Abstract
Smith-Lemli-Opitz syndrome (SLOS) is caused by mutations in the gene encoding 3β-hydroxysterol-Δ7-reductase and as a result of this defect, 7-dehydrocholesterol (7-DHC) and 8-dehydrocholesterol (8-DHC) accumulate in the fluids and tissues of patients with this syndrome. Both 7- and 8-DHC are susceptible to peroxidation reactions, and several biologically active DHC oxysterols are found in cell and animal models of SLOS. Ex vivo oxidation of DHCs can be a confounding factor in the analysis of these sterols and their esters, and we developed HPLC/MS methods that permit the direct analysis of cholesterol, 7-DHC, 8-DHC, and their esters in human plasma, thus avoiding ex vivo oxidation. In addition, three oxysterols were classified as endogenously formed products by the use of an isotopically-labeled 7-DHC (d7-7-DHC) added to the sample before workup, followed by MS analysis of products formed. Analysis of 17 SLOS plasma samples shows that 8-DHC linoleate correlates better with the SLOS severity score of the patients than other sterols or metabolites, including cholesterol and 7-DHC. Levels of 7-ketocholesterol also correlate with the SLOS severity score. 8-DHC esters should have utility as surrogate markers of severity in SLOS for prognostication and as endpoints in clinical trials.
Keywords: 7-dehydrocholesterol, 8-dehydrocholesterol, sterol ester, acyl-CoA:cholesterol acyltransferase, lecithin:cholesterol acyl transferase, oxysterol, free radical oxidation, lipid peroxidation
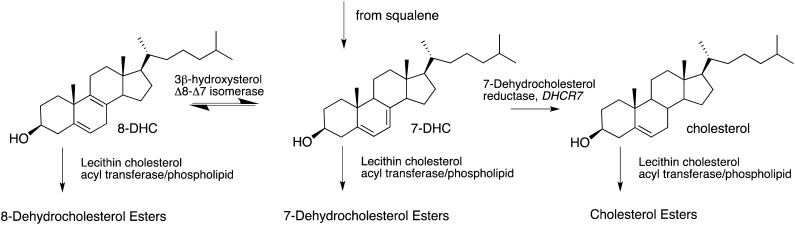
Mutations in the gene encoding 3β-hydroxysterol-Δ7-reductase (DHCR7; EC 1.3.1.21) give rise to a neurodevelopmental syndrome first characterized by Smith, Lemli, and Opitz. (Smith-Lemli-Opitz syndrome; SLOS, OMIM 270400) (1–3). DHCR7 catalyzes the reduction of 7-dehydrocholesterol (7-DHC) to cholesterol in the last step of cholesterol biosynthesis from squalene. Elevated concentrations of 7-DHC and lower-than-normal levels of cholesterol are typically observed in the plasma of patients with SLOS as a result of the reduced activity of DHCR7 (3, 4). Equilibration of 7-DHC and 8-dehydrocholesterol (8-DHC), as shown in Fig. 1, is promoted by 3β-hydroxysterol Δ8–Δ7 isomerase (D8D7I, EBP) (5), and, as a result, not only are levels of 7-DHC high, but levels of 8-DHC are also elevated in the plasma of SLOS patients. The effect of mutations in DHCR7 not only has consequences in the free sterol distributions of cholesterol, 7-DHC, and 8-DHC, but probably also affects those of the corresponding sterol esters in tissues and fluids of SLOS patients, which presumably reflect the free sterol levels in some way (see Fig. 1) (6, 7).
Fig. 1.
Biological transformations between 8-DHC, 7-DHC, cholesterol, and their fatty esters.
7-DHC is the most oxidizable lipid known (8, 9). 8-DHC is less reactive than 7-DHC, but is still approximately 90 times more oxidizable than cholesterol (10). Furthermore, oxysterol products derived from the DHCs have been shown to reduce cell viability by a combination of reduced proliferation and induced differentiation of Neuro2a cells (11). Overlapping gene expression changes in Dhcr7-deficient and 7-DHC oxysterol-treated cells indicate that the pathophysiological findings in the syndrome might be due to accumulated 7-DHC oxysterols. The propensity of the DHCs to undergo free radical chain oxidation suggests that analytical methods that minimize exposure to potential initiators of oxidation should be developed in order to minimize ex vivo oxidation and formation of oxysterols from the DHCs. Although formation of oxysterols in plasma of SLOS patients has previously been reported (12), recent studies in our laboratories suggest that these products are probably formed from ex vivo oxidation of 7-DHC (13).
Any plan to analyze the plasma of SLOS patients poses a challenge because sterol esters are important plasma constituents and standard methods of plasma lipid protocols usually involve harsh ester hydrolysis methods that probably degrade the sensitive dehydrocholesterols. Nevertheless, analysis of plasma sterol esters seems warranted in SLOS because novel compounds originating from 7- and 8-DHC esters could play a role in the pathogenesis of the disorder. Of note in this regard is a recent study that suggests that plasma sterol esterification by LCAT and the corresponding cellular ACAT is highly dependent on sterol structure, with the dehydrocholesterols esterified to different extents than cholesterol (7).
We report here analyses of plasma sterols, sterol esters, and oxysterols from 17 patients diagnosed with SLOS having severity scores that ranged from 9 to 41 (14–16). Our protocol includes a new method for collecting plasma that results in reduced ex vivo oxidation, and we utilize a reverse-phase HPLC/MS/MS to provide quantitation of individual esters of cholesterol, 7-DHC, and 8-DHC. We also report the identification of three novel endogenously formed oxysterols in SLOS plasma that are not detected at comparable levels in control patient plasma.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Co. HPLC-grade solvents (acetonitrile, dichloromethane, hexanes, and 2-propanol) were purchased from Thermo Fisher Scientific, Inc. Syntheses of [25,26,26,26,27,27,27 -d7]7-DHC, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, 7-ketocholesterol, and d7-7-ketocholesterol have been described elsewhere (13, 17). NH2-SPE cartridges (55 μm, 70Å, 500 mg/3 ml) were purchased from Phenomenex, Inc. 8-DHC was obtained by chemical synthesis as previously described (18). The synthesis of the 7- and 8-DHC esters was by standard methods and is described in the supplementary material.
