Abstract
We investigated the effect of weight loss, independent of change in diet composition, on HDL and apoAI metabolism in men with metabolic syndrome (MetS). Subjects (19 men with MetS [NCEP-ATPIII]) were fed an isoenergetic Mediterranean-style diet for 5 weeks (all foods provided). Participants then underwent a 20-week free-living period during which they were counseled to restrict energy intake, after which they were again fed an isoenergetic Mediterranean-style diet for 5 weeks. At the end of the two controlled diets, participants received a single bolus of [5,5,5-2H3] L-leucine, and fasting blood samples were collected over a 96 h period. ApoAI kinetic was assessed using multicompartmental modeling of the tracer enrichment data. Participants achieved a 9.1 ± 2.8% reduction in body weight (P < 0.001). Weight loss resulted in an increase in plasma HDL-cholesterol (HDL-C) concentrations of 6.0% (P = 0.059) and HDL3-C of 7.9% (P = 0.045), attributable to a reduction in apoAI fractional catabolic rate (−7.8%; P = 0.046) with no change in apoAI production rate (2.2%; P = 0.58). These data indicate that weight loss, independent of variation in diet composition, increases plasma HDL primarily by delaying the catabolism of apoAI.
Keywords: apolipoproteins, kinetics, high density lipoprotein metabolism, obesity
Typical features of metabolic syndrome (MetS) include abdominal obesity, hypertension, insulin resistance, high plasma triglyceride (TG) concentrations, and low HDL-cholesterol (HDL-C) concentrations, all of which are important risk factors for cardiovascular disease (1). The cardiometabolic risk profile associated with MetS is thought to be largely attributable to central obesity (2), and this is why weight loss is generally considered the primary target for the treatment of patients with MetS (3). Several studies have shown that weight loss of as little as 5% of initial body weight markedly improves several features of MetS (4, 5).
Kinetic studies have shown that weight loss reduces VLDL- apoB secretion in viscerally obese patients, probably owing to reduced visceral fat mass, enhanced insulin sensitivity, and decreased hepatic lipogenesis (6). Because plasma TG concentrations are so closely linked to HDL catabolism (7, 8), one would expect the TG-lowering effect of weight loss to be systematically associated with raised plasma HDL-C concentrations (9), but this has not always been a systematic observation (9–11). Diet composition is also an important determinant of HDL metabolism (12, 13), and it is possible that inconsistencies among weight loss studies may be attributable at least in part to important variations in diet during weight loss. Nevertheless, mechanisms underlying the impact of weight loss on HDL are not well understood, and we believe it is important to dissect out the effects of weight loss per se to those due to dietary changes during weight loss when investigating the mechanisms by which weight loss modulates HDL-C concentrations. To the best of our knowledge, this has not been done.
The aim of the present study was to investigate the impact of weight loss alone, independent of variation in diet composition, on HDL composition and metabolism by characterizing apoAI kinetics in men with MetS. Our hypothesis was that weight loss, independent of change in diet composition, increases plasma HDL-C concentrations primarily by delaying the catabolism of apoAI.
MATERIALS AND METHODS
Population, study design, and diet intervention
Details of the study design have been previously described (14). In the present study, we focused on data derived from the weight-loss phase of the main study protocol. Briefly, men (18–65 years) from the Québec City metropolitan area who met the NCEP-ATP III criteria for MetS (15) were recruited for the study. All study procedures were approved by the Research Ethics Committee of Laval University. Written informed consent was obtained from all participants enrolled in the study. Men with a previous history of cardiovascular disease or type 2 diabetes, those on lipid-lowering or hypertension medication, and smokers were excluded. The subjects also had to have a stable body weight for at least 6 months.
The subjects were provided with a control diet (5 weeks, all foods provided including red wine) that was formulated to be concordant with a Mediterranean-style diet (16) in quantities to maintain body weight constant (Table 1). Participants subsequently participated in a 20-week weight loss period (see next section). Finally, subjects were again provided with Mediterranean-style diet (5 weeks) in quantities to maintain their reduced body weight. This allowed us to investigate the impact of weight loss per se (i.e., independent of variation in diet composition) on HDL composition and metabolism.
TABLE 1.
