Abstract
The capacity of HDL to induce cell cholesterol efflux is considered one of its main antiatherogenic properties. Little is known about the impact of such HDL function on vascular physiology. We investigated the relationship between ABCA1-dependent serum cholesterol efflux capacity (CEC), an HDL functionality indicator, and pulse wave velocity (PWV), an indicator of arterial stiffness. Serum of 167 healthy subjects was used to conduct CEC measurement, and carotid-femoral PWV was measured with a high-fidelity tonometer. J774 macrophages, labeled with [3H]cholesterol and stimulated to express ABCA1, were exposed to sera; the difference between cholesterol efflux from stimulated and unstimulated cells provided specific ABCA1-mediated CEC. PWV is inversely correlated with ABCA1-dependent CEC (r = −0.183; P = 0.018). Moreover, controlling for age, sex, body mass index, mean arterial pressure, serum LDL, HDL-cholesterol, and fasting plasma glucose, PWV displays a significant negative regression on ABCA1-dependent CEC (β = −0.204; 95% confidence interval, −0.371 to −0.037). The finding that ABCA1-dependent CEC, but not serum HDL cholesterol level (r = −0.002; P = 0.985), is a significant predictor of PWV in healthy subjects points to the relevance of HDL function in vascular physiology and arterial stiffness prevention.
Keywords: ATP-binding cassette transporter, high density lipoprotein, arterial stiffness, vascular biology
Risk and prognostic markers for cardiovascular diseases (CVDs) include metabolic indexes and parameters relative to arterial structure and function. The inverse correlation between plasma HDL cholesterol levels and CVD is generally assumed to be an indication of the atheroprotective activity of HDL (1). An important emerging concept in this field is that HDL quality and function are more important than its circulating concentration for the antiatherosclerotic activity, as suggested by observational studies (2) and pharmacological HDL modulation attempts (3). However, the importance of HDL functionality has been assessed only in a case-control setting (4) and further investigations are needed.
The ability of serum HDL to promote cholesterol efflux from cells is thought to play a key role in HDL atheroprotection (5). This function, called cholesterol efflux capacity (CEC), measured through standardized in vitro techniques, has been recently demonstrated to be inversely related to intima-media thickness (IMT), an index of arterial structural changes in subclinical atherosclerosis, and coronary artery disease, independently of serum HDL cholesterol level (4). Moreover, hypoalphalipoproteinemic subjects with the apolipoprotein A-I (apoA-I)Milano mutation or lecithin cholesterol acyltransferase deficiency, who are not always at increased cardiovascular risk (6, 7), have an efficient serum CEC despite very low total HDL levels (8, 9). These observations are consistent with the notion of a specific ability of HDL, depending on its composition, to interact with plasma membrane receptors or transporters involved in cholesterol handling (10–12). Among these, ABCA1 is particularly relevant for macrophage cholesterol efflux because it promotes plasma membrane cholesterol availability for HDL and has been suggested to play a major role in cardioprotection (11). The interaction between HDL and ABCA1 is followed by the activation of distinct intracellular signaling pathways in macrophages and in endothelial cells, resulting in preservation of vessel health (13). Isolated aortic endothelial cells from transgenic mice overexpressing hABCA1 show enhanced cholesterol efflux and elevated levels of eNOS mRNA (14). Moreover, a more recent study demonstrated that binding of apoA-I to ABCA1 increases prostaglandin I-2 (PGI-2) secretion in endothelial cells, resulting in atheroprotection through vasodilation as well as the inhibition of platelet aggregation and monocyte adhesion (13, 15).
Pulse wave velocity (PWV), the propagation speed of the pulse pressure wave, is one of the major determinants of pulse pressure and is widely used as an index of arterial stiffness. Arterial stiffness is determined by several structural and functional factors, including the cross-sectional arrangement of cells and interstitial components (particularly collagen and elastin) in the vessel wall, the characteristics and number of smooth muscle cells, endothelium-mediated vasodilation, hormones, and electrolyte balance. The correlation between arterial stiffness and end-organ damage in cardiovascular diseases is widely accepted on the basis of clinical studies and pathophysiology mechanisms (16, 17). In particular, PWV has been demonstrated to be a predictor for adverse cardiovascular events in hypertension and CVD in many patient populations (18) and has been indicated as a useful diagnostic tool for increased cardiovascular risk by international guidelines (19, 20). The relevance of PWV derives not only from the fact that it reflects the structure of elastic arteries (i.e., the composition and organization of vessel wall) but also from its involvement in the evolution of heart and vessel function in time. In fact, if PWV increases, the backward pressure wave reflections return from the distal arterial compartment earlier than normal, during systole instead of diastole, increasing ventricular and aortic systolic pressure and decreasing aortic pressure during diastole. These changes augment left ventricular afterload and myocardial oxygen demand, reduce coronary perfusion, and cause mismatch between ventricle emptying and arterial pulse wave transmission, leading to ventricular hypertrophy (21, 22).
