Abstract
The synthesis of apoE by adipocytes has profound effects on adipose tissue lipid flux and gene expression. Using adipose tissue transplantation from wild-type (WT) to apoE knockout (EKO) mice, we show that adipose tissue also contributes to circulating apoE. Different from circulating apoE produced by bone marrow transplantation (BMT), however, adipose tissue-derived apoE does not correct hyperlipidemia or suppress atherosclerosis. ApoE secreted by macrophages has a more acidic isoform distribution, and it increases binding of reconstituted VLDL particles to hepatocytes and fibroblasts more effectively than apoE secreted by adipocytes. The incremental binding can be entirely accounted for by binding to the LDL receptor. After BMT into EKO hosts, plasma cholesterol and macrophage-derived apoE are largely within IDL/LDL- and HDL-sized particles. After adipose tissue transplantation, most cholesterol and adipocyte apoE remain in VLDL. After BMT, circulating apoE no longer demonstrates predominance of acidic isoforms compared with that circulating after fat transplantation. In conclusion, fat transplantation provides circulating apoE levels similar to those provided by bone marrow transplantation, but it does not suppress hyperlipidemia or atherosclerosis. A potential mechanism contributing to this difference is differential binding to cell surface lipoprotein receptors.
Keywords: apolipoprotein E, bone marrow transplantation, adipose tissue transplantation
Apolipoprotein E (apoE) is a multifunctional protein that is synthesized and secreted by several mammalian cells. These sources include hepatocytes, which account for approximately 90% of circulating apoE, as well as macrophages, astrocytes, endocrine cells, and smooth muscle cells (1–4). Especially relevant to the studies reported here, adipocytes produce abundant apoE (5, 6). It is generally accepted that apoE secreted by each of these tissues shares the same general systemic function; however, there is evidence that apoE derived from specific tissues may have additional functions unique to that tissue (1–6). Among the functions that have been attributed to apoE are the clearance of remnant lipoproteins in plasma (7), participation in reverse cholesterol transport (8), and the assembly of nascent VLDL particles (9). In adipocytes and adipose tissue, endogenous apoE expression regulates triglyceride flux and adipocyte gene expression (10, 11). Thus, adipocytes of apoE knockout (EKO) mice are smaller, contain less triglyceride, and internalize less triglyceride from extracellular, triglyceride-rich lipoprotein than WT adipocytes (12, 13). The deficiency in adipocyte triglyceride accumulation in EKO adipocytes is not corrected by the provision of extracellular apoE, but it can be corrected by viral-mediated expression of endogenous apoE in adipocytes (11). In vivo, the absence of endogenous adipocyte apoE expression is reflected by less weight gain and reduced adipose tissue compared with WT mice (11, 12).
EKO mice have been employed as a favored model for the study of atherosclerosis. These mice manifest significant hypercholesterolemia and accelerated atherosclerosis on a chow diet. Both atherosclerosis and hyperlipidemia can be significantly attenuated by the expression of apoE in macrophages derived from transplanted WT bone marrow (14, 15) and by increased transgenic expression of apoE by adrenal cells (16;17). These effects of bone marrow cell-derived and adrenal cell-derived apoE occur at relatively low levels of circulating apoE in comparison to normal circulating levels in wild-type animals. In addition, there is evidence that extrahepatic apoE expression, even at a level that produces very low circulating apoE concentration, can suppress the development and progression of atherosclerosis, even without correcting systemic hyperlipidemia (17, 18).
In this report, we confirm that provision of macrophage-derived apoE after transplantation of WT bone marrow into EKO mice leads to correction of hyperlipidemia. Unexpectedly, we show that transplantation of WT adipose tissue into EKO hosts produces an apoE plasma level above that reported to correct hyperlipidemia and suppress atherosclerosis (after bone marrow transplantation or adrenal transgenic expression of apoE) but that it has no impact on hyperlipidemia or atherosclerosis. We further explore potential mechanisms for the differential impact of macrophage-derived compared with adipocyte-derived apoE on hyperlipidemia.
MATERIALS AND METHODS
Materials
Cell culture medium and FBS were purchased from Invitrogen (Carlsbad, CA). Organic solvents were from Thermo-Fisher (Pittsburgh, PA). [3H] Cholesteryl hexadecyl ether was obtained from PerkinElmer (Wellesley, MA). Total cholesterol (TC) and triglyceride (TG) assay kits were obtained from Wako Chemicals USA (Richmond, VA) and Roche Molecular Diagnostics (Pleasanton, CA). Other chemicals were from Sigma (St. Louis, MO) unless noted.
Adipose tissue transplantation
All animal protocols and surgical procedures were approved by the Institutional Animal Care and Use Committees of the University of Illinois at Chicago and the University of Chicago. C57BL/6 mice (WT) were purchased from Charles River (Wilmington, MA). EKO breeder pairs on a C57BL/6J background were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in-house. Adipose tissue transplantation was performed as described previously (12). Briefly, epididymal white adipose tissue was isolated from 14-week-old male WT or EKO donor mice and rinsed in cold PBS. The recipient mice were 12- to 14-week-old male EKO mice (n = 8 per group). WT or EKO adipose tissue grafts (400 mg total donor adipose tissue per mouse) were inserted subcutaneously in equal portions into four subcutaneous dorsal skin incisions, which were closed with surgical clips. After transplantation, mice were housed individually and fed with chow diet for 12–14 weeks. On the day of experiments, mice were fasted for 4 h before euthanasia.
Bone marrow transplantation
Donor bone marrow was harvested from WT GFP-positive expressing mice (C57BL/6 background, stock 006567 obtained from Jackson Lab) by flushing femurs and tibias of donor mice with RPMI supplemented with 2% FBS and 10 U/ml heparin. Cells were extensively washed and injected to lethally irradiated (900 rads) EKO recipient mice (n = 7, male, 6 weeks old) at a concentration of 5 million cells/200 µl via the retro-orbital sinus. After 8 weeks, mice were euthanized for tissue harvesting.
Analysis of atherosclerotic lesions
Atherosclerotic lesions were quantified as described previously (19). Briefly, mice were exsanguinated via the retro-orbital sinus and perfused with PBS for 2 min followed by a 20 min perfusion with 4% paraformaldehyde and 5% sucrose in PBS. The upper half of the heart and the proximal aorta were embedded in OCT compound and frozen in dry ice for sectioning. Serial, 10 µm frozen sections were collected from the innominate artery through the aortic root. Lesions in the innominate artery were quantified as the average of three sections separated by 100 µm and located between 100 and 300 µm proximal to the apex of the lesser curvature of the aortic arch. Aortic root lesions were measured as the average of three sections separated by 100 µm, beginning at the site of appearance of the coronary artery and valve leaflets. All sections were stained with Oil Red O and counterstained with Fast Green. Atherosclerotic lesions were quantified using OpenLab Software, version 3.1.5 (Improvision, Lexington, MA).
Lipid and lipoprotein analysis
Plasma samples (100 μl) were fractionated on tandem Superose 6 fast protein liquid chromatography (FPLC) columns in 200 mmol/l sodium phosphate (pH 7.4), 50 mmol/l NaCl, 0.03% EDTA, and 0.02% sodium azide, and 400 μl fractions were collected (19). The amount of cholesterol in the even-numbered fractions and total cholesterol in plasma were determined using Roche Diagnostics kits.
ApoE protein analysis by one- and two-dimensional Western blot
Mouse peritoneal macrophages (MPM)-conditioned medium (MCM) and adipocyte-conditioned medium (ACM) were collected from cultured macrophages or adipocytes 10–14 days after isolation. MPM were isolated by peritoneal lavage with PBS (20), and adipocytes were prepared as previously described (11). Digestion of cell-derived apoE with neuraminidase (Sigma, recombinant α2-3,6,8,9-neuraminidase) was conducted over 4 h at 37°C as described (21).
ApoE protein levels were measured by quantitative Western blot as previously described (11). Experimental samples were run alongside purified apoE at various concentrations to generate a standard curve.
