Abstract
Lipid asymmetry, the difference in inner and outer leaflet lipid composition, is an important feature of biomembranes. By utilizing our recently developed MβCD-catalyzed exchange method, the effect of lipid acyl chain structure upon the ability to form asymmetric membranes was investigated. Using this approach, SM was efficiently introduced into the outer leaflet of vesicles containing various phosphatidylcholines (PC), but whether the resulting vesicles were asymmetric (SM outside/PC inside) depended upon PC acyl chain structure. Vesicles exhibited asymmetry using PC with two monounsaturated chains of >14 carbons; PC with one saturated and one unsaturated chain; and PC with phytanoyl chains. Vesicles were most weakly asymmetric using PC with two 14 carbon monounsaturated chains or with two polyunsaturated chains. To define the origin of this behavior, transverse diffusion (flip-flop) of lipids in vesicles containing various PCs was compared. A correlation between asymmetry and transverse diffusion was observed, with slower transverse diffusion in vesicles containing PCs that supported lipid asymmetry. Thus, asymmetric vesicles can be prepared using a wide range of acyl chain structures, but fast transverse diffusion destroys lipid asymmetry. These properties may constrain acyl chain structure in asymmetric natural membranes to avoid short or overly polyunsaturated acyl chains.
Keywords: cyclodextrin, lipid exchange, membrane structure, fluorescence, transverse diffusion, flip-flop
The eukaryotic plasma membrane exhibits asymmetry with respect to its lipid distribution across the bilayer; the majority of sphingomyelin (SM) and phosphatidylcholine (PC) is in the outer leaflet, and the aminophospholipids phosphatidylethanolamine (PE) and phosphatidylserine (PS) are in the cytoplasmic leaflet (1–3). It is known that the asymmetric distribution of lipids in the bilayer affects various biological properties, such as membrane permeability, membrane potential, surface charge, the mechanical stability of membranes, and membrane shape (4–10). Therefore, biologically realistic asymmetric vesicles could be widely useful in model membrane studies. However, artificial membranes produced in the laboratory usually have a symmetric distribution of lipids in the inner and outer leaflets of the bilayer. Asymmetric artificial membranes have been difficult to produce, although some important progress has been made (11–16). We recently developed a robust method in which methyl-β-cyclodextrin (MβCD)-induced lipid exchange is used to prepare model membrane vesicles with lipid asymmetry, and we have applied the method to produce asymmetric small, large, and giant unilamellar vesicles (17–19).
In the present study, we extended the range of this method by using it to construct asymmetric vesicles containing SM in the outer leaflet and various species of PCs in the inner leaflet. PC acyl chain structure was systematically varied, and how this variation affects the ability to form asymmetric membranes was assayed. We found that whether the resulting vesicles were asymmetric [SM outside/PC inside (SMo/PCi)] depended upon PC acyl chain structure. Despite this limitation, asymmetric vesicles with a wide range of acyl chain structures could be produced. This is an important advance that should allow new applications of asymmetric vesicles. These studies also allowed analysis of the effect of lipid type upon membrane structure and behavior. We investigated the origin of the acyl chain dependence of asymmetry by measuring the transverse diffusion rate of the various PCs used. There was a correlation between asymmetry and transverse diffusion. Asymmetric vesicles could be prepared only when PCs with slow transverse diffusion were used. It was found that the presence of two polyunsaturated acyl chains or overly short acyl chains prevented the maintenance of asymmetry. This finding may explain why such lipids are not generally abundant in biological membranes.
METHODS
Materials
Porcine brain SM; 1,2-dimyristoleoylphosphatidylcholine (di14:1PC); 1,2 dipalmitoleoylphosphatidylcholine (di16:1PC); 1,2-dioleoylphosphatidylcholine (DOPC or di18:1PC); 1,2-dieicosenoylphosphatidylcholine (di20:1PC); 1,2-dierucoylphosphatidylcholine (di22:1PC); 1,2-diphytanoyl-sn-glycero-3-phosphocholine (diphyPC); 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (16:0-18:2PC); 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (16:0-20:4PC); 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (di18:2PC); 1,2-dilinolenoyl-sn-glycero-3-phosphocholine (di18:3PC); 1,2-diarachidonoyl-sn-glycero-3-phosphocholine (di20:4PC); and 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl-sn-glycero-3-phosphocholine (C6-NBD-PC) were purchased from Avanti Polar Lipids (Alabaster, AL). 1,6-Diphenyl-1,3,5-hexatriene (DPH); methyl-β-cyclodextrin (MβCD); and sodium hydrosulfite (sodium dithionite) were from Sigma-Aldrich (St. Louis, MO). 1-(4-Trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMADPH) was from the Molecular Probes division of Invitrogen (Carlsbad, CA). Lipids were dissolved in chloroform and stored at −20°C. DPH and TMADPH were dissolved in ethanol and stored at −20°C.
Concentrations of lipids were measured by dry weight. Concentrations of DPH and TMADPH were determined by absorbance as described previously (17). Sepharose CL-4B and Sepharose CL-2B were purchased from Amersham Biosciences (Piscataway, NJ). High-performance thin-layer chromatography (HP-TLC) plates (Silica Gel 60) were purchased from VWR International (Batavia, IL). The digital thermometer was purchased from Fisher Scientific (Pittsburgh, PA).
Formation of asymmetric SUV
Asymmetric small unilamellar vesicles (SUV) were formed using a protocol adapted from that described in Cheng et al. (17). First, donor multilamellar vesicles (MLV), the population from which bSM would be exchanged with the outer leaflet of the SUVs, were prepared. Donor MLVs composed of 16 mM bSM were formed by drying the lipid in a film under nitrogen followed by high vacuum for at least 1 h, followed by hydration at 70°C with 500 µl of PBS (1.8 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, and 2.7 mM KCl, pH 7.4). This sample was vortexed in a multitube vortexer (VWR International) at 55°C for 15 min and then subjected to centrifugation at 11,000 g for 5 min at room temperature to remove any small vesicles present prior to lipid exchange. The supernatant was discarded, the pellet was resuspended with 500 μl PBS, and then 95 μl of 390 mM MβCD dissolved in PBS was added. This mixture was vortexed in the multitube vortexer at 55°C for 2 h.
Next, an acceptor solution, which contains sonicated PC SUV, was prepared. We formed 600 μl of 8 mM PC MLVs by drying the lipid in a film and rehydrating it with 70°C in PBS just like the SM MLVs described above. This mixture was also vortexed in a multitube vortexer (VWR International) at 55°C for 15 min and then sonicated in a bath sonicator (Special Ultrasonic Cleaner Model G1112SP1, Laboratory Supplies Co., Hicksville, NY) at room temperature for at least 15 min until the solution became nearly transparent, and then it was diluted to 4 mM with PBS.
Third, this 500 μl of 4 mM sonicated SUVs containing unsaturated glycerophospholipid were added to the SM MLV-MβCD mixture and vortexed at 55°C for 30 min. After cooling for 5 min, samples were centrifuged at 49,000 g for 5 min using an air-driven microultracentrifuge (Beckman Airfuge). The supernatant was applied onto a Sepharose CL2B column (dimensions, 25 cm length and 1 cm diameter). We found that Sepharose CL2B gave better resolution of small and large vesicles than did the Sepharose CL4B we used previously. Fractions of 1 ml were collected, with SUVs mainly eluting into fractions 16–19. These fractions were combined for further analysis. Generally the lipid concentration in the peak SUV fractions was ∼150 μM as measured by the intensity of lipid bands on HP-TLC relative to standards with known amounts of lipid (see below). In cases in which lipid concentration was not explicitly measured, a 150 μM lipid concentration was assumed unless otherwise noted.