The PWV, the vessel vasodilative function (23), and endothelial function (24) are interrelated through a complex network of cellular and biochemical factors, including PGI-2 and NO (13).
Because HDL function determination could be a useful tool to better define vessel health and preclinical vascular risk, the aim of this study was to evaluate the relationship between serum CEC, as a metabolic parameter reflecting HDL function, and PWV, as an index of arterial stiffness, in a population of healthy subjects in the absence of pharmacological treatment. The inverse correlation that we found between ABCA1-mediated CEC and PWV in a healthy population provides insights into the mechanisms of early atherosclerotic process and HDL atheroprotection.
MATERIALS AND METHODS
Study design
Healthy subjects were selected among those enrolled in the Brisighella Heart Study (BHS) (25). The general protocol of the BHS and of its substudies have been approved by the Ethical Committee of the University of Bologna and conform to the principles outlined in the Helsinki Declaration. Written informed consent was obtained from all study participants.
The BHS is a prospective, population-based, longitudinal, epidemiological investigation started in 1972 and involving randomly selected subjects, aged 14 to 84 years and free of CVD at enrolment, all resident in the rural town of Brisighella, an area characterized by life-style homogeneity and a very low migration rate from other countries (26). Participants were clinically evaluated at baseline and every 4 years thereafter by extensively assessing clinical and laboratory profiles according to a standardized protocol that has been described in detail elsewhere (27, 28).
Subjects
The final enrolled population sample consisted of 167 subjects (54 male and 113 female) who were nonsmokers; nondiabetics; untreated with antihypertensive, antihyperlipidaemic, or antidiabetic drugs; and free from echographically detectable atherosclerotic plaques (Table 1). Beyond the standard procedures enlisted in the BHS protocol, these subjects underwent serum cholesterol efflux capacity determination and carotid-femoral PWV measurement.
TABLE 1.
Mean and SD of age and of anthropometric and metabolic parameters of the healthy population studied
| Variable | Mean | SD | Variable | Mean | SD |
| Age (years) | 55.27 | 11.45 | TC (mg/dl) | 205.68 | 30.72 |
| BMI (kg/m2) | 25.51 | 4.75 | TG (mg/dl) | 88.51 | 40.00 |
| Waist C. (cm) | 87.80 | 12.74 | HDL-C (mg/dl) | 51.38 | 11.15 |
| Hip C. (cm) | 95.86 | 11.39 | LDL-C (mg/dl) | 136.60 | 27.16 |
| Waist/hip ratio | 0.92 | 0.08 | ApoB (mg/dl) | 96.23 | 23.36 |
| SBP (mm Hg) | 132.82 | 17.20 | ApoA1 (mg/dl) | 164.66 | 33.04 |
| DBP (mm Hg) | 79.15 | 8.29 | Lp(a) (mg/dl) | 8.03* | 14.09* |
| PP (mm Hg) | 53.66 | 14.23 | FPG (mg/dl) | 97.11 | 10.20 |
| MAP (mm Hg) | 97.04 | 9.97 | SUA (mg/dl) | 4.76 | 1.29 |
| HR (bpm) | 63.82 | 10.59 | Creatinine (mg/dl) | 0.89 | 0.15 |
BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-C, HDL cholesterol; Hip C, hip circumference; HR, heart rate; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides; Waist C, waist circumference.
* Lp(a) levels are expressed as median (interquartile range) because of their asymmetric distribution.
CEC
We quantified serum CEC by using a validated ex vivo system that involves incubation of macrophages with whole serum from the study participants (4, 8, 9). Efflux studies were performed using macrophages labeled with [1,2-3H] cholesterol in the presence of an ACAT inhibitor (Sandoz 58035) used at 2 μg/ml. Aqueous diffusion-dependent process was evaluated in J774 murine macrophages, which, under basal conditions, express low levels of ABCA1, ABCG1, and SR-BI and release membrane cholesterol to extracellular acceptors mainly by aqueous diffusion (4, 29). Stimulation of J774 murine macrophages with cAMP (0.3 mM) for 18 h up-regulates the ABCA1 protein (30, 31). In such conditions, total release of cholesterol occurs mainly by ABCA1 and aqueous diffusion (4, 32). ABCA1-mediated CEC was calculated as the difference in efflux between ABCA1 expressing J774 and J774 in basal conditions (33). The efflux was promoted for 4 h to 2% (v/v) serum samples. CEC was expressed as a percentage of the radioactivity released to the medium over the total radioactivity incorporated by cells (33). Whole serum was used to measure ABCA1-mediated CEC because the specific acceptor for ABCA1 is lipid free or lipid poor apoA-I, which characterizes nascent HDL (8, 34, 35); thus, the presence of apoB lipoprotein during the procedure does not affect the final result (29).