Composition of the experimental isoenergetic controlled diet and mean dietary intakes during the weight loss period
| Nutrients | Controlled Diet before Weight Lossa | Free-living Caloric Restriction Weight Loss Periodb | Controlled Diet afterWeight Lossa |
| Energy (kcal) | 3,181 ± 478 | 2,318 ± 682 | 2,865 ± 390 |
| Lipids (%) | 32.0 | 31.1 ± 6.3 | 32.0 |
| SFA (%) | 6.7 | 8.9 ± 2.5 | 6.7 |
| MUFA (%) | 18.1 | 13.3 ± 2.9 | 18.1 |
| PUFA (%) | 4.7 | 6.3 ± 1.8 | 4.7 |
| TFA (%) | 0.3 | 0.9 ± 0.4 | 0.3 |
| Carbohydrates (%) | 50.0 | 51.0 ± 7.5 | 50.0 |
| Proteins (%) | 17.0 | 20.7 ± 4.9 | 17.0 |
| Alcohol (%) | 5.0 | 3.9 ± 3.0 | 5.0 |
| Cholesterol (mg/d) | 368.6 ± 55.4 | 281.6 ± 120.0 | 332.0 ± 45.2 |
| Total fibers (g/d) | 53.7 ± 8.1 | 33.8 ± 10.2 | 48.4 ± 6.6 |
| Soluble dietary fiber (g/d) | 15.5 ± 2.3 | 9.6 ± 2.9 | 13.9 ± 1.9 |
| Sodium (mg/d) | 3,866 ± 581 | 4,389 ± 6240 | 3,483 ± 474 |
Values are presented as mean ± SD and percentages of daily energy intake. MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TFA, trans fatty acids.
Isoenergetic Mediterranean-style diet. There is no variation in nutrient intake (% of calories) because all subjects received the same diet regimen.
Nutritional composition based on three 3-day food journals collected at weeks 2, 8, and 16 of the weight loss period.
Weight loss period and weight stabilization
After the first phase in controlled feeding isoenergetic conditions, participants received instructions on how to reduce their usual energy intake by 500 kcal/day. The objective was to generate a 5–10% reduction in body weight over a 20 week period. A registered dietitian provided personalized advice on how to create a 500 kcal deficit in daily energy intake. The primary focus was on reducing portion size and promoting low energy-density food choices. Three 3-day food records were collected at weeks 2, 8, and 16 and averaged to document dietary intake during the weight loss period (Table 1). Because our objective was to investigate the impact of weight loss on HDL kinetics, only participants who had lost at least 5% of their body weight were eligible for the second kinetic studies after the 5 weeks of weight stabilization.
Plasma lipids and lipoproteins assessment
The lipid content of the various plasma lipoproteins was measured enzymatically on a modular Hitachi using Roche products (Roche Diagnostics GmbH, Mannheim, Germany) according to standardized procedures (17). The cholesterol and triglyceride content of the HDL2 and HDL3 subfractions was determined after sequential precipitation with dextran sulfate as previously described (18). The precipitation method in our laboratories showed very high intra-class correlations (ICCs) for repeated samples (ICC = 0.99 and 0.98 for HDL2-C and HDL3-C, respectively; n = 82) and low CV (5.7% and 3.2% for HDL2-C and HDL3-C, respectively) (19). Plasma apoAI concentrations were measured by nephelometry (Dade Behring, Mississauga, Ontario, Canada). Serum cholesteryl ester transfer protein (CETP; CV ≤ 10%) (ALPCO diagnosticsSalem, NH), hepatic lipase, and endothelial lipase (both CV ≤ 10%) (USCN Life Sciences Inc., Wuhan, PR, China) concentrations were determined by ELISA. Fasting insulin concentrations were determined by radioimmunoassay (20) and plasma concentrations of lathosterol using a GC method similar to that previously described (21).
Kinetic protocol
Participants underwent a kinetic study before and after weight loss during week 5 of each controlled feeding period. After a 12 h fast, participants received a single intravenous bolus of [5,5,5-2H3] L-leucine (11 mg/kg), and fasting blood samples (20 ml) were collected at predetermined time points (0, 0.5, 1, 2, 4, 6, 8, 10 h). Additional 12 h fasting blood samples were collected in the morning of the next 4 days (24, 48, 72, 96 h), during which participants remained on the experimental control diet, with all meals provided to them.
Quantification and isotopic enrichment of apoAI
Plasma apoAI was first isolated by sequential ultracentrifugation from the d < 1.25 g/ml fraction using a Beckman 50.4ti Rotor. Samples were then dialyzed overnight in a NaCl-Tris-EDTA buffer. After a cysteamine treatment for 4 h at 37°C, samples were delipidated according to standardized procedures (22). ApoAI bands isolated by isoelectric focusing on polyacrylamide-urea gels were hydrolyzed for 24 h at 110°C using 6 N HCl and dried before a derivatisation step. ApoAI isotopic enrichment (%) was determined by GC-MS (GC 6890N, MS 5973N; Agilent Technologies, Palo Alto, CA).
