Abstract
Cholesterol-metabolism-associated molecules, including scavenger receptor class A (SR-A), lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), CD36, ACAT1, ABCA1, ABCG1, and scavenger receptor class B type I, can modulate cholesterol metabolism in the transformation from macrophages to foam cells. Voltage-gated potassium channel Kv1.3 has increasingly been demonstrated to play an important role in the modulation of macrophage function. Here, we investigate the role of Kv1.3 in modulating cholesterol-metabolism-associated molecules in human acute monocytic leukemia cell-derived macrophages (THP-1 macrophages) and human monocyte-derived macrophages exposed to oxidized LDL (ox-LDL). Human Kv1.3 and Kv1.5 channels (hKv1.3 and hKv1.5) are expressed in macrophages and form a heteromultimeric channel. The hKv1.3-E314 antibody that we had generated as a specific hKv1.3 blocker inhibited outward delayed rectifier potassium currents, whereas the hKv1.5-E313 antibody that we had generated as a specific hKv1.5 blocker failed. Accordingly, the hKv1.3-E314 antibody reduced percentage of cholesterol ester and enhanced apoA-I-mediated cholesterol efflux in THP-1 macrophages and human monocyte-derived macrophages exposed to ox-LDL. The hKv1.3-E314 antibody downregulated SR-A, LOX-1, and ACAT1 expression and upregulated ABCA1 expression in THP-1 macrophages and human monocyte-derived macrophages. Our results reveal that specific Kv1.3 blockade represents a novel strategy modulating cholesterol metabolism in macrophages, which benefits the treatment of atherosclerotic lesions.
Keywords: macrophage, cholesterol, atherosclerosis, oxidized lipids
Atherosclerosis contributes chiefly to ischemic diseases, including coronary heart disease, cerebral infarction, and intermittent claudication. Lipid plaque is the main pathological presentation and is characterized by abundant foam cells, which are derived mostly from macrophages. In the transformation, cholesterol ester accumulation accelerates foam cell formation.
Macrophages possess an entrance-to-exit machinery for the modulation of cholesterol metabolism. Scavenger receptor class A (SR-A), CD36, and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) are largely responsible for cholesterol influx (1–8), and ABCA1, ABCG1 and scavenger receptor class B type I (SR-B I) facilitate cholesterol efflux (9–15). Free cholesterol inside macrophages is esterified by ACAT1, thereby promoting cholesterol ester accumulation (16–18). All the molecules form an integrated modulating system or network to maintain cellular cholesterol homeostasis in macrophages. It is clinic-promising to modulate the expression of these cholesterol-metabolism-associated molecules in macrophages.
In recent years, voltage-gated potassium channel Kv1.3 has increasingly been demonstrated to play a crucial role in controlling macrophage proliferation, activation, apoptosis (19–21), and inflammatory cytokine secretion (22–24). Notwithstanding preliminary evidence of selective Kv1.3 blockade by rMargatoxin, one selective Kv1.3 blocker, inhibited human monocytes derived macrophages differentiation into foam cells (25), it remains uncertain whether Kv1.3 blockage modulates the cholesterol-metabolism-associated molecules in macrophages. This unraveling would hold a potential target for atherosclerosis therapy.
To address the role of Kv1.3 in modulating the expression of SR-A, CD36, LOX-1, ACAT1, SR-B I, ABCG1, and ABCA1, we used the hKv1.3-E314 antibody as a novel and specific Kv1.3 blocker (26) that we generated to block Kv1.3 channels in THP-1 macrophages and human monocyte-derived macrophages (HMDMs) exposed to oxidized LDL (ox-LDL), which are typically established cell models mimicking the formation of foam cells (27, 28).
MATERIALS AND METHODS
Ethics statement
Our experiment involving fresh plasma and peripheral blood mononuclear cells of normolipidemic volunteers was approved by volunteers and Wuhan Blood Centre (authorizations: 2010-8) and conformed to the Declaration of Helsinki.
Antibody generation, LDL isolation, and oxidization
The antibody targeting the E314 peptide of human Kv1.3 pore region (named the hKv1.3-E314 antibody; China Patent Application Number of the E314 peptide: 201110044416.x) was previously generated and used as a specific blocker of hKv1.3 channels (26). In addition, we generated the antibody targeting the E313 peptide of human Kv1.5 pore region (named the hKv1.5-E313 antibody; China Patent Application Number of the E313 peptide: 201110293643.6) as a specific blocker of hKv1.5 channels following the same strategy as described previously (29–31).
Native LDLs (densities ranging from 1.006 to 1.063 g/ml) were isolated from fresh plasma of normolipidemic volunteers by sequential preparative ultracentrifugation according to published standard protocols (32). Then LDLs were oxidized with 10 μM CuSO4 to obtain ox-LDL.
Cell culture
THP-1 cells were purchased from American Type Culture Collection (ATCC) and maintained in RPMI 1640 medium supplemented with 10% FBS at 37°C. To induce monocyte-to-macrophage differentiation, THP-1 cells were cultured in the presence of 160 nM phorbol 12-myristate 13-acetate for 72 h.
Peripheral blood mononuclear cells were isolated from peripheral blood samples of normal volunteers by Ficoll density gradient centrifugation and cultured in RPMI 1640 medium with 10% FBS at 37°C in 5% CO2 for 7 days to induce differentiation into HMDMs.
After differentiation, THP-1 macrophages or HMDMs were exposed to 100 μg/ml ox-LDL in the presence of the hKv1.3-E314 antibody at varying concentrations of 37.5, 75, or 300 nM for 2 h. THP-1 macrophages or HMDMs were exposed to 100 μg/ml ox-LDL for 24 h to accelerate foam cell formation with less toxicity or apoptosis (33, 34). Meanwhile, to observe the action of the hKv1.3-E314 antibody during the transformation, THP-1 macrophages or HMDMs were exposed to 100 μg/ml ox-LDL for up to 36 or 48 h in the presence of the 300 nM hKv1.3-E314 antibody. In the experiments, THP-1 macrophages and HMDMs exposed to the hKv1.3-E314 antibody alone and 100 μg/ml ox-LDL alone were cultured for 24 h.
Immunofluorescent staining
THP-1 macrophages were fixed and blocked with a solution containing 1% BSA and 10% goat serum (Invitrogen, Carlsbad, CA). Fixed cells were incubated with the hKv1.3-E314 antibody or the hKv1.5-E313 antibody and then with the FITC-conjugated secondary anti-rabbit goat antibody (Alomone, Israel). Nuclear chromatin was stained with DAPI (eBioscience, San Diego, CA). Negative control was prepared by the primary antibody preincubated with an excess of corresponding antigenic peptides. Cell samples were imaged with a Nikon A1si confocal laser microscope (Nikon, Tokyo, Japan).