To minimize the intra-assay variability, every serum sample was run in triplicate, and average values and standard deviations were calculated for each percentage of efflux obtained (29). cAMP-induced ABCA1 expression was verified by the increase in efflux to 10 μg/ml apoA-I used as ABCA1 specific extracellular acceptor (31, 32). A pool of human sera as reference standard 1 (St1) was tested in each assay, and its efflux capacity was used to normalize the patient sample values from different experiments to correct for the interassay variability. A second pool of human sera as reference standard 2 (St2) was tested in each assay, and its efflux capacity, after normalization, was the index of the intra-assay variability (32). Calculated mean intra- and interassay coefficients of variation were 5.95% and 9.53%, respectively.
To prevent HDL remodeling at room temperature, all serum samples were immediately stored at −80°C after drawing, and the aliquots were defrosted in ice just before use. All sera used in the study underwent one cycle of freezing and thawing.
Vascular investigations
Carotid-femoral PWV was measured using the PulsePen device (DiaTecne srl, Milan, Italy), which is a validated, easy-to-use, high-fidelity tonometer that has been described in detail previously (36). PWV is determined by a single probe at two intervals in a highly rapid succession, using the electrocardiogram trace as reference: the detector is first positioned at the common carotid artery, the central detection site, simultaneously performing electrocardiogram and tonometry, and then on the femoral artery. When the difference between heart rate recorded during the carotid measurement and that recorded during the femoral measurement is ≥10%, the PWV evaluation is repeated (the difference in heart rate is indicated in the PulsePen software). The PWV is calculated as the distance between the measurement sites divided by transit time delay between femoral and carotid pulse wave. The distance of the pulse wave transit is the difference between the distance from suprasternal notch to femoral point of application of the tonometer and the distance from carotid point of tonometer application and the suprasternal notch. The time delay is measured between the foot of the femoral artery and carotid waveforms. The wave foot is defined at the end of diastole, when the steep rise of the waveform begins.
To avoid methodological bias, a single senior investigator performed all of the PWV measurements. The intraobserver coefficient of variation of PWV measurements with PulsePen device was 5.7% (37).
Statistical analysis
Statistical analyses were performed with STATA 11.0, Version for Windows. Mean and standard deviation were used to describe the studied variables except for lipoprotein(a), which was summarized by means of median and interquartile range due to its natural asymmetric distribution. A multiple linear regression analysis with nested design was then performed to investigate the relationship between PWV and CEC. A nested design allowed us to assess the effect of each variable and potential confounder and to evaluate the improvement in model fit produced by the introduction of such variable (or set of variables). This latter statistical element informs how well the model predicts the outcome. All the variables included in the models are continuous. Because age has a non-normal distribution, as emerged from application of the Shapiro-Wilk test for normality, all the models were estimated with Huber-White sandwich robust estimator of standard errors, which limits the effects of non-normality of explanatory variables. In the nested design, model 1 estimates the effect of individual characteristics (age and sex) on PWV, model 2 checks for the influence of cardio-circulatory risk factors (body mass index, mean arterial pressure, LDL-cholesterol, fasting plasma glucose), and models 3 and 4 assess, respectively, the effect of HDL-C and ABCA1 once controlled for the previous variables. A further model (model 5), including an interaction between ABCA1 and sex, was estimated. The improvement in model fit after the estimation of every model was assessed by means of adjusted R2 and a likelihood ratio test. Finally, a postestimation analysis was carried out to check for the presence of multicollinearity among variables using the variance inflation factor (VIF) statistic. Multicollinearity is a statistical problem that arises when two predictor variables are highly correlated and might bias the coefficient estimates of the variables involved. This statistic indicates the existence of multicollinearity for VIF ≥ 5. All reported P values are two-tailed, with a P value of 0.05 indicating statistical significance.