Analysis of lipid composition by HP-TLC
Lipids were extracted with chloroform/methanol (1:1 v/v) from ordinary and exchange vesicles as described (18). The extracted lipids or pure lipid standards were applied to HP-TLC (Silica Gel 60) plates and chromatographed in solvent as described (17). The lipids in the vesicles were quantified by measuring TLC spot intensity versus a standard curve for each lipid generated on the same TLC plate as described (17).
Fluorescence measurements
Fluorescence was measured on a SPEX FluoroLog 3 spectrofluorometer (Jobin-Yvon, Edison, NJ) using quartz semimicro cuvettes (excitation path length, 10 mm; emission path length, 4 mm) according to described protocols (17). DPH fluorescence was measured at an excitation wavelength of 358 nm and emission wavelength of 427 nm. TMADPH fluorescence was measured at an excitation wavelength of 364 nm and emission wavelength of 426 nm. C6-NBD-PC fluorescence was measured at an excitation wavelength of 465 nm and emission wavelength of 534 nm. The slit bandwidths for fluorescence measurements were generally set to 4.2 nm (2 mm physical size) for excitation and to 4.2 nm (2 mm physical size) for emission. The backgrounds were less than 1% of fluorescence-containing samples and were not subtracted from the sample signals. An exception to this was for measurements of TMADPH fluorescence anisotropy (see below).
Steady-state fluorescence anisotropy measurements
DPH or TMADPH dissolved in ethanol (stock solutions of 20 µM) were added to preformed vesicles to a concentration of 0.1 mol% of total lipid. Anisotropy measurements were made using a SPEX automated Glan-Thompson polarizer accessory according to previously described protocols (17). For DPH, the bandwidths of slit for anisotropy were set to 4.2 nm (2 mm physical size) for both excitation and emission. For TMADPH, the bandwidths of slit for anisotropy were set to 4.2 nm (2 mm physical size) for excitation and to 8.4 nm (4 mm physical size) for emission. For TMADPH, the anisotropy was calculated after subtraction of fluorescence intensity in background samples lacking fluorophore. The anisotropy was determined using the following equation: A = [((Ivv × Ihh)/(Ivh × Ihv)) – 1]/[((Ivv × Ihh)/(Ivh × Ihv)) + 2], where A represents anisotropy, I represents the fluorescence emission intensity, with the vertical (v) and horizontal (h) orientation of the excitation and emission polarization filters (20).
Vesicle scrambling experiments
The scrambling process, in which vesicles are destroyed and then reformed as symmetric vesicles, was carried out similarly to previously described protocols (17). Fractions 16–19 from CL2B column were combined and then divided into four tubes (1 ml each). Two of the samples were subjected to scrambling, and two were used without scrambling. The samples to be scrambled were dried by a nitrogen stream, dissolved in 20 μl of ethanol, and then dispersed at 70°C in 980 μl of distilled water (which should reconstitute the PBS as well as the lipid vesicles). After cooling to room temperature, DPH or TMADPH, which was dissolved in ethanol, was added as described above.
Measurement of lipid transverse diffusion (flip-flop)
The transverse diffusion of C6-NBD-PC was measured using the dithionite method (21). First, 1 ml of 100 μM SUVs were prepared by ethanol dilution, and then 1 mol% C6-NBD-PC (from a stock solution dissolved in ethanol) was added to the preformed vesicles, which were then incubated overnight at room temperature. Alternately, large unilamellar vesicles (LUV) were prepared by lipid extrusion technique using a Mini-Extruder set (Avanti Polar Lipids, Alabaster, AL). A sample containing 1 ml of MLVs (1 mM lipid) were disrupted by five freeze-thaw cycles and then forced 15 times through a polycarbonate filter with a pore size of 100 nm. The sample was then diluted to 100 µM lipid with PBS. Then C6-NBD-PC was added (to 1 mol% of lipid). Next, initial NBD fluorescence emission (F0) was measured. Then, for both SUV and LUV, 10 μl of 1.0 M sodium dithionite freshly prepared in 1.0 M Tris pH 10.0 was added, and the fluorescence emission intensity (FP) was recorded versus time for up to 1 h. The fraction of C6-NBD-PC protected from external dithionite due to location in the inner leaflet is given by the ratio FP/F0. The fraction of C6-NBD-PC that had flipped to the inner leaflet was estimated from the FP/F0 value at time = 0, determined by extrapolation from fluorescence values between 10 and 60 min after dithionite addition, assuming that the leakage of dithionite into the vesicles followed a first-order reaction. All calculations were performed by using the software SlideWrite Plus (Advanced Graphics Software, Inc., Encinitas, CA).
Prevention of lipid oxidation in polyunsaturated lipids
The possibility of lipid oxidation that might occur was determined by the increase in absorption at 233 nm. The polyunsaturated PCs that we used, such as 16:0-18:2PC, 16:0-20:4PC, di18:2PC, di18:3PC, and di20:4PC, are highly oxidation-prone especially during the process of sonication. Free radical autoxidation of polyunsaturated lipids is accompanied at an early stage by diene conjugation, which results in significant absorbance at 233 nm (22). To minimize oxidation during vesicle preparation, prior to sonication, the test tubes containing polyunsaturated PC MLVs were tightly sealed with teflon tape, after a nitrogen gas stream was directed down into the test tube. For all of our lipids, we estimated at most a small percentage of conjugated diene was present, indicating little oxidation. We also carried out the all experiments immediately after the formation of the exchange vesicles, so that all experiments on the exchange vesicles were completed within 1 h of their preparation.
Percentage SM in exchange vesicles calculation
The percentage of SM in exchange vesicles was calculated from HP-TLC and, for the outer leaflet, TMADPH anisotropy. TLC data gives total percentage of SM in the bilayer (both leaflets), and percentage of SM derived from the anisotropy of TMADPH incorporated into the outer leaflet, when compared with a standard curve (supplemental Fig. II), gives percentage of SM only in the outer leaflet. The percentage of SM by TLC was evaluated after calculating the intensity of SM band on the TLC plate. The samples for HP-TLC were from the exchange SUV-containing fractions from the Sepharose CL-2B column. TMADPH fluorescence probe was added into preformed vesicles at a concentration of 0.1 mol % of total lipid concentration.
Dynamic light scattering measurements
The measurement of SUV size was carried out at 23°C using Protein Solutions DynaPro 99 dynamic light -scattering instrument (Wyatt Technology, Santa Barbara, CA) (18). Fractions 16–19 from the Sepharose CL2B column were subjected to the dynamic light-scattering experiment 5 min and 1 day after eluting from the column. Data were analyzed with the software Dynamics version 5.25.44 (Protein Solutions, Inc. Charlottesville, VA).
