol synthesis and trafficking sites during sperm maturation. A limited quantity of cholesterol required to synthesize new plasma membranes during spermatogenesis in seminiferous tubules originates from de novo synthesis in spermatocytes (34). The stage-specific expression of cholesterogenic enzymes farnesyl diphosphate farnesyl transferase 1 (FDFT1), CYP51, and POR during spermatogenesis is displayed in gray boxes (left) (24). Supporting Sertoli cells provide an additional source of cholesterol for spermatogenesis. Sertoli cells acquire cholesterol by de novo synthesis from acetate (35) or import external cholesterol from HDL (37) by specialized cholesterol transporters (36). Another important source of cholesterol in Sertoli cells might be from the recycling of lipid-rich residual bodies and apoptotic germ cells (39). Excess cholesterol can be esterified to cholesterol esters (CE) and stored in lipid droplets, serving as cholesterol reservoirs (40). Some cholesterol can be effluxed to HDL by reverse cholesterol transport (41). Spermatozoa formed in the testis enter the caput epididymis and progress to the caudal region. The epididymis possesses the ability for de novo cholesterol synthesis (51), or it can import cholesterol from the circulation (59). Cholesterol might be effluxed into the epididymal lumen by ABCA1 and ATP-binding cassette sub-family G member 1 (ABCG1) (53). The principal cells of the epididymis secrete small membranous vesicles known as epididymosomes, which could serve as a source for cholesterol exchange with maturing sperm cells (128). The cholesterol content of sperm membranes is decreased during epididymal transit in several species, resulting in the decreased ratio of cholesterol (CH) to phospholipids (PL) (48). The loss of cholesterol results in an increase in sperm membrane fluidity and sperm motility (71). The content of cholesterol in the sperm membrane is further decreased in the female reproductive tract, mostly by the process of capacitation. Albumin serves as a cholesterol acceptor during capacitation. Prostasomes present in the ejaculate are able to fuse with the membranes of spermatozoa, to increase motility, and to prevent early acrosome reaction (73). Individual data were obtained from in vitro and in vivo experiments on different mammalian species and used to collate this scheme.
STAGE-SPECIFIC EXPRESSION OF CHOLESTEROGENIC GENES IN MALE GERM CELLS
An additional argument to support the role of MAS in spermatogenesis comes from the unique testicular expression pattern of other cholesterogenic genes upstream of CYP51. Besides Cyp51, testis-specific expression was observed also for farnesyl diphosphate synthetase (Fdps) and farnesyl diphosphate farnesyl transferase 1 or squalene synthase (Fdft1). Fdps is expressed as a longer, developmentally regulated testis-specific transcript that localizes specifically to postmeiotic round spermatids in rat (29). Similarly, a longer testis-specific transcript was described for Fdft1 (30). Fdft1 mRNA levels were highest in round spermatids and decreased in elongated spermatids (22). During spermatogenesis, Fdft1 expression peaked earlier than that of Cyp51 (Fig. 2), which is in line with the fact that Fdft1 acts upstream of Cyp51. A protein analysis study showed that Fdft1 translation is moderately delayed, similar to Cyp51 (24). As mentioned previously, POR is expressed exclusively in late stages of spermatogenesis. Taken collectively, these results demonstrate that cholesterogenic proteins are not constantly and coordinately expressed during spermatogenesis, as is the case in other tissues where they provide a housekeeping role in cholesterol synthesis. Indeed, the transcriptional regulation of cholesterogenic genes in the testis is different from cholesterol-dependent regulation by the sterol regulatory element binding protein pathway in the liver (23). One example is the unique germ cell-specific regulation of Cyp51 by cAMP-responsive element modulator τ (CREMτ). CREMτ acts as a master switch that is responsible for the cAMP-dependent transcriptional activation of specific genes in postmeiotic male germ cells, including Cyp51 (31). This regulatory mechanism is responsible for the presence of the shorter Cyp51 transcript in the testis (23). Crem−/− mice lacked germ cell-specific Cyp51 mRNA in testis, whereas the expression of somatic Cyp51 and testis-specific Fdft1 remained unaffected. In a recent large-scale expression analysis study of Crem−/− mice, Crem was found to be involved in several aspects of spermatogenesis (32). Based on these results, one could speculate that the role of Cyp51 in germ cell development is indispensable.
Another support for the role of MAS in spermatogenesis comes from the comparison of expression patterns between preMAS and postMAS genes (9). This study showed that during sexual development of the rat testis, preMAS genes were up-regulated, whereas postMAS genes were expressed at low levels, unchanged, or down-regulated with increasing age. These results also implied that other, yet-to-be-defined transcription factors besides cAMP/CREMτ might be involved in the control of cholesterogenic genes or sets of genes during spermatogenesis. Microarray data on the expression of cholesterogenic genes in mouse spermatogenic fractions (http://mrg.genetics.washington.edu) confirmed the high accumulation of presqualene Fdft1 and squalene epoxidase (Sqle) RNA in round spermatids (33). Based on the lack of a coordinated transcriptional regulation and a discrepancy between preMAS and postMAS gene expression, it was suggested that the primary role of male germ cells may not be to synthesize cholesterol but to produce MAS (9). This hypothesis is well supported by an 8-fold increase in T-MAS and a 43% decrease of cholesterol during sexual maturation in rat. The stage-specific expression pattern of Cyp51 and the differential expression of other cholesterogenic genes in germ cells suggest that a specific regulatory mechanism might exist to facilitate the accumulation of MAS intermediates in the testis.
SYNTHESIS OF CHOLESTEROL IN GERM CELLS DURING SPERMATOGENESIS
In contrast to MAS intermediates, the role of cholesterol in sperm maturation and male fertility has been well studied. The underlying imperative was that one could obtain insight into the role of cholesterol in sperm development by studying the dynamic cellular events that control its level during spermatogenesis and after spermiation. Due to the extensive production of germ cells throughout spermatogenesis, the requirement for cholesterol is significant. During sperm release from the seminiferous epithelium, sperm plasma membranes are believed to be highly loaded with cholesterol. Early experiments examining the role of de novo cholesterol synthesis revealed elevated rates of 14C acetate incorporation into cholesterol during the leptotene, zygotene, and pachytene spermatocyte stages of development (34). This implies that increased de novo cholesterol synthesis is ongoing in these germ cells, which correlates well with the increase in germ cell diameter and surface area during development, requiring the synthesis of new plasma membranes. The capacity to synthesize cholesterol decreased in late pachynema and remained low in all subsequent stages of spermatogenesis, including mature sperm. From the end of the pachytene stage, spermatocytes do not increase significantly in size but differentiate to diplotene spermatocytes, which undergo meiotic divisions to form round spermatids. In contrast to cholesterol, the rate of acetate incorporation into dolichol remained elevated in late pachytene spermatocytes and round spermatids and then decreased and remained low in mature sperm (34). This study suggests that the early stages of cholesterol synthesis in pachytene spermatocytes and round spermatids might precede the synthesis of dolichol, which is a crucial component in protein glycosylation and the production of specific membrane glycoproteins. However, the exact role of de novo cholesterol synthesis in germ cells has not been determined conclusively, mainly due to the complex relationship between the germ cells and supporting Sertoli cells.