Collection of human plasma
Blood taken in EDTA-coated tubes was wrapped with aluminum foil to minimize exposure to room light and was processed in a timely manner after the addition of 50 μl/ml plasma of a standard solution of butylated hydroxytoluene (BHT) and triphenylphosphine (TPP) made from 10 mg BHT and 25 mg TPP in 10 ml of ethanol. TPP reduces peroxides, and it is more effective in reducing hydrophobic peroxides, such as lipid-derived species, than are other reducing agents such as sodium borohydride (19). As little as 200 μl of plasma can be analyzed with success following this protocol. All plasma samples analyzed in this study were stored at −80°C and shipped on dry ice.
Total sterol analysis by GC
Human plasma (0.2 ml) was added to 3 ml of an ice-cold mixture of chloroform-methanol (2:1, v/v) containing 0.25 mg/ml TPP and 0.005% BHT. 5β-Cholestan-3α-ol was added as an internal standard. After addition of 2 ml of 0.9% NaCl, the sample was vigorously vortexed and then centrifuged. The bottom organic layer was dried under a stream of nitrogen and subjected to hydrolysis at high temperature (70°C) for 1 h with 4% KOH in ethanol. The mixture was cooled on ice and then extracted with hexane (2 ml × 2). Extracts were dried under nitrogen and converted to trimethylsilyl ether derivatives that were analyzed by GC.
GC was performed using a Hewlett-Packard HP6890 GC instrument equipped with an SPB-5 column (30 m, 0.32 mm i.d., 250 μm film thickness; Supelco, Sigma-Aldrich) and an flame ionization detector. The injector was set at 220°C, and the following gradient was used: 220°C for 5 min, followed by 15°C/min up to 275°C, 1°C/min up to 280°C, 5°C/min up to 290°C, and 290°C for 10 min (20).
Free sterols and sterol ester analysis by HPLC-MS/MS or GC
All samples were processed under dim red light. Human plasma (0.2 ml) prepared as described above was added to a 3 ml ice-cold mixture of chloroform-methanol (2:1, v/v) containing 0.25 mg/ml TPP and 0.005% BHT. d7-7-DHC, d7-7-DHC-LA, d7-Chol, and d7-Chol-LA were added as internal standards. The sample was placed at room temperature under nitrogen with occasional shaking for 30 min. After addition of 2 ml of 0.9% NaCl, the sample was vigorously vortexed and centrifuged. The organic layer was separated and dried under a stream of nitrogen. The residue was reconstituted in 0.2 ml acetonitrile-CH2Cl2 (1:1, v/v) for LC-MS/MS analysis.
LC-MS/MS was conducted using a Thermofinnigan TSQ Quantum Ultra equipped with a Finnigan Surveyor Autosampler Plus. Reversed-phase HPLC was performed on a Phenomenex Luna C18 (2) column (150 × 2 mm, 3 µM), preequilibrated in ACN (solvent A)/CH2Cl2 (solvent B) (87.5/12.5). The samples were injected and eluted (0.2 ml/min) with an increasing concentration of solvent B [time (min)/per percentage of solvent B: 4/12.5, 7/47.5, 28/50.0, 32/12.5, 35/12.5] (21). The MS was operated in the positive-ion mode using atmospheric pressure chemical ionization (APCI) in the selective reaction monitoring (SRM) mode. MS parameters were optimized for d7-7-DHC and d7-7-DHC-LA and were as follows: auxiliary gas pressure was set at 5 psi, sheath gas pressure was 30 psi, utilizing nitrogen for both. Discharge current was set at 20 eV, and the vaporizer temperature was set at 230°C. Collision-induced dissociation was optimized at 16 eV under 1.0 mTorr of argon. Data acquisition and analysis were performed using Xcalibur software, version 2.0 (San Jose, CA).
For GC analysis of free sterol, the sample was processed in a way similar to the above procedure for sterol ester analysis, but using 5β-cholestan-3α-ol as the internal standard. GC analysis was the same as described above (20).
Analysis of oxysterols by HPLC-MS/MS
An appropriate amount of d7-7-ketocholesterol standard was added to each sample before sample processing. Lipid extraction from plasma (200 μl) was carried out in a procedure similar to that described above. Thus-obtained organic layer from plasma extraction was blown dry with nitrogen and was reconstituted in methanol (0.5 ml) and 1M KOH in water (0.5 ml), and the resulting mixture was incubated at 37°C for 30 min. The hydrolyzed mixture was directly extracted with hexanes (3 ml × 3), and the combined organic layers were dried under nitrogen, reconstituted in methylene chloride (200 μl), and stored at −80°C until analysis. Oxysterols in all samples were analyzed by normal-phase HPLC-APCI-MS/MS following the previously reported method with a slightly modified HPLC condition (13, 17). HPLC conditions: silica 150 × 4.6 mm column (Phenomenex, Inc.), 3 μm; 1.0 ml/min; elution solvent: 10% 2-propanol in hexanes. For MS analysis, SRM was employed to monitor the dehydration process of the ion [M+H]+ or [M+H-H2O]+ in the mass spectrometry (13, 17).
Test of ex vivo oxidation of 7-DHC in plasma of SLOS patients
An appropriate amount of d7-7-DHC (to reach a level that is close to the endogenous level of d0-7-DHC) was added to each sample, and the sample was processed and analyzed as described above. Both d0- and d7-oxysterols were monitored in order to examine the ex vivo oxidation of 7-DHC.
SLOS patients
SLOS was confirmed biochemically and by molecular analysis of the DHCR7 gene. Because SLOS shows a wide clinical variation, the clinical degree of severity was determined by a scoring system that takes account of the anatomical abnormalities, first suggested by Bialer et al. (22) and then modified by Kelley and Hennekam (4). The severity scores in our patients ranged from 9 to 41. Most of the patients were taking dietary cholesterol supplements; some were receiving simvastatin treatment. The age range of patients was 1.4 to 28.3 years; nine males and eight females were included in the study. Sample collection for this study was approved by the ethics committee of the medical faculty, University of Heidelberg, Germany (No. S-071/2012) and by the Institutional Review Board of Oregon Health and Science University (No. 6790).