RESULTS
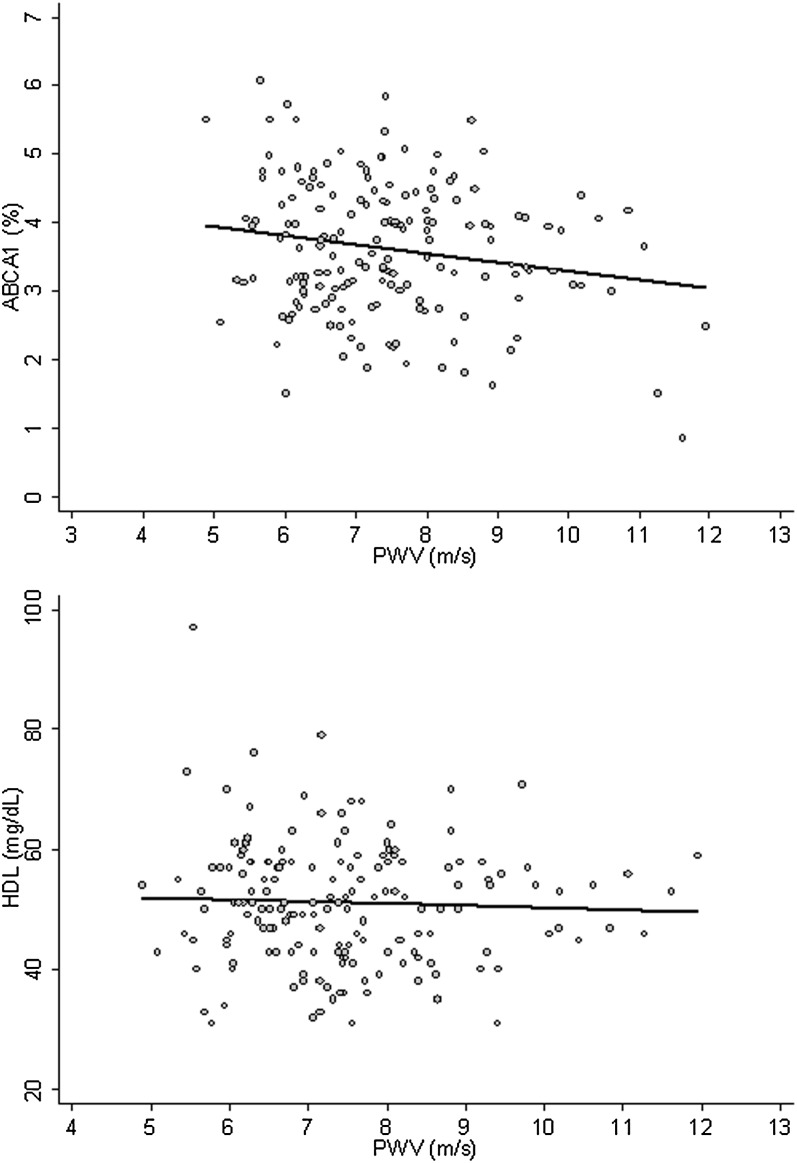
The baseline characteristics of the study population are given in Table 1. An inverse correlation between PWV and ABCA1-dependent CEC was found (r = −0.183; P = 0.018) (Fig. 1). No correlation was found between PWV and aqueous diffusion-dependent CEC (r = 0.129; P = 0.095). Total HDL-C serum levels were not correlated to PWV (r = −0.002; P = 0.985) (Fig. 1) or to ABCA1-dependent CEC (r = −0.075; P = 0.333).
Fig. 1.
Correlation between carotid-femoral PWV and ABCA1-dependent CEC or serum HDL-C. Upper panel: ABCA1, ABCA1-dependent CEC (%) after a 4 h incubation with 2% individual serum. Lower panel: HDL (mg/dl), serum HDL-C.
In the nested linear regression (Table 2), all the models show a significant improvement in model fit except models 3 and 5 (likelihood ratio test returns P values of 0.475 and 0.441, respectively). This means that HDL-C and the interaction term do not increase the explanatory power of the model. On the other hand, model 4 has the best fit (adjusted R2 = 0.440). In this model, as expected, age is the only significant individual characteristics, with PWV increasing by 0.042 m/s (95% confidence interval [CI], 0.026–0.058) per year of age. Regarding risk factors, BMI (β = 0.052; 95% CI, 0.019–0.085) and mean arterial pressure (β = 0.056; 95% CI 0.039–0.074) show a significant impact on PWV. All these effects are robust across models. Finally, ABCA1-mediated CEC shows a significant negative relationship with PWV. Once controlled for the previous factors, a one-unit change in ABCA1 produces a significant decrease of 0.179 m/s in PWV (95% CI, −0.363 to 0.006) and a 0.015 increase in adjusted R2 with respect to model 3. The VIF statistic does not support the hypothetic existence of multicollinearity issues (Table 3).
TABLE 2.