CHOLESTEROL SYNTHESIS AND TRANSPORT IN SERTOLI CELLS
The majority of nutrients, including lipids, needed for spermatogenesis are provided by supporting Sertoli cells, which have the capacity to synthesize cholesterol from acetate in vitro (35). Because the amount of cholesterol required to support spermatogenesis far exceeds the biosynthetic capacity of Sertoli cells, specialized cholesterol transporters are likely to facilitate the influx of cholesterol from the circulation (36). The basal membrane, which separates seminiferous tubules from blood capillaries present in the interstitial space, blocks the entry of LDLs but allows the entry of HDLs, the major source of cholesterol for Sertoli cells (37, 38). Rat Sertoli cells have the ability to uptake HDL cholesterol mainly by APOE-dependent pathways (38). Another important source of cholesterol might be the recycling of lipid-rich residual bodies and apoptotic germ cells that are constantly phagocytosed by Sertoli cells (39). To avoid toxicity, excess cholesterol is esterified and stored in lipid droplets, which are highly mobile and dynamic structures involved in the maintenance of cholesterol equilibrium. Lipid droplets are known to communicate with intracellular organelles and cell junctions through stable or transient surface contacts, enabling the efficient transfer of cholesterol from the site of synthesis and uptake to the site of usage or elimination (40). Another important aspect of cholesterol homeostasis in the Sertoli cell is the efflux of excess cholesterol to HDL in a process called “reverse cholesterol transport.” This process relies on the presence of cholesterol transporters of the ATP-binding cassette superfamily, mainly ATP-binding cassette transporter 1 (ABCA1) (41). Perturbations in Sertoli cell cholesterol homeostasis resulted in complete infertility or subfertility caused by the excessive accumulation of cholesterol esters. This was demonstrated by knockout mouse models lacking functional nuclear receptors involved in cellular cholesterol homeostasis, such as retinoid X receptor β (gene symbol Rxrb) (42, 43), and by Abca1 knockout mice (41). In the case of liver X receptor (synonym symbol Lxr, official symbol Nr1h) double knockout mice (Lxrα;β−/−), the accumulation of cholesterol esters was due to an ABCA1-dependent decrease in cholesterol efflux from Sertoli cells, ABCA1 being a well-known LXR target gene (44). Therefore, the role of Sertoli cells in the regulation of cholesterol homeostasis seems to be very dynamic and to involve mechanisms for cholesterol uptake, storage, recycling, and efflux, which we schematically summarize in Fig. 2. To conclude, Sertoli cells should be considered in the interpretation of results relating to spermatogenesis, including the potential effects of MAS intermediates on germ cell development.
IMPORTANCE OF CHOLESTEROL DURING SPERM MATURATION IN THE EPIDIDYMIS
When sperm is released from the seminiferous epithelium, it is immotile and unable to fertilize an oocyte. Full fertilizing capacity of spermatozoa is acquired during their transport through the epididymis and in the female reproductive tract by capacitation. Capacitation is a multistep process whereby activation of bicarbonate-dependent adenylyl cyclase results in the elevation of cAMP and protein kinase A-mediated tyrosine phosphorylation of a subset of flagellar proteins, leading to changes in sperm motility and acrosomal responsiveness (45–47). These events are highly dependent upon changes in plasma membrane cholesterol (48). Spermatozoa formed in the testis enter the caput epididymis, progress to the corpus, and finally reach the caudal region, where they are stored. Only sperm cells collected from the cauda epididymis of different species have full fertilizing capacity (49). An accepted hypothesis is that cholesterol excreted from the epididymal epithelium contributes to the maturation of transiting sperm (50). Furthermore, the epididymis possesses the ability to synthesize de novo cholesterol (51, 52). Similar to Sertoli cells, cholesterol trafficking was shown to involve an LXR-regulated mechanism in the mouse epididymis. Efflux of cholesterol into the lumen is likely to be mediated by the complementary action of ABCA1 and ABCG1, which are well known LXR targets (53). It is interesting to note that epididymal epithelial cells use a unique apocrine mode of secretion to produce small membranous vesicles called epididymosomes (Fig. 2) (54–56), mainly composed of sphingomyelin and various amounts of cholesterol (57, 58). Recently, an important role for dietary cholesterol in epididymal sperm maturation was suggested (59). Dietary cholesterol overload in an LXR-deficient mouse model resulted in complete infertility caused by the accumulation of cholesterol ester lipid droplets in smooth muscle cells lining the epididymal duct. Spermatogenesis was not affected, as shown by normal sperm count, testicular weight, and histology. It should also be noted that a high cholesterol diet significantly reduced testosterone levels in double knockout Nr1h2−/−;Nr1h3−/− animals (59). Unfortunately, the direct effect of dietary cholesterol intake on the endogenous cholesterol concentration in sperm plasma membranes of wild-type animals has not been demonstrated, as it was for fatty acids (60–62).
Cholesterol content in sperm traveling through the epididymal tract may be regulated by human epididymis-specific proteins (HE1–HE6), initially described in the human epididymis (63). The best studied member of this group is HE1 or Niemman-Pick C2, a secreted sterol-binding glycoprotein involved in sterol trafficking (64). The HE1 homolog has been suggested to be one of the important regulators of cholesterol content in sperm membranes at least in some species (65). During epididymal transit, the content of cholesterol in sperm membranes decreased by approximately 50% in several species (57, 66–68). In some species, cholesterol content increased during sperm maturation (69) or remained unchanged (70). The loss of cholesterol is known to increase the fluidity of sperm membranes, as demonstrated in human spermatozoa (71), which might be necessary for the final steps of sperm maturation in the female reproductive tract. The importance of the stage-specific sterol content of sperm membranes is additionally demonstrated by the presence of vesicular formations produced and secreted by the prostate, known as prostatic secretory granules or prostasomes. Prostasomes have a different lipid composition from sperm plasma membranes (72). In particular, they contain large amounts of sterols and sphingomyelin. However, their physiological function has been thoroughly debated by many investigators. Prostasomes present within sperm are able to fuse with the plasma membrane (Fig. 2), where they are thought to increase motility and prevent early acrosome reaction (73). The various mechanisms that regulate cholesterol content in sperm membranes during their epididymal transit indicate the functional relevance of cholesterol in sperm function.
DYNAMICS OF SPERM MEMBRANE CHOLESTEROL IN THE FEMALE REPRODUCTIVE TRACT
Ejaculated sperm is motile but remains unable to fertilize the oocyte. Sperm is activated during the process of capacitation, which occurs in the female reproductive tract (74). During capacitation, the surface of the sperm head is remodeled, priming it to bind to the zona pellucida (ZP) and to undergo the ZP-induced acrosomal reaction. An important first step enabling capacitation is an organized destabilization of the plasma membrane, which also involves the redistribution of cholesterol, lipids, and proteins. During capacitation, ZP-binding proteins cluster within lipid rafts present in the apical ridge area of the sperm head plasma membrane (75). An obligatory event during capacitation is the loss of cholesterol from the sperm plasma membrane (76). Cholesterol efflux is promoted by albumin (Fig. 2) and high bicarbonate levels, which facilitate lipoprotein-mediated cholesterol efflux (77–79). Cholesterol removal was restricted only to the nonraft membrane fraction of the sperm plasma membrane, whereas the content of cholesterol in the raft membrane fraction remained unchanged (75). These data suggest that cholesterol depletion is important in the remodeling of lipid rafts on the sperm surface (80). Simultaneous with cholesterol efflux, phospholipids inserted into the sperm membrane and decreased the cholesterol to phospholipid ratio (Fig. 2) (48). The fatty acid composition of sperm during maturation is also highly modified, similar to sterols (81). These combined events increase membrane fluidity and facilitate Ca2+ influx into sperm, triggering the acrosomal reaction and fusion with the oocyte (82).
Cholesterol content within sperm plasma membranes varies significantly among different species. In humans, the amount of cholesterol in sperm varies considerably even among ejaculates (83). However, current evidence suggests that the proportion of cholesterol present within sperm membranes is directly related to human sperm morphology (84) and fertility potential (83). The role of cholesterol in reproduction was also demonstrated in a mouse knockout model with complete ablation of the 24-dehydrocholesterol reductase gene (Dhcr24), which is involved in the conversion of desmosterol to cholesterol. These animals contained only trace amounts of cholesterol and were unable to reproduce (85).