RESULTS
HPLC/MS analyses of plasma
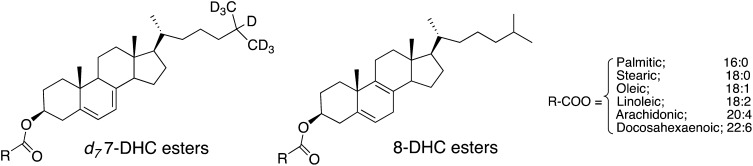
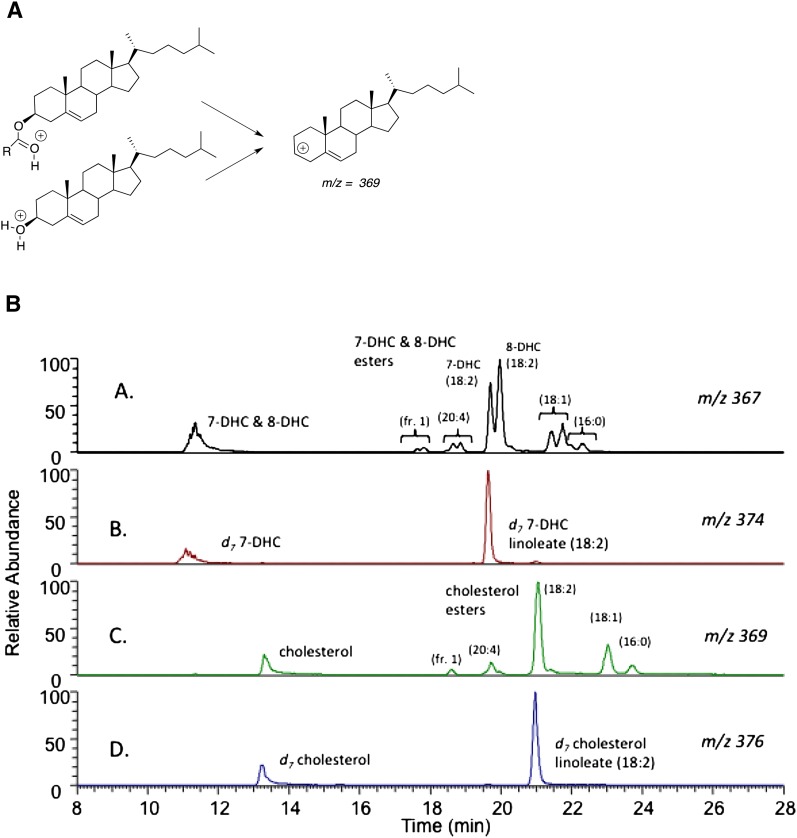
Several compounds were synthesized for use as standards for sterols and sterol esters found in plasma (Fig. 2). Methods for the separation and analysis of individual molecular species of the esters of cholesterol, 7-DHC, and 8-DHC could be achieved in one chromatographic run using reverse-phase chromatography coupled with a mass spectrometer operating in the APCI mode. Cholesterol and its esters undergo fragmentation to give the same carbocation with m/z = 369 daltons, formed by loss of either water or the fatty ester constituent. 7-DHC and its esters undergo fragmentations to give a carbocation m/z = 367 daltons, as does 8-DHC and its esters. SRM of the parent molecular ion undergoing fragmentation to the common carbocation, m/z = 369 daltons for cholesterol and its esters and m/z = 367 daltons for 7-DHC and 8-DHC and their esters, can be used to provide partial evidence for the sterol plasma lipid constituents in the HPLC/MS/MS chromatograms. Independent syntheses of the 7-DHC and 8-DHC esters provide the final link to the identification of the chromatographic fractions. Figure 3B shows a typical chromatogram from a SLOS patient. In panel A are shown all lipids that give rise to an m/z = 367 dalton fragment. These lipids include 7-DHC, 8- DHC, and all of the common esters of these sterols. Deuterated (d7) analogs of 7-DHC and 7-DHC linoleate show peaks in panel B of Fig. 3B, both compounds giving fragment ions having m/z = 374 daltons. The corresponding cholesteryl esters in SLOS plasma give a chromatogram as shown in panel C of Fig. 3B, and d7-cholesterol and its linoleate ester give the chromatogram presented in panel D of the figure. The major cholesteryl esters present in plasma, including linoleate (18:2), oleate (18:1), palmitate (16:0), and arachidonate (20:4), all separate well under the chromatography conditions used. The peak labeled as fraction 1 in panels A and C of Fig. 3B contains both the 22:6 and 20:5 esters because these compounds do not separate under our best conditions.
Fig. 2.
Structures of d7-7-DHC linoleate and d0-8-DHC fatty esters that are under study.
Fig. 3.
Free sterol and sterol ester analysis by LC-MS/MS. A: Fragmentation pattern of protonated free sterols or sterol esters in MS analysis; B: Typical chromatogram of HPLC-MS/MS analysis of sterol esters in human plasma. Human plasma was added to chloroform:methanol containing triphenylphosphine and BHT along with deuterated standards,. d7-7DHC, d7-7DHC-LA, d7-Chol, and d7-Chol-LA. LC-MS/MS was conducted using a Thermofinnigan TSQ Quantum Ultra equipped with an HPLC. Chromatography was performed on a Phenomenex Luna C18 column (150 × 2 mm, 3 µM). The MS was operated in the positive ion mode using APCI in the SRM mode. A: Chromatogram showing products that fragment to give an ion with m/z = 367, the dehydrocholesterols and their esters. B: Chromatogram showing products that fragment to give an ion with m/z = 374, the d7-dehydrocholesterols and their d7-esters. C: Chromatogram showing products that fragment to give an ion with m/z = 369, cholesterol, and its esters. D: Chromatogram showing products that fragment to give an ion with m/z = 376, d7-cholesterols, and its d7-esters.
It is noteworthy that 7-DHC and 8-DHC esters are well separated under the chromatography conditions. Thus, panel A shows that 7-DHC linoleate elutes earlier than 8-DHC linoleate. This is a general pattern for the other esters as well; the 7-DHC compounds elute before the 8-DHC esters in every case. For the linoleates, oleates, palmitates, and arachidonates, this conclusion is based on the independent synthesis of either the 7-DHC or 8-DHC ester. The elution order for the 20:5 and 22:6 DHC esters is inferred by comparison.
Table 1 shows SLOS severity scores for 17 SLOS patients. We anticipated that any attempted correlations of sterol analytical data with the SLOS severity scores should take into account any ongoing interventions at the time plasma was donated; those interventions are noted in the table. All but one of the patients were taking dietary supplements of cholesterol, and four of them were receiving simvastatin.
TABLE 1.