Linear regression with nested design. Coefficients for the association between cfPWV and other parameters in the healthy population studied
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
|||||||||||
| Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | Coeff. | SE | P value | |
| Indiv. characteristics | |||||||||||||||
| Age | 0.059 | 0.008 | 0.000 | 0.044 | 0.008 | 0.000 | 0.043 | 0.008 | 0.000 | 0.042 | 0.008 | 0.000 | 0.042 | 0.008 | 0.000 |
| Female (ref. male) | −0.202 | 0.201 | 0.317 | −0.005 | 0.202 | 0.980 | −0.036 | 0.213 | 0.868 | −0.031 | 0.213 | 0.885 | −0.499 | 0.863 | 0.564 |
| Risk factors | |||||||||||||||
| BMI | 0.052 | 0.016 | 0.001 | 0.055 | 0.017 | 0.002 | 0.052 | 0.017 | 0.002 | 0.051 | 0.017 | 0.003 | |||
| MAP | 0.056 | 0.009 | 0.000 | 0.056 | 0.009 | 0.000 | 0.056 | 0.009 | 0.000 | 0.056 | 0.009 | 0.000 | |||
| FPG | −0.009 | 0.010 | 0.377 | −0.009 | 0.010 | 0.383 | −0.008 | 0.010 | 0.419 | −0.008 | 0.010 | 0.417 | |||
| LDL-C | −0.004 | 0.003 | 0.168 | −0.004 | 0.003 | 0.153 | −0.004 | 0.003 | 0.244 | −0.004 | 0.003 | 0.218 | |||
| Efflux parameter | |||||||||||||||
| HDL-C | 0.005 | 0.009 | 0.525 | 0.004 | 0.009 | 0.677 | 0.004 | 0.009 | 0.663 | ||||||
| ABCA1-CEC | −0.179 | 0.083 | 0.033 | −0.261 | 0.182 | 0.154 | |||||||||
| Interaction Sex × ABCA1 | 0.129 | 0.210 | 0.538 | ||||||||||||
| Constant | 4.514 | 0.584 | 0.000 | −0.254 | 1.479 | 0.864 | −0.529 | 1.496 | 0.724 | 0.152 | 1.550 | 0.922 | 0.955 | 2.267 | 0.674 |
| Observations | 167 | 167 | 167 | 167 | 167 | ||||||||||
| R2adj | 0.247 | 0.426 | 0.425 | 0.440 | 0.439 | ||||||||||
| Log-likelihood | −264.5 | −239.2 | −238.9 | −236.5 | −236.3 | ||||||||||
| LR test (P value) | <0.001 | <0.001 | 0.475 | 0.029 | 0.441 | ||||||||||
ABCA1-CEC, ABCA1-dependent cholesterol efflux capacity; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MAP, mean arterial pressure.
TABLE 3.
Variance inflation factor for assessing the existence of multicollinearity among the variables used in Table 2
| Variables | VIF |
| Age | 1.14 |
| Female | 1.21 |
| BMI | 1.19 |
| MAP | 1.20 |
| FPG | 1.28 |
| LDL-C | 1.07 |
| HDL-C | 1.18 |
| ABCA1-CEC | 1.03 |
ABCA1-CEC, ABCA1-dependent cholesterol efflux capacity; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MAP, mean arterial pressure.
DISCUSSION
The main findings of this work are that serum ABCA1-mediated CEC inversely correlates with PWV independently of total HDL-C serum concentration and is a strong independent predictor of arterial stiffness in a healthy population not receiving pharmacological therapy. These findings point to the relevance of HDL function in the maintenance of vessel health.
Our new observation that ABCA1-dependent serum CEC is the first strong parameter that follows those well known to be predictive of PWV (age, blood pressure, and BMI) (38, 39) is consistent with the idea that functionally conserved HDL, influencing cholesterol trafficking in the arterial wall, may contribute to limit vessel stiffness. A very recent work showed that arterial stiffness, evaluated as PWV, correlates with circulating oxidized LDL in healthy subjects, pointing to the importance of lipid metabolism on this parameter (40). Our findings support the emerging concept that HDL-mediated atheroprotection is dependent on its function rather than quantity and, most importantly, is relevant in clinically healthy subjects. Indeed, only ABCA1-dependent serum CEC, an index of HDL functionality (8), and not aqueous diffusion-dependent CEC, which reflects a bidirectional nonspecific efflux with variable impact on cellular cholesterol content (31), is a good indicator of arterial compliance in our study. The measurement of ABCA1-mediated CEC is a direct index of HDL functionality strictly dependent on serum preβ HDL content, as widely demonstrated (8, 34, 35, 41), so it renders redundant HDL composition analyses such as HDL preβ content measurement in the present study. The relationship between HDL composition/function and arterial stiffness seems to be underlined also by the recent observation that reduction of paraoxonase-1, an atheroprotective HDL antioxidant component, predicts PWV in renal transplant recipients (42). The demonstration that PWV in adulthood is influenced by atherogenic lifestyle factors in childhood (43, 44) confirms the existence of a large number of determinants, other than blood pressure and age, able to modify arterial wall over time in asymptomatic subjects. The idea that HDL promotion of cholesterol efflux may be an important determinant of vessel health is supported by increasing evidence that cholesterol efflux mediated by ABCA1 and other ABC transporters, particularly ABCG1, are associated with intracellular signaling resulting, for example, in endothelial eNOS activation, PGI-2 secretion, and inhibition of various proinflammatory molecules (13).