OTHER STEROL INTERMEDIATES IN MALE REPRODUCTION
T-MAS was suggested to have an important biological role in male reproduction because it was found to be a predominant intermediate in the testis, but only in a few cases (3, 9, 13). By contrast, desmosterol (cholesta-5,24-dien-3β-ol) was identified as a major sterol intermediate in guinea pig (12) and mouse (Keber et al., unpublished data) testes as well as in human (86) and boar (70) spermatozoa. Furthermore, monkey (87) and rabbit (88) sperm membranes were found to be significantly richer in desmosterol than in cholesterol. Desmosterol was the predominant sterol found in all subfractions of rabbit semen (56.7% of total sterols in prostatic secretory granules, 63.8% in the seminal plasma, and 60% in spermatozoa) (88). The proportion of desmosterol in sperm varies depending on the species, representing 59% of total sterols in rhesus monkey and rabbit (87, 88) and 25% of total sterols in human (89). In rhesus monkey testes, the desmosterol concentration was found to increase with animal age (90). High levels of desmosterol might be the result of low levels or low activity of 24-dehydrocholesterol reductase, which converts desmosterol to cholesterol, or the presence of an inhibitor of this enzyme in the testis and epididymis. Microarray studies using male mouse germ cell fractions (http://mrg.genetics.washington.edu) show that Dhcr24 is indeed expressed at very low levels compared with other genes involved in earlier steps of cholesterol synthesis (33). A possible reason for the accumulation of desmosterol in the testis might be the specific progestin-mediated inhibition of 24-dehydrocholesterol reductase, as demonstrated by Lindenthal et al. (10). Similar to cholesterol, the proportion of desmosterol changed during the epididymal transit of sperm. The amount of desmosterol in sperm membranes increases as sperm travel from the caput to the cauda epididymis in boar (70), hamster (67), and rat (10), resulting in a higher desmosterol to cholesterol ratio. By contrast, desmosterol is present in ram spermatozoa entering the epididymis but is barely detected in mature sperm collected from the cauda epididymis (68). Based on the differential expression of cholesterogenic genes in the epididymis (91) and the presence of sterol-loaded epididymosomes, it is very unlikely that synthesis within sperm alone is responsible for changes in sterol composition during epididymal transit.
A unique sterol composition of spermatozoa was demonstrated in some mammalian species. The predominant sterol intermediate in hamster spermatozoa is cholesta-7,24-dien-3β-ol, a direct precursor of 7-dehydrocholesterol (92, 93). The amount of cholesta-7,24-dien-3β-ol increased during the transit of spermatozoa through the epididymis, similar to desmosterol (67). The predominant sterol in the testis of guinea pig is lathosterol, with concentrations similar to that of desmosterol (12). Other sterol compounds found in the semen are sterol sulfates. The sterol sulfate fraction of human ejaculated spermatozoa contains 85% cholesterol sulfate (94), which represents about 6% of total cholesterol in spermatozoa (89). By contrast, the sterol sulfate fraction of hamster spermatozoa is composed almost exclusively of desmosterol sulfate (95). Both sterol sulfates were also present in monkey spermatozoa (96). Another modification of sterol compounds during sperm transit through epididymis is esterification, which was shown to occur in ram (97). Extreme diversity of sterol content during sperm maturation in different species is further demonstrated by high levels of 7- and 8-dehydrocholesterol in the rat caput epididymis. This intermediate is undetectable in mature sperm collected from the cauda epididymis (98). An on-going study examining the expression of cholesterogenic genes and an array of sterol intermediates in Crem-deficient mice reported that the profile of sterol intermediates in the testis could not be explained by an established model of cholesterol biosynthesis (Ačimovič et al., unpublished data). The existence of new sterol elimination pathways was suggested based on mathematical model analyses. This study indicates that cholesterol biosynthesis in germ cells might involve another yet-to-be-defined regulatory mechanism.
FUNCTIONAL ROLE OF STEROLS IN SPERM DEVELOPMENT
The development of highly specialized spermatozoa requires extensive remodeling of membrane structure, involving stage-specific changes in membrane permeability and fluidity. During the maturation process, a sperm cell needs to maintain integrity and to acquire a high level of motility. In the female reproductive tract, before fusion with the oocyte, a drastic change in membrane structure enables the extrusion of acrosomal content and eventually fusion with the oocyte. Highly specialized membrane function is mostly acquired with precise regulation of sperm membrane lipid content at distinct stages of the maturation process. The functional importance of sterol composition during sperm maturation was clearly demonstrated by unique molecular events that resulted in stage-specific modifications of sperm membrane sterol content. In addition to cholesterol, high levels of different sterol intermediates are known to accumulate in the testis. Their exact function is unknown, but they are speculated to have important roles in the modification of membrane properties. The biological basis for selecting cholesterol as the major sterol in the majority of animal cells is unclear; however, it is generally accepted that cholesterol is the most effective sterol at modulating membrane properties. Its structure allows the tight packing of cholesterol alongside the fatty acid tails of neighboring phospholipids. This can increase order within liquid membranes through the effects of its rigid ring system and the ability to fill interstitial spaces. A higher order of lipid packing results in a specialized type of membrane organization that provides a functional permeability barrier (99). Generally, the highest concentration of cholesterol is present within immature and immotile sperm, collected from the testis or caput epididymis. At this point, cholesterol is believed to function as a membrane stabilizer to provide protection against the premature release of proteolytic enzymes from the acrosome. In the majority of species, cholesterol content during sperm maturation decreased, whereas the content of other sterols, such as desmosterol, increased. Depletion of cholesterol is crucial for normal sperm function, as demonstrated in patients with idiopathic infertility that display a higher cholesterol to phospholipid ratio (83). High cholesterol levels in sperm are negatively correlated with sperm quality in humans (86). Moreover, cholesterol is believed to have an important specific role in sperm raft function. During capacitation, a preferential efflux of cholesterol from the nonraft pool might serve as a stimulus to promote coalescence of separate microdomains into larger rafts over the anterior sperm head (100).
The second most common sterol in the testis is desmosterol. One basic function of desmosterol and cholesterol in the sperm membrane is the inhibition of sperm capacitation. A sufficient level of both sterols within the sperm membrane is therefore constantly maintained by the presence of epididymosomes in the epididymis and prostasomes in ejaculated sperm. Both types of vesicles contain high levels of cholesterol and desmosterol and may prevent the acrosomal reaction by supplying endogenous sterols. This is an important aspect for the fertilizing capacity of spermatozoa in species where spermatozoa have to wait a long time to interact with the oocyte without losing membrane integrity. During this period, prostasomes might remain in contact with sperm (88). Therefore, the loss of cholesterol and desmosterol is the initial obligatory step for capacitation (48, 101). Desmosterol is the predominant sterol in monkey and rabbit spermatozoa and was found to accumulate preferentially in the sperm tail. The difference in membrane desmosterol composition between sperm heads and tails might underlie the different functions of these two structures. Desmosterol has an additional double bond between carbon atoms 24 and 25 and might contribute to membrane fluidity, which is necessary for the motility of flagella (90). Another possible explanation for the preferential use of desmosterol that was previously not considered is the high desorption efficiency of desmosterol from the plasma membrane. Desmosterol was shown to be transferred between large unilamellar vesicles two to three times faster than cholesterol (102). This resulted in a higher efflux of biosynthetic desmosterol from cells (103). It is plausible that a high desorption efficiency of desmosterol, facilitating its eventual efflux from the membrane, represents the main underlying reason for the higher accumulation of desmosterol in the testis and spermatozoa. The double bond at the C24 position in desmosterol significantly weakens sterol ordering potential and the formation of lipid rafts. The weaker effect of desmosterol on bilayer properties is probably due to the different tilt of the steroid ring when compared with cholesterol (Fig. 1). Because desmosterol cannot replace cholesterol within lipid rafts, it was suggested that the ability of cells to follow the desmosterol synthesis route over the cholesterol synthesis route might provide a physiological mechanism to modulate raft-dependent functions (104). Similar to cholesterol and desmosterol, cholesterol and desmosterol sulfate were shown to prevent capacitation and inhibit acrosomal enzyme acrosin (105, 106). Moreover, both are present in low proportions. Sulfatase activity in the female tract suggests a key role of sterol sulfates in the process of fertilization (106).
CONCLUSIONS AND PERSPECTIVES
Based on current literature, the source of cholesterol needed for spermatogenesis has not been shown conclusively, mainly due to the complexity of the process and lack of a suitable in vitro systems. Cholesterol might originate from de novo synthesis in germ or Sertoli cells, or it might be transported from the interstitial compartment with lipoprotein particles, as discussed previously. This question can be addressed by carefully examining the phenotypes of cell conditional knockouts of genes involved in cholesterol synthesis and transport in different types of testicular cells. To our knowledge, no such model has been developed. A few studies that have examined mouse models with systemic inactivation of genes involved in cholesterol transport suggest that the transport of cholesterol may play an essential role in spermatogenesis. Mice with complete ablation of type 2 apolipoprotein E receptor (Lrp8) (107) and mice with partial ablation of apolipoprotein B (Apob) (108) exhibited severely compromised fertility. Based on systemic knockout models, we cannot conclude with certainty that infertility is solely due to perturbations in spermatogenesis or sperm maturation because the underlying cause could also be of systemic origin. However, infertility in the Lrp8 knockout was ascribed to defects in sperm maturation, mainly due to a high expression of Lrp8 in the epididymis and the specific nature of the sperm phenotype (107). A list of genes involved in different cholesterol-related biological processes that are also implicated in male fertility as demonstrated by mouse models is shown in Table 1. In addition to knockout models, the role of cholesterol transport in sperm development is further demonstrated by the negative effects of dietary cholesterol overload on fertility in rats (109, 110). The importance of de novo cholesterol synthesis during spermatogenesis is currently being studied by our group by examining conditional knockouts of Cyp51 in different cells of the testis. Characterizing testis and fertility phenotypes in these conditional knockout models is likely to provide insight on the importance of de novo cholesterol synthesis and/or accumulating intermediates in germ, Sertoli, and Leydig cells as well as in male reproductive function.