Electrophysiological recording
THP-1-derived macrophages preincubated with various concentrations of the hKv1.3-E314 antibody were plated onto glass coverlids for measuring whole cell currents using the patch clamp technique. An Axon-200B (Molecular Devices) amplifier with pClamp 9.0 software was used for data recording and analysis. Patch electrodes (filled resistance 2–5 MΩ) were fabricated in a P-97 puller (Sutter Instruments) from borosilicate glass (outer diameter 1.5 mm and inner diameter 1.05 mm; VitalSense Instruments, Wuhan, China) and filled with solution containing (in mM) 20 KCl, 110 K-aspartate, 1 MgCl2, 10 HEPES, 5 EGTA, 0.1 Na2-GTP, 5 Na2-phosphocreatine, and 5 Mg-ATP, adjusted to pH 7.2 with KOH. The extracellular solution contained (in mM) 120 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 D-glucose, adjusted to pH 7.4 with NaOH.
Cholesterol content and efflux analysis
Cells were counterstained with hematoxylin and oil red O (ORO) following the routine procedure. Cells with a lipid droplet area no less than the width of the nucleus were designated ORO positive (ORO+). The ORO+ cells were counted (35).
HPLC was conducted as follows. Briefly, cells were sonicated and lysed before triglycerides and proteins were eliminated from cell lysates. Dissolved in a solution of n-hexane and isopropanol (4:1, V/V), free cholesterol (FC) was extracted. One aliquot sample was treated with cholesterol esterase to obtain total cholesterol (TC). Samples were dried through a vacuum degasser and dissolved in a mobile phase containing isopropanol:n-heptane:acetonitrile (35:12:52, v/v). TC and FC were measured by a chromatographer system (VARIAN Prostar 210). Cholesterol ester (CE) was calculated through the subduction of FC from TC.
Percentage of cholesterol efflux was measured by liquid scintillation counting. Treated THP-1 macrophages or HMDMs were labeled with 1.0 μCi/ml [3H]cholesterol. ApoA-I (10 μg/ml), HDL2 (50 μg/ml), or HDL3 (50 μg/ml) was also added to media. The percentage of cholesterol efflux was calculated by dividing media-derived radioactivity by the sum of the radioactivity in media and cells: [media counts/ (media counts + cellular counts)] × 100%.
Real-time quantitative RT-PCR
Total cellular RNA was isolated, and cDNA was synthesized by reverse transcription reaction. Real-time quantitative PCR was performed with SYBR® Premix Ex TaqTM (Takara, Japan) using Applied Biosystems StepOne Realtime PCR System. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. Fold changes in mRNA expression level normalized to GAPDH were calculated by the comparative Ct method formula 2−ΔΔCt. The sequences of the PCR primers are listed in Table 1.
TABLE 1.
Sequences of Real-Time Quantitative RT-PCR
| Molecules | Sequence (5′–3′) |
| SR-A sense | GCAGTTCTCATCCCTCTCAT |
| SR-A anti-sense | GGTATTCTCTTGGATTTTGCC |
| LOX-1 sense | CGGCAACAAGCAGAAGAAGC |
| LOX-1 anti-sense | TGAGCCCGAGGAAAATAGGTAA |
| CD36 sense | TGCCTCTCCAGTTGAAAACCC |
| CD36 anti-sense | GCAACAAACATCACCACACCA |
| ACAT1 sense | TGGGCAATGGAGTCTTACTCTGCT |
| ACAT1 anti-sense | AAACAGCTGGCTCCAAATCAGGGA |
| ABCA1 sense | TACAGCCAGAAAGACACCAG |
| ABCA1 anti-sense | CACAGTAGACTTTGGGAGAG |
| ABCG1 sense | CAGGAAGATTAGACACTGTGG |
| ABCG1 anti-sense | GAAAGGGGAATGGAGAGAAGA |
| SR-B I sense | AACAACTCCGACTCTGGGCTCT |
| SR-B I anti-sense | CATTTGCCCAGAAGTTCCATTG |
| GAPDH sense | ATGGTGGTGAAGACGCCAGTA |
| GAPDH anti-sense | GGCACAGTCAAGGCTGAG AATG |
Western blotting analysis
Total protein extracts were prepared and subjected to Western blotting analysis. After SDS-PAGE, proteins were transferred onto nitrocellulose membrane and detected by the corresponding primary antibodies against human Kv1.3 (commercially from Abcam, Cambridge, UK, or the hKv1.3-E314 antibody), Kv1.5 (commercially from Millipore/Chemicon, Billerica, MA, or the hKv1.5-E313 antibody), SR-A (Santa Cruz Biotechnology, Santa Cruz, CA), LOX-1 (R&D, Minneapolis, MN), CD36 (Santa Cruz Biotechnology), ACAT1 (Cayman, Ann Arbor, MI), ABCA1 (Abcam), ABCG1 (Epitomics, Burlingame, CA) and SR-B I (Epitomics), and GAPDH (Beyotime, China), and the following HRP-conjugated secondary antibodies. The proteins were visualized with the Enhance chemiluminescence kit (Thermo, Rockford, IL). Semiquantitative analysis of film was performed with the Image-pro Plus analysis software.
Statistics
All data are presented as the means ± SEM. SPSS 13.0 software was used for statistical analysis. Direct comparisons between two groups were made using unpaired t-test. Data from more than two groups were available for ANOVA. P < 0.05 was considered as statistically significant.
RESULTS
Human Kv1.3 and Kv1.5 channels are expressed in THP-1 macrophages and THP-1-derived foam cells
hKv1.3 and hKv1.5 expression in THP-1 macrophages and THP-1 derived foam cells were detected by Western blotting using the commercial antibodies (supplementary Fig. I). At the protein level, both channels were identified in THP-1 macrophages and THP-1-derived foam cells. In the transformation from macrophages to foam cells, hKv1.3 or hKv1.5 expression showed no significant difference.
The hKv1.3-E314 antibody or the hKv1.5-E313 antibody specifically recognizes human Kv1.3 or Kv1.5 channels and binds to plasma membrane in THP-1 macrophages
By Western blotting and immunofluorescent staining, we confirmed specificity and plasma membrane binding of both the antibodies (the hKv1.3-E314 antibody and the hKv1.5-E313 antibody) that we had generated in THP-1 macrophages. The hKv1.3-E314 antibody or the hKv1.5-E313 antibody, respectively, recognized 64 kDa or 75 kDa protein, whereas both the antibodies preincubated with corresponding antigenic peptides were unable to recognize identical molecular weight proteins (supplementary Fig. IIA, B). Immunofluorescent staining results indicated that only plasma membrane was stained with green fluorescence in THP-1 macrophages (supplementary Fig. IIC, D).