Kinetic analysis
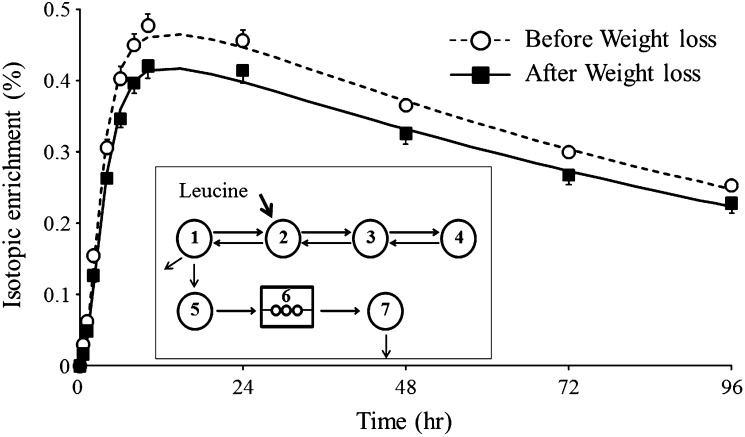
The apoAI fractional catabolic rate (FCR) was obtained by multicompartmental modeling of the isotopic enrichment data over time using SAAM II software (University of Washington, Department of Bioengineering, Seattle, WA). Fig. 1 (insert) shows the multicompartmental model used to describe the kinetic of plasma apoAI and mean isotopic enrichment in apoAI over time in the d < 1.25 g/l plasma fraction. Plasma volume was estimated using the equation suggested by Nikkila and Kekki for overweight and obese subjects (23). Plasma volume estimated using this equation has been strongly correlated with plasma volume measured by the standard isotopic dilution technique (24). The pool size (PS) of apoAI was estimated by multiplying plasma apoAI concentrations by plasma volume. ApoAI production rate (PR) was calculated by multiplying the FCR by the PS of apoAI and by dividing by body weight.
Fig. 1.
Mean isotopic enrichment over time of plasma apoAI for the 19 subjects before and after weight loss and multicompartmental model used to determine the kinetics of apoAI (insert). Compartments 1–4 describe the kinetic of leucine in plasma. Compartment 5 represents the intracellular pool of leucine (hepatic and other tissues) from which apoAI is synthesized and appears into the circulation (compartment 7) after a delay (compartment 6). Enrichment data in compartment 5 were used as a forcing function for the enrichment data of apoAI in the circulation.
Statistical analysis
Data are reported as means ± SD and percentage changes from the first controlled diet unless stated otherwise. Data were analyzed using the PROC MIXED procedure for repeated measures in SAS with time (before vs. after weight loss) as the main repeated effect (v9.2; SAS, Cary, NC). Spearman correlation analyses were used to examine the contribution of various factors to metabolic changes induced by weight loss. Predictors of the weight loss-induced variations in apoAI FCR were also evaluated using stepwise regression analysis. P ≤ 0.10 and P ≤ 0.05 were used as criteria for variables entry and maintenance in the model, respectively. The model included weight loss-induced variations in waist circumference, VLDL-TG, HDL-TG, CETP, hepatic lipase, endothelial lipase, and fasting insulin. Variables with a skewed distribution were log-10 transformed before statistical analysis. Differences at P ≤ 0.05 (two-sided) were considered significant.
RESULTS
Of the 26 men entering this study, seven did not achieve the 5% body weight loss prescription that was defined a priori to undergo the second kinetic study. The mean weight loss in these seven subjects was −1.2 ± 1.7%. Thus, comparisons before and after weight loss are based on n = 19. The overall compliance to the predetermined control diets before and after the weight loss periods calculated from the food checklist in completers was 97.1 ± 6.2%. Characteristics at screening of the 19 participants are shown in Table 2. All men were characterized as having the MetS at screening.
TABLE 2.
Physical characteristics and plasma lipid profile at screening of the 19 men included in analyses
| Variable | Mean ± SD | Frequency of MetS criteria |
| Age (years) | 50.8 ± 10.8 | — |
| Weight (kg) | 99.5 ± 19.2 | — |
| BMI (kg/m2) | 33.4 ± 5.5 | — |
| Waist circumference (cm) | 112.5 ± 11.7 | 100% |
| Systolic blood pressure (mm Hg) | 124.7 ± 10.0 | 15.8% |
| Diastolic blood pressure (mm Hg) | 81.8 ± 7.1 | 42.1% |
| Total-C (mmol/l) | 5.46 ± 1.06 | — |
| LDL-C (mmol/l) | 3.53 ± 0.97 | — |
| HDL-C (mmol/l) | 1.00 ± 0.26 | 42.1% |
| Triacylglycerol (mmol/l) | 2.02 ± 0.78 | 57.9% |
| Fasting glucose (mmol/l) | 5.64 ± 0.56 | 63.2% |
| MetS (%) | 100% | — |
Values are presented as mean ± SD and percentage of metabolic syndrome's criteria (NCEP ATP III). BMI, body mass index; C, cholesterol; MetS, metabolic syndrome.