TABLE 1.
Genes involved in cholesterol-related biological processes that are essential for normal male fertility.
| Gene name(synonym) | Gene Product | Biological Process Related to Cholesterol | Reference | Inbred Mouse Line or Hybrids (×) |
| Abca1 | ATP-binding cassette, subfamily A, member 1 | Cholesterol efflux, metabolic process, reverse transport | (41) | DBA1/J |
| Abcg5 | ATP-binding cassette, subfamily G (WHITE), member 5 | Cholesterol efflux, homeostasis, negative regulation of intestinal absorption | (117) | A/J |
| ApoB | Apolipoprotein B | Cholesterol homeostasis, metabolic process, transport, positive regulation of storage, regulation of biosynthetic process | (118) | 129S4/SvJae × C57BL/6J |
| Cftr | Cystic fibrosis transmembrane conductance regulator | Cholesterol biosynthetic process, transport | (119) | 129P2/OlaHsd |
| Crem | cAMP-responsive element modulator | Regulation of transcription, DNA dependent | (120) | 129/Sv × C57BL/6 |
| Dhcr24 | 24-dehydrocholesterol reductase | Cholesterol biosynthetic process, metabolic process | (85) | C57BL/6J × 129S5 |
| Ehd1 | EH-domain containing 1 | Cholesterol homeostasis, positive regulation of storage | (121) | 129;B6 |
| Lep | Leptin | Cholesterol metabolic process, regulation of intestinal absorption | (122) | C57BL/6J |
| Lepr | Leptin receptor | Cholesterol metabolic process | (123) | 129 × C57BL/6 |
| Lipe | Lipase, hormone-sensitive | Cholesterol metabolic process | (124) | B6 × 129S7 |
| Lrp8 (Apoe2) | LDL receptor-related protein 8, apolipoprotein e receptor | Positive regulation of CREB transcription factor activity | (107) | 129S6/SvEvTac |
| Npc1 | Niemann Pick type C1 | Cholesterol efflux, homeostasis | MGI direct data submission 2004–2007 | BALB/C |
| Nr1h2 (Lxrb) | Nuclear receptor subfamily 1, group H, member 2 | Cholesterol homeostasis, negative regulation of storage, positive regulation of efflux, positive regulation of transport | (125) | 129S6/SvEvTac × C57BL/6 |
| Nr1h3 (Lxra) | Nuclear receptor subfamily 1, group H, member 3 | Cholesterol homeostasis, negative regulation of storage, positive regulation of efflux, positive regulation of transport and homeostasis, homeostasis of sterol | (126) | 129S6/SvEvTac × C57BL/6 |
| Rxrb | Retinoid X receptor β | Regulation of transcription, DNA dependent | (42) | 129S2/SvPas |
| Sirt1 | Sirtuin1 | Cholesterol homeostasis, response to cholesterol | (127) | 129/Sv × 129S6/Sv |
Targeted, spontaneous, or ENU-induced inactivation of these genes in mouse models results in reduced fertility or infertility in males. Data are adopted from Mouse Genome Informatics database.
Based on various accumulation patterns of sterol intermediates in the testis of different mammalian species, one could speculate that the main function of sterols is stage- and species-specific regulation of membrane function. Studies on cell-based systems suggest that sterol synthesis might be accompanied by the accumulation of several sterol intermediates that do not remain confined to the endoplasmic reticulum but rather are incorporated into the outer leaflet of the plasma membrane along with cholesterol (103). Lange and coworkers reported that the transport of newly synthesized zymosterol to the plasma membrane in fibroblasts was two times faster than that of newly synthesized cholesterol (111). The mobilization rate of different sterol precursors from the cell membrane differs not only from that of cholesterol but also from each other, with more polar intermediates being more avidly effluxed (112). Based on these experiments, we conclude that sterol intermediates can be readily incorporated into the membrane. Different sterol intermediates differ in the number of methyl groups, number and position of double bonds, and conformation of the sterol ring system (Fig. 1). Each sterol intermediate therefore possesses unique structural features and can affect membrane structure in a specific way. Different membrane properties might be especially beneficial during sperm development. Additionally, species-specific differences in sterol composition during sperm development could be important for unique reproductive characteristics of individual species.
Transport and distribution of cholesterol and sterol intermediates play an important role in spermatogenesis, but these processes are not well understood. Recently developed methods (113) for high-resolution lipid imaging in vivo or in vitro may be helpful if applied to studies in the testis. For instance, one of the first studies for directly monitoring sterol movement in living cells has been achieved by fluorescently labeling sterol derivatives (114). Additionally, fluorescent and photoreactive sterol probes may reveal not only the location and movement of these lipids but also reveal their interactions with proteins as demonstrated in a recent study by Gimpl and Gehrig-Burger (115). Although these new approaches have not been extensively used in the testis, they hold great promise in functional studies of sterol trafficking and distribution during spermatogenesis.
Among the several sterol intermediates shown to accumulate in the testis and spermatozoa of different species, MAS are considered unique because of their potential role in the regulation of meiosis. A thorough examination of the role of MAS in oocyte development has led to the conclusion that MAS possess a nonspecific receptor-independent positive effect on oocyte maturation. A similar effect was demonstrated with closely related sterols (6). The role of MAS in spermatogenesis has been very poorly studied, mainly due to the inability to establish an in vitro spermatogenic cell culture system. To investigate the specific role of MAS during spermatogenesis in vivo, we will use conditional knockout models of the MAS-producing gene Cyp51 in various cell types in testis. A thorough characterization of such models is likely to reveal the function of MAS and other intermediates upstream of the CYP51 enzymatic step in male germ development. Despite the long-term availability of the conditional knockout technology, germ cell-specific knockout of genes involved in cholesterol synthesis has not yet been developed. Although some conditional knockout models have provided answers to important physiological questions, others have shown unexpected complexities or technical limitations that have confounded interpretation of the results in testis, such as the case of the conditional knockout of the androgen receptor. Moreover, improved and more sensitive sterol measurement methods are needed to analyze quantitatively levels of intermediates in minute samples from various cell types or testis cell fractions (116). New-generation RNA sequencing technology should also allow determination of the absolute amounts of various testis-specific RNA species from genes involved in cholesterol production and homeostasis. Such studies have not been possible by microarray analyses, which can only provide relative but not absolute amount comparisons. In addition, sensitive proteomic analyses are required to determine complete RNA-protein relationships of cholesterogenic genes and to discover possible protein modifications specific to testis. Diet perturbation experiments combined with conditional knockout models and sensitive high-throughput techniques should help to reveal the role of sterols in highly specific mechanisms of sperm development. In addition to mouse, important information might be acquired from mutant Drosophila strains, which are ideal for in vivo studies of membrane remodeling during spermatogenesis. A combination of these approaches is likely to result in a better understanding of sterol function in sperm formation. It may also reveal that some cases of unexplained male infertility are the result of cholesterol and sterol intermediate perturbations in the testis due to genetic or environmental factors.
Footnotes
Abbreviations:
- CREMτ
- cAMP-responsive element modulator tao
- CYP
- cytochrome P450
- CYP51
- cytochrome P450 lanosterol 14α-demethylase
- FF-MAS
- follicular fluid meiosis-activating sterol
- HE
- human epididymis-specific protein
- LXR
- liver X receptor
- MAS
- meiosis-activating sterols
- ppm
- parts per million
- POR
- cytochrome P450 oxidoreductase
- T-MAS
- testis meiosis-activating sterol
- ZP
- zona pellucida
This research was supported by grants from the Slovenian Research Agency (core funding program P4-0220, project N5-0003 Syntol and J4-4306) (S.H.), by a Young Scientist Fellowship (R.K.), and by project J7-4053 (D.R.).