SLOS patients: medication and severity score
| Patient | Treatment | Severity score | M/F | Age (y) |
| 1 | Cholesterol 100 mg/kg/d | 9 | M | 7.6 |
| 2 | Cholesterol 125 mg/kg/d | 27 | M | 6.3 |
| 3 | Cholesterol 139 mg/kg/d | 27 | F | 2 |
| 4 | Cholesterol 51 mg/kg/d | 18 | M | 283 |
| 5 | Cholesterol 150 mg/kg/d | 32 | M | 4.8 |
| 6 | Cholesterol 70 mg/kg/d | 23 | M | 236 |
| 7 | Cholesterol 128 mg/kg/d Simvastatin 0.85 mg/kg/d | 14 | M | 111 |
| 8 | Cholesterol 116 mg/kg/d | 14 | F | 1.4 |
| 9 | Cholesterol 128 mg/kg/d | 41 | F | 2.1 |
| 10 | Cholesterol 28 mg/kg/d Simvastatin 0.37 mg/kg/d | 9 | F | 276 |
| 11 | Cholesterol 46–80 mg/kg/d Simvastatin 0.48 mg/kg/d | 9 | M | 7.9 |
| 12 | No medication | 9 | F | 6.2 |
| 13 | Cholesterol 43 mg/kg/d Simvastatin 0.43 mg/kg/d | 23 | M | 158 |
| 14 | Cholesterol 42 mg/kg/d | 14 | F | 19 |
| 15 | Cholesterol 30 mg/kg/d | 10 | F | 174 |
| 16 | Cholesterol 30 mg/kg/d | 10 | F | 165 |
| 17 | Cholesterol 30 mg/kg/d | 20 | M | 5.6 |
When the chromatographic method described in Fig. 3B was used in conjunction with internal standards or deuterated compounds with standardization of responses, quantitative information about all of the sterol esters in our sample collection became possible. Total cholesterol levels reported here were determined by GC analysis of plasma subsequent to KOH hydrolysis, and the same method was used for analysis of total 8-DHC. We found that the analysis of total 7-DHC was more reliable if the amount of free 7-DHC determined by GC (without KOH hydrolysis) was added to the amount of 7-DHC esters determined by HPLC/MS with appropriate deuterated internal standards.
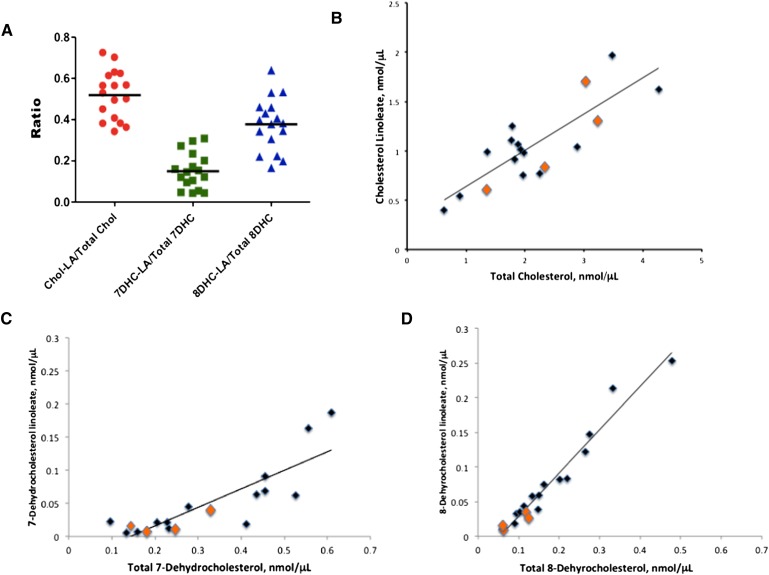
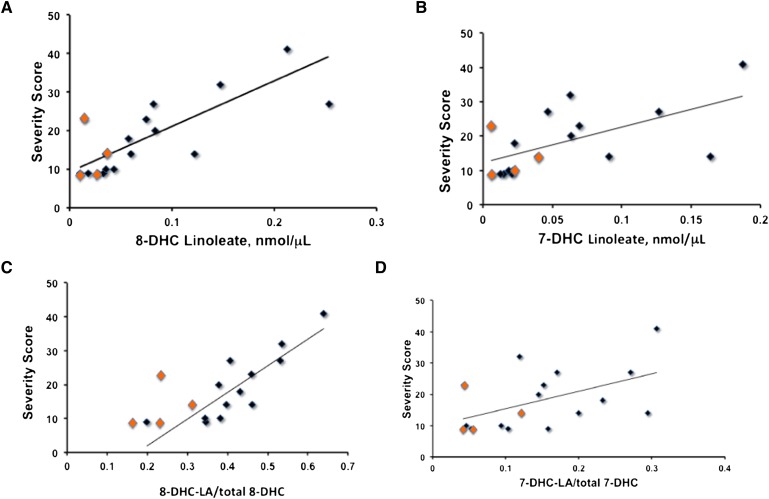
Figure 4A shows the ratio of sterol linoleate to total sterol in plasma for cholesterol, 7-DHC, and 8-DHC, an analysis similar to that given in the report by Lin et al. for the ratio of the sum of all sterol esters to total sterol (7). Figure 4B–D show more revealing correlations of the sterol ester-total sterol distribution in plasma. As expected, the amount of sterol linoleate present in plasma depends directly on the total sterol level. But the relationship between sterol ester and total sterol levels is highly dependent on sterol structure and the correlation is substantially better for 8-DHC (r2 =0.96) than either cholesterol (r2 =0.70) or 7-DHC (r2 =0.69).
Fig. 4.
Linoleate ester:free sterol ratio determined for SLOS plasmas by LC-MS-MS. A: Ratio for cholesterol linoleate:total cholesterol, 7-DHC-linoleate/total 7-DHC, and 8-DHC-linoleate/total 8-DHC in plasma of SLOS patients. B: Plot of cholesterol linoleate versus total cholesterol in plasma of SLOS patients. C: Plot of 7-dehydrocholesterol linoleate versus total 7-dehydrocholesterol in plasma of SLOS patients. D: Plot of 8-dehydrocholesterol linoleate versus total 8-dehydrocholesterol in plasma of SLOS patients. Samples shown in orange are from patients undergoing simvastatin treatment. Removal of these samples from the data does not significantly alter the correlations.