The hKv1.3-E314 antibody inhibits outward delayed rectifier potassium currents in THP-1 macrophages
The effect of the hKv1.3-E314 antibody or the hKv1.5-E313 antibody on outward delayed rectifier potassium currents in THP-1 macrophages was examined by the whole-cell patch clamp technique. THP-1 macrophages were exposed to the hKv1.3-E314 antibody or the hKv1.5-E313 antibody 37°C for 2 h before the patch clamp experiment. To evoke voltage-dependent potassium currents, all cells were clamped to a holding potential of −80 mV and stimulated with 400-ms square pulses ranging from −60 to +60 mV in 10-mV increments (supplementary Fig. IIIA). The hKv1.3-E314 antibody at varying concentrations of 37.5, 75, or 300 nM decreased current densities significantly compared with control. The inhibition showed concentration dependence (supplementary Fig. IIIA). At the depolarizing pulse +60 mV, the hKv1.3-E314 antibody at concentrations ranging from 37.5 nM to 300 nM decreased current densities by 44%, 56%, or 85% (8.4474 ± 0.9329 pA/pF, 6.6156 ± 0.6049 pA/pF, 2.3365 ± 0.3514 pA/pF, vs. 15.1561 ± 1.4485 pA/pF) (supplementary Fig. IIIB). In contrast, the hKv1.5-E313 antibody at a concentration of 300 nM, which was identical to the hKv1.3-E314 antibody, exerted no significant effect on outward delayed rectifier potassium currents in THP-1 macrophages (supplementary Fig. IIIC, D).
The hKv1.3-E314 antibody reduces cholesterol content in THP-1 macrophages and HMDMs exposed to ox-LDL and enhances apoA-I-mediated cholesterol efflux
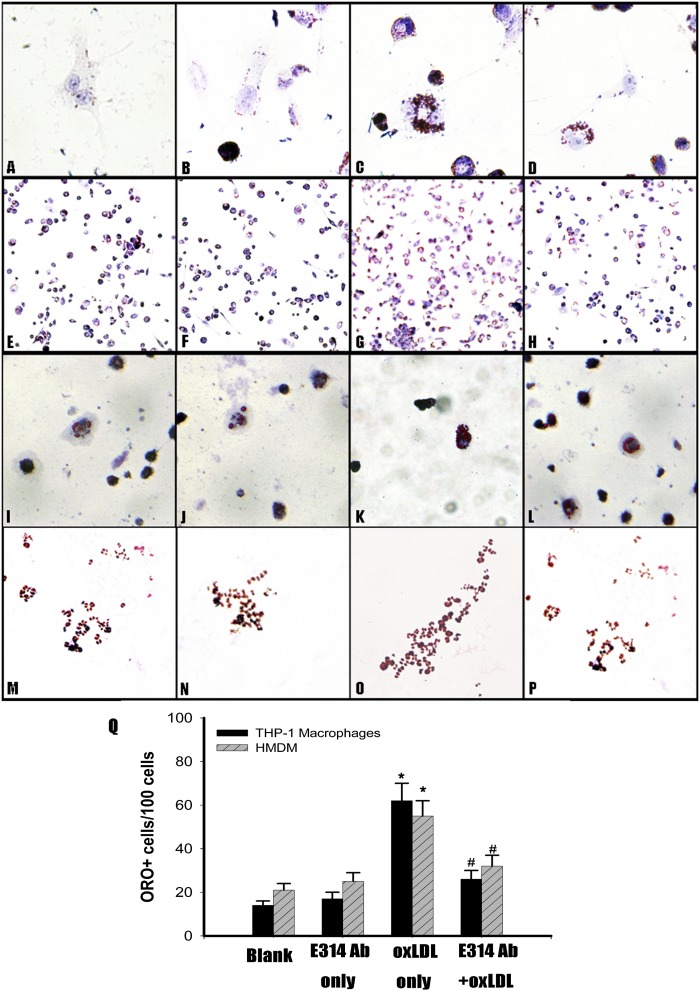
We had a direct-viewing of cholesterol content in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the presence or absence of the hKv1.3-E314 antibody by ORO staining. When THP-1 macrophages and HMDMs were exposed to 100 μg/ml ox-LDL, lipid droplets increased (Fig. 1C, K). In the presence of the 300 nM hKv1.3-E314 antibody, lipid droplets in THP-1 macrophages and HMDMs decreased markedly (Fig. 1D, L). The amount of ORO+ cells increased when THP-1 macrophages and HMDMs were exposed to 100 μg/ml ox-LDL(Fig. 1G, O), and the amount decreased significantly in the presence of the 300 nM hKv1.3-E314 antibody (Fig. 1H, P, Q).
Fig. 1.
Effect of the hKv1.3-E314 antibody on cellular cholesterol content and cholesterol efflux in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL. Intracellular lipid droplets were observed by ORO staining. Lipid droplets were stained red and nuclei blue (from A to P), and ORO+ cells were counted (Q). A, E and I, M: THP-1 macrophages and HMDMs were cultured for 24 h (original magnification: ×800 and ×100). B, F and J, N: THP-1 macrophages and HMDMs exposed to the 300 nM hKv1.3-E314 antibody alone were cultured for 24 h (original magnification: ×800 and ×100). C, G and K, O: THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL alone were cultured for 24 h (original magnification: ×800 and ×100). D, H, and L, P: THP-1 macrophages and HMDM cells exposed to 100 μg/ml ox-LDL in the presence of the 300 nM hKv1.3-E314 antibody were cultured for 24 h (original magnification: ×800 and ×100). Q: The number of ORO+ cells in each group (n = 3). *P < 0.05 versus control group; #P < 0.05 versus macrophages exposed to 100 μg/ml ox-LDL in the absence of the 300 nM hKv1.3-E314 antibody.
By HPLC, TC, FC, and CE in treated THP-1 macrophages and HMDMs were quantified. In THP-1 macrophages and HMDMs, there were significant decreases of TC and CE in the presence of the hKv1.3-E314 antibody in a concentration-dependent manner, compared with the absence of the hKv1.3-E314 antibody. Increases of FC were also observed in the presence of the hKv1.3-E314 antibody, whereas TC decreased. The homeostasis of FC, TC, and CE did not alter with ox-LDL exposure times ranging from 24 h to 48 h (Table 2).