REFERENCES
- 1.Goldstein J. L., Brown M. S. 1990. Regulation of the Mevalonate Pathway. Nature. 343: 425–430 [DOI] [PubMed] [Google Scholar]
- 2.Clayton R. B., Bloch K. 1956. Biological synthesis of lanosterol and agnosterol. J. Biol. Chem. 218: 305–318 [PubMed] [Google Scholar]
- 3.Byskov A. G., Andersen C. Y., Nordholm L., Thogersen H., Xia G., Wassmann O., Andersen J. V., Guddal E., Roed T. 1995. Chemical structure of sterols that activate oocyte meiosis. Nature. 374: 559–562 [DOI] [PubMed] [Google Scholar]
- 4.Byskov A. G., Andersen C. Y., Leonardsen L., Baltsen M. 1999. Meiosis activating sterols (MAS) and fertility in mammals and man. J. Exp. Zool. 285: 237–242 [PubMed] [Google Scholar]
- 5.Vaknin K. M., Lazar S., Popliker M., Tsafriri A. 2001. Role of meiosis-activating sterols in rat oocyte maturation: effects of specific inhibitors and changes in the expression of lanosterol 14alpha-demethylase during the preovulatory period. Biol. Reprod. 64: 299–309 [DOI] [PubMed] [Google Scholar]
- 6.Grondahl C. 2008. Oocyte maturation. Basic and clinical aspects of in vitro maturation (IVM) with special emphasis of the role of FF-MAS. Dan. Med. Bull. 55: 1–16 [PubMed] [Google Scholar]
- 7.Byskov A. G., Saxen L. 1976. Induction of meiosis in fetal mouse testis in vitro. Dev. Biol. 52: 193–200 [DOI] [PubMed] [Google Scholar]
- 8.Byskov A. G., Fenger M., Westergaard L., Andersen C. Y. 1993. Forskolin and the meiosis inducing substance synergistically initiate meiosis in fetal male germ cells. Mol. Reprod. Dev. 34: 47–52 [DOI] [PubMed] [Google Scholar]
- 9.Tacer K. F., Haugen T. B., Baltsen M., Debeljak N., Rozman D. 2002. Tissue-specific transcriptional regulation of the cholesterol biosynthetic pathway leads to accumulation of testis meiosis-activating sterol (T-MAS). J. Lipid Res. 43: 82–89 [PubMed] [Google Scholar]
- 10.Lindenthal B., Holleran A. L., Aldaghlas T. A., Ruan B., Schroepfer G. J., Jr, Wilson W. K., Kelleher J. K. 2001. Progestins block cholesterol synthesis to produce meiosis-activating sterols. FASEB J. 15: 775–784 [DOI] [PubMed] [Google Scholar]
- 11.Bogh I. B., Baltsen M., Byskov A. G., Greve T. 2001. Testicular concentration of meiosis-activating sterol is associated with normal testicular descent. Theriogenology. 55: 983–992 [DOI] [PubMed] [Google Scholar]
- 12.Lindenthal B., Bertsch T., Fassbender K., Stroick M., Kuhl S., Lutjohann D., von Bergmann K. 2002. Influence of simvastatin, pravastatin, and BM 15.766 on neutral sterols in liver and testis of guinea pigs. Metabolism. 51: 492–499 [DOI] [PubMed] [Google Scholar]
- 13.Fon Tacer K., Pompon D., Rozman D. 2010. Adaptation of cholesterol synthesis to fasting and TNF-alpha: profiling cholesterol intermediates in the liver, brain, and testis. J. Steroid Biochem. Mol. Biol. 121: 619–625 [DOI] [PubMed] [Google Scholar]
- 14.Režen T., Debeljak N., Kordiš D., Rozman D. 2004. New aspects on lanosterol 14 alpha-demethylase and cytochrome P450 evolution: lanosterol/cycloartenol diversification and lateral transfer. J. Mol. Evol. 59: 51–58 [DOI] [PubMed] [Google Scholar]
- 15.Lepesheva G. I., Waterman M. R. 2011. Structural basis for conservation in the CYP51 family. Biochim. Biophys. Acta. 1814: 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strushkevich N., Usanov S. A., Park H. W. 2010. Structural basis of human CYP51 inhibition by antifungal azoles. J. Mol. Biol. 397: 1067–1078 [DOI] [PubMed] [Google Scholar]
- 17.Hargrove T. Y., Wawrzak Z., Liu J., Nes W. D., Waterman M. R., Lepesheva G. I. 2011. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14 alpha-demethylase (CYP51) from Leishmania infantum. J. Biol. Chem. 286: 26838–26848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C. K., Leung S. S., Guilbert C., Jacobson M. P., McKerrow J. H., Podust L. M. 2010. Structural characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei bound to the antifungal drugs posaconazole and fluconazole. PLoS Negl. Trop. Dis. 4: e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepesheva G. I., Park H. W., Hargrove T. Y., Vanhollebeke B., Wawrzak Z., Harp J. M., Sundaramoorthy M., Nes W. D., Pays E., Chaudhuri M., et al. 2010. Crystal structures of Trypanosoma brucei sterol 14alpha-demethylase and implications for selective treatment of human infections. J. Biol. Chem. 285: 1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keber R., Motaln H., Wagner K. D., Debeljak N., Rassoulzadegan M., Acimovic J., Rozman D., Horvat S. 2011. Mouse knockout of the cholesterogenic cytochrome P450 lanosterol 14 alpha-demethylase (Cyp51) resembles Antley-Bixler syndrome. J. Biol. Chem. 286: 29086–29097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stromstedt M., Rozman D., Waterman M. R. 1996. The ubiquitously expressed human CYP51 encodes lanosterol 14 alpha-demethylase, a cytochrome P450 whose expression is regulated by oxysterols. Arch. Biochem. Biophys. 329: 73–81 [DOI] [PubMed] [Google Scholar]
- 22.Stromstedt M., Waterman M. R., Haugen T. B., Tasken K., Parvinen M., Rozman D. 1998. Elevated expression of lanosterol 14 alpha-demethylase (CYP51) and the synthesis of oocyte meiosis-activating sterols in postmeiotic germ cells of male rats. Endocrinology. 139: 3771–3771 [DOI] [PubMed] [Google Scholar]
- 23.Rozman D., Fink M., Fimia G. M., Sassone-Corsi P., Waterman M. R. 1999. Cyclic adenosine 3 ‘,5 ‘-monophosphate(cAMP)/cAMP-responsive element modulator (CREM)-dependent regulation of cholesterogenic lanosterol 14 alpha-demethylase (CYP51) in spermatids. Mol. Endocrinol. 13: 1951–1962 [DOI] [PubMed] [Google Scholar]
- 24.Majdič G., Parvinen M., Bellamine A., Harwood H. J., Ku W. W., Waterman M. R., Rozman D. 2000. Lanosterol 14 alpha-demethylase (CYP51), NADPH-cytochrome P450 reductase and squalene synthase in spermatogenesis: late spermatids of the rat express proteins needed to synthesize follicular fluid meiosis activating sterol. J. Endocrinol. 166: 463–474 [DOI] [PubMed] [Google Scholar]
- 25.Cotman M., Jezek D., Tacer K. F., Frangez R., Rozman D. 2004. A functional cytochrome P450 lanosterol 14 alpha-demethylase CYP51 enzyme in the acrosome: transport through the Golgi and synthesis of meiosis-activating sterols. Endocrinology. 145: 1419–1426 [DOI] [PubMed] [Google Scholar]
- 26.Syed V., Stephan J. P., Gerard N., Legrand A., Parvinen M., Bardin C. W., Jegou B. 1995. Residual bodies activate Sertoli cell interleukin-1 alpha (IL-1 alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology. 136: 3070–3078 [DOI] [PubMed] [Google Scholar]
- 27.Jegou B. 1993. The Sertoli-germ cell communication network in mammals. Int. Rev. Cytol. 147: 25–96 [PubMed] [Google Scholar]
- 28.Kruczynski D., Passia D., Haider S. G., Glassmeyer M. 1985. Zinc transport through residual bodies in the rat testis: a histochemical study. Andrologia. 17: 98–103 [DOI] [PubMed] [Google Scholar]
- 29.Teruya J. H., Salido E. C., Edwards P. A., Clarke C. F. 1991. Testis-specific transcripts of rat farnesyl pyrophosphate synthetase are developmentally regulated and localized to haploid germ cells. Biol. Reprod. 44: 663–671 [DOI] [PubMed] [Google Scholar]