RESULTS
Preparation of asymmetric (exchange) SUVs and their SM content in the inner and outer leaflets
We used MβCD-induced lipid exchange methods to produce asymmetric SUVs with SM in the outer leaflet and PC in the inner leaflet. In this protocol, the lipid to be introduced into the outer leaflet is in donor MLV, whereas the lipid to be located in the inner leaflet is in acceptor SUV. One change made from our previous protocol was to fractionate vesicles using Sepharose CL2B, which gave better resolution of vesicles by size than the Sepharose CL4B used previously (17). Using this approach, the properties of acceptor SUV having PCs with various acyl chains were investigated to determine whether the capability to form asymmetric vesicles is affected by lipid acyl chain structure. The PCs tested had monounsaturated acyl chains of different lengths (di14:1PC, di16:1PC, di18:1PC, di20:1PC, and di22:1PC); diphytanoyl chains, which have four methyl groups per chain (diphyPC); one saturated and one polyunsaturated chain (16:0-18:2PC and 16:0-20:4PC); and two polyunsaturated chains (di18:2PC, di18:3PC, and di20:4PC). HP-TLC analysis of the acceptor vesicles after exchange (i.e., exchange vesicles) revealed that they contained both SM and PC. Based on HP-TLC, the SM content in exchange vesicles was in the range 50–70% (Table 1). Because the theoretical value for complete exchange of the entire outer leaflet of small SUV is about 66%, this indicates that the MβCD-induced lipid exchange method is efficient for all PCs tested (e.g., see supplemental Fig. I).
TABLE 1.
Percentage of SM in exchange vesicles and in the outer leaflet of exchange vesicles
| Sample Composition | % SM in Vesicle | % SM in Outer Leaflet |
| SMo / di 14:1PC in | 68 ± 4.5 (3) | 83.2 ± 7.9 (7) |
| SMo / di 16:1PC in | 57.2 ± 6.7 (3) | 89.8 ± 8.0 (11) |
| SMo / di 18:1PC in | 60 ± 4.2 (3) | 95.6 ± 3.7 (5) |
| SMo / di 20:1PC in | 51.6 ± 2.3 (3) | 87.7 ± 3.7 (10) |
| SMo / di 22:1PC in | 52.4 ± 1.9 (3) | 91.8 ± 7.9 (11) |
| SMo / diphyPC in | 62.8 ± 1.2 (3) | 94.5 ± 7.7 (14) |
| SMo / 16:0-18:2PC in | 61.9 ± 7.6 (3) | 92.3 ± 8.4 (10) |
| SMo / 16:0-20:4PC in | 68.2 ± 10 (3) | 90.6 ± 7.6 (17) |
| SMo / di 18:2PC in | 66.8 ± 6.7 (3) | 76.7 ± 4.3 (14) |
| SMo / di 18:3PC in | 65.8 ± 3.1 (3) | 62.2 ± 8.2 (18) |
| SMo / di 20:4PC in | 68.6 ± 1.0 (3) | 69.6 ± 8.5 (9) |
%SM calculated as described in Methods. Average (mean) and SD for the number of samples shown in parentheses are shown. %SM in exchange vesicles determined by TLC versus standard curves in which different amounts of lipids were loaded on the TLC plate. %SM in outer leaflet was calculated from the fluorescence anisotropy of TMADPH incorporated into the outer leaflet of the exchange vesicles and standard curves of anisotropy versus % SM in vesicles as described in supplemental Fig. II (see also supplemental Table I) Measurements made at 23°C. %SM in vesicle refers to the % of lipids in the vesicle that are SM. %SM in outer leaflet refers to the % of outer leaflet lipids that are SM.
To determine the percentage of outer leaflet lipids that were SM after exchange, we examined the steady-state fluorescence anisotropy of TMADPH. This probe is localized to the outer leaflet of the bilayer when it is added to the preformed vesicles due to its charged quaternary amino group. First, a standard curve of anisotropy versus SM content was generated in ordinary, symmetric vesicles for each of the PCs used. Anisotropy increases with SM content because at room temperature SM tends to form the solid-like gel state, in which TMADPH is highly ordered and has high anisotropy, whereas by themselves, the PCs used are in the liquid disordered (Ld) state, in which TMADPH exhibits low anisotropy. Next, the anisotropy in the exchange vesicles (supplemental Table I) was compared with the standard curves (supplemental Fig. II) to estimate the percentage of SM in the outer leaflet. This showed the outer leaflet of exchange vesicles was composed of 62–96% SM, depending upon the PC used (Table 1 and Fig. 1).
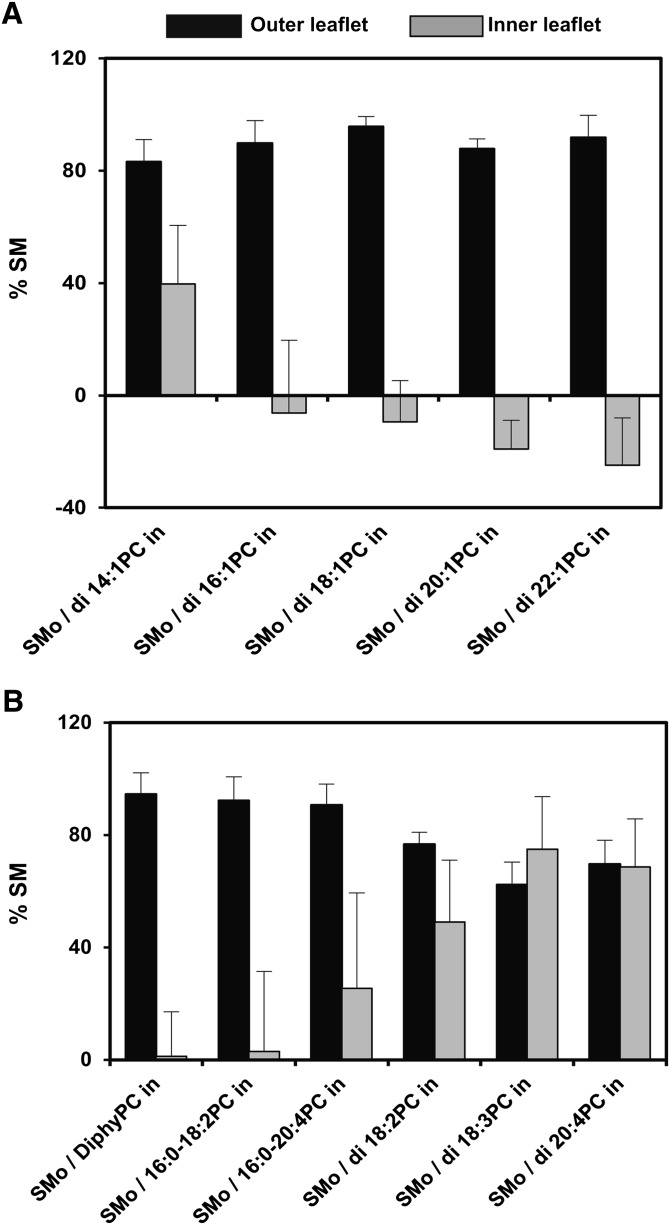
Fig. 1.