TABLE 2.
Effect of the hKv1.3-E314 antibody on TC, FC, and CE in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL
| E314 Ab (nM) | 0 | 300 | 0 | 37.5 | 75 | 300 | 300 | 300 | |
| Exposure time inox-LDL (h) | — | — | 24 | 24 | 24 | 24 | 36 | 48 | |
| TC (mg/dl) | |||||||||
| THP-1 | 226.3 ± 9.5 | 226.0 ± 15.5 | 488.7 ± 15.0 | 445.0 ± 13.3* | 426.3 ± 9.8** | 298.3 ± 7.4** | 316.3 ± 13.0** | 306.3 ± 11.0** | |
| HMDM | 165.3 ± 27.5 | 177.3 ± 15.5 | 277.7 ± 33.3 | 232.7 ± 24.1* | 209.6 ± 20.4* | 188.3 ± 19.9* | 226.3 ± 33.5* | 209.9 ± 31.9* | |
| FC (mg/dl) | |||||||||
| THP-1 | 182.0 ± 10.1 | 178.7 ± 13.3 | 188.7 ± 11.3 | 230.0 ± 7.2* | 263.3 ± 11.9** | 223.0 ± 7.5* | 234.0 ± 4.4** | 232.0 ± 11.4* | |
| HMDM | 106.5 ± 25.8 | 119.4 ± 13.3 | 106.4 ± 19.3 | 115.8 ± 19.5 | 121.2 ± 21.9* | 129.7 ± 7.5* | 138.1 ± 21.3* | 140.6 ± 30.1* | |
| CE (mg/dl) | |||||||||
| THP-1 | 44.3 ± 1.2 | 47.3 ± 2.7 | 300.0 ± 9.6 | 215.0 ± 12.0** | 163.0 ± 3.8** | 75.3 ± 5.0** | 82.3 ± 8.7** | 74.3 ± 4.5** | |
| HMDM | 58.8 ± 11.1 | 57.9 ± 12.8 | 171.3 ± 24.6 | 116.9 ± 29.7* | 88.4 ± 13.9** | 58.6 ± 15.0** | 88.2 ± 18.2** | 69.3 ± 23.7** |
Percentage of CE and FC in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the absence (macrophages and macrophages exposed to 100 μg/ml ox-LDL or the 300 nM hKv1.3-E314 antibody alone) or presence of the hKv1.3-E314 antibody at a varying concentrations of 37.5, 75, or 300 nM. In the presence of the 300 nM hKv1.3-E314 antibody, macrophages were exposed to 100 μg/ml ox-LDL, respectively, for 24, 36, or 48 h. HPLC was performed to determine TC, FC, and CE. Data represent the means ± SEM of three independent experiments (n = 3). *P > 0.05, **P < 0.01 versus macrophages exposed to 100 μg/ml ox-LDL alone. There was no significant difference in percentage of cholesterol ester when macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h in the presence of the 300 nM hKv1.3-E314 antibody.
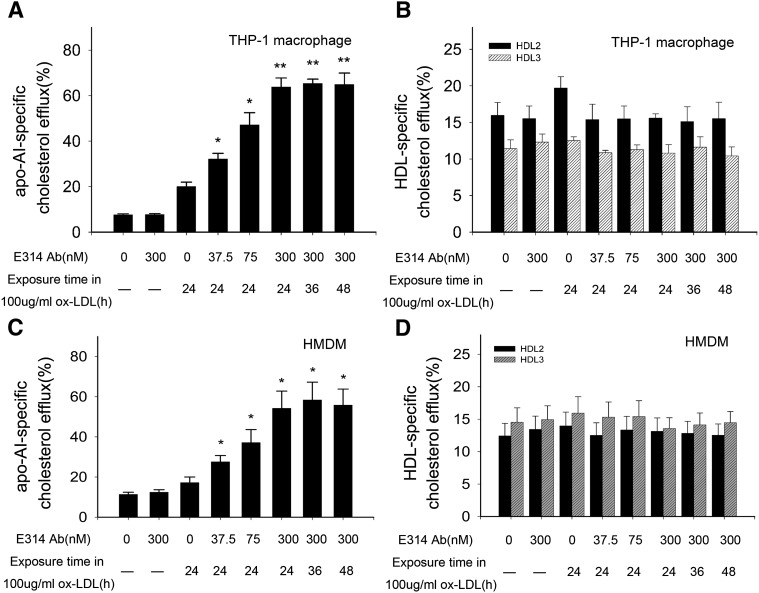
With CE and FC alterations, there were significant enhancements of apoA-I-mediated [3H]cholesterol efflux in THP-1 macrophages and HMDMs (Fig. 2A, C), whereas there was no significant alteration of mature HDL(HDL2 or HDL3)-mediated [3H]cholesterol efflux (Fig. 2B, D). The enhancement showed a concentration-dependent manner and did not change with ox-LDL exposure time from 24 h to 48 h (Fig. 2A, C).
Fig. 2.
Percentage of cholesterol efflux from THP-1 macrophages or HMDMs exposed to 100 μg/ml ox-LDL in the absence (macrophages and macrophages exposed to 100 μg/ml ox-LDL or the 300 nM hKv1.3-E314 antibody alone) or presence of the hKv1.3-E314 antibody at varying concentrations of 37.5, 75, or 300 nM. In the presence of the 300 nM hKv1.3-E314 antibody, macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h. Medium and cell-associated apoA-I or HDL-mediated [3H]cholesterol were measured by liquid scintillation counting (A–D). A and C: Percentage of apoA-I-mediated cholesterol efflux in THP-1 macrophages and HMDMs,. B and D: Percentage of HDL-mediated cholesterol efflux in THP-1 macrophages and HMDM cells (n = 3). *P < 0.05, **P < 0.01, and P > 0.05 versus macrophages exposed to 100 μg/ml ox-LDL alone. There was no significant difference in percentage of apoA-I or HDL-mediated cholesterol efflux when macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h in the presence of the 300 nM hKv1.3-E314 antibody.
The hKv1.3-E314 antibody downregulates SR-A, LOX-1, and ACAT1 expression and upregulates ABCA1 expression in THP-1 macrophages and HMDMs exposed to ox-LDL
By real-time PCR and Western blotting, we assayed the mRNA and protein level of cholesterol-metabolism-associated molecules in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the absence or presence of the hKv1.3-E314 antibody, which include SR-A, CD36, LOX-1, ACAT1, ABCA1, ABCG1, and SR-B I. Some of these molecules were downregulated or upregulated.