ApoE isoprotein distribution was analyzed by two-dimensional (2D) gel electrophoresis. The resultant samples were analyzed by Western blot using an antibody to apoE. Samples (plasma, conditioned medium, or isolated VLDL) were extracted with rehydration buffer consisting of 8 M urea, 4% CHAPS, 65 mM DTE, 0.5% ampholytes, and a trace of bromophenol blue; the final volume was 350 μl. The protein solution was then spun at 100,000 RPM for 30 min at 4°C. Then 340 μl was absorbed into an 18 cm immobilized pH gradient (pH 4–7) IPG strip (GE Bioscience, Piscataway, NJ) overnight. The first isoelectric focusing dimension was conducted for a total of 60 kVh (PROTEAN IEF cell, Bio-Rad) at room temperature. Before SDS-PAGE electrophoresis, IPG strips were equilibrated with 3 ml of an equilibrium solution containing 50 mM Tris-HCl (pH 8.8), 6 M urea, 30% glycerol, 2% SDS, a trace of bromophenol blue, and DTE (1% w/v) for 20 min, followed by a second equilibration for 20 min in the same equilibrium solution containing iodoacetamide (2.5% w/v) instead of DTE. Finally, the strips were transferred to the top of 4–12% Bis-Tris Zoom gels and run at constant voltage of 200 V for 50 min. Proteins were transferred to nitrocellulose membrane and blotted with anti-apoE antibodies. ApoE isoprotein distribution is presented as percentage of total apoE protein.
VLDL reconstitution and binding to HepG2 cells
ApoE-deficient VLDL was isolated from the plasma of EKO mice (n = 50, 12–18 weeks old). VLDL was reconstituted with macrophage-derived or adipocyte-derived apoE by incubation in apoE free lipoprotein deficient serum (LPDS) with MCM or ACM containing 15 µg of secreted apoE for 4 h at 37°C. For some experiments reconstituted particles were radiolabeled by incubation in 125 µCi [3H] cholesteryl hexadecyl ether in apoE-free LPDS for 2 h at room temperature (22, 23). Reconstituted VLDL preparations (VLDL-E) were reisolated by overnight centrifugation at 38,000 rpm. Total protein mass and specific activity of the label in the reconstituted particles was measured. ApoE content of the particles was also measured and was not significantly different between particles reconstituted with macrophage-derived or adipocyte-derived apoE.
VLDL binding to HepG2 cells was performed as described previously (22, 23). HepG2 cells seeded in 12-well plates were washed with cold PBS and 4 IU/ml heparinase/PBS and two more washes with cold PBS. MCM/ACM reconstituted [3H]VLDL-E was added to the cells at 0, 5, 10, 20, and 50 µg/ml in DMEM with 0.1% BSA for 2 h on melting ice in the presence or absence of 20-fold excess of each unlabeled VLDL-E. After incubation, cells were washed two times with PBS, and cell protein and radioactivity were determined. Specific binding was calculated by subtracting nonspecific binding from the total binding. Binding curve was fit with nonlinear regression, and Bmax and Kd were calculated using Sigmaplot 11.0 (San Jose, CA). The binding assay was repeated in a separate set of HepG2 cells at a single concentration of [3H]VLDL (25 µg/ml). VLDL-E uptake in fibroblast derived from C57BL6 (WT) mice and LDL receptor-deficient mice (LDLR KO) was also evaluated by apoE Western blot. Mouse skin fibroblasts were prepared from newborn pups as described previously (24)
ApoE-mediated efflux in J774 macrophages
Cholesterol efflux to apoE containing conditioned medium was measured as previously described (25). J774 macrophages were seeded in 6-well plates and cultured in DMEM with 10% FBS plus 1 μCi/ml [3H]cholesterol, 80 μg/ml acLDL and 2 μg/ml ACAT inhibitor for 48 h. Cells were washed three times with DMEM and 0.1% BSA, and then incubated in DMEM and 0.2% BSA for 3 h. After washing, ACM and MCM were added to the cells based on equal amount of apoE in the conditioned medium (3.2 μg/well). Aliquots of medium samples were collected at 0, 1, 2, 4, and 24 h and centrifuged at 14,000 rpm for 15 min to remove detached cells. Radioactivity in the supernatants was quantified by a β-counter. Cells were washed and lysed with 0.1N NaOH, and cell radioactivity was measured.
Characterization of VLDL-E particles and apoE containing conditioned medium
Protein composition of VLDL-E particles and apoE containing conditioned medium samples were analyzed by quantitative Western blot for apoE and apoAI or by ELISA analysis for apoCIII (Assaypro, St. Charles, MO). Lipids were extracted with the Bligh Dyer method, total and free cholesterol were measured with Amplex Red cholesterol assay kit (Life Technologies, Grand Island, NY), TG was quantified with Wako TG kit (Wako USA, Richmond, VA), and total phosphorus in phospholipids was determined by the modified Bartlett procedure (26).
Analysis of F4/80-positive cells derived from bone marrow
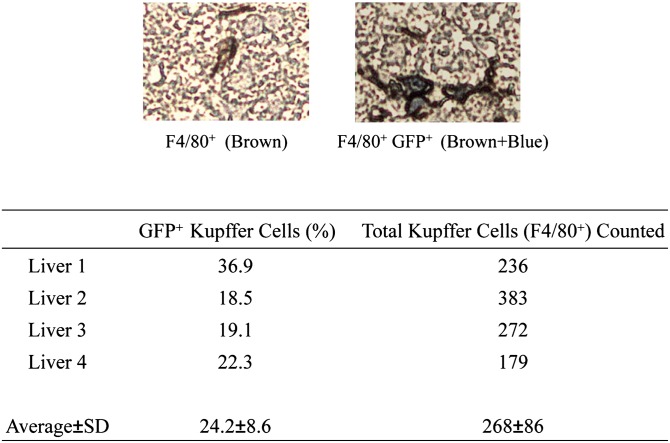
Liver samples were harvested from EKO recipient mice 8 weeks after receiving bone marrow transplantation from WT GFP-positive donors. Liver specimens were fixed in formalin, and paraffin sections were generated. The sections were stained with anti-F4/80 or anti-GFP antibodies (Abcam, Cambridge, MA) followed by alkaline phosphatase-conjugated IgG and DAB. Total Kupffer cells (F4/80-positive) in each liver were counted, and the proportion of GFP-positive Kupffer cells was estimated.
Lipoprotein agarose gel electrophoresis
Lipoprotein VLDL-E preparations were subject to Titan Gel electrophoresis according to the manufacturer's instructions (Helena Laboratories, Beaumont, TX). Ten microliters of VLDL-apoE samples were applied to Titan gel and electrophoresed at 80 V for 45 min, and then the gel was stained with Fat Red 7B.
Lipid and protein estimation
TC and TG levels were estimated with Wako or Roche TC and TG kits. Protein levels were quantified with Bio-Rad protein DC kit (Bio-Rad, Hercules, CA).
Statistics
Unless otherwise indicated, results are presented as mean ± SD of triplicate determinations. The results shown are from experiments representative of 2–3 experiments with similar results. Statistical differences were analyzed using Student t-test or ANOVA (PASW 18.0, IBM SPSS, Armonk, NY). P < 0.05 was considered significant. Differences in atherosclerotic lesion area were analyzed by the Mann-Whitney test.
RESULTS
Adipose tissue-derived apoE does not correct hyperlipidemia or attenuate atherosclerosis in EKO recipient mice
In the first set of experiments, EKO recipient mice received transplants of adipose tissue or bone marrow from WT donors. After 8–12 weeks, plasma was obtained from recipient mice to measure TC, total TG, and plasma apoE levels. The adipose transplantation experiment was performed twice. As shown in Table 1, provision of WT-type bone marrow to EKO recipients produced a circulating apoE level of 0.54 mcg per ml and substantially corrected the hyperlipidemia of the recipients. Provision of WT adipose tissue to EKO recipients produced a circulating apoE level of 0.48 mcg per ml in one experiment (14-week-old recipients) and 0.32 mcg per ml in the other (12-week-old recipients) The plasma apoE levels in both adipose tissue transplantation experiments are above the threshold apoE levels reported to correct hyperlipidemia and suppress atherosclerosis in EKO mice after BMT or transgenic expression of apoE in adrenal glands (14–17). In spite of this and contrary to our expectation, hyperlipidemia was not corrected by provision of apoE derived from WT adipose tissue.
TABLE 1.