- 30.Collins B. S., Tansey T. R., Shechter I. 2001. Comparative squalene synthase gene expression in mouse liver and testis. Arch. Biochem. Biophys. 395: 253–258 [DOI] [PubMed] [Google Scholar]
- 31.Foulkes N. S., Mellstrom B., Benusiglio E., Sassone-Corsi P. 1992. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 355: 80–84 [DOI] [PubMed] [Google Scholar]
- 32.Kosir R., Juvan P., Perse M., Budefeld T., Majdic G., Fink M., Sassone-Corsi P., Rozman D. 2012. Novel insights into the downstream pathways and targets controlled by transcription factors CREM in the testis. PLoS ONE. 7: e31798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shima J. E., McLean D. J., McCarrey J. R., Griswold M. D. 2004. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol. Reprod. 71: 319–330 [DOI] [PubMed] [Google Scholar]
- 34.Potter J. E. R., Millette C. F., James M. J., Kandutsch A. A. 1981. Elevated cholesterol and dolichol synthesis in mouse pachytene spermatocytes. J. Biol. Chem. 256: 7150–7154 [PubMed] [Google Scholar]
- 35.Wiebe J. P., Tilbe K. S. 1979. De novo synthesis of steroids (from acetate) by isolated rat Sertoli cells. Biochem. Biophys. Res. Commun. 89: 1107–1113 [DOI] [PubMed] [Google Scholar]
- 36.Akpovi C. D., Yoon S. R., Vitale M. L., Pelletier R. M. 2006. The predominance of one of the SR-BI isoforms is associated with increased esterified cholesterol levels not apoptosis in mink testis. J. Lipid Res. 47: 2233–2247 [DOI] [PubMed] [Google Scholar]
- 37.Fofana M., Travert C., Carreau S., Le Goff D. 2000. Evaluation of cholesteryl ester transfer in the seminiferous tubule cells of immature rats in vivo and in vitro. J. Reprod. Fertil. 118: 79–83 [DOI] [PubMed] [Google Scholar]
- 38.Fofana M., Maboundou J. C., Bocquet J., Le Goff D. 1996. Transfer of cholesterol between high density lipoproteins and cultured rat Sertoli cells. Biochem. Cell Biol. 74: 681–686 [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi Y., Shiratsuchi A. 2004. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol. Pharm. Bull. 27: 13–16 [DOI] [PubMed] [Google Scholar]
- 40.Pelletier R. M. 2011. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog. Histochem. Cytochem. 46: 49–127 [DOI] [PubMed] [Google Scholar]
- 41.Selva D. M., Hirsch-Reinshagen V., Burgess B., Zhou S., Chan J., McIsaac S., Hayden M. R., Hammond G. L., Vogl A. W., Wellington C. L. 2004. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J. Lipid Res. 45: 1040–1050 [DOI] [PubMed] [Google Scholar]
- 42.Kastner P., Mark M., Leid M., Gansmuller A., Chin W., Grondona J. M., Decimo D., Krezel W., Dierich A., Chambon P. 1996. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 10: 80–92 [DOI] [PubMed] [Google Scholar]
- 43.Robertson K. M., Schuster G. U., Steffensen K. R., Hovatta O., Meaney S., Hultenby K., Johansson L. C., Svechnikov K., Soder O., Gustafsson J. A. 2005. The liver X receptor-{beta} is essential for maintaining cholesterol homeostasis in the testis. Endocrinology. 146: 2519–2530 [DOI] [PubMed] [Google Scholar]
- 44.Volle D. H., Mouzat K., Duggavathi R., Siddeek B., Dechelotte P., Sion B., Veyssiere G., Benahmed M., Lobaccaro J. M. 2007. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol. Endocrinol. 21: 1014–1027 [DOI] [PubMed] [Google Scholar]
- 45.Visconti P. E., Bailey J. L., Moore G. D., Pan D., Olds-Clarke P., Kopf G. S. 1995. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 121: 1129–1137 [DOI] [PubMed] [Google Scholar]
- 46.Visconti P. E., Moore G. D., Bailey J. L., Leclerc P., Connors S. A., Pan D., Olds-Clarke P., Kopf G. S. 1995. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 121: 1139–1150 [DOI] [PubMed] [Google Scholar]
- 47.Wennemuth G., Carlson A. E., Harper A. J., Babcock D. F. 2003. Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development. 130: 1317–1326 [DOI] [PubMed] [Google Scholar]
- 48.Cross N. L. 1998. Role of cholesterol in sperm capacitation. Biol. Reprod. 59: 7–11 [DOI] [PubMed] [Google Scholar]
- 49.Cooper T. G. 2007. Sperm maturation in the epididymis: a new look at an old problem. Asian J. Androl. 9: 533–539 [DOI] [PubMed] [Google Scholar]
- 50.Saez F., Ouvrier A., Drevet J. R. 2011. Epididymis cholesterol homeostasis and sperm fertilizing ability. Asian J. Androl. 13: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans R. R., Johnson A. D. 1975. The metabolic activity of the bovine epididymis. III. Cholesterol and esterified cholesterol metabolism. J. Reprod. Fertil. 43: 527–530 [DOI] [PubMed] [Google Scholar]
- 52.Hamilton D. W., Jones A. L., Fawcett D. W. 1969. Cholesterol biosynthesis in the mouse epididymis and ductus deferens: a biochemical and morphological study. Biol. Reprod. 1: 167–184 [DOI] [PubMed] [Google Scholar]
- 53.Ouvrier A., Cadet R., Vernet P., Laillet B., Chardigny J. M., Lobaccaro J. M., Drevet J. R., Saez F. 2009. LXR and ABCA1 control cholesterol homeostasis in the proximal mouse epididymis in a cell-specific manner. J. Lipid Res. 50: 1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fornes W. M., Sosa M. A., Bertini F., Burgos M. H. 1995. Vesicles in rat epididymal fluid. Existence of two populations differing in ultrastructure and enzymatic composition. Andrologia. 27: 233–237 [DOI] [PubMed] [Google Scholar]
- 55.Frenette G., Sullivan R. 2001. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. 59: 115–121 [DOI] [PubMed] [Google Scholar]
- 56.Gatti J. L., Metayer S., Belghazi M., Dacheux F., Dacheux J. L. 2005. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol. Reprod. 72: 1452–1465 [DOI] [PubMed] [Google Scholar]
- 57.Rejraji H., Sion B., Prensier G., Carreras M., Motta C., Frenoux J. M., Vericel E., Grizard G., Vernet P., Drevet J. R. 2006. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol. Reprod. 74: 1104–1113 [DOI] [PubMed] [Google Scholar]
- 58.Sullivan R., Frenette G., Girouard J. 2007. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 9: 483–491 [DOI] [PubMed] [Google Scholar]
- 59.Ouvrier A., Alves G., Damon-Soubeyrand C., Marceau G., Cadet R., Janny L., Brugnon F., Kocer A., Pommier A., Lobaccaro J. M., et al. 2011. Dietary cholesterol-induced post-testicular infertility. PLoS ONE. 6: e26966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cerolini S., Zaniboni L., Maldjian A., Gliozzi T. 2006. Effect of docosahexaenoic acid and alpha-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 66: 877–886 [DOI] [PubMed] [Google Scholar]
- 61.Gliozzi T. M., Zaniboni L., Maldjian A., Luzi F., Maertens L., Cerolini S. 2009. Quality and lipid composition of spermatozoa in rabbits fed DHA and vitamin E rich diets. Theriogenology. 71: 910–919 [DOI] [PubMed] [Google Scholar]
- 62.Mitre R., Cheminade C., Allaume P., Legrand P., Legrand A. B. 2004. Oral intake of shark liver oil modifies lipid composition and improves motility and velocity of boar sperm. Theriogenology. 62: 1557–1566 [DOI] [PubMed] [Google Scholar]
- 63.Kirchhoff C., Osterhoff C., Habben I., Ivell R. 1990. Cloning and analysis of mRNAs expressed specifically in the human epididymis. Int. J. Androl. 13: 155–167 [DOI] [PubMed] [Google Scholar]