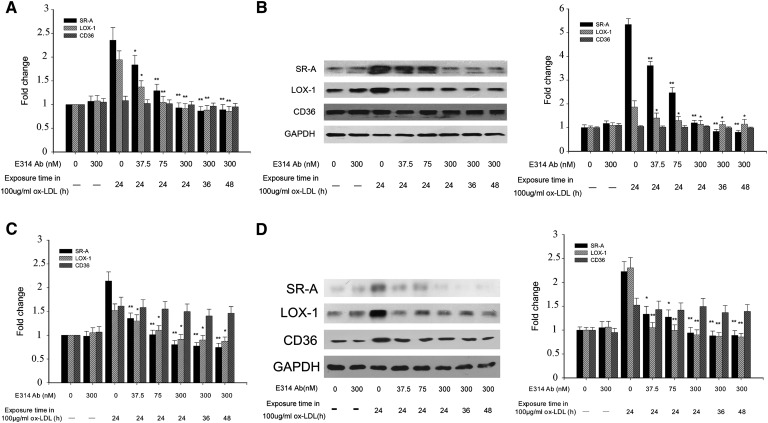
Compared with SR-A and LOX-1 expression levels in THP-1 macrophages or HMDMs exposed to 100 μg/ml ox-LDL alone, which were elevated in THP-1 macrophages and in HMDMs, the mRNA and protein levels of SR-A and LOX-1 were downregulated in the presence of the hKv1.3-E314 antibody in a concentration-dependent manner. The SR-A and LOX-1 expressions did not change with ox-LDL exposure time ranging from 24 h to 48 h. The mRNA and protein level of CD36 were elevated in HMDMs but not in THP-1 macrophages. There was no significant alteration of CD36 expression level in cells preincubated with various concentrations of the hKv1.3-E314 antibody (Fig. 3).
Fig. 3.
mRNA and protein expression levels of SR-A, LOX-1, and CD36 in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the absence (macrophages and macrophages exposed to 100 μg/ml ox-LDL or the 300 nM hKv1.3-E314 antibody alone) or presence of the hKv1.3-E314 antibody at varying concentrations of 37.5, 75, or 300 nM. In the presence of the 300 nM hKv1.3-E314 antibody, macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h. mRNA and protein levels of SR-A, LOX-1, and CD36 were assayed by real-time quantitative RT-PCR and Western blotting. A and C: mRNA levels of SR-A, LOX-1, and CD36 in THP-1 macrophages and HMDMs. B and D: Protein levels of SR-A, LOX-1, and CD36 in THP-1 macrophages and HMDMs (n = 3). *P < 0.05, **P < 0.01, and P > 0.05 versus macrophages exposed to 100 μg/ml ox-LDL alone. There was no significant difference in mRNA and protein levels when macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h in the presence of the 300 nM hKv1.3-E314 antibody.
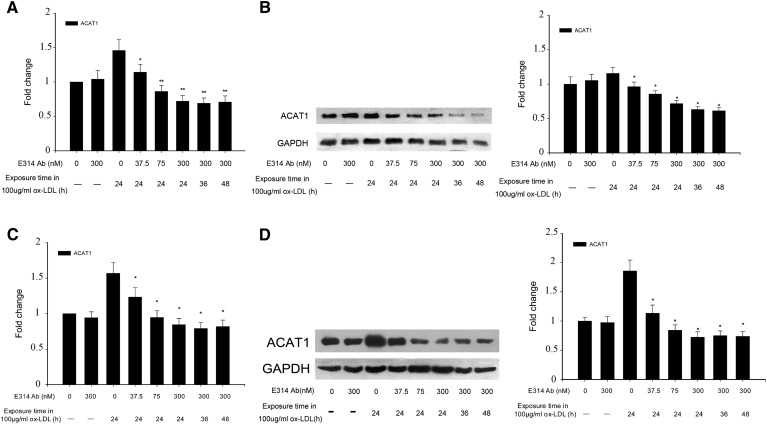
The hKv1.3-E314 antibody also downregulated, in a concentration-dependent manner, ACAT1 expression in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL. The ACAT1 expression did not alter with ox-LDL exposure time ranging from 24 h to 48 h (Fig. 4).
Fig. 4.
mRNA and protein expression levels of ACAT1 in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the absence (macrophages and macrophages exposed to 100 μg/ml ox-LDL or the 300 nM hKv1.3-E314 antibody alone) or presence of the hKv1.3-E314 antibody at varying concentrations of 37.5, 75, or 300 nM. In the presence of the 300 nM hKv1.3-E314 antibody, macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h. mRNA and protein levels of ACAT1 were assayed, respectively, by real-time quantitative RT-PCR and Western blotting. A and C: mRNA levels of ACAT1 in THP-1 macrophages and HMDMs,. B and D: Protein levels of ACAT1 in THP-1 macrophages and HMDM cells (n = 3). *P < 0.05, **P < 0.01, and P > 0.05 versus macrophages exposed to 100 μg/ml ox-LDL alone. There was no significant difference in mRNA and protein levels when macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h in the presence of the 300 nM hKv1.3-E314 antibody.
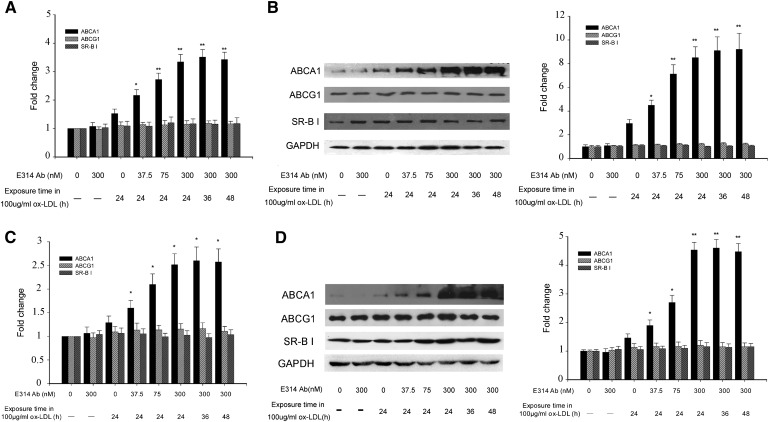
Of all the molecules mediating cholesterol efflux, including ABCA1, ABCG1, and SR-B I, only ABCA1 expression was upregulated in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the absence or presence of the hKv1.3-E314 antibody in a concentration-dependent manner. In line with ABCA1 mRNA level, ABCA1 protein level was significantly elevated compared with its level in THP-1 macrophages exposed to 100 μg/ml ox-LDL alone. The hKv1.3-E314 antibody at a varying concentration caused a 1.5- to 3-fold increase in THP-1 macrophages and a 1.3 to 3.1-fold increase in HMDMs. And the ABCA1 expression did not alter with ox-LDL exposure time ranging from 24 h to 48 h (Fig. 5).