Plasma lipid and apoE levels in fat-transplanted or bone marrow-transplanted mice
| Total TC (mg/dl) | Total TG (mg/dl) | Plasma apoE (µg/ml) | |
| mean ± SD | mean ± SD | mean ± SD | |
| Fat transplantation | |||
| EKO-EKO | 672 ± 163 | 146 ± 58 | 0 |
| WT-EKO | 657 ± 87 | 177 ± 38 | 0.48 ± 0.16 |
| EKO-EKO | 388 ± 49 | 68.2 ± 21 | 0 |
| WT-EKO | 443 ± 106 | 69.2 ± 17 | 0.32 ± 0.06 |
| Bone marrow transplantation | |||
| EKO-EKO | 358 ± 59a | 81 ± 34 | 0 |
| WT (GFP)-EKO | 94 ± 19 | 45 ± 28 | 0.54 ± 0.09 |
In each row, the first indicated genotype represents the donor and the second the recipient of the indicated transplanted tissue (n = 7–8 for each transplantation pair). Plasma was obtained for analysis 8–12 weeks after transplantation.
P< 0.01 for EKO-EKO to WT-EKO comparison.
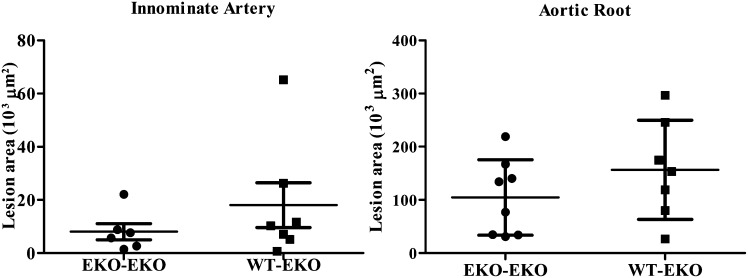
Provision of WT bone marrow to EKO recipient mice or the transgenic expression of apoE in adrenal gland has been shown to suppress atherosclerosis in EKO mice independently of an effect on hyperlipidemia (14, 16, 17). By contrast, the provision of WT adipose tissue to EKO recipient mice fails to suppress atherosclerosis at either the innominate artery or the aortic root (Fig. 1), in spite of circulating apoE levels comparable to those that have been shown to influence atherogenesis when the apoE is derived from other extrahepatic sources (i.e., macrophage or adrenal cells).
Fig. 1.
Quantitation of atherosclerotic lesions in EKO recipients of EKO or WT adipose tissue. WT or EKO adipose tissue was transplanted into EKO recipient mice at 12 weeks. At 24 weeks, mice were sacrificed for analysis of atherosclerotic lesions in the innominate artery (IA) or the aortic root (AR) as described in Materials and Methods.
Charge differences in macrophage and adipose tissue-derived apoE
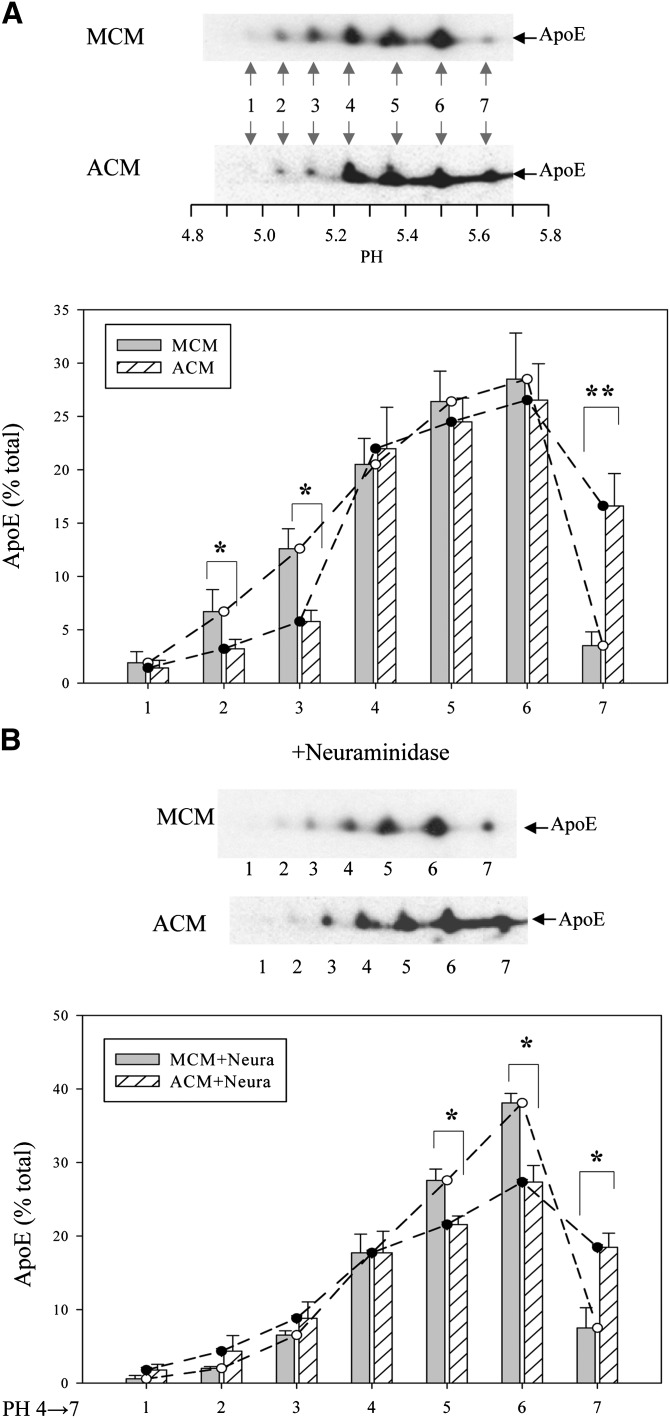
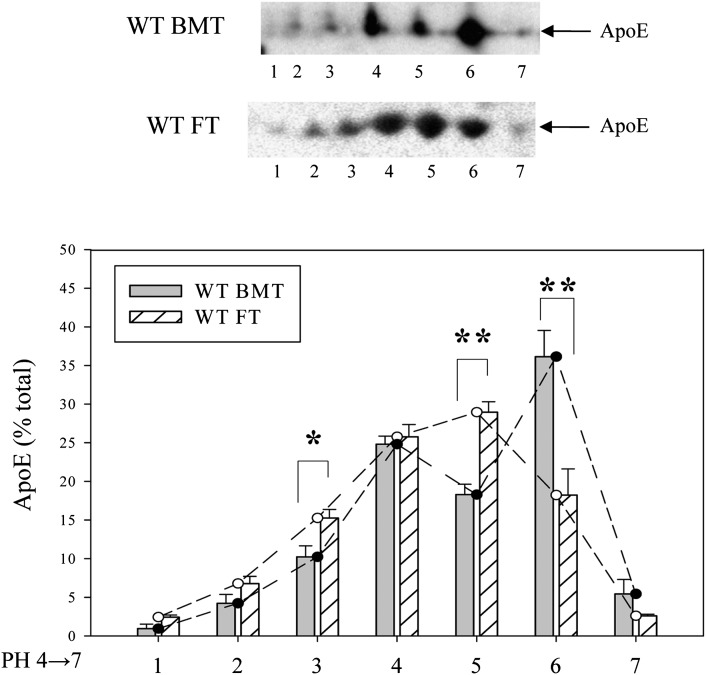
The results in Table 1 and Fig. 1 establish important in vivo functional differences between the apoE produced by adipose tissue and macrophages derived from bone marrow. To obtain insight into potential structural correlates of these functional differences we performed 2D gel electrophoresis of the apoE secreted by primary cultures of mouse peritoneal macrophages and mouse adipocytes. Fig. 2A shows that the apoE can be resolved into seven distinct isoforms and that apoE secreted by macrophages is significantly more acidic than that secreted by adipocytes. Substantially more macrophage-derived apoE is found in spots 1, 2, and 3 (corresponding to a more acidic isoelectric point), whereas more adipocyte-derived apoE is found in spot 7. A potential source of charge heterogeneity in apoE relates to the posttranslational addition of sialic acid residues (21, 27, 28). We tested this potential explanation by subjecting macrophage-derived and adipocyte-derived apoE to neuraminidase digestion prior to isoelectric focusing. As shown in Fig. 2B, digestion with neuraminidase substantially reduced the difference in the relative abundance of acidic isoforms between macrophage-derived and adipocyte-derived apoE, confirming the importance of sialic acid residues for contributing to this difference. After neuraminidase digestion, there was no difference between the relative abundance of the most acidic isoforms, whereas the macrophage-derived apoE had a larger fraction in spots 5 and 6 after neuraminidase treatment, suggesting that this was the result of the conversion of the more acidic forms to forms with more basic isoelectric points.
Fig. 2.