After the first isoenergetic feeding period, 15 out of 19 participants were still characterized as having MetS as defined by NCEP-ATP III. However, mean plasma TG and HDL-C concentrations were not significantly modified by the 5 week consumption of the Mediterranean-style diet (14). Body weight was reduced by an average of 9.1 ± 2.8% (P < 0.001) and waist circumference by 7.9 ± 2.8 cm (P < 0.001) after caloric restriction and weight stabilization. Body weight was stable during the last 4 weeks of the second weight-stabilizing feeding period (not shown). Anthropometric characteristics, lipid profiles, and plasma apoAI kinetic parameters before and after stabilized weight loss are presented in Table 3. Weight loss increased plasma HDL-C concentrations (6.0%), although the difference did not reach statistical significance (P = 0.059). This was mainly attributable to a 7.9% increase in plasma HDL3-C concentrations (P = 0.045). Plasma apoAI concentrations and apoAI PS remained essentially unchanged after weight loss. The FCR of apoAI decreased significantly with weight loss (−7.8%; P = 0.046), with no change in apoAI PR. A significant reduction in plasma VLDL-TG (−24.8%; P = 0.007) and a trend toward a reduction in plasma endothelial lipase (−12.4%; P = 0.078) concentrations with weight loss were also observed. Weight loss led to significant reductions in plasma fasting insulin (−16.9%) and glucose (−4.2%) concentrations as well as in the HOMA-IR score (−20.7%; all P < 0.05).
TABLE 3.
Lipid profiles and plasma apoAI kinetic parameters before and after weight loss
| Variables | Before Weight Loss | After Weight Loss | % Change | Pa |
| Weight (kg)b | 98.3 ± 19.6 | 89.4 ± 18.2 | −9.1% | <0.001 |
| Waist circumference (cm)b | 112.2 ± 12.2 | 104.3 ± 13.4 | −7.0% | <0.001 |
| VLDL-TG (mmol/l) | 1.37 ± 0.53 | 1.03 ± 0.48 | −24.8% | 0.007 |
| HDL-C (mmol/l)b | 0.88 ± 0.19 | 0.93 ± 0.22 | 6.0% | 0.059 |
| HDL2-C (mmol/l)b | 0.29 ± 0.09 | 0.30 ± 0.10 | 2.3% | 0.684 |
| HDL3-C (mmol/l) | 0.58 ± 0.15 | 0.63 ± 0.15 | 7.9% | 0.045 |
| HDL-TG (mmol/l) | 0.13 ± 0.02 | 0.14 ± 0.02 | 2.4% | 0.625 |
| HDL2-TG (mmol/l)b | 0.03 ± 0.01 | 0.03 ± 0.01 | −0.5% | 0.975 |
| HDL3-TG (mmol/l)b | 0.11 ± 0.02 | 0.11 ± 0.02 | 1.5% | 0.805 |
| HDL-ApoAI (g/l) | 1.03 ± 0.17 | 1.06 ± 0.17 | 2.6% | 0.190 |
| Apo-AI | ||||
| PS (mg)b | 4,224 ± 749 | 4,208 ± 702 | −0.4% | 0.808 |
| Concentration (g/l) | 1.18 ± 0.16 | 1.20 ± 0.16 | 2.3% | 0.126 |
| PR (mg/kg/d) | 14.2 ± 2.4 | 14.5 ± 3.0 | 2.2% | 0.583 |
| FCR (pool/d)b | 0.33 ± 0.06 | 0.30 ± 0.04 | −7.8% | 0.046 |
| CETP (μg/ml) | 2.81 ± 0.54 | 2.81 ± 0.46 | −0.1% | 0.978 |
| Hepatic lipase (U/l)b | 38.3 ± 93.5 | 36.3 ± 92.6 | −5.3% | 0.345 |
| Endothelial lipase (ng/ml)b | 249.7 ± 70.5 | 218.8 ± 52.2 | −12.4% | 0.078 |
| ApoB (g/l) | 1.00 ± 0.21 | 0.94 ± 0.20 | −6.0% | 0.008 |
| Glucose (mmol/)b | 6.08 ± 0.48 | 5.83 ± 0.41 | −4.2% | <0.001 |
| Insulin (pmol/l)b | 128.4 ± 62.6 | 106.6 ± 50.2 | −17.0% | 0.034 |
| HOMA-IR | 34.5 ± 16.6 | 27.4 ± 12.2 | −20.7% | 0.030 |
| Lathosterol/C ratio (10 μmol/mmol C) | 280.4 ± 75.1 | 229.4 ± 85.6 | −18.2% | 0.002 |
Values are presented as mean ± SD and percent change versus the control diet before weight loss. The HOMA-IR score, apoB, glucose, and insulin concentrations have been published previously (14). Lathosterol/C ratio data have been published previously (21). C, cholesterol; CETP, cholesteryl ester transfer protein; FCR, fractional catabolic rate; HOMA-IR score = (insulin × glucose)/22.5; PR, production rate; PS, pool size; TG, triglycerides; VLDL, very low-density lipoprotein.
P value from the main effect of time in the mixed model (n = 19).