Fig. 5.
mRNA and protein expression levels of ABCA1, ABCG1, and SR-B I in THP-1 macrophages and HMDMs exposed to 100 μg/ml ox-LDL in the absence (macrophages and macrophages exposed to 100 μg/ml ox-LDL or the 300 nM hKv1.3-E314 antibody alone) or presence of the hKv1.3-E314 antibody at varying concentrations of 37.5, 75, or 300 nM. In the presence of the 300 nM hKv1.3-E314 antibody, macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h. mRNA and protein levels of ABCA1, ABCG1, and SR-B I were assayed by real-time quantitative RT-PCR and Western blotting. A and C: mRNA levels of ABCA1, ABCG1, and SR-B I in THP-1 macrophages and HMDMs,. B and D: Protein levels of ABCA1, ABCG1, and SR-B I in THP-1 macrophages and HMDMs, (n = 3). *P < 0.05, **P < 0.01, and P > 0.05 versus macrophages exposed to 100 μg/ml ox-LDL alone. There was no significant difference in mRNA and protein levels when macrophages were exposed to 100 μg/ml ox-LDL for 24, 36, or 48 h in the presence of the 300 nM hKv1.3-E314 antibody.
DISCUSSION
Our study confirms that blockade of Kv1.3 prevents foam cell formation. We have, using a novel antibody-based approach, provided the first evidence for some of the molecular changes that contribute to this effect.
Outward delayed rectifier potassium currents are elicited and elevated when membranes are depolarized accompanied by macrophage activation (19, 36). The currents were long thought to be carried by the Kv1.3 channel (19, 20). However, in recent years, the Kv1.3 and Kv1.5 channels have been identified, forming a heteromultimeric complex in mouse macrophages (37–39). Herein our data provide supportive evidence that the complex is present in THP-1 macrophages. Owing to the homogeneous structural features of the entire voltage-gated potassium channel superfamily and the conservation of drug-binding sites of the Kv1.3 and Kv1.5 channels (40, 41), the coexistence makes it difficult to discriminate the dominance of hKv1.3 or hKv1.5 channels in THP-1 macrophages, at which possible pharmaceutical targets would be aimed.
We generated two antibodies directed against the extracellular peptides of hKv1.3 or hKv1.5 pore region, which can specifically block hKv1.3 or hKv1.5 channels, respectively, named the hKv1.3-E314 antibody and the hKv1.5-E313 antibody. These antibodies are not able to cross-react to other closely related Kv1 channels but can react with themselves. The hKv1.3-E314 antibody significantly inhibited outward delayed rectifier potassium currents in THP-1 macrophages in a concentration-dependent manner, whereas the hKv1.5-E313 antibody failed to show an inhibiting tendency at the concentration identical to the hKv1.3-E314 antibody, which indicates that hKv1.3-containing subunit regulates the permeability of the heteromultimeric channel.
Initially, when the heteromultimeric Kv1.3 channel was blocked, cholesterol content decreased pronouncedly in THP-1 macrophages and HMDMs exposed to ox-LDL, which was generally consistent with Lei's result by another Kv1.3 blocker (25). Furthermore, a novel and interesting finding emerged that apoA-I-mediated cholesterol efflux from THP-1 macrophages and HMDMs was greatly enhanced. These findings enabled us to investigate the underlying mechanism in human macrophages, including THP-1 macrophages and HMDMs.
In this study, we presented an expression pattern of key cholesterol-metabolism-associated molecules in THP-1 macrophages or HMDMs preincubated with various concentrations of the hKv1.3-E314 antibody. The mRNA and protein expression of SR-A and LOX-1 was downregulated, thereby terminating the positive feedback of ox-LDL uptake. ACAT1 downregulation also resulted in the reduction of cholesterol ester synthesis. The downregulation of ACAT1 produced a large amount of free cholesterol in macrophages, which is harmful to cells and facilitates plaque destabilization (42–45). Surplus FC efflux from macrophages could be mediated by ABCA1 upregulation interacting with lipid-free apoA-I (46–48), unlike ABCG1 and SR-B I interacting with mature HDL (47, 49–51).
The expression pattern represents a comprehensive system or network modulating cholesterol influx, synthesis, and efflux. The modulating system or network caused a significant reduction of cholesterol accumulation in macrophages by downregulating SR-A, LOX-1, and ACAT1 expression and upregulating ABCA1 expression, of which ACAT1 and ABCA1 are considered to be candidate targets for the treatment of atherosclerotic lesions due to their roles in macrophage cholesterol metabolism (18, 52–55). ACAT1 inhibition or downregulation was shown to be atheroprotective in animal models (18, 56–58). ABCA1 acts as the primary gatekeeper for eliminating excess tissue cholesterol and represents the first and rate-controlling step in reverse cholesterol transport (59, 60). The paramount importance of ABCA1 is exemplified by Tangier disease, which is characterized by loss-of-function mutations in the ABCA1 gene (61), and is highlighted by an ABCA1-deficient or ABCA1-overexpression mouse model, which disabled or enhanced removal of intracellular free cholesterol (54, 62). However, mere ACAT inhibition is not an effective strategy for ameliorating atherosclerosis and may promote atherogenesis, which was validated by the ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) study (63). Moreover, the ACTIVATE study and a clinical trial about apoA-IMilano suggest that ACAT1 inhibitors when used in combination with those compounds, which increase reverse cholesterol transport may benefit the treatment of atherosclerosis (63–66). ACAT1 downregulation and ABCA1 upregulation by specific Kv1.3 blockade in macrophages conform to the strategy.
Although our study presented attractive results that the hKv1.3-E314 antibody can prevent foam cell formation, for the sake of clinical practice, it is necessary to investigate the effect of the antibody on foam cells after exposure to ox-LDL through further research.
In previous studies, Kv1.3 has been validated to play a key role in the modulation of pathogenic T subset function (24, 40, 67–69). Moreover, selective Kv1.3 blockade can reach equilibrium between efficacy and safety in animal models, revealing no systemic toxicity (68, 69). Increasing evidence shows that a specified pathogenic T subset can aggravate atherosclerosis (70, 71). These results encourage us to investigate an atheroprotective effect of the hKv1.3-E314 antibody on T lymphocytes in our ongoing efforts.