Percentage of SM in the outer leaflet and inner leaflet of exchange vesicles. The average (mean) % of the outer leaflet lipids that are SM (black bars), the % of inner leaflet lipids that are SM (gray bars), and SD are shown. Notice that this is not the same as the % of SM in a vesicle that is in the outer leaflet or inner leaflet, respectively. Values for the outer leaflet are from Table 1 and for the inner leaflet calculated from data in Table 1 using the equation: %SM in inner leaflet = (%SM total – 0.67 × %SM outer leaflet) / 0.33. This assumes the % of total surface area in SUV is ∼67% outer leaflet and ∼33% inner leaflet. A: PC with different acyl chain lengths. B: PC with different number and/or position of double bonds. Measurements made at 23°C. In this and all following figures, vesicles were dispersed in PBS, pH 7.4. o, outside (outer leaflet); in, inside (inner leaflet).
The percentage of inner leaflet lipids that were SM in the exchange vesicles was then calculated from the total SM in the vesicles (Fig. 1). The SM content in the inner leaflet of exchange SUVs was found to be highly dependent on the type of PC present. The inner leaflet SM values were close to zero in many cases (di16:1PC, di18:1PC, di20:1PC, di22:1PC, diphyPC, and 16:0-18:2PC), indicating that SM in those vesicles was largely restricted to the outer leaflet (i.e., they were SMo/PCi vesicles). In contrast, SM was less asymmetrically distributed (i.e., located to a substantial extent in both inner and outer leaflets of the bilayer) for PC with short acyl chains (di14:1PC) or highly polyunsaturated acyl chains (16:0-20:4PC, di18:2PC, di18:3PC, and di20:4PC). In fact, in the case of lipids with two polyunsaturated chains, the inner and outer leaflet SM concentrations were almost the same, indicative of no asymmetry.
Assessment of asymmetry in exchange vesicles compared with scrambled vesicles with the same lipid composition
It is important to prove that exchange vesicles are or are not asymmetric with additional methods. When exchange vesicles are asymmetric, they have different properties than the corresponding symmetric vesicles (17, 18). The presence or absence of such differences was used to confirm the presence or absence of asymmetry. To prepare ordinary symmetric vesicles with the same lipid compositions as those in the exchange vesicles, a protocol was used in which the exchange vesicles were dissolved in organic solvent and the lipids were scrambled by reforming vesicles from them to yield ordinary symmetric vesicles (i.e., scrambled vesicles).
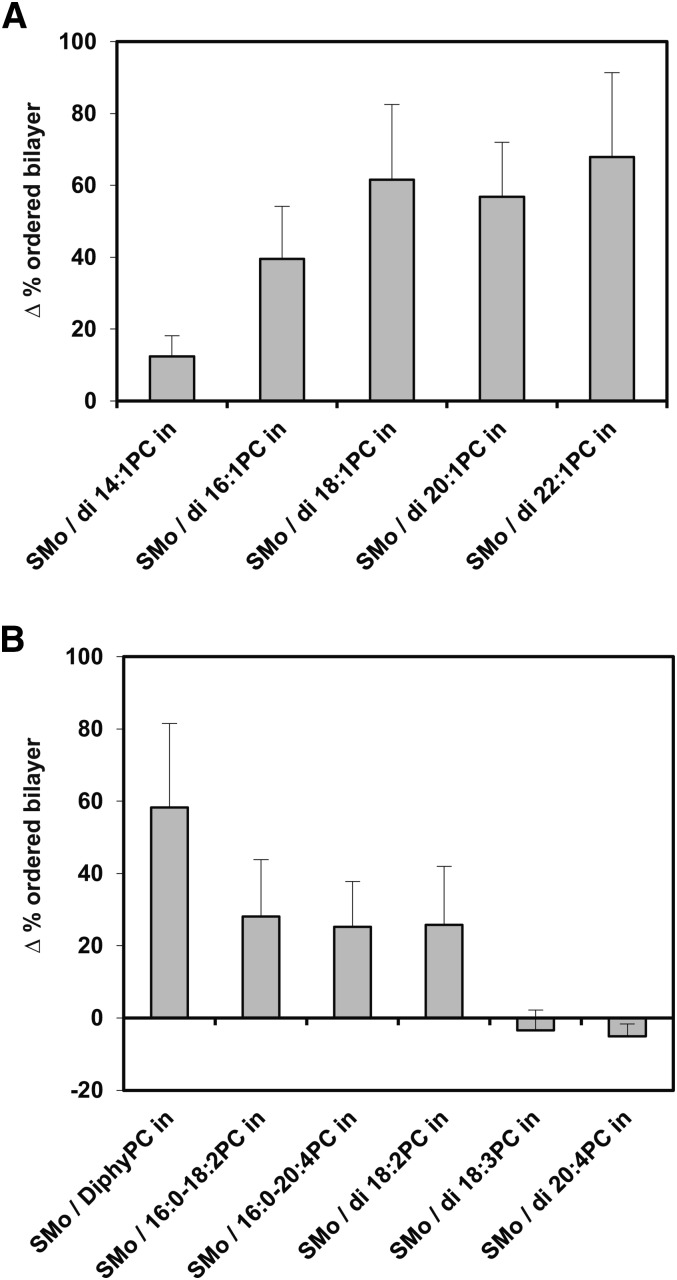
The first assay used to compare physical properties of exchange and scrambled vesicles was outer leaflet membrane order measured by TMADPH anisotropy (supplemental Table I and Fig. 2). As noted above, in asymmetric vesicles containing SM in their outer leaflet, the outer leaflet membrane order is especially high at 23°C because the SM is primarily in the solid-like gel state. For the lipid compositions studied, outer leaflet order is lower when vesicles are symmetric due to the introduction of a low melting temperature (Tm) PC into the outer leaflet. As shown in Fig. 2, relative to exchange vesicles, there was no decrease in outer leaflet order for scrambled vesicles containing di18:3PC and di20:4PC. This indicates that the exchange vesicles prepared from these lipids are not asymmetric, in agreement with the estimates above showing that there are similar fractions of SM in the inner and outer leaflets of these vesicles. In addition, there was only a very small decrease in order for scrambled vesicles versus exchange vesicles containing di14:1PC, suggesting vesicles with this lipid have very little asymmetry. For the other PCs studied, Fig. 2 shows there was a significant decrease in order in scrambled vesicles relative to exchange vesicles, indicative of a significant degree of lipid asymmetry in the exchange vesicles, which is in agreement with the conclusions based upon inner and outer leaflet SM content in these vesicles.
Fig. 2.
Difference between TMADPH fluorescence anisotropy in exchange and scrambled vesicles of identical composition. The average (mean) difference in % ordered bilayer and SD between exchange vesicles and scrambled vesicles formed from the exchange vesicles by the scrambling process is shown on the y-axis. Positive values indicate a higher % ordered in the exchange vesicles. Y axis values calculated from values in supplemental Table I. Measurements made at 23°C. A: PC with different acyl chain lengths. B: PC with different number and/or position of double bonds.
However, for vesicles containing 16:0-18:2PC, 16:0-20:4PC, and di18:2PC, the decrease in outer leaflet order in scrambled vesicles relative to exchange vesicles was not as large as for the other lipids. By itself, changes in overall membrane order are not conclusive, because if SM is very laterally immiscible with PC in symmetric vesicles, the scrambled vesicles could appear to be as ordered as asymmetric vesicles. However, in our studies, relatively small changes in order suggest incomplete asymmetry in the exchange vesicles, as it is in agreement with conclusions based upon SM content in the inner and outer leaflets.