Isoform distribution of ApoE secreted by macrophages and adipocytes. (A) Cultures of primary mouse peritoneal macrophages or adipocytes were incubated overnight in serum-free medium to collect secreted ApoE. ApoE in the MCM and the ACM was separated by isoelectric focusing followed by SDS PAGE. Proteins were transferred to nitrocellulose membranes and blotted with antibodies to ApoE. (B) ApoE in conditioned medium was digested with neuraminidase prior to isoelectric focusing as described in Materials and Methods. Representative Western blots are shown. For quantitation, ApoE isoprotein distribution is expressed as a percentage of a total of seven isoproteins, and the results shown are the mean ± SD of three separate experiments. The pH values of isoproteins in discrete spots are #1, 4.95; #2, 5.04; #3, 5.12; #4, 5.23; #5, 5.37; #6, 5.50; and #7, 5.65. *P < 0.05; **P < 0.01 for the comparison of the ApoE isoprotein in MCM to ACM.
Macrophage and adipocyte apoE binding to cell surface receptors
Normal lipoprotein metabolism depends on lipoprotein particle binding to cell surface receptors, for which apoE serves as an important ligand. Therefore, differences in receptor binding could contribute to the differing potencies of macrophage and adipocyte apoE for correcting hyperlipidemia and suppressing atherosclerosis that we observed in vivo. To examine the receptor binding properties of the macrophage and adipocyte apoE, we reconstituted VLDL particles from EKO mice with conditioned media containing apoE secreted from cultured macrophages or adipocytes (VLDL-E). The composition of the two sets of conditioned media is shown in Table 2. Because apoC proteins are thought to modify the function of apoE on VLDL particles, both apoE and apoCIII levels were measured. Conditioned media have similar amounts of apoE, but adipocyte-conditioned media (ACM) has more apoCIII. Consistent with our previous observations demonstrating the importance of endogenous macrophage apoE for mediating macrophage lipid efflux, there is more lipid per milligram of apoE in macrophage-conditioned media (MCM).
TABLE 2.
Characterization of conditioned medium
| ACM | MCM | |
| Proteins | ||
| ApoE (µg/µg cell protein) | 4.33 ± 0.4 | 4.03 ± 0.70 |
| ApoCIII (µg/mg cell protein) | 6.71 ± 0.74a | 4.92 ± 0.45 |
| Lipids | ||
| TC (µg/µg ApoE) | 0.45 ± 0.01a | 0.70 ± 0.04 |
| FC (µg/µg ApoE) | 0.37 ± 0.07a | 0.67 ± 0.05 |
| TG (µg/µg ApoE) | 0.38 ± 0.05a | 0.69 ± 0.08 |
| PL (µmol/µg ApoE) | 0.056 ± 0.005a | 0.081 ± 0.006 |
P< 0.05 ACM versus MCM.
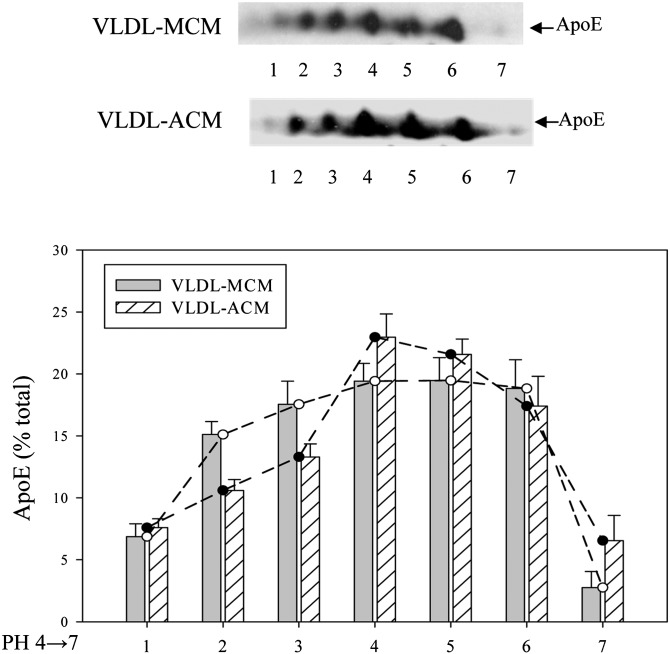
After reconstitution of the VLDL particles harvested from EKO mice with equal amounts of apoE from the two types of conditioned media, VLDL-E particles were reisolated by density gradient ultracentrifugation. The reisolated VLDL-E particles contained similar amounts of apoE and apoA1 per total amount of VLDL protein (Table 3) but differed in the amount of apoCIII, and the VLDL reconstituted with ACM had a higher apoCIII to apoE molar ratio. Figure 3 shows the results of 2D gel electrophoresis of apoE incorporated into VLDL-E particles and confirms the predominance of more acidic apoE isoforms in VLDL particles reconstituted with macrophage-derived apoE compared with those reconstituted with adipocyte-derived apoE. The entire agarose gel electrophoresis of the reconstituted VLDL-E particles demonstrates slightly faster migration to the positive electrode of the particle reconstituted with macrophage-derived apoE, consistent with a more negative overall VLDL particle charge (not shown).
TABLE 3.
Characterization of VLDL-E particles reconstituted with ACM or MCM
| VLDL-ACM | VLDL-MCM | |
| Proteins | ||
| Total protein (mg/ml) | 1.8 ± 0.2 | 1.6 ± 0.1 |
| ApoE (µg/mg protein) | 1.33 ± 0.08 | 1.94 ± 0.5 |
| ApoAI (scanning units/mg protein) | 1.89 ± 0.24 | 1.91 ± 0.38 |
| ApoCIII (µg/mg protein) | 126.9 ± 17.9a | 111.4 ± 9.3 |
| ApoCIII/ApoE molar ratio | 401.3 ± 56.7a | 241.5 ± 20.2 |
| Lipids | ||
| TC (µg/mg protein) | 178.1 ± 13.5a | 143.5 ± 14.2 |
| FC (µg/mg protein) | 161.2 ± 14.8a | 137.3 ± 18.3 |
| TG (µg/mg protein) | 4.77 ± 0.11 | 6.50 ± 0.54 |
| PL (µmol/mg protein) | 1.33 ± 0.13a | 1.35 ± 0.08 |
P< 0.05 VLDL-ACM versus VLDL-MCM.
Fig. 3.
Isoform distribution of macrophage- or adipocyte-derived ApoE incorporated into VLDL isolated from EKO mice. Serum-free medium containing ApoE secreted by mouse peritoneal macrophages or adipocytes was incubated with VLDL isolated from the plasma of EKO mice as described in Materials and Methods. VLDL was re -isolated by density gradient ultracentrifugation and subjected to isoelectric focusing followed by SDS PAGE. Proteins were transferred to nitrocellulose membranes and blotted with antibodies to ApoE. A representative Western blot is shown. The quantitative results represent results averaged from two separate experiments.