Overall, specific Kv1.3 blockade exerts an atheroprotective effect in vitro, which signifies potential value in the treatment of atherosclerotic lesions as a novel strategy. In the future, the monoclonal antibody derived from the E314 peptide of human Kv1.3 pore region will be used in in vivo studies due to its specificity and long circulating biological half-life (72, 73).
Supplementary Material
Acknowledgments
The authors thank Dr. Chun-Li Mei (Union Hospital, Huazhong University of Science and Technology) for technical assistance.
Footnotes
Abbreviations:
- CE
- cholesterol ester
- FC
- free cholesterol
- HMDM
- human monocyte-derived macrophage
- LOX-1
- lectin-like oxidized low-density lipoprotein receptor-1
- ORO
- oil red O
- ox-LDL
- oxidized LDL
- SR-A
- scavenger receptor class A
- SR-B I
- scavenger receptor class B type I
- TC
- total cholesterol
This work was supported by National Natural Science Foundation of China grant 30700747/C08); by Natural Science Foundation of Shandong Province, China grant ZR2010HL032); and by Natural Science Foundation of Hubei Province, China grant 2010CDB07901.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Rensen P. C., Gras J. C., Lindfors E. K., van Dijk K. W., Jukema J. W., van Berkel T. J., Biessen E. A. 2006. Selective targeting of liposomes to macrophages using a ligand with high affinity for the macrophage scavenger receptor class A. Curr. Drug Discov. Technol. 3: 135–144 [DOI] [PubMed] [Google Scholar]
- 2.Dhaliwal B. S., Steinbrecher U. P. 1999. Scavenger receptors and oxidized low density lipoproteins. Clin. Chim. Acta. 286: 191–205 [DOI] [PubMed] [Google Scholar]
- 3.Collot-Teixeira S., Martin J., McDermott-Roe C., Poston R., McGregor J. L. 2007. CD36 and macrophages in atherosclerosis. Cardiovasc. Res. 75: 468–477 [DOI] [PubMed] [Google Scholar]
- 4.Thorne R. F., Mhaidat N. M., Ralston K. J., Burns G. F. 2007. CD36 is a receptor for oxidized high density lipoprotein: implications for the development of atherosclerosis. FEBS Lett. 581: 1227–1232 [DOI] [PubMed] [Google Scholar]
- 5.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., et al. 2002. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 277: 38517–38523 [DOI] [PubMed] [Google Scholar]
- 6.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., et al. 2002. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 277: 38503–38516 [DOI] [PubMed] [Google Scholar]
- 7.Reiss A. B., Anwar K., Wirkowski P. 2009. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) in atherogenesis: a brief review. Curr. Med. Chem. 16: 2641–2652 [DOI] [PubMed] [Google Scholar]
- 8.Mehta J. L., Chen J., Hermonat P. L., Romeo F., Novelli G. 2006. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 69: 36–45 [DOI] [PubMed] [Google Scholar]
- 9.Mauldin J. P., Nagelin M. H., Wojcik A. J., Srinivasan S., Skaflen M. D., Ayers C. R., McNamara C. A., Hedrick C. C. 2008. Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation. 117: 2785–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oram J. F., Vaughan A. M. 2006. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 99: 1031–1043 [DOI] [PubMed] [Google Scholar]
- 11.Clee S. M., Zwinderman A. H., Engert J. C., Zwarts K. Y., Molhuizen H. O., Roomp K., Jukema J. W., van Wijland M., van Dam M., Hudson T. J., et al. 2001. Common genetic variation in ABCA1 is associated with altered lipoprotein levels and a modified risk for coronary artery disease. Circulation. 103: 1198–1205 [DOI] [PubMed] [Google Scholar]
- 12.Yvan-Charvet L., Wang N., Tall A. R. 2010. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30: 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldan A., Tarr P., Lee R., Edwards P. A. 2006. ATP-binding cassette transporter G1 and lipid homeostasis. Curr. Opin. Lipidol. 17: 227–232 [DOI] [PubMed] [Google Scholar]
- 14.Zhang W., Yancey P. G., Su Y. R., Babaev V. R., Zhang Y., Fazio S., Linton M. F. 2003. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 108: 2258–2263 [DOI] [PubMed] [Google Scholar]
- 15.Braun A., Trigatti B. L., Post M. J., Sato K., Simons M., Edelberg J. M., Rosenberg R. D., Schrenzel M., Krieger M. 2002. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 90: 270–276 [DOI] [PubMed] [Google Scholar]
- 16.Kusunoki J., Hansoty D. K., Aragane K., Fallon J. T., Badimon J. J., Fisher E. A. 2001. Acyl-CoA:cholesterol acyltransferase inhibition reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 103: 2604–2609 [DOI] [PubMed] [Google Scholar]
- 17.Dove D. E., Su Y. R., Swift L. L., Linton M. F., Fazio S. 2006. ACAT1 deficiency increases cholesterol synthesis in mouse peritoneal macrophages. Atherosclerosis. 186: 267–274 [DOI] [PubMed] [Google Scholar]
- 18.Yoshinaka Y., Shibata H., Kobayashi H., Kuriyama H., Shibuya K., Tanabe S., Watanabe T., Miyazaki A. 2010. A selective ACAT-1 inhibitor, K-604, stimulates collagen production in cultured smooth muscle cells and alters plaque phenotype in apolipoprotein E-knockout mice. Atherosclerosis. 213: 85–91 [DOI] [PubMed] [Google Scholar]
- 19.Vicente R., Escalada A., Coma M., Fuster G., Sanchez-Tillo E., Lopez-Iglesias C., Soler C., Solsona C., Celada A., Felipe A. 2003. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J. Biol. Chem. 278: 46307–46320 [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie A. B., Chirakkal H., North R. A. 2003. Kv1.3 potassium channels in human alveolar macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 285: L862–L868 [DOI] [PubMed] [Google Scholar]
- 21.Dallaporta B., Marchetti P., de Pablo M. A., Maisse C., Duc H. T., Metivier D., Zamzami N., Geuskens M., Kroemer G. 1999. Plasma membrane potential in thymocyte apoptosis. J. Immunol. 162: 6534–6542 [PubMed] [Google Scholar]
- 22.Price M., Lee S. C., Deutsch C. 1989. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA. 86: 10171–10175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desir G. V. 2005. Kv1.3 potassium channel blockade as an approach to insulin resistance. Expert Opin. Ther. Targets. 9: 571–579 [DOI] [PubMed] [Google Scholar]