The absence of a significant correlation between PWV and HDL cholesterol levels in our population supports the idea that HDL serum concentration does not always reflect HDL function. The discrepancy between our data and previous reports (45–47) could be related to the type of population analyzed. Our observation adds to the recently reported correlation between serum CEC and IMT as a marker of early atherosclerosis (4). However, the data presented in our work represent new information because IMT and PWV are considered different entities of vascular damage, with IMT believed to reflect more advanced structural atherosclerotic changes in the arterial wall compared with PWV (48). Moreover, PWV has been recently demonstrated to inversely correlate with flow-mediated dilation, a sensitive parameter for endothelial function (24). Our results support the idea that the predictive power of ABCA1-mediated CEC with respect to arterial stiffness may be an adjunctive tool to understand and detect early functional and structural vascular derangement.
One of the strengths of our study is the accurate selection of pharmacologically untreated subjects with a known clinical history. Moreover, the role of other confounding factors was minimized through the careful exclusion of variables that could interfere with PWV, such as smoking habit and diabetes, or controlled through a specific statistical analysis. The main limitation of this study is the relatively low number of subjects enrolled. This was mainly due to the strict definition of “healthy subject” that we applied and to the search for a representative sample of a general population cohort.
In conclusion, we have shown for the first time that ABCA1-dependent serum CEC is inversely related to PWV in pharmacologically untreated healthy subjects, independently of total HDL-C serum levels, and is a powerful predictor of arterial stiffness. This finding points to the relevance of HDL function in vascular physiology and arterial stiffness prevention.
Supplementary Material
Footnotes
Abbreviations:
- BHS
- Brisighella Heart Study
- CEC
- cholesterol efflux capacity
- CVD
- cardiovascular disease
- IMT
- intima-media thickness
- PGI-2
- prostaglandin I-2
- PWV
- pulse wave velocity
- VIF
- variance inflation factor
This work was supported by a grant from Istituto Nazionale per le Ricerche Cardiovascolari (INRC), Bologna, Italy; by a grant from the Health Service of Emilia-Romagna Region (ADSL/DiAL-ER project), Parma, Italy; and by a local funding grant from the University of Bologna, Bologna, Italy.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables and two figures.
REFERENCES
- 1.Duffy D., Rader D. J. 2009. Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6: 455–463 [DOI] [PubMed] [Google Scholar]
- 2.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756 [DOI] [PubMed] [Google Scholar]
- 3.Briel M., Ferreira-Gonzalez I., You J. J., Karanicolas P. J., Akl E. A., Wu P., Blechacz B., Bassler D., Wei X., Sharman A., et al. 2009. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 338: b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuchel M., Rader D. J. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 113: 2548–2555 [DOI] [PubMed] [Google Scholar]
- 6.Calabresi L., Baldassarre D., Castelnuovo S., Conca P., Bocchi L., Candini C., Frigerio B., Amato M., Sirtori C. R., Alessandrini P., et al. 2009. Functional lecithin: cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 120: 628–635 [DOI] [PubMed] [Google Scholar]
- 7.Hovingh G. K., Hutten B. A., Holleboom A. G., Petersen W., Rol P., Stalenhoef A., Zwinderman A. H., de Groot E., Kastelein J. J., Kuivenhoven J. A. 2005. Compromised LCAT function is associated with increased atherosclerosis. Circulation. 112: 879–884 [DOI] [PubMed] [Google Scholar]
- 8.Calabresi L., Favari E., Moleri E., Adorni M. P., Pedrelli M., Costa S., Jessup W., Gelissen I. C., Kovanen P. T., Bernini F., et al. 2009. Functional LCAT is not required for macrophage cholesterol efflux to human serum. Atherosclerosis. 204: 141–146 [DOI] [PubMed] [Google Scholar]
- 9.Favari E., Gomaraschi M., Zanotti I., Bernini F., Lee-Rueckert M., Kovanen P. T., Sirtori C. R., Franceschini G., Calabresi L. 2007. A unique protease-sensitive high density lipoprotein particle containing the apolipoprotein A-I(Milano) dimer effectively promotes ATP-binding Cassette A1-mediated cell cholesterol efflux. J. Biol. Chem. 282: 5125–5132 [DOI] [PubMed] [Google Scholar]