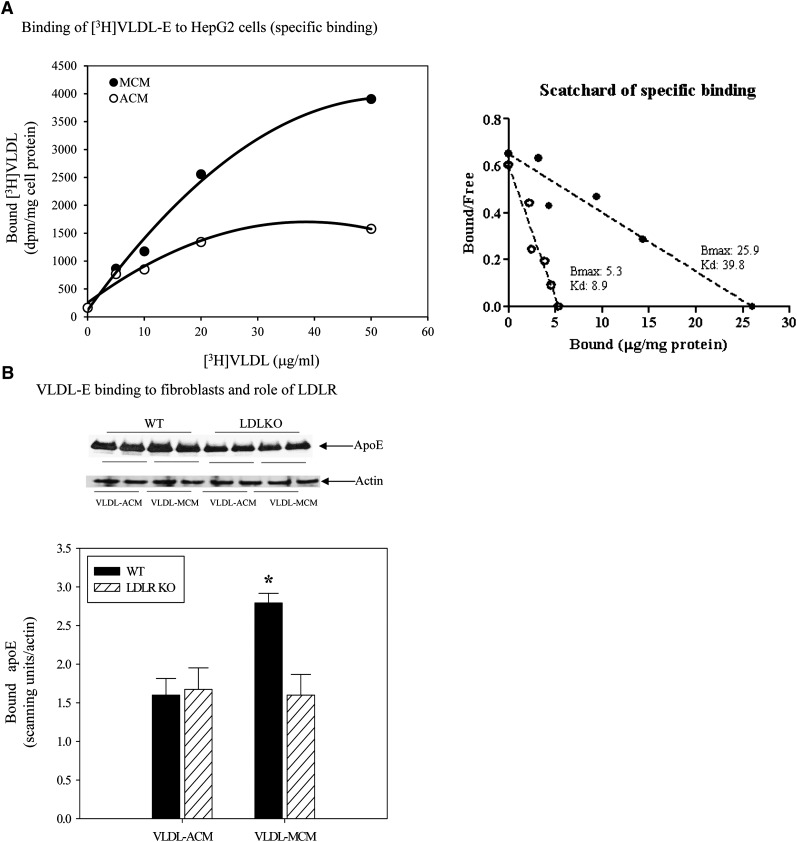
To determine whether the two apoE derived from different sources differ in their binding to cells, we evaluated the binding of the reconstituted VLDL-E particles to monolayers of HepG2 cells or skin fibroblasts. Fig. 4A shows binding of cholesteryl ether-labeled reconstituted VLDL-E particles to HepG2 cells. Binding of each type of reconstituted particle was saturable and specific, but the binding of the particles reconstituted with macrophage-derived apoE was significantly higher than that observed with adipocyte-derived apoE. A Scatchard plot of the binding of the VLDL-E particles suggests that the VLDL-E reconstituted with ACM binds to fewer sites on the surface of HepG2 cells than does MCM-reconstituted VLDL (Bmax for VLDL-ACM = 5.3 versus 25.9 for VLDL-MCM), yet the former exhibits higher affinity (Kd = 8.9 versus 39.9 for the VLDL-MCM). To gain additional insight into the basis for this difference in binding, we next measured binding of VLDL-E to fibroblasts derived from either wild-type mice or from mice lacking the LDL receptor (LDLR) (Fig. 4B). Because these cells produce no endogenous apoE, we utilized unlabeled particles and performed Western blot to measure the amount of apoE remaining associated with the fibroblast cells after extensive washing. Consistent with the observation in HepG2 cells, binding of the VLDL-E particles reconstituted with macrophage-derived apoE to wild-type fibroblasts was significantly higher than that observed for the particles reconstituted with adipocyte-derived apoE. However, based on the binding of the VLDL-E particles to fibroblasts from LDLR-deficient mice, it appeared that the LDLR had no apparent role in the binding of VLDL-ACM, whereas it appeared to account for about half the binding of the VLDL-MCM. Based on these results, it seems that the difference in binding between VLDL-E reconstituted with MCM and ACM can be completely accounted for by the latter's failure to bind to cell surface LDLR.
Fig. 4.
Binding of EKO VLDL reconstituted with macrophage or adipocyte ApoE to HepG2 cells and murine skin fibroblasts. VLDL particles were isolated from the plasma of EKO mice and reconstituted with ApoE secreted from macrophages or adipocytes. [3H]cholesteryl ether-labeled (for HepG2 experiments) or unlabeled (for fibroblast experiments) reconstituted VLDL particles were used for binding experiments as described in Materials and Methods. (A) Binding curve measuring saturable binding of [3H]cholesteryl ether-reconstituted particles to HepG2 cells. (B) Binding of reconstituted particles to fibroblasts from wild-type and LDLR-deficient mice. *P < 0.05 for comparing the binding of particles reconstituted with macrophage-derived to adipocyte-derived ApoE.
To determine whether the difference in sialic acid content was responsible for the increased binding of VLDL-E particles reconstituted with macrophage apoE, we reconstituted EKO VLDL with macrophage-derived and adipocyte-derived apoE after neuraminidase digestion. Contrary to our expectation, the binding of the macrophage-derived particle remained 2-fold higher (not shown). These differential binding results support the hypothesis that one potential mechanism for the ability of bone marrow transplantation to normalize hyperlipidemia in EKO mice, whereas adipose tissue transplantation does not, relates to the ability of macrophage-derived compared with adipocyte-derived apoE to enhance binding of VLDL particles to cell surface lipoprotein receptors. However, this differential binding is most likely not due to differences in sialic acid content.
Repopulation of liver macrophages following bone marrow transplantation
There is data in the literature to suggest that apoE produced in the liver is more effective for correcting hyperlipidemia than that produced extrahepatically (29, 30). Others have reported that macrophages in bone marrow could migrate to the liver as precursors of hepatic Kupffer cells (31). Such migration could provide a source of local hepatic apoE production and provide another potential mechanism for the greater efficacy of macrophage-derived apoE for correcting hyperlipidemia. We therefore next determined whether we could demonstrate significant migration of bone-marrow-derived macrophages to the liver using our BMT protocol and assay time points. We evaluated the migration of bone marrow-derived macrophages into the liver by transplanting bone marrow from WT GFP-positive donors into EKO recipients. After 8 weeks, the livers of recipient mice were harvested to evaluate the percentage of GFP-positive cells among F4/80-positive cells in liver sections. As shown in Fig. 5, in four different liver preparations in which an average of 268 total F4/80-positive cells were counted, approximately 25% were positive for GFP. This observation confirms that donor bone marrow macrophage-derived cells contribute significantly to total hepatic macrophage-like cells.
Fig. 5.
Analysis of the percentage of hepatic F4/80-positive cells derived from donor tissue after bone marrow transplantation. EKO mice (n = 4) received bone marrow from WT GFP-positive donors. After 8 weeks, livers of recipient mice were harvested for analysis of GFP positivity in hepatic F4/80-positive cells.
Lipoproteins in transplantation recipients
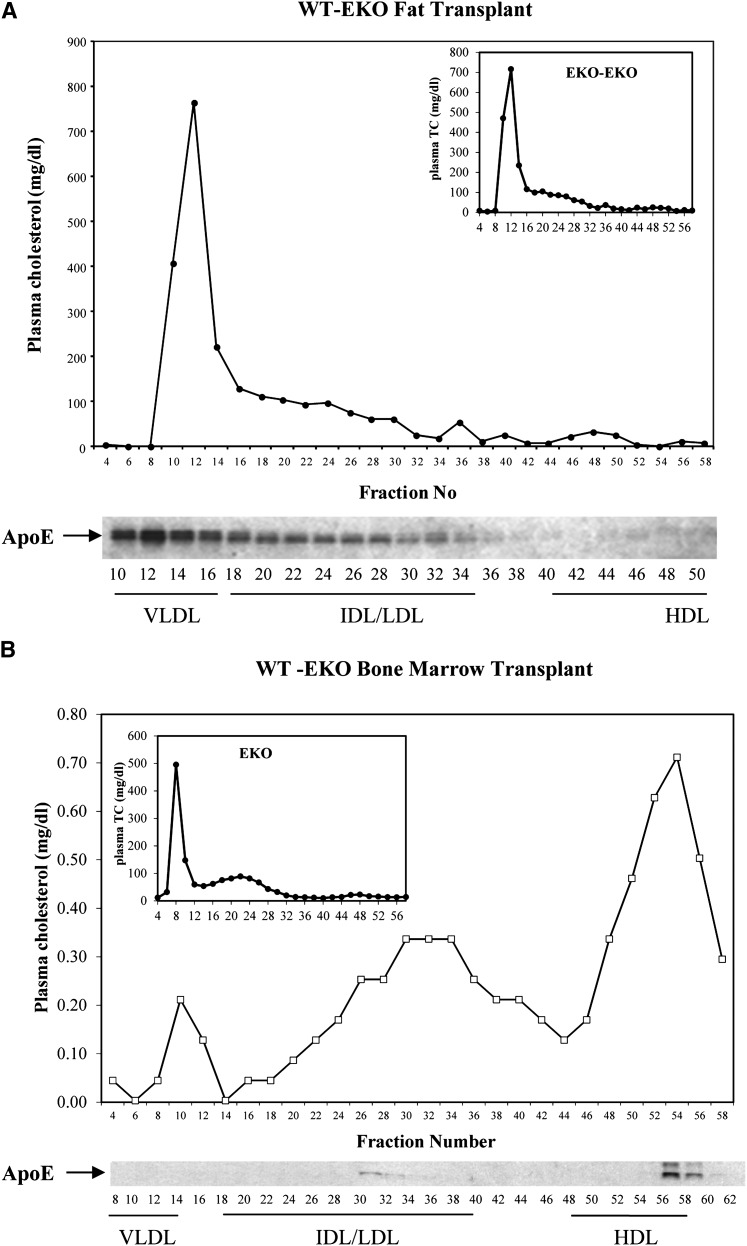
The enhanced binding of VLDL reconstituted with macrophage-derived apoE to cell surface receptors or the facilitated removal of EKO VLDL particles by the acquisition of apoE secreted by resident hepatic apoE-secreting macrophages after bone marrow transplantation predicts differential metabolism of VLDL particles containing macrophage-derived or adipocyte-derived apoE. As shown in Fig. 6A, after fat transplantation from WT to EKO, both plasma cholesterol and apoE are predominantly carried in large VLDL particles, which are also the predominant particles in the apoE-deficient hosts (Fig. 6A, insert). After bone marrow transplantation, however, there is significant reduction of cholesterol in VLDL, with most of the cholesterol now carried in IDL, LDL, and HDL particles. The apoE is found primarily in HDL particles (Fig. 6B). Fig. 7 shows the results of 2D electrophoresis of the apoE harvested from the plasma of EKO recipients of bone marrow or fat transplantation. ApoE from the plasma of EKO recipients of WT bone marrow compared with that from recipients of fat demonstrates no predominance in the abundance of the acidic apoE isoforms that characterize the VLDL reconstituted with macrophage-derived apoE in vitro. Although this observation could be the result of selective in vivo removal of sialic residues from VLDL-associated apoE, the overall decrease in plasma cholesterol and the specific reduction in VLDL cholesterol favor a model in which large VLDL particles that carry these macrophage-derived isoforms are preferentially removed from the circulation.