SLOS severity score correlations
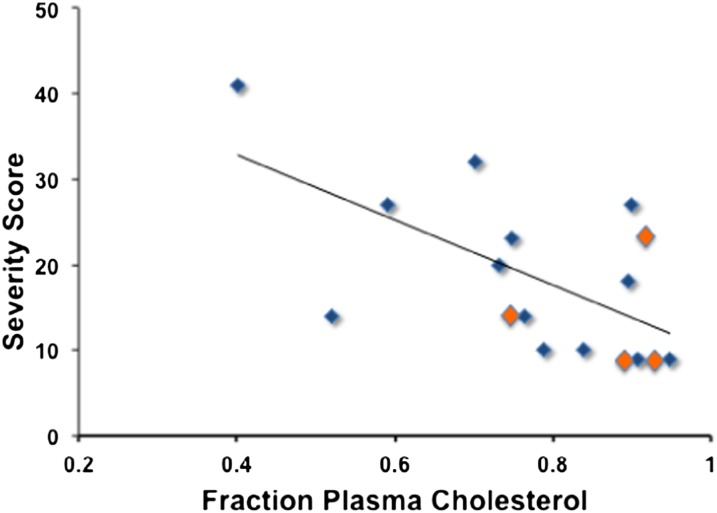
The SLOS severity score was found to correlate negatively with the fraction of plasma sterol found as cholesterol, i.e., correlation score = y = −38 × 48, r2 = 0.37 and P = 0.009 (Fig. 5). Removal of data from patients receiving simvastatin therapy in the DHC-severity score analysis did not change the correlation. Data using sterol esters as potential biomarkers for SLOS severity are presented in Fig. 6. Figure 6A shows that the correlation of 8-DHC linoleate and severity score is significantly better than correlations obtained with DHC or cholesterol fractions; severity score = 114 (8-DHC-LA) + 9.5 with r2 = 0.57 for all data, P = 0.0004 and r2 = 0.68 if patients receiving simvastatin are not included in the analysis. On the other hand, correlation of 7-DHC linoleate and severity score shown in Fig. 6B, while significant (P = 0.01), does not improve on the use of DHC fraction as a biomarker. Cholesterol linoleate and total cholesterol do not correlate with severity score, whereas free cholesterol does give a marginal correlation (P = 0.03). Analysis of various combinations of lipid molecular species versus severity score was carried out and those correlations having P < 0.05 are presented in the supplementary materials (see supplementary Table I). Of the various combinations attempted, the best correlation of severity score with plasma sterol lipid obtained was with 8-DHC linoleate/total 8-DHC, shown in Fig. 6C. This analysis gives severity score = 78 (8-DHC-LA/total 8-DHC) – 13.7 with r2 = 0.58 for all data, and r2 = 0.72 and P = 0.0001 if patients receiving simvastatin are not included in the analysis.
Fig. 5.
Plasma cholesterol fraction of total sterols versus SLOS severity score. Cholesterol and 8-DHC determined by GC after KOH hydrolysis. Free 7-DHC determined by GC before hydrolysis, 7-DHC esters determined by HPLC/MS, y = −38 × +48, r2 = 0.37. Samples shown in orange are from patients undergoing simvastatin treatment. Removal of these samples from the data does not significantly alter the correlations.
Fig. 6.
Plasma fraction dehydrocholesterol linoleate versus SLOS severity score. 8-DHC linoleate determined by HPLC/MS, total 8-DHC determined by GC after KOH hydrolysis. 7-DHC linoleate determined by HPLC/MS, total 7-DHC is sum of free 7-DHC determined by GC before hydrolysis and all 7-DHC esters determined by HPLC/MS. Samples shown in orange are from patients undergoing simvastatin treatment. A: 8-DHC-linoleate versus SLOS severity score, r2 = 0.57 for all data, P = 0.0004; y = 114 × +9.5, r2 = 0.68 if simvastatin data are not included. B: 7-DHC-linoleate versus SLOS severity score, y = 104 × +12 r2 = 0.36 removal of simvastatin data does not improve the correlation. C: 8-DHC-linoleate/total 8-DHC versus SLOS severity score, r2 = 0.58 for all data, y = 78 × −13.7 r2 = 0.72 if simvastatin data are not included. D: 7-DHC-linoleate/total 7-DHC versus SLOS severity score, y = 56 × +9.8, r2 = 0.26. Removal of simvastatin data does not alter the correlation.
Stability of sterols and sterol esters, ex vivo and endogenous oxysterol formation
If steps are not taken to stabilize plasmas from SLOS patients that contain appreciable amounts of 7-DHC or its esters, peroxidation products appear in the plasmas and appreciable loss of 7-DHC occurs (23–25). We carried out experiments to examine the stability of 7-DHC in human plasma. A known amount of 7-DHC was added to human plasma samples of normal individuals and the amount of 7-DHC was determined by GC under different storage conditions. We found that when the samples were stored at room temperature, the combination of BHT and TPP protected 7-DHC from oxidation for as long as 6 h while significant loss of 7-DHC was observed over the same time period when only BHT or no antioxidant was present in the samples. When the samples were stored at -80°C, 7-DHC appears to be stable for up to 4 weeks with or without the protection of BHT and TPP, but appreciable loss of 7-DHC was observed at 8 weeks when the antioxidants were not present. On the other hand, samples stored in the presence of BHT and TPP at -80°C are stable over the 8-week period (see supplementary Fig. I). We also found significant loss of 7-DHC when plasma samples of SLOS patients were saponified in 4% KOH in ethanol at 70°C. In some of the samples analyzed, the levels of 7-DHC measured by GC after saponification are similar to or even lower than the levels without saponification, suggesting significant decomposition of 7-DHC or its esters during the reaction at elevated temperatures.