Analysis was performed on log-transformed values.
Reduction in apoAI FCR with weight loss was positively correlated with concurrent reductions in plasma VLDL-TG and fasting insulin concentrations (Table 4). Variations in HDL-C concentrations with weight loss tended to be negatively associated with changes in plasma VLDL-TG and fasting insulin concentrations and were correlated with variations in plasma endothelial lipase (r = −0.64; P = 0.003). Variations in plasma apoAI concentrations with weight loss were positively correlated with variations in total-C, LDL-C, and HDL-C concentrations and tended to be negatively associated with reductions in insulin concentrations. In multiple regression analysis, reductions in plasma VLDL-TG with weight loss predicted change in apoAI FCR (shared variance, 38.6%; P = 0.005). Finally, the reduction in VLDL-TG concentrations with weight loss was positively correlated in univariate analyses with the reduction in fasting insulin concentrations (r = 0.52; P = 0.021) and in the lathosterol/C ratio (r = 0.43; P = 0.068).
TABLE 4.
Spearman correlation coefficients among changes in metabolic variables induced by weight loss
| Variables | ΔHDL-C (mmol/l) | ΔApoAI (g/l) | ΔApoAI FCR (pool/day) | ΔApoAI PR(mg/kg/day) |
| ΔWaist circumference (cm) | 0.04 | −0.13 | 0.02 | −0.20 |
| ΔVLDL-TG (mmol/l) | −0.35c | 0.00 | 0.61a | 0.62a |
| ΔTotal-C (mmol/l) | 0.08 | 0.54b | 0.15 | 0.32 |
| ΔHDL-C (mmol/l) | – | 0.41c | −0.31 | −0.19 |
| ΔFasting insulin (pmol/l) | −0.37c | −0.38c | 0.46b | 0.24 |
| ΔEndothelial lipase (ng/ml) | −0.64a | −0.19 | 0.19 | 0.18 |
C, cholesterol; FCR, fractional catabolic rate; PR, production rate; TG, triglyceride.
P < 0.01.
P < 0.05.
P < 0.15.
DISCUSSION
To our knowledge, this is the first study designed specifically to characterize the impact of weight loss per se, independent of concurrent variation in diet composition, on HDL and apoAI kinetic in men with MetS. Our main findings are that weight loss alone increases plasma HDL-C and HDL3-C concentrations primarily by decreasing apoAI FCR with no change in apoAI PR. Results must be interpreted while considering that there was no control group to assess changes over time, which is an important limitation. The possibility that change in HDL metabolism over time may have occurred independent of the intervention cannot be excluded.
A meta-analysis of 70 studies published before 1992 assessed the effect of weight loss by caloric restriction on HDL-C concentrations. Data indicated that plasma HDL-C concentrations are reduced by 0.007 mmol/l for each kilogram of body weight loss during the active weight loss period and increased by 0.009 mmol/l for each kilogram of weight loss at a stabilized reduced weight (10). Few studies in this meta-analysis presented data pertaining to changes in dietary fat quantity or quality during weight loss. Thus, it was impossible for the authors to identify the independent influence of changes in dietary fat intake during caloric restriction from those due to weight loss per se on changes in lipids and lipoproteins. In our study, each kg of body weight loss after the 5-week stabilization period, in the absence of variation in diet composition before versus after weight loss, was associated with a 0.0056 mmol/l increase in plasma HDL-C concentrations. This value is 35% lower than the 0.009 mmol/l increase for each kg of weight loss predicted by data from the previous meta-analysis on this topic (10). This is consistent with the possibility that the specific contribution of weight loss per se to raising HDL-C concentrations in previous studies may have been overestimated due to the significant confounding effect of changes in diet composition. The increase in HDL-C in our study was mostly attributed to increased HDL-3C rather than in HDL2-C concentrations. Although some studies have shown that weight loss is associated with increased concentrations of large HDL particles (25), this has not been a consistent finding (26). Williams et al. (26) have shown that the 18% increase in plasma HDL-C concentrations after a 1-year weight loss study in men with low HDL at baseline (vs. a control group) was mostly attributable to an increase in HDL3 mass rather than to changes in HDL2 mass. This is consistent with our data because men in our study were also characterized as having relatively low HDL-C concentrations at baseline.