Fig. 6.
Cholesterol and ApoE distribution in circulating lipoproteins. Plasma samples were obtained from EKO recipients of WT fat (A) or WT bone marrow (B). Samples were fractionated by FPLC, and cholesterol concentration was measured in indicated fractions. ApoE was analyzed by Western blot in indicated fractions. (A) Insert shows distribution of cholesterol in lipoproteins of EKO recipients of EKO fat. (B) Insert shows cholesterol distribution in lipoproteins of EKO mice.
Fig. 7.
ApoE isoprotein distribution in the plasma of EKO recipients of WT fat or WT bone marrow. Plasma samples from EKO recipients of WT fat (FT) or WT bone marrow (BMT) were analyzed by two-dimensional electrophoresis and immunoblotted for ApoE as described in Materials and Methods. Plasma samples were pooled from 7–8 mice in each transplantation group prior to analysis. The quantitative results presented are the mean ± SD of three separate experiments.*P < 0.05, **P < 0.01 for comparison of apoE in recipients of WT bone marrow to recipients of WT fat.
Cholesterol efflux to ACM and MCM
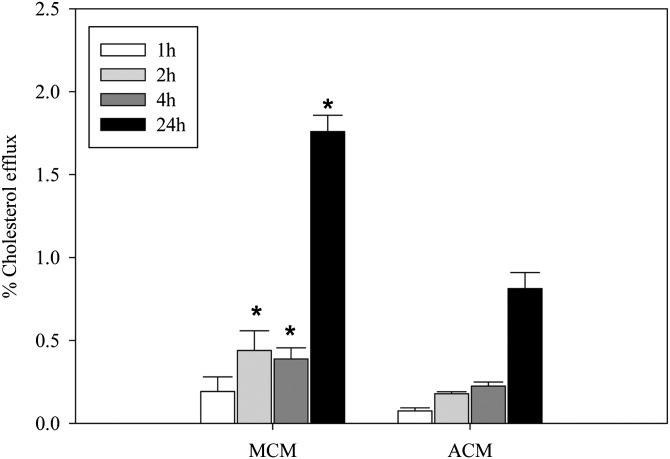
Finally, a potential mechanism that may differentiate the functions of the two sources of apoE is cholesterol efflux. We assessed this by loading J774 cells with acLDL and radioactive cholesterol, and then measured the capacity of the two conditioned media containing similar amounts of apoE to accept cholesterol from the loaded cells. As shown in Fig. 8, the MCM was more effective in accepting cholesterol than was the ACM at all time points after 1 h of incubation.
Fig. 8.
Cholesterol efflux by MCM and ACM. MCM and ACM containing similar amounts of apoE were incubated with J774 cells loaded with AcLDL and [3H] cholesterol. The percentage of total [3H]cholesterol in the media was determined at various times for comparison of efflux to MCM and ACM. *P < 0.05 for comparing ACM to MCM for each time point.
DISCUSSION
In this study, we establish important functional differences between the apoE derived from macrophages compared with apoE derived from adipose tissue. In vivo manifestations of this functional difference are reflected in the inability of apoE derived from subcutaneously transplanted adipose tissue to lower plasma cholesterol or attenuate atherosclerosis in the innominate artery and aortic root of apoE-deficient mice. Others have also reported that transplantation of intra-abdominal adipose tissue into EKO mice can increase atherosclerosis (32). This contrasts with the activity of macrophage-derived apoE derived from bone marrow transplantation, which is capable of both lowering plasma cholesterol as shown in this study and in previous studies and attenuating atherosclerosis as shown in previous studies (14, 15). Additional functional differences in the apoE derived from these two sources are seen in their respective binding characteristics to cell surface binding sites and in their differential ability to promote cholesterol efflux.
The lipoprotein profile of recipient animals expressing adipose tissue-derived apoE and those expressing bone marrow-derived apoE are quite different (Fig. 6). The animals receiving the apoE-expressing adipose tissue transplantation exhibit a lipoprotein profile that is almost identical to that of global apoE-deficient animals. By contrast, the lipoprotein profile of the animals receiving apoE-expressing bone marrow approximates that of normal animals. In vivo turnover studies of apoE produced by isolated macrophages or adipocytes in EKO mice were not feasible due to the inability to isolate sufficient quantities of purified secreted apoE that could be labeled to sufficient specific activity. However, based on our experimental observations and established pathways for lipoprotein metabolism, we believe it most likely that the improved lipid levels after bone marrow transplantation compared with adipose tissue transplantation relate to differences in the clearance of VLDL particles bearing apoE secreted from macrophages or adipose tissue. The results in Fig. 5 suggest that one mechanism that could contribute to the efficacy of bone marrow transplantation for correcting hyperlipidemia in EKO could relate to repopulation of Kupffer cells by WT macrophages with subsequent local hepatic production of apoE. ApoE production by hepatocytes has already been shown to more effectively reduce hyperlipidemia compared with apoE production by transplanted bone marrow, an effect that has been ascribed to the greater efficacy of apoE produced in the liver to facilitate lipoprotein clearance (29, 30). It has already been demonstrated that after bone marrow transplantation, a small amount of apoE can be detected on the liver of EKO recipients (29). However, this apoE could represent uptake of circulating apoE or the local production of apoE by WT macrophages that have migrated to the liver as precursors for hepatic Kupffer cells. Our studies using GFP-positive bone marrow provide evidence that bone marrow-derived macrophages represent a significant portion of F4/80-positive cells in the liver after bone marrow transplantation. Subcutaneous adipose transplantation will not provide precursors for Kupffer cells. Thus, local hepatic production of apoE by bone marrow-derived cells could be a potential mechanism for the greater efficacy of bone marrow transplantation for reducing hyperlipidemia in EKO mice. Although this mechanism may contribute to reduce plasma lipid levels after bone marrow transplantation, it is unlikely to be the only mechanism by which bone marrow-derived apoE suppresses hyperlipidemia and atherosclerosis, based upon results of experiments with adrenal expression of apoE. Furthermore, for both adrenal and macrophage origins of apoE, low levels of circulating apoE have been shown to suppress atherosclerosis independently of changes in circulating lipid levels (17, 18).
We believe it most likely that the functional difference between macrophage and adipocyte apoE for correcting hyperlipidemia relates to the differences we have established in their ability to bind to cell surface lipoprotein receptors. ApoE-deficient VLDL complemented with macrophage-derived apoE is much more effective than that complemented with adipose tissue-derived apoE, as measured by the ability of the VLDL-E to bind to HepG2 cells and skin fibroblasts (Fig. 4). Furthermore, the difference in binding appears to be explained by the failure of VLDL reconstituted with apoE in ACM to bind to the LDL receptor.
We sought to understand the basis for these functional differences. The first to be considered is a possible structural difference. Unfortunately the amount of apoE practically obtainable from isolated mouse adipose tissue or adipocytes is insufficient to allow for a complete structural analysis including mass spectrometry. Instead, we employed alternative approaches, relying mostly on isoelectric focusing to examine charge heterogeneity. This approach to apoE heterogeneity has been studied for a number of tissues. In the mouse, there is no evidence for allelic variation as there is in humans. Thus most of the observed heterogeneity is thought to arise as a result of posttranslational modification. Here we show that murine macrophage and adipose tissue culture secrete different proportions of apoE isoforms, with the acidic forms being more prominent in the macrophage culture medium relative to that secreted by the adipocytes. Sialylation of apoE has been reported to be altered with chronic alcohol intake (33) and in acquired immune deficiency syndrome (34). Some of the difference we detect between macrophage-derived and adipocyte-derived apoE, which is most likely the result of posttranslational glycosylation, can be reduced by the removal of the terminal sialic residues on the carbohydrate chains as shown in Fig. 2.