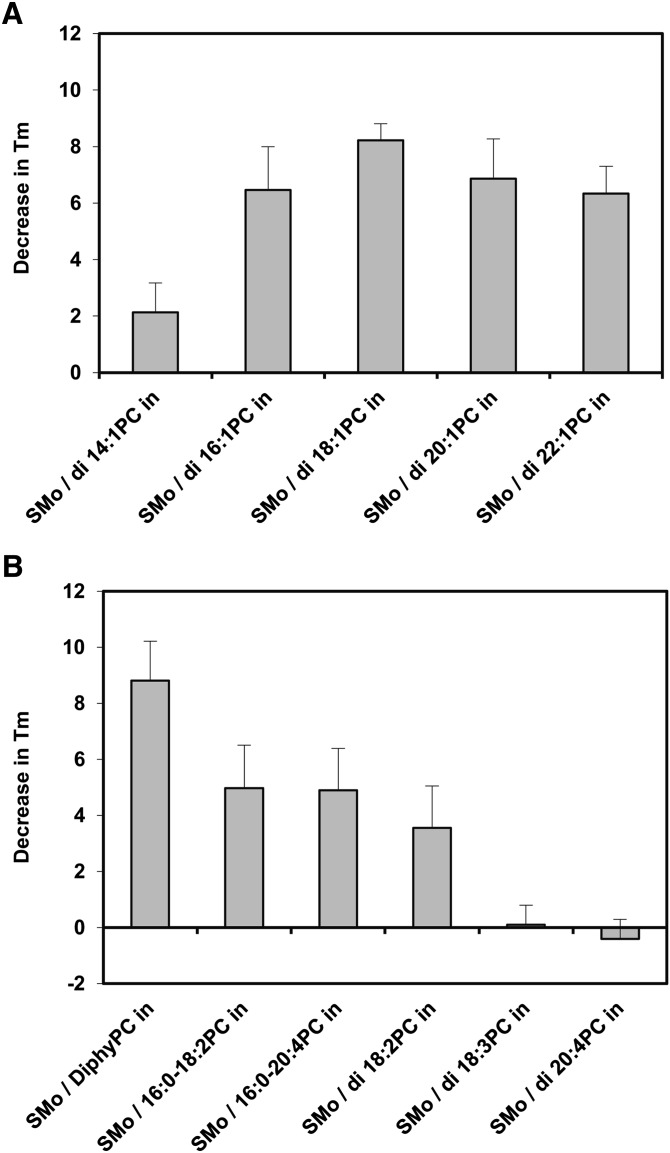
The second assay used to evaluate the difference between the properties of exchange and scrambled vesicles was the thermal stability of gel-state domains in the vesicles. The bilayer of SM-containing vesicles undergoes a readily detected phase transition from the ordered gel phase to the liquid-disordered phase as temperature is increased. The Tm can be defined as the midpoint of this phase transition (23, 24). It was previously found that Tm values are higher in asymmetric SMo/PCi vesicles than symmetric vesicles of the same composition (17). This reflects the fact that the nearly pure SM outer leaflets in asymmetric vesicles melt at a temperature similar to that of SM in pure SM vesicles, whereas in symmetric vesicles, the mixture of SM with low-Tm PC in each leaflet results in a Tm value lower than that of pure SM (17, 18). Table 2 shows Tm values of exchange vesicles both before and after the scrambling process, and the difference between Tm in exchange vesicles and scrambled vesicles prepared from them is shown in Fig. 3. The results were virtually identical to those obtained from measurements of outer leaflet order in terms of the relationship between PC acyl chain structure and asymmetry at 23°C.
TABLE 2.
Melting temperature of exchange and scrambled vesicles
| Melting Temperature (°C) |
||
| Sample Composition | Before Scrambling | After Scrambling |
| SMo / di 14:1PC in | 37.7 ± 1.0 (6) | 35.6 ± 0.8 (6) |
| SMo / di 16:1PC in | 37.9 ± 1.5 (7) | 31.4 ± 0.7 (7) |
| SMo / di 18:1PC in | 36.9 ± 1.0 (4) | 28.7 ± 0.4 (4) |
| SMo / di 20:1PC in | 35.2 ± 1.5 (6) | 28.4 ± 1.1 (6) |
| SMo / di 22:1PC in | 34.7 ± 1.8 (6) | 28.3 ± 2.2 (6) |
| SMo / diphyPC in | 41.8 ± 0.6 (7) | 33.0 ± 1.8 (7) |
| SMo / 16:0-18:2PC in | 36.1 ± 1.2 (10) | 31.1 ± 1.6 (10) |
| SMo / 16:0-20:4PC in | 37.7 ± 1.4 (10) | 32.8 ± 1.6 (10) |
| SMo / di 18:2PC in | 33.5 ± 0.6 (7) | 30.0 ± 1.7 (7) |
| SMo / di 18:3PC in | 36.0 ± 1.1 (6) | 35.9 ± 0.8 (6) |
| SMo / di 20:4PC in | 36.9 ± 1.0 (11) | 37.3 ± 1.2 (11) |
Fig. 3.
Difference between bilayer melting point in exchange and scrambled vesicles of identical composition. The average (mean) difference and SD between bilayer melting point (Tm) in exchange vesicles and scrambled vesicles formed from the exchange vesicles by the scrambling process is shown. Positive values indicate a higher Tm in the exchange vesicles. Y axis values calculated from values in Table 2. Measurements made at 23°C. A: PC with different acyl chain lengths. B: PC with different number and/or position of double bonds.
Relationship between PC acyl chain structure and lipid transverse diffusion
By definition, the ability to maintain membrane asymmetry, in which the inner and outer leaflets have different lipid compositions, requires, in the absence of active lipid transport catalyzed by a protein, that the movement of lipids between inner and outer leaflets is slow. Therefore, we determined whether the different extents of asymmetry observed could be explained by differences in lipid transverse diffusion (i.e., flip-flop). The transverse diffusion assay used was based on the ability of sodium dithionite to reduce C6-NBD-PC into a nonfluorescent derivative. C6-NBD-PC was incorporated into the outer leaflet of preformed vesicles, and translocation of C6-NBD-PC into the inner leaflet was detected by the amount of NBD fluorescence that was protected from reduction by dithionite added to the external solution. If the extent of transverse diffusion is slow, there should have been very little protection, but if transverse diffusion is fast, there should have been significant protection from dithionite (supplemental Figs. III and IV).
To identify the best conditions in which to carry out this assay, the effects of varying time over which transverse diffusion could occur and varying lipid composition were measured (supplemental Figs. V and VI). Transverse diffusion was both time and composition dependent, and ultimately, mixtures (1:1 mol:mol) of the PCs of interest with DOPC or SM were used together with a 24 h incubation time for transverse diffusion because these conditions showed the most sensitivity to PC acyl chain structure (see supplemental materials for details).