- 64.Sturley S. L., Patterson M. C., Pentchev P. 2009. Unraveling the sterol-trafficking defect in Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA. 106: 2093–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okamura N., Kiuchi S., Tamba M., Kashima T., Hiramoto S., Baba T., Dacheux F., Dacheux J. L., Sugita Y., Jin Y. Z. 1999. A porcine homolog of the major secretory protein of human epididymis, HE1, specifically binds cholesterol. Biochim. Biophys. Acta. 1438: 377–387 [DOI] [PubMed] [Google Scholar]
- 66.Aveldano M. I., Rotstein N. P., Vermouth N. T. 1992. Lipid remodelling during epididymal maturation of rat spermatozoa. Enrichment in plasmenylcholines containing long-chain polyenoic fatty acids of the n-9 series. Biochem. J. 283: 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Awano M., Kawaguchi A., Mohri H. 1993. Lipid composition of hamster epididymal spermatozoa. J. Reprod. Fertil. 99: 375–383 [DOI] [PubMed] [Google Scholar]
- 68.Parks J. E., Hammerstedt R. H. 1985. Development changes occurring in the lipids of ram epididymal spermatozoa plasma membrane. Biol. Reprod. 32: 653–668 [DOI] [PubMed] [Google Scholar]
- 69.Rana A. P., Majumder G. C., Misra S., Ghosh A. 1991. Lipid changes of goat sperm plasma membrane during epididymal maturation. Biochim. Biophys. Acta. 1061: 185–196 [DOI] [PubMed] [Google Scholar]
- 70.Nikolopoulou M., Soucek D. A., Vary J. C. 1985. Changes in the lipid content of boar sperm plasma membranes during epididymal maturation. Biochim. Biophys. Acta. 815: 486–498 [DOI] [PubMed] [Google Scholar]
- 71.Haidl G., Opper C. 1997. Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum. Reprod. 12: 2720–2723 [DOI] [PubMed] [Google Scholar]
- 72.Castellini C., Cardinali R., Dal Bosco A., Minelli A., Camici O. 2006. Lipid composition of the main fractions of rabbit semen. Theriogenology. 65: 703–712 [DOI] [PubMed] [Google Scholar]
- 73.Burden H. P., Holmes C. H., Persad R., Whittington K. 2006. Prostasomes: their effects on human male reproduction and fertility. Hum. Reprod. Update. 12: 283–292 [DOI] [PubMed] [Google Scholar]
- 74.Chang M. C. 1951. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 168: 697–698 [DOI] [PubMed] [Google Scholar]
- 75.Boerke A., Tsai P. S., Garcia-Gil N., Brewis I. A., Gadella B. M. 2008. Capacitation-dependent reorganization of microdomains in the apical sperm head plasma membrane: functional relationship with zona binding and the zona-induced acrosome reaction. Theriogenology. 70: 1188–1196 [DOI] [PubMed] [Google Scholar]
- 76.Travis A. J., Kopf G. S. 2002. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Invest. 110: 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flesch F. M., Brouwers J. F., Nievelstein P. F., Verkleij A. J., van Golde L. M., Colenbrander B., Gadella B. M. 2001. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J. Cell Sci. 114: 3543–3555 [DOI] [PubMed] [Google Scholar]
- 78.Lin Y., Kan F. W. 1996. Regionalization and redistribution of membrane phospholipids and cholesterol in mouse spermatozoa during in vitro capacitation. Biol. Reprod. 55: 1133–1146 [DOI] [PubMed] [Google Scholar]
- 79.Visconti P. E., Galantino-Homer H., Ning X., Moore G. D., Valenzuela J. P., Jorgez C. J., Alvarez J. G., Kopf G. S. 1999. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J. Biol. Chem. 274: 3235–3242 [DOI] [PubMed] [Google Scholar]
- 80.Kawano N., Yoshida K., Miyado K., Yoshida M. 2011. Lipid rafts: keys to sperm maturation, fertilization, and early embryogenesis. J. Lipids. 2011: 264706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wathes D. C., Abayasekara D. R., Aitken R. J. 2007. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 77: 190–201 [DOI] [PubMed] [Google Scholar]
- 82.Neild D. N., Gadella B. M., Aguero A., Stout T. A., Colenbrander B. 2005. Capacitation, acrosome function and chromatin structure in stallion sperm. Anim. Reprod. Sci. 89: 47–56 [DOI] [PubMed] [Google Scholar]
- 83.Sugkraroek P., Kates M., Leader A., Tanphaichitr N. 1991. Levels of cholesterol and phospholipids in freshly ejaculated sperm and Percoll-gradient-pelletted sperm from fertile and unexplained infertile men. Fertil. Steril. 55: 820–827 [PubMed] [Google Scholar]
- 84.Meseguer M., Garrido N., Martinez-Conejero J. A., Simon C., Pellicer A., Remohi J. 2004. Relationship between standard semen parameters, calcium, cholesterol contents, and mitochondrial activity in ejaculated spermatozoa from fertile and infertile males. J. Assist. Reprod. Genet. 21: 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wechsler A., Brafman A., Shafir M., Heverin M., Gottlieb H., Damari G., Gozlan-Kelner S., Spivak I., Moshkin O., Fridman E., et al. 2003. Generation of viable cholesterol-free mice. Science. 302: 2087. [DOI] [PubMed] [Google Scholar]
- 86.Zalata A., Hassan A., Christophe A., Comhaire F., Mostafa T. 2010. Cholesterol and desmosterol in two sperm populations separated on Sil-Select gradient. Int. J. Androl. 33: 528–535 [DOI] [PubMed] [Google Scholar]
- 87.Lin D. S., Connor W. E., Wolf D. P., Neuringer M., Hachey D. L. 1993. Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. J. Lipid Res. 34: 491–499 [PubMed] [Google Scholar]
- 88.Mourvaki E., Cardinali R., Roberti R., Dal Bosco A., Castellini C. 2010. Desmosterol, the main sterol in rabbit semen: distribution among semen subfractions and its role in the in vitro spermatozoa acrosome reaction and motility. Asian J. Androl. 12: 862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sion B., Grizard G., Boucher D. 2001. Quantitative analysis of desmosterol, cholesterol and cholesterol sulfate in semen by high-performance liquid chromatography. J. Chromatogr. A. 935: 259–265 [DOI] [PubMed] [Google Scholar]
- 90.Connor W. E., Lin D. S., Neuringer M. 1997. Biochemical markers for puberty in the monkey testis: desmosterol and docosahexaenoic acid. J. Clin. Endocrinol. Metab. 82: 1911–1916 [DOI] [PubMed] [Google Scholar]
- 91.Johnston D. S., Jelinsky S. A., Bang H. J., DiCandeloro P., Wilson E., Kopf G. S., Turner T. T. 2005. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol. Reprod. 73: 404–413 [DOI] [PubMed] [Google Scholar]
- 92.Legault Y., VandenHeuvel W. J., Arison B. H., Bleau G., Chapdelaine A., Roberts K. D. 1978. 5alpha-Cholesta-7,24-dien-3beta-ol as a major sterol of the male hamster reproductive tract. Steroids. 32: 649–658 [DOI] [PubMed] [Google Scholar]
- 93.Legault Y., Bouthillier M., Bleau G., Chapdelaine A., Roberts K. D. 1979. The sterol and sterol sulfate content of the male hamster reproductive tract. Biol. Reprod. 20: 1213–1219 [DOI] [PubMed] [Google Scholar]
- 94.Lalumiere G., Bleau G., Chapdelaine A., Roberts K. D. 1976. Cholesteryl sulfate and sterol sulfatase in the human reproductive tract. Steroids. 27: 247–260 [DOI] [PubMed] [Google Scholar]
- 95.Bleau G., VandenHeuvel W. J. 1974. Desmosteryl sulfate and desmosterol in hamster epididymal spermatozoa. Steroids. 24: 549–556 [DOI] [PubMed] [Google Scholar]
- 96.Connor W. E., Lin D. S., Wolf D. P., Alexander M. 1998. Uneven distribution of desmosterol and docosahexaenoic acid in the heads and tails of monkey sperm. J. Lipid Res. 39: 1404–1411 [PubMed] [Google Scholar]
- 97.Quinn P. J., White I. G. 1967. Phospholipid and cholesterol content of epididymal and ejaculated ram spermatozoa and seminal plasma in relation to cold shock. Aust. J. Biol. Sci. 20: 1205–1215 [DOI] [PubMed] [Google Scholar]