ApoE is a glycoprotein with O-linked carbohydrates, and evidence supports addition of carbohydrate as an important posttranslational modification of apoE (21, 27, 28). No evidence exists for the N-glycosylation of apoE. There are two positions in human apoE that are subject to O-glycosylation: threonine 194 and serine 290 (21). Although these sites are not conserved in mouse, there are serine or threonine residues within 1–4 residues of these sites in mouse apoE. The studies of Lee et al. (21) using mass spectrometry on human macrophage apoE indicate that at the threonine 194 the glycosylation chains can be quite complex; they identified eight different glycoforms, with (HexNAc)2- Hex2-(NeuAc)2 being the most complex. Their data suggest that individual molecules of apoE may carry different glycosylated forms; in other words, there is likely heterogeneity of glycosylation at this single site. In addition, four additional glycosylation substituents were detected related to the serine 290 position. The relative proportion of each of these glycosylated forms in the apoE of different tissues remains to be determined. However the proportion may differ from tissue to tissue as reflected in the studies of Williams et al. (35), who examined the apoE produced by Cynomolgus organ cultures from liver, spleen, lymph node, testis, and lung.
Because neuraminidase digestion prior to VLDL reconstitution did not eliminate the difference in binding to HepG2 cells it seems unlikely that the heterogeneity in isoforms contributed by sialic underlies all of the functional differences between the two sources of apoE. Given the complexity of the glycosylation pattern reported for human apoE (21) it remains possible that there is still glycosylation heterogeneity even after the removal of sialic acid residues. This clearly requires further study. It is of interest, however, that the more acidic forms do not appear in circulating VLDL after bone marrow transplantation. We cannot rule out the possibility that this results from the selective removal of sialic acid from apoE in vivo. The significant fall in TC and the large reduction of both the apoE and cholesterol in large VLDL particles, considered along with the in vitro binding data, support a model in which large lipoprotein particles containing macrophage-derived apoE are more efficiently removed from the circulation.
Alternative explanations for the ineffectiveness of apoE derived from the adipose tissue transplantation for suppressing hyperlipidemia and atherosclerosis could result from other elements derived from the adipose tissue or from the nonfunctional conformation of the apoE derived from adipose tissue. ACM contains more apoCIII than does MCM. This difference was maintained in the VLDL-E particles reconstituted with these conditioned media. ApoCIII is generally thought of as originating from the liver and intestine and to be associated with either VLDL or HDL. There is, however, evidence that adipose tissue makes apoCIII (36), confirmed by our findings. There is a complex interaction between apoE and apoCIII in the clearance and uptake of VLDL. The presence of a high relative ratio of apoCIII to apoE inhibits the uptake of lipoprotein particles, as first pointed out by Havel and colleagues (37, 38). With the available data, it is not possible to say whether the difference in the apoCIII:apoE ratio in the reconstituted VLDL particles (Table 3) is physiologically significant in the context of our transplantation models. The molar ratio of apoCIII:apoE in the reconstituted VLDL is very high in both cases, but the apparent significant difference between them may not be physiologically important. The apoE-deficient VLDL used for these reconstitution experiments had a high initial content of apoCIII (data not shown); the differences in apoCIII: apoE ratios in the reconstituted particles may result from the acquisition of more apoCIII or the remodeling of the particles. An additional function of apoCIII relevant to our results has been reported. It has been shown that apoCIII, whether free or associated with VLDL, influences monocyte adhesion to the endothelium of the vessel wall (39, 40), which could influence atherogenesis.
The functional distinction between the two apoE could also be attributable to a conformational difference. The most clear-cut distinction is that adipose tissue apoE does not appear to interact with the LDLR in contrast to the macrophage-derived apoE. Puzzling is our observation that, although the adipose tissue apoE does not bind to the LDLR, it binds to relatively few hepatocyte binding sites with a higher affinity than is exhibited by macrophage-derived apoE. The nature of this binding site(s) is unknown. This result suggests that the apoE derived from adipose tissue may be heterogeneous in its conformational states, with a small pool having a high affinity for a small pool of receptors but with most of the apoE being conformationally inactive. This would be compatible with two properties: the relatively lower activity of adipose tissue apoE in promoting cholesterol efflux and the inability of adipose tissue-conditioned VLDL to bind to the LDLR. It has previously been reported that rabbit β-VLDL containing apoE is not a good ligand for LRP on hepatocytes (41). However when the apoE was extracted and purified from this lipoprotein, it was quite an effective ligand. In our system, we cannot obtain an amount of material needed to directly test this hypothetical basis for the inactivity of the adipose tissue apoE.
We cannot resolve the basis for this intriguing functional distinction between macrophage and adipocyte apoE with the present experimental system; additional experiments in a different model in which we can obtain larger quantities of protein will be required. Preliminary experiments using enzyme digests and labeled precursors provide no evidence for phosphorylation, sulfation, or methylation as contributors to the structural heterogeneity (not shown). On the basis of our current information, we favor a model in which the more effective resolution of hyperlipidemia and suppression of atherosclerosis after bone marrow transplantation is mechanistically related to both the more avid binding of VLDL particles reconstituted with macrophage-derived apoE and to the migration of macrophages expressing apoE to the liver where they could facilitate removal of large VLDL particles (29, 30). In this scenario, it is necessary to consider the possibility that the similar circulating apoE levels in plasma after fat transplantation compared with bone marrow transplantation may not reflect secreting activity of the transplanted cells as some fraction of macrophage-derived apoE could be removed prior to its appearance in the plasma.
Our results provide clear evidence for a functional distinction between adipose tissue apoE and macrophage apoE with respect to lipoprotein clearance, atheroprotection, and VLDL binding to cell surface receptors. Liver apoE can perform all these functions as can macrophage and adrenal apoE. Whereas apoE is widely expressed, the systemic function of extrahepatic apoE has been previously studied only for macrophages and the adrenal gland. To this we add the systemic impact of adipose tissue-derived apoE, which does not follow the function of the apoE derived from these other extrahepatic sources.
Acknowledgments
The authors thank Stephanie Thompson and Laura Metz for assistance with manuscript preparation.