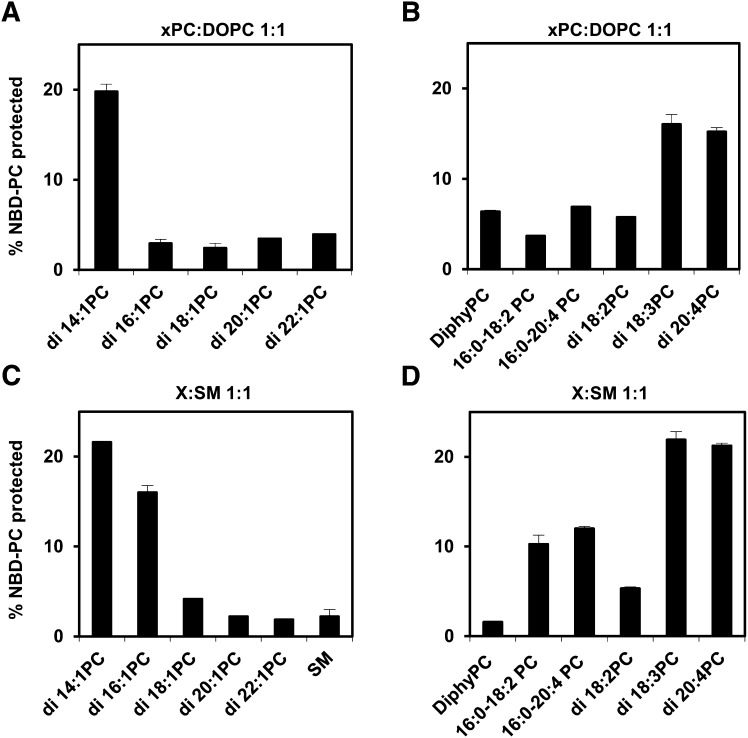
Fig. 4 shows the degree of protection of C6-NBD-PC fluorescence in symmetric SUVs containing PCs with different acyl chain structures. Varying the PC structure gave an extent of transverse diffusion that closely paralleled the effect of PC structure on asymmetry in exchange vesicles. Transverse diffusion was low for vesicles containing any of the PCs that were able support full asymmetry, whereas transverse diffusion was high with vesicles containing di14:1PC, di18:3PC, and di20:4PC, which do not support asymmetry. The behavior of vesicles containing di18:2PC was surprising in that they seemed to allow only slow C6-NBD-PC transverse diffusion even though the di18:2PC cannot support the formation of highly asymmetric vesicles. Possible explanations of this are considered in the Discussion.
Fig. 4.
Different levels of transverse lipid diffusion in symmetric vesicles of different compositions. The effect of PC acyl chain structure on transverse diffusion was determined from the amount of C6-NBD-PC (1 mol% of vesicles) that moved overnight into the inner leaflet, as judged by protection from reduction by dithionite. See Methods for details. Vesicles were prepared by ethanol dilution. Unlabeled lipids were mixed 1:1 with DOPC (A and B) or SM (C and D) A, C: PC with different acyl chain lengths. B, D: PC with different number and/or position of double bonds. Value for vesicles in which the unlabeled lipid was 100% SM is also shown. Average values and range derived from duplicate samples are shown. Other details are given in Methods.
Alternate explanations of the acyl chain dependence of transverse diffusion were tested. One possible factor could be vesicle size/curvature. However, exchange vesicles are isolated from the same, fixed fractions of a size exclusion chromatography column, so there should be no dependence of exchange vesicle size upon PC acyl chain structure. Nevertheless, transverse diffusion in LUV was compared with that in SUV to investigate the possibility that curvature strongly impacts lipid transverse diffusion (supplemental Fig. VII). The pattern in which lipids supported less or more C6-NBD-PC transverse diffusion was very similar in SUV and LUV, indicating that the effect of bilayer curvature is not great.
Another possibility is that lipid randomization during spontaneous vesicle fusion could cause loss of asymmetry. However, using dynamic light scattering, no vesicle fusion was detected over 24 h for di18:3PC or di14:1PC, which failed to form asymmetric vesicles (data not shown).
Asymmetric lipid distribution is associated with a difference between order in the inner and outer leaflet for SM outside/PC inside vesicles
Previous studies using asymmetric vesicles have shown that when the outer leaflet of a vesicle is SM in the gel state (100% ordered) and the inner leaflet is composed of DOPC or POPC, the inner leaflet remains largely in the Ld state (17, 18). This means that the physical states of the inner and outer leaflets are not strongly coupled to each other (17, 18). To see how general this behavior is, we examined the degree of coupling between the inner and outer leaflet physical states for exchange vesicles with a wider variety of PC acyl chains. To do this, the degree of order in the inner leaflet was calculated from the fluorescence anisotropy of DPH (which measures order in both leaflets) and externally added TMADPH (which measures outer leaflet order) (18), as shown in Fig. 5.
Fig. 5.
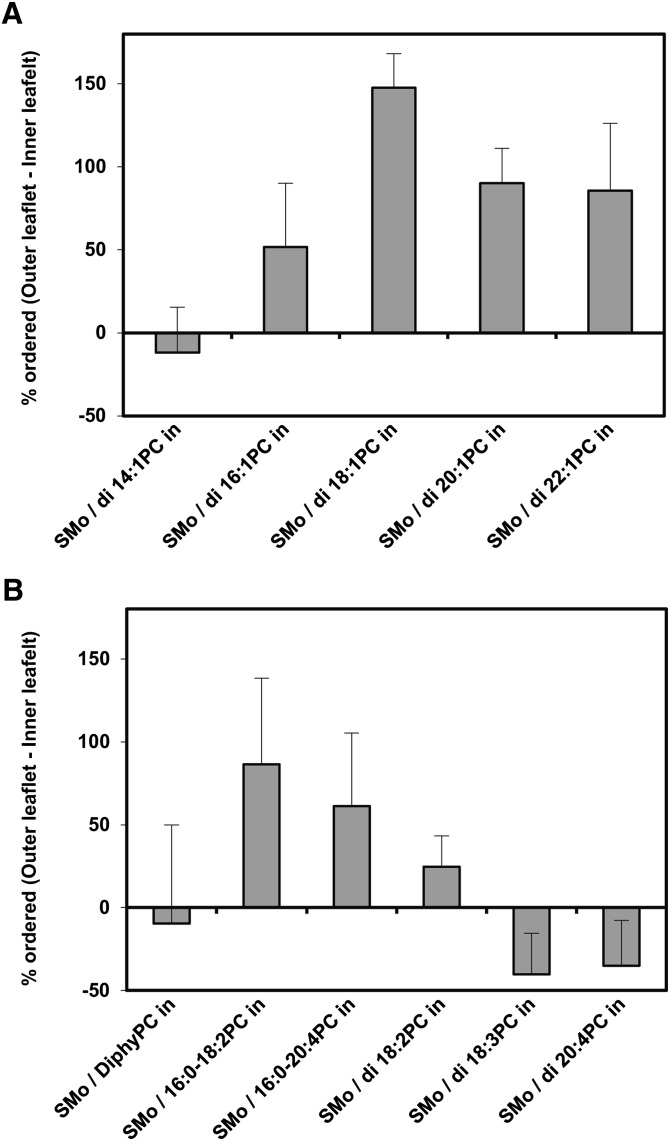
Coupling between inner and outer leaflet physical states at 23°C estimated from inner and outer leaflet bilayer order. Outer leaflet % ordered lipid and total (both leaflet) % ordered lipid were calculated from TMADPH and DPH anisotropy, respectively, as described in supplemental Tables I and II. Inner leaflet % ordered lipid was calculated using the following equation: Inner leaflet % ordered = (total % ordered lipid – outer leaflet % ordered lipid × 0.67) / 0.33. This assumes the % of total surface area in SUV is ∼67% outer leaflet and ∼33% inner leaflet. If inner and outer leaflets have a symmetric composition or have coupled physical states, the difference between the outer and inner leaflet order should be zero. If the vesicles are fully asymmetric and have uncoupled inner and outer leaflet physical states (i.e., outer leaflet SM in gel state, inner leaflet PC in Ld state), the difference between order in the inner and outer leaflet should be 100%. Average (mean) values and SD shown for the number of samples given in supplemental Tables I and II. A: PC with different acyl chain lengths. B: PC with different number and/or position of double bonds.