To our knowledge, only one kinetic study has assessed the effect of weight loss on HDL-apoAI metabolism using stable isotope technique in men with MetS (11). The hypocaloric, low-fat diet resulted in a 12.2% decrease in body weight and a 13% decrease in apoAI FCR and PR, resulting in no change in plasma HDL-C and apoAI concentrations (11). Weight loss was achieved by reducing energy intake and shifting the fat composition of the diet from 37% of energy to 26% of energy and by increasing the dietary carbohydrate intake from 37% of energy to 48% of energy (11). Our study in men with MetS showed that weight loss alone (i.e., when there is no confounding effect of change in diet composition) reduces the FCR of apoAI but has no influence on its PR. Previous studies have shown that hypercatabolism of apoAI is the primary mechanism underlying the unfavorable low HDL-C phenotype in obese insulin-resistant individuals (27–29). The fact that weight loss primarily lowered apoAI FCR in our study is consistent with that concept. ApoA-I FCR values in men with MetS nevertheless remained high after weight loss (0.30 pools/day on average) compared with values generally seen in normal nonMetS subjects (0.20–0.25 pools/day) (28), and this is consistent with the fact that HDL-C levels, although increased after weight loss (from 0.88 to 0.93 mmol/l on average), remained below normal values. The reduction in apoA-I FCR with weight loss, in the absence of change in PR, should have been associated with a significant increase in plasma apoA-I concentrations, but the change did not reach statistical significance (P = 0.12). We suspect that single-point assessment of apoAI concentrations versus multipoint assessment of apoAI kinetic may have resulted in different capacities to detect significant changes in response to weight loss.
In the present study, changes in endothelial lipase with weight loss were negatively correlated with changes in HDL-C concentrations. Hydrolysis of phospholipids by endothelial lipase has been associated with reductions in HDL particles size, and small HDL that are the product of the intravascular lipolysis of triglyceride-enriched HDL have been shown to be catabolized more rapidly than large HDL (30, 31). It has also been reported that endothelial lipase overexpression enhances renal and hepatic apoAI catabolism (32). However, weight loss-induced reduction in VLDL-TG concentrations was most strongly correlated with the reduction in apoAI FCR in our study. TG in VLDL are substrates for CETP-mediated exchange of neutral lipids to HDL particles, and this, combined with an increased hepatic or endothelial lipase activity often seen in obesity (33, 34), leads to a remodeling of HDL that are susceptible to being cleared more rapidly (35, 36). Our data based on correlation analyses are therefore consistent with direct evidence indicating that variations in plasma TG concentrations, along with intravascular lipase activities, are a key determinant of HDL catabolism (7). Insulin resistance has been shown to drive the secretion of TG enriched-apoB-containing lipoprotein particles primarily by increasing free fatty acid flux to the liver and enhancing hepatic lipogenesis (37). Hepatic availability of cholesterol has also been shown to regulate secretion of plasma apoB-containing lipoprotein (38). Consistent with this, we observed a positive association between changes in VLDL-TG and changes in fasting insulin concentrations and in the lathosterol/C ratio, a surrogate marker of endogenous cholesterol synthesis, with weight loss. We hypothesize that the reduction in plasma VLDL-TG concentrations with weight loss may be explained, at least in part, by the concurrent reduction in fasting insulin concentrations reflecting improved insulin sensitivity and reduced endogenous cholesterol synthesis.
Although the number of subjects in this study may be considered limited (n = 19), the controlled feeding nature of our protocol, the achievement of a stabilized weight for the kinetic study, and the use of tracers to characterize HDL metabolism in response to weight loss are important strengths. The use of a bolus has also been shown to be preferable to the primed constant infusion for the study of lipoproteins with a slower turnover such as HDL (39).
In conclusion, data from this carefully controlled feeding study suggest that the HDL-C raising effect of weight loss in MetS is smaller in magnitude than expected based on data from previous studies that have not controlled for changes in dietary intake that generally occur with weight loss. Our data also indicated that weight loss per se, independent of variation in diet composition, increases HDL primarily by delaying the catabolism of apoAI.
Acknowledgments
The authors thank the staff of the metabolic kitchen,the nurses and laboratory staff of the Institute of Nutraceuticals and Functional Food for their technical assistance and the expert care provided to the participants, and the participants of this study.
Footnotes
Abbreviations:
- CETP
- cholesteryl estertransfer protein
- FCR
- fractional catabolic rate
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- MetS
- metabolic syndrome
- PR
- production rate
- PS
- pool size
- TG
- triglyceride
This study was supported by an operating grant from the Canadian Institutes of Health Research (MOP-68866). Provigo/Loblaws donated the foods used in this study.