- 10.Rye K. A., Barter P. J. 2004. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24: 421–428 [DOI] [PubMed] [Google Scholar]
- 11.Rader D. J. 2006. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 116: 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favari E., Calabresi L., Adorni M. P., Jessup W., Simonelli S., Franceschini G., Bernini F. 2009. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 48: 11067–11074 [DOI] [PubMed] [Google Scholar]
- 13.Prosser H. C., Ng M. K., Bursill C. A. 2012. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr. Opin. Lipidol. 23: 182–189 [DOI] [PubMed] [Google Scholar]
- 14.Liao H., Langmann T., Schmitz G., Zhu Y. 2002. Native LDL upregulation of ATP-binding cassette transporter-1 in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 22: 127–132 [DOI] [PubMed] [Google Scholar]
- 15.Liu D., Ji L., Tong X., Pan B., Han J. Y., Huang Y., Chen Y. E., Pennathur S., Zhang Y., Zheng L. 2011. Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. Am. J. Physiol. Cell Physiol. 301: C739–C748 [DOI] [PubMed] [Google Scholar]
- 16.Laurent S., Boutouyrie P., Asmar R., Gautier I., Laloux B., Guize L., Ducimetiere P., Benetos A. 2001. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 37: 1236–1241 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell G. F., Hwang S. J., Vasan R. S., Larson M. G., Pencina M. J., Hamburg N. M., Vita J. A., Levy D., Benjamin E. J. 2010. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 121: 505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlachopoulos C., Aznaouridis K., Stefanadis C. 2010. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 55: 1318–1327 [DOI] [PubMed] [Google Scholar]
- 19.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A. M., Kjeldsen S. E., Laurent S., et al. 2007. 2007 guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 25: 1105–1187 [DOI] [PubMed] [Google Scholar]
- 20.Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H. 2006. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 27: 2588–2605 [DOI] [PubMed] [Google Scholar]
- 21.Safar M. E., Levy B. I., Struijker-Boudier H. 2003. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 107: 2864–2869 [DOI] [PubMed] [Google Scholar]
- 22.Weber T., Auer J., O'Rourke M. F., Kvas E., Lassnig E., Berent R., Eber B. 2004. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 109: 184–189 [DOI] [PubMed] [Google Scholar]
- 23.Yamashina A., Tomiyama H., Takeda K., Tsuda H., Arai T., Hirose K., Koji Y., Hori S., Yamamoto Y. 2002. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 25: 359–364 [DOI] [PubMed] [Google Scholar]
- 24.Figueiredo V. N., Yugar-Toledo J. C., Martins L. C., Martins L. B., de Faria A. P., de Haro Moraes C., Sierra C., Coca A., Moreno H. 2012. Vascular stiffness and endothelial dysfunction: Correlations at different levels of blood pressure. Blood Press. 21: 31–38 [DOI] [PubMed] [Google Scholar]
- 25.Descovich G. C.1990. The Brisighella Heart Study: an interim report. Eur. Heart J. 11(Suppl H): 32–37.
- 26.Cicero A. F. G., D'Addato S., Borghi C., on behalf of the Brisighella Heart Study Staff 2011. From risk factor assessment to cardiovascular disease risk and mortality modification: the first 40 years of the Brisighella Heart Study. Clin. Lipidol. 6: 11 [Google Scholar]
- 27.Borghi C., Dormi A., D'Addato S., Gaddi A., Ambrosioni E. 2004. Trends in blood pressure control and antihypertensive treatment in clinical practice: the Brisighella Heart Study. J. Hypertens. 22: 1707–1716 [DOI] [PubMed] [Google Scholar]
- 28.Cicero A. F., Dormi A., Nascetti S., Panourgia M. P., Grandi E., D'Addato S., Gaddi A. 2005. Relative role of major risk factors for Type 2 diabetes development in the historical cohort of the Brisighella Heart Study: an 8-year follow-up. Diabet. Med. 22: 1263–1266 [DOI] [PubMed] [Google Scholar]
- 29.Pisciotta L., Favari E., Magnolo L., Simonelli S., Adorni M. P., Sallo R., Fancello T., Zavaroni I., Ardigo D., Bernini F., et al. 2012. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ. Cardiovasc. Genet. 5: 42–50 [DOI] [PubMed] [Google Scholar]
- 30.Favari E., Zanotti I., Zimetti F., Ronda N., Bernini F., Rothblat G. H. 2004. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 24: 2345–2350 [DOI] [PubMed] [Google Scholar]