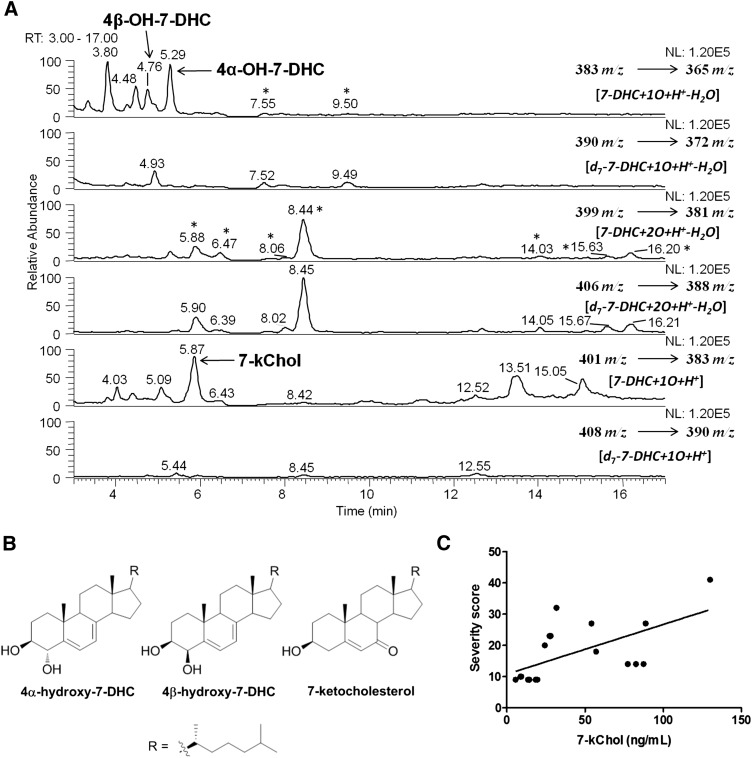
Analysis of 7-DHC-derived oxysterols in human plasma proved to be particularly challenging due to the occurrence of ex vivo oxidation of 7-DHC. As oxysterols have normally been observed at levels that are less than one thousandth of the levels of the corresponding sterol precursors, even a small extent of ex vivo oxidation could contribute significantly to the overall oxysterol pool. Thus, the d7-7-DHC synthetic compound we prepared was used to assess ex vivo oxidation during sample preparation. Even though a mild saponification condition (0.5 M KOH in 50% methanol at 37°C) was used (13, 19), oxysterol products derived from d7-7-DHC were observed when human samples were processed in the presence of d7-7-DHC (Fig. 7). As seen in the figure, if an oxysterol peak was observed at a comparable level in both the d0- and d7-MS panels, this particular oxysterol was assigned to formation from ex vivo oxidation of 7-DHC. On the other hand, if a peak was observed only or predominantly in the d0-MS panel, this peak (oxysterol or not) was designated as an endogenous product. Using a similar protocol, we previously reported that oxysterols were formed from 7-DHC ex vivo photo-oxidation in serum of AY9944-treated rats (a pharmacological model of SLOS) when the samples were collected and processed under light (13). Thus, elimination of light is also a necessary precaution when analyzing SLOS samples.
Fig. 7.
Oxysterols present in plasma of SLOS patients. A: A typical chromatogram of normal-phase HPLC-MS-MS analysis of an SLOS plasma sample that was processed in the presence of d7-7-DHC [HPLC conditions: silica 150 × 4.6 mm column (Phenomenex, Inc.); 3 μm; 1.0 ml/min; elution solvent: 10% 2-propanol in hexanes]. d7-7-DHC was added to the samples before workup. Chromatographic fractions that have d7:d0 ratios comparable to the d7:d0 ratio of the sterol precursor (7-DHC) present in the fluid or tissue are formed from ex vivo oxidation, whereas endogenous products have d7:d0 ratios close to zero. B: Structures of three endogenously formed oxysterols found in SLOS plasmas. C: 7-Ketocholesterol measured in SLOS plasmas versus SLOS severity scores.
By comparing the profiles of d0- and d7-oxysterols in Fig. 7A, we identified three oxysterols that are formed endogenously, namely 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC and 7-ketocholesterol (Fig. 7B), by comparing the retention time and MS characteristics with those of synthetic standards (13). These products are not formed from exogenous d7-7-DHC added to the plasma at the time it is taken and they are not products normally observed in the product mixture formed from free radical or photochemical oxidation of 7-DHC. Note that there are other unidentified endogenously formed peaks that could correspond to 7-DHC-derived oxysterols, but the identification of those peaks is less likely without appropriate standards. Of the three identified compounds, 7-ketocholesterol levels correlate with the patient's severity score (y = 0.168× + 11; r2 = 0.37; P = 0.008), as shown in Fig. 7C.
DISCUSSION
Plasma sterol esters
For the SLOS patients studied, over 50% of total cholesterol in plasma is present as the linoleate ester while nearly 40% of 8-DHC is in the form of the linoleate ester and less than 20% of 7-DHC is present as 7-DHC linoleate. Lin et al. (7) have reported that plasma cholesterol and the DHCs have different distributions between free and esterified forms, with the percentage of total sterol present in the esterified form decreasing from 83% for cholesterol to 68% for 8-DHC and 58% for 7-DHC. The data shown in Fig. 4A report on only the fraction of sterol that is the linoleate ester, the major plasma ester, and the figure shows that direct analysis of the sterol esters by HPLC/MS gives results that are consistent with the earlier report on total sterol ester (7). Including the18:1, 18:0, 16:0, 20:4, 20:5, and 22:6 esters in our analyses gives ester:total sterol ratios that are essentially the same as those reported by Lin et al. (7)
The data presented in Fig. 4B–D and Table 2 show that cholesterol, 7-DHC, and 8-DHC have a substantially different response to the plasma enzymes controlling the distribution of sterol and sterol ester.
TABLE 2.
Correlations of linoleate ester with total sterol
| [Chol-LA] = 0.37 [total Chol] + 0.27, r2 = 0.70 |
| [7-DHC-LA] = 0.28 [total 7-DHC] − 0.04, r2 = 0.69 |
| [8-DHC-LA] = 0.62 [total 8-DHC] − 0.03, r2 = 0.96 |
Although cholesterol levels in plasma track reasonably well with total cholesterol, and 7-DHC linoleate has a similar correlation with total 7-DHC, the data for 8-DHC linoleate versus total 8-DHC give a remarkably good correlation. The correlations also show that both 7- and 8-DHC exist entirely as the free sterol at concentrations below about 0.1 nmol/μl (∼4 mg/dl), suggesting a saturation limit for free DHCs that must be exceeded before incorporation into the ester pool occurs. No such saturation limit is apparent for cholesterol. The correlations also show that above this saturation limit, 8-DHC is more likely to be incorporated in the ester fraction than either cholesterol or 7-DHC. As described previously, supplementation with cholesterol, as well as additional treatment with simvastatin, significantly reduces the dehydrocholesterol fraction of total sterols (26). Although cholesterol supplementation results in an increase of cholesterol concentration, the effect of simvastatin on the dehydrocholesterol fraction is mainly due to a reduction of dehydrocholesterols. A similar trend can also be seen in Fig. 4B–D.
Both total cholesterol and cholesterol linoleate levels for patients receiving cholesterol supplementation and additional simvastatin treatment (orange data points) showed a wide variation (Fig. 4B), whereas their levels of total and linoleate esters of 7-DHC (Fig. 4C), and particularly 8-DHC (Fig. 4D), were much below the average levels of all of the samples assayed. However, inasmuch as simvastatin use was mainly in patients with mild biochemical abnormalities, this could also reflect a less-severe phenotype instead of reduced biosynthesis of dehydrocholesterol.