Supplemental Tables I and II contain the raw anisotropy data for TMADPH and DPH, and supplemental Table III shows the calculated percentage order in the outer and inner leaflets. Fig. 5 illustrates the difference between the level of order in the inner and outer leaflets. In most cases, the percentage order in the outer leaflet was higher than that in the inner leaflet, as expected for asymmetric vesicles In the case of almost all of the vesicles that were fully or mostly asymmetric (inside leaflet composed of di16:1PC, di18:1PC, di20:1PC, di22:1PC, 16:0-18:2PC, and 16:0-20:4PC), the difference was in the range 50–100%. Because the outer leaflet was almost fully ordered (∼90%) in these vesicles (supplemental Table III), this means that the inner leaflet was largely disordered (supplemental Table III) and thus that the physical states of the inner and outer leaflets are largely uncoupled. (The experimental error in anisotropy values makes it difficult to make a precise statement concerning low levels of coupling.)
The behavior of diphytanoyl PC was surprising because it is in vesicles that are fully asymmetric, but there did not appear to be any difference in the order of the inner and outer leaflet. This means either that the inner and outer leaflet physical states are coupled in SMo/diphyPCi vesicles or that DPH does not partition well into the inner leaflet composed of diphytanoyl PC. We suspect the latter possibility is more likely because the methyl groups of diphytanoyl PC would interfere with van der Waals interactions between lipid acyl chains and the flat DPH molecule.
In the cases of di14:1PC and lipids with two polyunsaturated chains, the percentage ordered in the outer leaflet was at most modestly higher than in the inner leaflet. This was expected, because it reflects the lack of asymmetry in these vesicles. In the absence of asymmetry, the percentage ordered should be almost equal in the inner and outer leaflets. [The observation that the calculated value of order is higher for the inner leaflet than the outer leaflet in some of these cases is an artifact of fact that TMADPH does not partition into ordered domains as well as does DPH, and was also observed previously (17, 18).] We cannot make any statement about coupling in these vesicles.
DISCUSSION
The acyl chain dependence of asymmetric vesicle formation is explained by lipid transverse diffusion (flip-flop)
This article shows that asymmetric vesicles can be prepared from lipids having a wide range of acyl chain structures. This ability should be useful in future studies of the effect of acyl chain structure upon the properties and function of asymmetric bilayers. However, the ability to form asymmetric bilayers was not a universal property of lipids. A question that arises from this is why asymmetric vesicles cannot be formed specifically from lipids with two short acyl chains or two polyunsaturated acyl chains. Because maintenance of asymmetry requires, by definition, that lipids do not equilibrate between inner and outer leaflets by transverse diffusion across the bilayer, the logical implication is that rapid transverse diffusion for lipids with two short or polyunsaturated acyl chains explains their inability to form asymmetric vesicles. This was confirmed by the measurements of the extent of transverse diffusion for a probe lipid in vesicles with various lipid compositions. The lipid mixtures that allowed most rapid transverse diffusion of the probe lipid were the same ones in which lipid asymmetry was not present, which means that the lipid compositions were such that they increased the rate of transverse diffusion for both labeled and unlabeled lipids.
A fundamental question that arises from these observations is why lipids with certain acyl chains impart rapid transverse diffusion to all of the lipids in a membrane. Armstrong et al. proposed that lipid packing was the important parameter determining transverse diffusion rates (25). Packing should become looser as the number of double bonds increases. This could increase the spaces in which a molecule crossing the bilayer might dissolve. Another factor arises from the fact that the barrier to transverse diffusion is very likely to be the movement of the polar headgroup of a lipid across the hydrophobic core of the bilayer. In the case of shorter acyl chains, the width of this hydrophobic barrier is small, which should allow more rapid transverse diffusion. In the case of lipids with polyunsaturated acyl chains, the lesser hydrophobicity of the bilayer core, due to the presence of multiple carbon-carbon double bonds (26), may be a property that allows faster transverse diffusion. In the case of polyunsaturated lipids, the issue of acyl chain oxidation must also be considered. This is important because it has been shown that oxidized lipids exhibit fast transverse diffusion across bilayers (27). Indeed, when a preparation of polyunsaturated lipid that had been stored for an extended period of time was used, we noted very fast transverse diffusion, suggesting oxidation could have been involved (data not shown). For this reason, we took precautions against oxidation of the lipids with polyunsaturated acyl chains, and no or very little acyl chain oxidation was detected (see Methods). Nevertheless, we cannot rule out a small degree of oxidation.
We also observed that mixtures of lipids with slow and fast transverse diffusion tended to show an intermediate extent of transverse diffusion, although generally they did not show a linear relationship between lipid composition and extent of transverse diffusion (supplemental Fig. VI). This suggests that transverse diffusion during the lipid exchange process might follow a complex course of events. At the outset of the lipid exchange process, the acceptor vesicles are composed only of the intended inner leaflet lipid, so SM molecules would be expected to flip across the bilayer with a rate reflecting that of the PC present. However, toward the end of the SM exchange process, the membrane composition will be very different, and it is likely that transverse diffusion rates will have changed. This may explain why the extent of transverse diffusion observed using symmetric vesicles did not always exactly parallel the loss of asymmetry observed in the exchange vesicles. Another factor could be a difference between the transverse diffusion of the labeled C6-NBD-PC molecules and that of the unlabeled lipids.
It should be noted that previous studies of the acyl chain dependence of lipid transverse diffusion (in symmetric vesicles) are consistent with our results. In the study of Armstrong et al., it was found that increased unsaturation increased transverse diffusion rates for a headgroup labeled NBD-lipid (25). The study of Volinsky et al. found that oxidized PC transverse diffusion was much more rapid than that of unoxidized PC (27).
Fast lipid transverse diffusion also helps explain the tendency to have the highest extent of SM exchange in cases in which the lipid composition did not result in asymmetric vesicle formation. In vesicles that have low transverse diffusion, once the outer leaflet is nearly 100% SM, there can be no further increase in SM content. However, if transverse diffusion is fast, then SM introduced into the outer leaflet could flip into the inner leaflet, allowing exchange to continue, so that both leaflets would end up with a high SM concentration.