- 98.Lindenthal B., Aldaghlas T. A., Kelleher J. K., Henkel S. M., Tolba R., Haidl G., von Bergmann K. 2001. Neutral sterols of rat epididymis. High concentrations of dehydrocholesterols in rat caput epididymidis. J. Lipid Res. 42: 1089–1095 [PubMed] [Google Scholar]
- 99.Maxfield F. R., Tabas I. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621 [DOI] [PubMed] [Google Scholar]
- 100.Shadan S., James P. S., Howes E. A., Jones R. 2004. Cholesterol efflux alters lipid raft stability and distribution during capacitation of boar spermatozoa. Biol. Reprod. 71: 253–265 [DOI] [PubMed] [Google Scholar]
- 101.Osheroff J. E., Visconti P. E., Valenzuela J. P., Travis A. J., Alvarez J., Kopf G. S. 1999. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol. Hum. Reprod. 5: 1017–1026 [DOI] [PubMed] [Google Scholar]
- 102.Phillips J. E., Rodrigueza W. V., Johnson W. J. 1998. Basis for rapid efflux of biosynthetic desmosterol from cells. J. Lipid Res. 39: 2459–2470 [PubMed] [Google Scholar]
- 103.Johnson W. J., Fischer R. T., Phillips M. C., Rothblat G. H. 1995. Efflux of newly synthesized cholesterol and biosynthetic sterol intermediates from cells. Dependence on acceptor type and on enrichment of cells with cholesterol. J. Biol. Chem. 270: 25037–25046 [DOI] [PubMed] [Google Scholar]
- 104.Vainio S., Jansen M., Koivusalo M., Rog T., Karttunen M., Vattulainen I., Ikonen E. 2006. Significance of sterol structural specificity. Desmosterol cannot replace cholesterol in lipid rafts. J. Biol. Chem. 281: 348–355 [DOI] [PubMed] [Google Scholar]
- 105.Burck P. J., Zimmerman R. E. 1980. The inhibition of acrosin by sterol sulphates. J. Reprod. Fertil. 58: 121–125 [DOI] [PubMed] [Google Scholar]
- 106.Roberts K. D. 1987. Sterol sulfates in the epididymis; synthesis and possible function in the reproductive process. J. Steroid Biochem. 27: 337–341 [DOI] [PubMed] [Google Scholar]
- 107.Andersen O. M., Yeung C. H., Vorum H., Wellner M., Andreassen T. K., Erdmann B., Mueller E. C., Herz J., Otto A., Cooper T. G., et al. 2003. Essential role of the apolipoprotein E receptor-2 in sperm development. J. Biol. Chem. 278: 23989–23995 [DOI] [PubMed] [Google Scholar]
- 108.Huang L. S., Voyiaziakis E., Chen H. L., Rubin E. M., Gordon J. W. 1996. A novel functional role for apolipoprotein B in male infertility in heterozygous apolipoprotein B knockout mice. Proc. Natl. Acad. Sci. USA. 93: 10903–10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bataineh H. N., Nusier M. K. 2005. Effect of cholesterol diet on reproductive function in male albino rats. Saudi Med. J. 26: 398–404 [PubMed] [Google Scholar]
- 110.Shalaby M. A., el-Zorba H. Y., Kamel G. M. 2004. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol. Res. 50: 137–142 [DOI] [PubMed] [Google Scholar]
- 111.Lange Y., Echevarria F., Steck T. L. 1991. Movement of zymosterol, a precursor of cholesterol, among three membranes in human fibroblasts. J. Biol. Chem. 266: 21439–21443 [PubMed] [Google Scholar]
- 112.Lusa S., Heino S., Ikonen E. 2003. Differential mobilization of newly synthesized cholesterol and biosynthetic sterol precursors from cells. J. Biol. Chem. 278: 19844–19851 [DOI] [PubMed] [Google Scholar]
- 113.Maxfield F. R., Wustner D. 2012. Analysis of cholesterol trafficking with fluorescent probes. Methods Cell Biol. 108: 367–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holtta-Vuori M., Uronen R. L., Repakova J., Salonen E., Vattulainen I., Panula P., Li Z., Bittman R., Ikonen E. 2008. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic. 9: 1839–1849 [DOI] [PubMed] [Google Scholar]
- 115.Gimpl G., Gehrig-Burger K. 2011. Probes for studying cholesterol binding and cell biology. Steroids. 76: 216–231 [DOI] [PubMed] [Google Scholar]
- 116.Ačimovič J., Lovgren-Sandblom A., Monostory K., Rozman D., Goličnik M., Lutjohann D., Bjorkhem I. 2009. Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2081–2086 [DOI] [PubMed] [Google Scholar]
- 117.Chase T. H., Lyons B. L., Bronson R. T., Foreman O., Donahue L. R., Burzenski L. M., Gott B., Lane P., Harris B., Ceglarek U., et al. 2010. The mouse mutation “thrombocytopenia and cardiomyopathy” (trac) disrupts Abcg5: a spontaneous single gene model for human hereditary phytosterolemia/sitosterolemia. Blood. 115: 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang L. S., Voyiaziakis E., Markenson D. F., Sokol K. A., Hayek T., Breslow J. L. 1995. apo B gene knockout in mice results in embryonic lethality in homozygotes and neural tube defects, male infertility, and reduced HDL cholesterol ester and apo A-I transport rates in heterozygotes. J. Clin. Invest. 96: 2152–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Snouwaert J. N., Brigman K. K., Latour A. M., Malouf N. N., Boucher R. C., Smithies O., Koller B. H. 1992. An animal model for cystic fibrosis made by gene targeting. Science. 257: 1083–1088 [DOI] [PubMed] [Google Scholar]
- 120.Blendy J. A., Kaestner K. H., Weinbauer G. F., Nieschlag E., Schutz G. 1996. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 380: 162–165 [DOI] [PubMed] [Google Scholar]
- 121.Rainey M. A., George M., Ying G., Akakura R., Burgess D. J., Siefker E., Bargar T., Doglio L., Crawford S. E., Todd G. L., et al. 2010. The endocytic recycling regulator EHD1 is essential for spermatogenesis and male fertility in mice. BMC Dev. Biol. 10: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hong C. J., Tsai P. J., Cheng C. Y., Chou C. K., Jheng H. F., Chuang Y. C., Yang C. N., Lin Y. T., Hsu C. W., Cheng I. H., et al. 2010. ENU mutagenesis identifies mice with morbid obesity and severe hyperinsulinemia caused by a novel mutation in leptin. PLoS ONE. 5: e15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McMinn J. E., Liu S. M., Dragatsis I., Dietrich P., Ludwig T., Eiden S., Chua S. C., Jr 2004. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm. Genome. 15: 677–685 [DOI] [PubMed] [Google Scholar]
- 124.Osuga J., Ishibashi S., Oka T., Yagyu H., Tozawa R., Fujimoto A., Shionoiri F., Yahagi N., Kraemer F. B., Tsutsumi O., et al. 2000. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc. Natl. Acad. Sci. USA. 97: 787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Repa J. J., Turley S. D., Lobaccaro J. A., Medina J., Li L., Lustig K., Shan B., Heyman R. A., Dietschy J. M., Mangelsdorf D. J. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 289: 1524–1529 [DOI] [PubMed] [Google Scholar]
- 126.Peet D. J., Turley S. D., Ma W. Z., Janowski B. A., Lobaccaro J. M. A., Hammer R. E., Mangelsdorf D. J. 1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 93: 693–704 [DOI] [PubMed] [Google Scholar]
- 127.Seifert E. L., Caron A. Z., Morin K., Coulombe J., He X. H., Jardine K., Dewar-Darch D., Boekelheide K., Harper M. E., McBurney M. W. 2012. SirT1 catalytic activity is required for male fertility and metabolic homeostasis in mice. FASEB J. 26: 555–566 [DOI] [PubMed] [Google Scholar]
- 128.Cornwall G. A. 2009. New insights into epididymal biology and function. Hum. Reprod. Update. 15: 213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]