SLOS severity scoring versus plasma lipid molecular species
Since the severity scoring system for SLOS was first proposed, efforts were made to link the score, which is based on anatomical patient characteristics, with biomarkers related to the biochemical defect. To date, there has been only modest success in those efforts to link the severity score with a sterol lipid biomarker. SLOS severity scores have been found to correlate positively with the fraction of plasma sterol found as dehydrocholesterol [score = 45 (DHC fraction) +14, r2 = 0.40] (27), whereas a negative correlation of severity score with cholesterol fraction was reported by Yu et al. (r2 = 0.43) (15). Consistent with these literature reports, assays of our patient group showed that the only cholesterol data that provided any significant correlation with severity score is the cholesterol fraction of total sterol, see Fig. 5.
In our assay, 8-DHC linoleate plasma levels give by far the best correlations with the SLOS severity score of any species measured. Indeed, 8-DHC linoleate itself gives a correlation with SLOS severity score having r2 = 0.57 and P = 0.0004 (Fig. 6A), and if the severity score is plotted against the ratio of 8-DHC linoleate/total 8-DHC, the correlation improves to r2 = 0.72 and P = 0.0001 for those patients not receiving simvastatin therapy (Fig. 4C). This same correlation noticeably differentiates those samples of the patients receiving simvastatin, the orange data points in Fig. 6C, from those not undergoing this therapy. Data obtained from assay of the 7-DHC linoleate or total 7-DHC give correlations that are marginal or do not reach the level of significance (Fig. 6B, D).
Taken together, the data presented in Figs. 4 and 6 suggest that 8-DHC and its esters may be the preferred default sterol biomarkers for SLOS. Analysis of 7-DHC is complicated by its extreme reactivity toward peroxidation, whereas cholesterol dietary variability compromises its use as a biomarker. 8-DHC, which is linked to 7-DHC by the isomerase enzyme, is much less reactive toward oxygen free radicals than is the 7-isomer, and 8-DHC and its esters are essentially absent from dietary lipid sources. For these reasons, 8-DHC and its esters are apparently more reliable indicators of the degree of compromise of the DHCR7 enzyme than either 7-DHC or cholesterol. In addition, 8-DHC and its esters should have utility as surrogate markers of severity in SLOS for prognostication and related purposes, as endpoints in clinical trials in SLOS.
Ex vivo oxidation and sterol oxysterols in SLOS plasma
Analytical protocols for determining levels of cholesterol as well as 7- and 8-DHC usually involve analysis of plasma lipid extracts by GC or GC/MS. This analysis is normally followed by KOH hydrolysis of the lipids and GC or GC/MS to determine total sterol present in the blood, inasmuch as a high percentage of the sterols are found as esters in circulating lipoproteins such as LDL and HDL. 7-DHC and its esters are, however, extremely susceptible to free radical chain oxidation reactions, and treatment of plasma under the vigorous conditions required for ester hydrolysis (4% KOH at 70°C or 0.5 M KOH at 37°C), leads to extensive ex vivo oxidation and thus compromises an analysis. Conditions in which light and heme iron are present, as is the case in most plasma workups, are even more likely to promote reactions of the isomeric DHCs and their esters with oxygen.
If deuterated 7-DHC is added to a plasma sample when it is taken, the ratio of a deuterated (d7) to undeuterated (d0) oxysterol in the sample, when compared with the ratio of d7:d0 for 7-DHC measured in the same sample, provides information about the genesis of the oxysterol. Oxysterols formed by ex vivo oxidation have d7:d0 ratios that are similar to the d7:d0 ratio of the sterol precursor (7-DHC) present in the fluid or tissue, whereas endogenous products have d7:d0 ratios close to zero. Figure 7A illustrates such an analysis. The compound eluting at 8.45 min is observed in both the d0 panel (399 → 381 m/z) and the d7 panel (406 → 388 m/z) at comparable levels, indicating that this product was not formed endogenously. In contrast, 7-ketocholesterol gives a strong signal in the d0 panel (401 → 383 m/z), but is not detected in the d7 panel (408 → 390 m/z).
Indeed, three oxysterols in SLOS plasmas, 4α- and 4β-hydroxy-7DHC and 7-ketocholesterol, are not products of ex vivo oxidation, and these compounds are also not products that have been identified in the mixture formed from free radical chain oxidation of 7-DHC. The genesis of the compounds is probably via P-450 oxidation of 7-DHC in the liver. Recent studies have shown that 7-ketocholesterol is a product of CYP7A1 action on 7-DHC, and although the 4α- and 4β-hydroxy-7-DHC products have not been shown to be products of enzymatic oxidation of 7-DHC, CYP3A4 is known to give an analogous product from cholesterol, 4β-hydroxycholesterol (28). The oxidation of 7-DHC with CYP7A1 gives 7-ketocholesterol as a major product, with the 7α,8α-epoxide as only a minor product.
7-Ketocholesterol is cytotoxic and it has a variety of biological activities that include control of cholesterol homeostasis as well as induction of inflammation and vascular endothelial growth factor (29–31). Levels of 7-ketocholesterol are also elevated in patients with Niemann-Pick disease type C1 (32). 4α- and 4β-hydroxy-7DHC and 7-ketocholesterol, along with 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), have been identified in tissues and fluids of a rat model of SLOS (13, 33) and in brain and liver tissues of a Dhcr7-null mouse model (17, 34). 7-DHC-derived oxysterols have been associated with the retinal degeneration in the SLOS rat model and in SLOS patients (33). The fact that the levels of 7-ketocholesterol in plasma of SLOS patients have a positive correlation with the severity scores suggests that this oxysterol may play some important roles in the pathogenesis of SLOS.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Zeljka Korade and Dr. Steven Fliesler for helpful discussions.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- BHT
- butylated hydroxytoluene
- Chol
- cholesterol
- 7-DHC
- 7-dehydrocholesterol
- 8-DHC
- 8-dehydrocholesterol
- DHCR7
- 7-dehydrocholesterol reductase
- SLOS
- Smith-Lemli-Opitz syndrome
- SRM
- selective reaction monitoring
- TPP
- triphenylphosphine
This work was supported by National Institutes of Health Grants ES-013125, HD-064727, HD-061939, and HL-073980. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and one figure.