Biological implications of the dependence of asymmetry upon acyl chain structure
Asymmetry is often an important, if poorly understood, aspect of membrane structure and function. Therefore, acyl chains or combinations of acyl chains that do not allow asymmetry to be easily maintained may be disfavored in living organisms. This could be one reason that membrane lipids having short acyl chains or two polyunsaturated acyl chains are rarely found in biological membranes. However, in small amounts, these lipids might not greatly destabilize asymmetry, and there are other reasons that might also be important for their absence, including for short acyl chains, the inability for form a sufficiently stable bilayer, or for two polyunsaturated acyl chains, extreme sensitivity to oxidation. Furthermore, the presence of such lipids, especially in small amounts, might not prevent maintenance of asymmetry because a suitably specific active transport system should be able to counteract the effects of spontaneous diffusion. However, the need for such a transport system might be wasteful of metabolic energy (e.g., ATP). Finally, it is not known whether the acyl chains differ for a particular species (e.g., PC) in the inner and outer leaflet. This could also affect the stability of asymmetry.
Potential application of asymmetric and symmetric exchange vesicles
One of the most promising potential applications of exchange vesicles is their use in studies of the relationship between lipid composition and membrane protein structure and function. The exchange protocol will allow investigation of lipid asymmetry upon proteins in membranes with a wide range of lipid compositions. However, there are some cases in which it may be desirable to examine the effect of lipid structure upon proteins in symmetric membranes. The observations in this article suggest that this can also be accomplished in an improved fashion using lipid exchange. The approach would be to reconstitute a protein in a vesicle rich in highly polyunsaturated PC, which does not support asymmetry, and then carry out lipid exchange with various lipids. The ability to exchange lipids into preformed vesicles in situ in this fashion would avoid many complications that arise when trying to compare samples in which membrane proteins are individually reconstituted into different vesicle populations with different lipid compositions (e.g., different orientations of a membrane protein when reconstituted separately into different lipid vesicles, varying vesicle size in different reconstitution experiments, and different vesicle homogeneity in terms of protein molecules per vesicle).
Supplementary Material
Footnotes
Abbreviations:
- HP-TLC
- high-performance thin-layer chromatography
- Ld
- liquid disordered
- LUV
- large unilamellar vesicle
- MβCD
- methyl-beta-cyclodextrin
- MLV
- multilamellar vesicle
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- SMo/PCi
- SM outside/PC inside
- SUV
- small unilamellar vesicle
- Tm
- melting temperature
This work was supported by National Science Foundation Grant DMR-1104367.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three tables and seven figures.
REFERENCES
- 1.Bretscher M. S. 1972. Asymmetrical lipid bilayer structure for biological membranes. Nat. New Biol. 236: 11–12 [DOI] [PubMed] [Google Scholar]
- 2.Devaux P. F. 1991. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 30: 1163–1173 [DOI] [PubMed] [Google Scholar]
- 3.Williamson P., Schlegel R. A. 1994. Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol. Membr. Biol. 11: 199–216 [DOI] [PubMed] [Google Scholar]
- 4.Hill W. G., Rivers R. L., Zeidel M. L. 1999. Role of leaflet asymmetry in the permeability of model biological membranes to protons, solutes, and gases. J. Gen. Physiol. 114: 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holzer M., Momm J., Schubert R. 2010. Lipid transfer mediated by a recombinant pro-sterol carrier protein 2 for the accurate preparation of asymmetrical membrane vesicles requires a narrow vesicle size distribution: a free-flow electrophoresis study. Langmuir. 26: 4142–4151 [DOI] [PubMed] [Google Scholar]
- 6.Farge E., Devaux P. F. 1992. Shape changes of giant liposomes induced by an asymmetric transmembrane distribution of phospholipids. Biophys. J. 61: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill W. G., Zeidel M. L. 2000. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J. Biol. Chem. 275: 30176–30185 [DOI] [PubMed] [Google Scholar]
- 8.Haest C. W., Oslender A., Kamp D. 1997. Nonmediated flip-flop of anionic phospholipids and long-chain amphiphiles in the erythrocyte membrane depends on membrane potential. Biochemistry. 36: 10885–10891 [DOI] [PubMed] [Google Scholar]
- 9.Manno S., Takakuwa Y., Mohandas N. 2002. Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA. 99: 1943–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiantia S., London E.2012. Lipid Bilayer Asymmetry In Encyclopedia of Biophysics. G. C. K. Roberts, editor. Springer-Verlag, Berlin. 1250–1253.
- 11.Pagano R. E., Martin O. C., Schroit A. J., Struck D. K. 1981. Formation of asymmetric phospholipid membranes via spontaneous transfer of fluorescent lipid analogues between vesicle populations. Biochemistry. 20: 4920–4927 [DOI] [PubMed] [Google Scholar]
- 12.Pautot S., Frisken B. J., Weitz D. A. 2003. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA. 100: 10718–10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmond D. L., Schmid E. M., Martens S., Stachowiak J. C., Liska N., Fletcher D. A. 2011. Forming giant vesicles with controlled membrane composition, asymmetry, and contents. Proc. Natl. Acad. Sci. USA. 108: 9431–9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins M. D., Keller S. L. 2008. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA. 105: 124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hope M. J., Redelmeier T. E., Wong K. F., Rodrigueza W., Cullis P. R. 1989. Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients. Biochemistry. 28: 4181–4187 [DOI] [PubMed] [Google Scholar]
- 16.Kiessling V., Crane J. M., Tamm L. K. 2006. Transbilayer effects of raft-like lipid domains in asymmetric planar bilayers measured by single molecule tracking. Biophys. J. 91: 3313–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng H. T., Megha , London E. 2009. Preparation and properties of asymmetric vesicles that mimic cell membranes: effect upon lipid raft formation and transmembrane helix orientation. J. Biol. Chem. 284: 6079–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H. T., London E. 2011. Preparation and properties of asymmetric large unilamellar vesicles: interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophys. J. 100: 2671–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiantia S., Schwille P., Klymchenko A. S., London E. 2011. Asymmetric GUVs prepared by M beta CD-mediated lipid exchange: an FCS study. Biophys. J. 100: L1–L3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakowicz J. R.1991. Principles of Fluorescence Spectroscopy, Plenum Press, New York.
- 21.McIntyre J. C., Sleight R. G. 1991. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 30: 11819–11827 [DOI] [PubMed] [Google Scholar]
- 22.Klein R. A. 1970. The detection of oxidation in liposome preparations. Biochim. Biophys. Acta. 210: 486–489 [DOI] [PubMed] [Google Scholar]
- 23.Lentz B. R., Barenholz Y., Thompson T. E. 1976. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 15: 4521–4528 [DOI] [PubMed] [Google Scholar]
- 24.Wenz J. J., Barrantes F. J. 2003. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 42: 14267–14276 [DOI] [PubMed] [Google Scholar]
- 25.Armstrong V. T., Brzustowicz M. R., Wassall S. R., Jenski L. J., Stillwell W. 2003. Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch. Biochem. Biophys. 414: 74–82 [DOI] [PubMed] [Google Scholar]
- 26.Smith M., Jungalwala F. B. 1981. Reversed-phase high performance liquid chromatography of phosphatidylcholine: a simple method for determining relative hydrophobic interaction of various molecular species. J. Lipid Res. 22: 697–704 [PubMed] [Google Scholar]
- 27.Volinsky R., Cwiklik L., Jurkiewicz P., Hof M., Jungwirth P., Kinnunen P. K. 2011. Oxidized phosphatidylcholines facilitate phospholipid flip-flop in liposomes. Biophys. J. 101: 1376–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.