Footnotes
Abbreviations:
- ACM
- adipocyte-conditioned medium
- BMT
- wild-type bone marrow
- EKO
- apoE knockout
- FPLC
- fast protein liquid chromatography
- FT
- wild-type fat
- LDLR
- LDL receptor
- LPDS
- lipoprotein-deficient serum
- MCM
- macrophage-conditioned medium
- MPM
- mouse peritoneal macrophage
- TC
- total cholesterol
- TG
- triglyceride
- WT
- wild-type
- VLDL-E
- reconstituted VLDL
This work was supported by National Institutes of Health Grants DK-071711 (to T.M.) and HL-68585 (to P.V.S.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Kraft H. G., Menzel H. J., Hoppichler F., Vogel W., Utermann G. 1989. Changes of genetic apolipoprotein phenotypes caused by liver transplantation. Implications for apolipoprotein synthesis. J. Clin. Invest. 83: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtiss L. K., Boisvert W. A. 2000. Apolipoprotein E and atherosclerosis. Curr. Opin. Lipidol. 11: 243–251 [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Weisgraber K. H., Mucke L., Mahley R. W. 2004. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer's disease. J. Mol. Neurosci. 23: 189–204 [DOI] [PubMed] [Google Scholar]
- 4.Mazzone T. 1996. Apolipoprotein E secretion by macrophages: its potential physiological functions. Curr. Opin. Lipidol. 7: 303–307 [DOI] [PubMed] [Google Scholar]
- 5.Mazzone T. 2007. Adipose tissue and the vessel wall. Curr. Drug Targets. 8: 1190–1195 [DOI] [PubMed] [Google Scholar]
- 6.Fantuzzi G., Mazzone T. 2007. Adipose tissue and atherosclerosis - exploring the connection. Arterioscler. Thromb. Vasc. Biol. 27: 996–1003 [DOI] [PubMed] [Google Scholar]
- 7.van Dijk K. W., Hofker M. H., Havekes L. M. 1999. Dissection of the complex role of apolipoprotein E in lipoprotein metabolism and atherosclerosis using mouse models. Curr. Atheroscler. Rep. 1: 101–107 [DOI] [PubMed] [Google Scholar]
- 8.Zanotti I., Pedrelli M., Poti F., Stomeo G., Gomaraschi M., Calabresi L., Bernini F. 2011. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 31: 74–80 [DOI] [PubMed] [Google Scholar]
- 9.Mensenkamp A. R., Jong M. C., van Goor H., van Luyn M. J., Bloks V., Havinga R., Voshol P. J., Hofker M. H., van Dijk K. W., Havekes L. M., et al. 1999. Apolipoprotein E participates in the regulation of very low density lipoprotein-triglyceride secretion by the liver. J. Biol. Chem. 274: 35711–35718 [DOI] [PubMed] [Google Scholar]
- 10.Yue L., Rassouli N., Ranganathan G., Kern P. A., Mazzone T. 2004. Divergent effects of PPARγ agonists and TNFα on adipocyte apoE expression. J. Biol. Chem. 279: 47626–47632 [DOI] [PubMed] [Google Scholar]
- 11.Huang Z. H., Reardon C. A., Mazzone T. 2006. Endogenous apoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 55: 3394–3402 [DOI] [PubMed] [Google Scholar]
- 12.Huang Z. H., Gu D., Mazzone T. 2009. Role of adipocyte-derived apoE for modulating adipocyte size, lipid metabolism, and gene expression in vivo. Am. J. Physiol. Endocrinol. Metab. 296: E1110–E1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z. H., Minshall R. D., Mazzone T. 2009. Mechanism for endogenously expressed APOE modulation of adipocyte VLDL metabolism: role in endocytic and lipase-mediated metabolic pathways. J. Biol. Chem. 284: 31512–31522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasty A. H., Linton M. F., Swift L. L., Fazio S. 1999. Determination of the lower threshold of apolipoprotein E resulting in remnant lipoprotein clearance. J. Lipid Res. 40: 1529–1538 [PubMed] [Google Scholar]
- 15.Linton M. F., Atkinson J. B., Fazio S. 1995. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 267: 1034–1037 [DOI] [PubMed] [Google Scholar]
- 16.Wientgen H., Thorngate F. E., Omerhodzic S., Rolnitzky L., Fallon J. T., Williams D. L., Fisher E. A. 2004. Subphysiologic apolipoprotein E (ApoE) plasma levels inhibit neointimal formation after arterial injury in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 24: 1460–1465 [DOI] [PubMed] [Google Scholar]
- 17.Thorngate F. E., Rudel L. L., Walzem R. L., Williams D. L. 2000. Low levels of extrahepatic nonmacrophage ApoE inhibit atherosclerosis without correcting hypercholesterolemia in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20: 1939–1945 [DOI] [PubMed] [Google Scholar]
- 18.Raffai R. L., Loeb S. M., Weisgraber K. H. 2005. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 25: 436–441 [DOI] [PubMed] [Google Scholar]
- 19.Reardon C. A., Blachowicz L., White T., Cabana V., Wang Y., Lukens J., Bluestone J., Getz G. S. 2001. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 1011–1016 [DOI] [PubMed] [Google Scholar]
- 20.Huang Z. H., Fitzgerald M. D., Mazzone T. 2006. Distinct cellular loci for the ABCA1-dependent and independent efflux mediated by endogenous apoE expression. Arterioscler. Thromb. Vasc. Biol. 26: 157–162 [DOI] [PubMed] [Google Scholar]
- 21.Lee Y., Kockx M., Raftery M. J., Jessup W., Griffith R., Kritharides L. 2010. Glycosylation and sialylation of macrophage-derived human apolipoprotein E analyzed by SDS-PAGE and mass spectrometry: evidence for a novel site of glycosylation on Ser290. Mol. Cell. Proteomics. 9: 1968–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krempler F., Kostner G. M., Friedl W., Paulweber B., Bauer H., Sandhofer F. 1987. Lipoprotein binding to cultured human hepatoma cells. J. Clin. Invest. 80: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holder J. C., Zammit V. A., Robinson D. S. 1990. The preferential uptake of very-low-density lipoprotein cholesteryl ester by rat liver in vivo. Biochem. J. 272: 735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. 2006. Current Protocols in Cell Biology. 2.0.1–2.0.2.
- 25.Lin C. Y., Huang Z. H., Mazzone T. 2001. Interaction with proteoglycans enhances the sterol efflux produced by endogenous expression of macrophage apoE. J. Lipid Res. 42: 1125–1133 [PubMed] [Google Scholar]
- 26.Marinetti G. V. 1962. Chromatographic separation, identification and analysis of phosphatides. J. Lipid Res. 3: 1–20 [Google Scholar]
- 27.Reardon C. A., Driscoll D. M., Davis R. A., Borchardt R. A., Getz G. S. 1986. The charge polymorphism of rat apoprotein E. J. Biol. Chem. 261: 4638–4645 [PubMed] [Google Scholar]
- 28.Zhao Y., Mazzone T. 2000. Transport & processing of endogenously synthesized apoE on the macrophage cell surface. J. Biol. Chem. 275: 4759–4765 [DOI] [PubMed] [Google Scholar]
- 29.Raffaï R. L., Hasty A. H., Wang Y., Mettler S. E., Sanan D. A., Linton M. F., Fazio S., Weisgraber K. H. 2003. Hepatocyte-derived ApoE is more effective than non-hepatocyte-derived ApoE in remnant lipoprotein clearance. J. Biol. Chem. 278: 11670–11675 [DOI] [PubMed] [Google Scholar]
- 30.Linton M. F., Hasty A. H., Babaev V. R., Fazio S. 1998. Hepatic apo E expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J. Clin. Invest. 101: 1726–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paradis K., Sharp H. L., Vallera D. A., Blazer B. R. 1990. Kupffer cell engraftment across the major histocompatibility barrier in mice: bone marrow origin, class II antigen expression, and antigen-presenting capacity. J. Pediatr. Gastroenterol. Nutr. 11: 525–533 [DOI] [PubMed] [Google Scholar]
- 32.Ohman M. K., Shen Y., Obimba C. I., Wright A. P., Warnock M., Lawrence D. A., Eitzman D. T. 2008. Visceral adipose tissue inflammation accelertes atherosclerosis in apolipoprotein E-deficent mice. Circulation. 117: 798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh P., Hale E. A., Mayur K., Seddon J., Lakshman M. R. 2000. Effects of chronic alcohol treatment on the synthesis, sialylation, and disposition of nascent apolipoprotein E by peritoneal macrophages of rats. Am. J. Clin. Nutr. 72: 190–198 [DOI] [PubMed] [Google Scholar]
- 34.Grunfeld C., Doerrler W., Pang M., Jensen P., Weisgraber K. H., Feingold K. R. 1997. Abnormalities of apoliproprotein E in the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 82: 3734–3740 [DOI] [PubMed] [Google Scholar]
- 35.Williams D. L., Dawson P. A., Newman T. C., Rudel L. L. 1985. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J. Biol. Chem. 260: 2444–2451 [PubMed] [Google Scholar]
- 36.Takahashi Y., Inoue J., Kagechika H., Satol R. 2009. ApoC-III gene expression is sharply increased during adipogenesis and is augmented by retinoid X receptor. FEBS Lett. 583: 493–497 [DOI] [PubMed] [Google Scholar]
- 37.Windler E., Havel R. J. 1985. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J. Lipid Res. 26: 556–565 [PubMed] [Google Scholar]
- 38.Windler E., Chao Y., Havel R. J. 1980. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J. Biol. Chem. 255: 8303–8307 [PubMed] [Google Scholar]
- 39.Kawakami A., Aikawa M., Libby P., Alcaide P., Luscinskas F. W., Sacks F. M. 2006. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 113: 691–700 [DOI] [PubMed] [Google Scholar]
- 40.Kawakami A., Aikawa M., Nitta N., Yoshida M., Libby P., Sacks F. M. 2007. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler. Thromb. Vasc. Biol. 27: 219–225 [DOI] [PubMed] [Google Scholar]
- 41.Kowal R. C., Herz J., Weisgraber K. H., Mahley R. W., Brown M. S., Goldstein J. L. 1990. Opposing effects of apolipoprotein E and C on lipoprotein binding to low density lipoprotein related protein. J. Biol. Chem. 265: 10771–10779 [PubMed] [Google Scholar]