REFERENCES
- 1.National Cholesterol Education Program 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421 [PubMed] [Google Scholar]
- 2.Despres J. P., Lemieux I. 2006. Abdominal obesity and metabolic syndrome. Nature. 444: 881–887 [DOI] [PubMed] [Google Scholar]
- 3.Grundy S. M., Hansen B., Smith S. C., Jr, Cleeman J. I., Kahn R. A. 2004. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 109: 551–556 [DOI] [PubMed] [Google Scholar]
- 4.Case C. C., Jones P. H., Nelson K., O'Brian Smith E., Ballantyne C. M. 2002. Impact of weight loss on the metabolic syndrome. Diabetes Obes. Metab. 4: 407–414 [DOI] [PubMed] [Google Scholar]
- 5.Lofgren I. E., Herron K. L., West K. L., Zern T. L., Brownbill R. A., Ilich J. Z., Koo S. I., Fernandez M. L. 2005. Weight loss favorably modifies anthropometrics and reverses the metabolic syndrome in premenopausal women. J. Am. Coll. Nutr. 24: 486–493 [DOI] [PubMed] [Google Scholar]
- 6.Chan D. C., Barrett H. P., Watts G. F. 2004. Dyslipidemia in visceral obesity: mechanisms, implications, and therapy. Am. J. Cardiovasc. Drugs. 4: 227–246 [DOI] [PubMed] [Google Scholar]
- 7.Lamarche B., Rashid S., Lewis G. F. 1999. HDL metabolism in hypertriglyceridemic states: an overview. Clin. Chim. Acta. 286: 145–161 [DOI] [PubMed] [Google Scholar]
- 8.Watts G. F., Barrett P. H., Chan D. C. 2008. HDL metabolism in context: looking on the bright side. Curr. Opin. Lipidol. 19: 395–404 [DOI] [PubMed] [Google Scholar]
- 9.Sacks F. M., Bray G. A., Carey V. J., Smith S. R., Ryan D. H., Anton S. D., McManus K., Champagne C. M., Bishop L. M., Laranjo N., et al. 2009. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360: 859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dattilo A. M., Kris-Etherton P. M. 1992. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am. J. Clin. Nutr. 56: 320–328 [DOI] [PubMed] [Google Scholar]
- 11.Ng T. W., Watts G. F., Barrett P. H., Rye K. A., Chan D. C. 2007. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care. 30: 2945–2950 [DOI] [PubMed] [Google Scholar]
- 12.Desroches S., Paradis M. E., Perusse M., Archer W. R., Bergeron J., Couture P., Bergeron N., Lamarche B. 2004. Apolipoprotein A-I, A-II, and VLDL-B-100 metabolism in men: comparison of a low-fat diet and a high-monounsaturated fatty acid diet. J. Lipid Res. 45: 2331–2338 [DOI] [PubMed] [Google Scholar]
- 13.Velez-Carrasco W., Lichtenstein A. H., Welty F. K., Li Z., Lamon-Fava S., Dolnikowski G. G., Schaefer E. J. 1999. Dietary restriction of saturated fat and cholesterol decreases HDL ApoA-I secretion. Arterioscler. Thromb. Vasc. Biol. 19: 918–924 [DOI] [PubMed] [Google Scholar]
- 14.Richard C., Couture P., Desroches S., Charest A., Lamarche B. 2011. Effect of the Mediterranean diet with and without weight loss on cardiovascular risk factors in men with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 21: 628–635 [DOI] [PubMed] [Google Scholar]
- 15.Grundy S. M., Cleeman J. I., Daniels S. R., Donato K. A., Eckel R. H., Franklin B. A., Gordon D. J., Krauss R. M., Savage P. J., Smith S. C., Jr, et al. 2005. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 112: 2735–2752 [DOI] [PubMed] [Google Scholar]
- 16.Willett W. C., Sacks F., Trichopoulou A., Drescher G., Ferro-Luzzi A., Helsing E., Trichopoulos D. 1995. Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr. 61: 1402S–1406S [DOI] [PubMed] [Google Scholar]
- 17.Moorjani S., Dupont A., Labrie F., Lupien P. J., Brun D., Gagne C., Giguere M., Belanger A. 1987. Increase in plasma high-density lipoprotein concentration following complete androgen blockage in men with prostatic carcinoma. Metabolism. 36: 244–250 [DOI] [PubMed] [Google Scholar]
- 18.Gidez L. I., Miller G. J., Burstein M., Slagle S., Eder H. A. 1982. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J. Lipid Res. 23: 1206–1223 [PubMed] [Google Scholar]
- 19.Despres J. P., Gagnon J., Bergeron J., Couillard C., Leon A. S., Rao D. C., Skinner J. S., Wilmore J. H., Bouchard C. 1999. Plasma post-heparin lipase activities in the HERITAGE Family Study: the reproducibility, gender differences, and associations with lipoprotein levels. HEalth, RIsk factors, exercise Training and GEnetics. Clin. Biochem. 32: 157–165 [DOI] [PubMed] [Google Scholar]
- 20.Desbuquois B., Aurbach G. D. 1971. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J. Clin. Endocrinol. Metab. 33: 732–738 [DOI] [PubMed] [Google Scholar]