- 31.Yancey P. G., Bortnick A. E., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Rothblat G. H. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 712–719 [DOI] [PubMed] [Google Scholar]
- 32.Adorni M. P., Zimetti F., Puntoni M., Bigazzi F., Sbrana F., Minichilli F., Bernini F., Ronda N., Favari E., Sampietro T. 2012. Cellular cholesterol efflux and cholesterol loading capacity of serum: effects of LDL-apheresis. J. Lipid Res. 53: 984–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanotti I., Favari E., Bernini F. 2012. Cellular cholesterol efflux pathways: impact on intracellular lipid trafficking and methodological considerations. Curr. Pharm. Biotechnol. 13: 292–302 [DOI] [PubMed] [Google Scholar]
- 34.Favari E., Lee M., Calabresi L., Franceschini G., Zimetti F., Bernini F., Kovanen P. T. 2004. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J. Biol. Chem. 279: 9930–9936 [DOI] [PubMed] [Google Scholar]
- 35.de la Llera-Moya M., Drazul-Schrader D., Asztalos B. F., Cuchel M., Rader D. J., Rothblat G. H. 2010. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvi P., Lio G., Labat C., Ricci E., Pannier B., Benetos A. 2004. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J. Hypertens. 22: 2285–2293 [DOI] [PubMed] [Google Scholar]
- 37.Kis E., Cseprekal O., Kerti A., Salvi P., Benetos A., Tisler A., Szabo A., Tulassay T., Reusz G. S. 2011. Measurement of pulse wave velocity in children and young adults: a comparative study using three different devices. Hypertens. Res. 34: 1197–1202 [DOI] [PubMed] [Google Scholar]
- 38.Nichols W. W. 2005. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am. J. Hypertens. 18: 3S–10S [DOI] [PubMed] [Google Scholar]
- 39.Steppan J., Barodka V., Berkowitz D. E., Nyhan D. 2011. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol. Res. Pract. 2011: 263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zagura M., Kals J., Serg M., Kampus P., Zilmer M., Jakobson M., Unt E., Lieberg J., Eha J. 2012. Structural and biochemical characteristics of arterial stiffness in patients with atherosclerosis and in healthy subjects. Hypertens. Res. 35: 1032–1037 [DOI] [PubMed] [Google Scholar]
- 41.de la Llera-Moya M., Drazul-Schrader D., Asztalos B. F., Cuchel M., Rader D. J., Rothblat G. H. 2010. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 30: 796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gungor O., Kircelli F., Demirci M. S., Tuncel P., Sisman A. R., Tatar E., Hur E., Asci G., Ok E., Toz H. 2011. Serum paraoxonase 1 activity predicts arterial stiffness in renal transplant recipients. J. Atheroscler. Thromb. 18: 901–905 [DOI] [PubMed] [Google Scholar]
- 43.Aatola H., Hutri-Kahonen N., Juonala M., Viikari J. S., Hulkkonen J., Laitinen T., Taittonen L., Lehtimaki T., Raitakari O. T., Kahonen M. 2010. Lifetime risk factors and arterial pulse wave velocity in adulthood: the cardiovascular risk in young Finns study. Hypertension. 55: 806–811 [DOI] [PubMed] [Google Scholar]
- 44.Aatola H., Koivistoinen T., Hutri-Kahonen N., Juonala M., Mikkila V., Lehtimaki T., Viikari J. S., Raitakari O. T., Kahonen M. 2010. Lifetime fruit and vegetable consumption and arterial pulse wave velocity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 122: 2521–2528 [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Meng S. Y., Meng C. C., Yu W. G., Wang R. T. 2012. Decreased serum bilirubin is associated with arterial stiffness in men. Nutr. Metab. Cardiovasc. Dis. In press [DOI] [PubMed] [Google Scholar]
- 46.Matsumae T., Ueda K., Abe Y., Nishimura S., Murakami G., Saito T. 2010. What factors accelerate aortic stiffening in hemodialysis patients? An observational study. Hypertens. Res. 33: 243–249 [DOI] [PubMed] [Google Scholar]
- 47.Wang R. T., Li Y., Zhu X. Y., Zhang Y. N. 2011. Increased mean platelet volume is associated with arterial stiffness. Platelets. 22: 447–451 [DOI] [PubMed] [Google Scholar]
- 48.Koivistoinen T., Virtanen M., Hutri-Kahonen N., Lehtimaki T., Jula A., Juonala M., Moilanen L., Aatola H., Hyttinen J., Viikari J. S., Raitakari O. T., Kahonen M. 2012 doi: 10.1016/j.atherosclerosis.2011.08.007. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: The Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis 220: 387–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.