REFERENCES
- 1.Smith D. W., LemIi L., Opitz J. M. 1964. A newly recognized syndrome of multiple congenital anomalies. J. Pediatr. 64: 210–217 [DOI] [PubMed] [Google Scholar]
- 2.Irons M., Elias E. R., Salen G., Tint G. S., Batta A. K. 1993. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 341: 1414. [DOI] [PubMed] [Google Scholar]
- 3.Porter F. D., Herman G. E. 2011. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52: 6–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley R. I., Hennekam R. C. 2000. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 37: 321–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paik Y., Billheimer J., Magolda R., Gaylor L. 1986. Microsomal enzymes of cholesterol biosynthesis from lanosterol. Solubilization and purification of steroid 8-isomerase. J. Biol. Chem. 261: 6470–6477 [PubMed] [Google Scholar]
- 6.Schwartz C. C., VandenBroek J. M., Cooper P. S. 2004. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J. Lipid Res. 45: 1594–1607 [DOI] [PubMed] [Google Scholar]
- 7.Lin D. S., Steiner R. D., Merkens L. S., Pappu A. S., Connor W. E. 2010. The effects of sterol structure upon sterol esterification. Atherosclerosis. 208: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Davis T. A., Porter N. A. 2009. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131: 13037–13044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L., Korade Z., Porter N. A. 2010. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 132: 2222–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin H., Xu L., Porter N. A. 2011. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111: 5944–5972 [DOI] [PubMed] [Google Scholar]
- 11.Korade Z., Xu L., Porter N. A. 2010. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J. Lipid Res. 51: 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Fabiani E., Caruso D., Cavaleri M., Galli Kienle M., Galli G. 1996. Cholesta-5,7,9(11)-trien-3 beta-ol found in plasma of patients with Smith-Lemli-Opitz syndrome indicates formation of sterol hydroperoxide. J. Lipid Res. 37: 2280–2287 [PubMed] [Google Scholar]
- 13.Xu L., Liu W., Sheflin L. G., Fliesler S. J., Porter N. A. 2011. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52: 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tint G., Salen G., Batta A., Shefer S., Irons M., Elias E., Abuelo D., Johnson V., Lambert M., Lutz R., et al. 1995. Correlation of severity and outcome with plasma sterol levels in variants of the Smith-Lemli-Opitz syndrome. J. Pediatr. 127: 82–87 [DOI] [PubMed] [Google Scholar]
- 15.Yu H., Lee M. H., Starck L., Elias E. R., Irons M., Salen G., Patel S. B., Tint G. S. 2000. Spectrum of delta(7)-dehydrocholesterol reductase mutations in patients with the Smith-Lemli-Opitz (RSH) syndrome. Hum. Mol. Genet. 9: 1385–1391 [DOI] [PubMed] [Google Scholar]
- 16.Cunniff C., Kratz L. E., Moser A., Natowicz M. R., Kelley R. I. 1997. Clinical and biochemical spectrum of patients with RSH/Smith-Lemli-Opitz syndrome and abnormal cholesterol metabolism of clinical trials. Am. J. Med. Genet. A. 68: 263–269 [PubMed] [Google Scholar]
- 17.Xu L., Korade Z., Dale A. Rosado J., Liu W., Lamberson C. R., Porter N. A. 2011. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 52: 1222–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anastasia M., Fiecchi A., Galli G. 1981. Synthesis of cholesta-5,8-diene-3-beta-ol. J. Org. Chem. 46: 3421–3422 [Google Scholar]
- 19.Liu W., Yin H., Akazawa Y., Yoshida Y., Niki E., Porter N. 2010. Ex vivo oxidation in tissue and plasma assays of hydroxyoctadecadienoates: Z,E/E,E stereoisomer ratios. Chem. Res. Toxicol. 23: 986–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen T. Y., Inbaraj B. S., Chien J. T., Chen B. H. 2010. Gas chromatography-mass spectrometry determination of conjugated linoleic acids and cholesterol oxides and their stability in a model system. Anal. Biochem. 400: 130–138 [DOI] [PubMed] [Google Scholar]
- 21.Do T. Q., Moshkani S., Castillo P., Anunta S., Pogosyan A., Cheung A., Marbois B., Faull K. F., Ernst W., Chiang S. M., et al. 2008. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J. Immunol. 181: 4177–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bialer M., Penchaszadeh V., Kahn E., Libes R., Krigsman G., Lesser M. 1987. Female external genitalia and müllerian duct derivatives in a 46,XY infant with the Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 28: 723–731 [DOI] [PubMed] [Google Scholar]
- 23.Richards M. J., Nagel B. A., Fliesler S. J. 2006. Lipid hydroperoxide formation in the retina: correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp. Eye Res. 82: 538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaoua W., Chevy F., Roux C., Wolf C. 1999. Oxidized derivatives of 7-dehydrocholesterol induce growth retardation in cultured rat embryos: a model for antenatal growth retardation in the Smith-Lemli-Opitz syndrome. J. Lipid Res. 40: 456–463 [PubMed] [Google Scholar]
- 25.Gelzo M., Dello Russo A., Corso G. 2012. Stability study of dehydrocholesterols in dried spot of blood from patients with Smith-Lemli-Opitz syndrome, using filter-paper treated with butylated hydroxytoluene. Clin. Chim. Acta. 413: 525–526 [DOI] [PubMed] [Google Scholar]
- 26.Haas D., Garbade S. F., Vohwinkel C., Muschol N., Trefz F. K., Penzien J. M., Zschocke J., Hoffmann G. F., Burgard P. 2007. Effects of cholesterol and simvastatin treatment in patients with Smith-Lemli-Opitz syndrome (SLOS). J. Inherit. Metab. Dis. 30: 375–387 [DOI] [PubMed] [Google Scholar]
- 27.Witsch-Baumgartner M., Fitzky B. U., Ogorelkova M., Kraft H. G., Moebius F. F., Glossmann H., Seedorf U., Gillessen-Kaesbach G., Hoffmann G. F., Clayton P., et al. 2000. Mutational spectrum in the delta7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 66: 402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinkyo R., Xu L., Tallman K. A., Cheng Q., Porter N. A., Guengerich F. P. 2011. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 286: 33021–33028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vejux A., Lizard G. 2009. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Aspects Med. 30: 153–170 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez I. R., Larrayoz I. M. 2010. Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J. Lipid Res. 51: 2847–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown A. J., Jessup W. 2009. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Aspects Med. 30: 111–122 [DOI] [PubMed] [Google Scholar]
- 32.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L., Sheflin L. G., Porter N. A., Fliesler S. J. 2012. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta. 1821: 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korade Z., Xu L., Mirnics K., Porter N. A. 2012. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit Metab Dis: Epub ahead of print; Jun 21, 2012; doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.