- 21.Richard C., Couture P., Desroches S., Benjannet S., Seidah N. G., Lichtenstein A. H., Lamarche B. 2012. Effect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndrome. Br. J. Nutr. 107: 705–711 [DOI] [PubMed] [Google Scholar]
- 22.Batal R., Tremblay M., Krimbou L., Mamer O., Davignon J., Genest J., Jr, Cohn J. S. 1998. Familial HDL deficiency characterized by hypercatabolism of mature apoA-I but not proapoA-I. Arterioscler. Thromb. Vasc. Biol. 18: 655–664 [DOI] [PubMed] [Google Scholar]
- 23.Nikkilä E. A., Kekki M. 1972. Plasma triglyceride metabolism in thyroid disease. J. Clin. Invest. 51: 2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riches F. M., Watts G. F., Naoumova R. P., Kelly J. M., Croft K. D., Thompson G. R. 1998. Hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 studied with a stable isotope technique in men with visceral obesity. Int. J. Obes. Relat. Metab. Disord. 22: 414–423 [DOI] [PubMed] [Google Scholar]
- 25.Aron-Wisnewsky J., Julia Z., Poitou C., Bouillot J. L., Basdevant A., Chapman M. J., Clement K., Guerin M. 2011. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J. Clin. Endocrinol. Metab. 96: 1151–1159 [DOI] [PubMed] [Google Scholar]
- 26.Williams P. T., Stefanick M. L., Vranizan K. M., Wood P. D. 1994. The effects of weight loss by exercise or by dieting on plasma high-density lipoprotein (HDL) levels in men with low, intermediate, and normal-to-high HDL at baseline. Metabolism. 43: 917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji J., Watts G. F., Johnson A. G., Chan D. C., Ooi E. M., Rye K. A., Serone A. P., Barrett P. H. 2006. High-density lipoprotein (HDL) transport in the metabolic syndrome: application of a new model for HDL particle kinetics. J. Clin. Endocrinol. Metab. 91: 973–979 [DOI] [PubMed] [Google Scholar]
- 28.Watts G. F., Barrett P. H., Ji J., Serone A. P., Chan D. C., Croft K. D., Loehrer F., Johnson A. G. 2003. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 52: 803–811 [DOI] [PubMed] [Google Scholar]
- 29.Chan D. C., Barrett P. H., Watts G. F. 2004. Lipoprotein transport in the metabolic syndrome: pathophysiological and interventional studies employing stable isotopy and modelling methods. Clin. Sci. (Lond.). 107: 233–249 [DOI] [PubMed] [Google Scholar]
- 30.Jahangiri A., Rader D. J., Marchadier D., Curtiss L. K., Bonnet D. J., Rye K. A. 2005. Evidence that endothelial lipase remodels high density lipoproteins without mediating the dissociation of apolipoprotein A-I. J. Lipid Res. 46: 896–903 [DOI] [PubMed] [Google Scholar]
- 31.Lamarche B., Uffelman K. D., Steiner G., Barrett P. H., Lewis G. F. 1998. Analysis of particle size and lipid composition as determinants of the metabolic clearance of human high density lipoproteins in a rabbit model. J. Lipid Res. 39: 1162–1172 [PubMed] [Google Scholar]
- 32.Maugeais C., Tietge U. J., Broedl U. C., Marchadier D., Cain W., McCoy M. G., Lund-Katz S., Glick J. M., Rader D. J. 2003. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 108: 2121–2126 [DOI] [PubMed] [Google Scholar]
- 33.Arai T., Yamashita S., Hirano K., Sakai N., Kotani K., Fujioka S., Nozaki S., Keno Y., Yamane M., Shinohara E. 1994. Increased plasma cholesteryl ester transfer protein in obese subjects. A possible mechanism for the reduction of serum HDL cholesterol levels in obesity. Arterioscler. Thromb. 14: 1129–1136 [DOI] [PubMed] [Google Scholar]
- 34.Paradis M. E., Badellino K. O., Rader D. J., Tchernof A., Richard C., Luu-The V., Deshaies Y., Bergeron J., Archer W. R., Couture P., et al. 2006. Visceral adiposity and endothelial lipase. J. Clin. Endocrinol. Metab. 91: 3538–3543 [DOI] [PubMed] [Google Scholar]
- 35.Lamarche B., Uffelman K. D., Carpentier A., Cohn J. S., Steiner G., Barrett P. H., Lewis G. F. 1999. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J. Clin. Invest. 103: 1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis G. F., Rader D. J. 2005. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96: 1221–1232 [DOI] [PubMed] [Google Scholar]
- 37.Krauss R. M., Siri P. W. 2004. Dyslipidemia in type 2 diabetes. Med. Clin. North Am. 88: 897–909 [DOI] [PubMed] [Google Scholar]
- 38.Thompson G. R., Naoumova R. P., Watts G. F. 1996. Role of cholesterol in regulating apolipoprotein B secretion by the liver. J. Lipid Res. 37: 439–447 [PubMed] [Google Scholar]
- 39.Chan D. C., Barrett P. H., Watts G. F. 2004. Lipoprotein transport in the metabolic syndrome: methodological aspects of stable isotope kinetic studies. Clin. Sci. (Lond.). 107: 221–232 [DOI] [PubMed] [Google Scholar]