- 24.Beeton C., Pennington M. W., Wulff H., Singh S., Nugent D., Crossley G., Khaytin I., Calabresi P. A., Chen C. Y., Gutman G. A., et al. 2005. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 67: 1369–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei X. J., Ma A. Q., Xi Y. T., Zhang W., Yao Y., Du Y. 2006. Inhibitory effects of blocking voltage-dependent potassium channel 1.3 on human monocyte-derived macrophage differentiation into foam cells. Beijing Da Xue Xue Bao. 38: 257–261 [PubMed] [Google Scholar]
- 26.Yang X. F., Yang Y., Lian Y. T., Wang Z. H., Li X. W., Cheng L. X., Liu J. P., Wang Y. F., Gao X., Liao Y. H., et al. 2012. The antibody targeting the e314 Peptide of human kv1.3 pore region serves as a novel, potent and specific channel blocker. PLoS ONE. 7: e36379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auwerx J. 1991. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 47: 22–31 [DOI] [PubMed] [Google Scholar]
- 28.Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. 1980. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc. Natl. Acad. Sci. USA. 77: 2214–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S. Z., Zeng F., Lei M., Li J., Gao B., Xiong C., Sivaprasadarao A., Beech D. J. 2005. Generation of functional ion-channel tools by E3 targeting. Nat. Biotechnol. 23: 1289–1293 [DOI] [PubMed] [Google Scholar]
- 30.Zhou B. Y., Ma W., Huang X. Y. 1998. Specific antibodies to the external vestibule of voltage-gated potassium channels block current. J. Gen. Physiol. 111: 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panyi G., Possani L. D., Rodriguez de la Vega R. C., Gaspar R., Varga Z. 2006. K+ channel blockers: novel tools to inhibit T cell activation leading to specific immunosuppression. Curr. Pharm. Des. 12: 2199–2220 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y. F., Yang X. F., Cheng B., Mei C. L., Li Q. X., Xiao H., Zeng Q. T., Liao Y. H., Liu K. 2010. Protective effect of Astragalus polysaccharides on ATP binding cassette transporter A1 in THP-1 derived foam cells exposed to tumor necrosis factor-alpha. Phytother. Res. 24: 393–398 [DOI] [PubMed] [Google Scholar]
- 33.Tian L., Luo N., Zhu X., Chung B. H., Garvey W. T., Fu Y. 2012. Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis. 221: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao S., Sang H., Song G., Yang N., Liu Q., Zhang Y., Jiao P., Zong C., Qin S. 2012. Quercetin protects macrophages from oxidized low-density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress-C/EBP homologous protein pathway. Exp. Biol. Med. (Maywood). 237: 822–831 [DOI] [PubMed] [Google Scholar]
- 35.Wada Y., Sugiyama A., Yamamoto T., Naito M., Noguchi N., Yokoyama S., Tsujita M., Kawabe Y., Kobayashi M., Izumi A., et al. 2002. Lipid accumulation in smooth muscle cells under LDL loading is independent of LDL receptor pathway and enhanced by hypoxic conditions. Arterioscler. Thromb. Vasc. Biol. 22: 1712–1719 [DOI] [PubMed] [Google Scholar]
- 36.Vicente R., Escalada A., Soler C., Grande M., Celada A., Tamkun M. M., Solsona C., Felipe A. 2005. Pattern of Kv beta subunit expression in macrophages depends upon proliferation and the mode of activation. J. Immunol. 174: 4736–4744 [DOI] [PubMed] [Google Scholar]
- 37.Vicente R., Villalonga N., Calvo M., Escalada A., Solsona C., Soler C., Tamkun M. M., Felipe A. 2008. Kv1.5 association modifies Kv1.3 traffic and membrane localization. J. Biol. Chem. 283: 8756–8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villalonga N., Escalada A., Vicente R., Sanchez-Tillo E., Celada A., Solsona C., Felipe A. 2007. Kv1.3/Kv1.5 heteromeric channels compromise pharmacological responses in macrophages. Biochem. Biophys. Res. Commun. 352: 913–918 [DOI] [PubMed] [Google Scholar]
- 39.Vicente R., Escalada A., Villalonga N., Texido L., Roura-Ferrer M., Martin-Satue M., Lopez-Iglesias C., Soler C., Solsona C., Tamkun M. M., et al. 2006. Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages. J. Biol. Chem. 281: 37675–37685 [DOI] [PubMed] [Google Scholar]
- 40.Wulff H., Beeton C., Chandy K. G. 2003. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discov. Devel. 6: 640–647 [PubMed] [Google Scholar]
- 41.Decher N., Pirard B., Bundis F., Peukert S., Baringhaus K. H., Busch A. E., Steinmeyer K., Sanguinetti M. C. 2004. Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. J. Biol. Chem. 279: 394–400 [DOI] [PubMed] [Google Scholar]
- 42.Kellner-Weibel G., Geng Y. J., Rothblat G. H. 1999. Cytotoxic cholesterol is generated by the hydrolysis of cytoplasmic cholesteryl ester and transported to the plasma membrane. Atherosclerosis. 146: 309–319 [DOI] [PubMed] [Google Scholar]
- 43.Tabas I. 2004. Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death Differ. 11(Suppl 1): S12–S16 [DOI] [PubMed] [Google Scholar]
- 44.Kellner-Weibel G., Jerome W. G., Small D. M., Warner G. J., Stoltenborg J. K., Kearney M. A., Corjay M. H., Phillips M. C., Rothblat G. H. 1998. Effects of intracellular free cholesterol accumulation on macrophage viability: a model for foam cell death. Arterioscler. Thromb. Vasc. Biol. 18: 423–431 [DOI] [PubMed] [Google Scholar]
- 45.Warner G. J., Stoudt G., Bamberger M., Johnson W. J., Rothblat G. H. 1995. Cell toxicity induced by inhibition of acyl coenzyme A:cholesterol acyltransferase and accumulation of unesterified cholesterol. J. Biol. Chem. 270: 5772–5778 [DOI] [PubMed] [Google Scholar]
- 46.Wang N., Silver D. L., Thiele C., Tall A. R. 2001. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 276: 23742–23747 [DOI] [PubMed] [Google Scholar]
- 47.Kennedy M. A., Barrera G. C., Nakamura K., Baldan A., Tarr P., Fishbein M. C., Frank J., Francone O. L., Edwards P. A. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1: 121–131 [DOI] [PubMed] [Google Scholar]
- 48.Oram J. F., Lawn R. M., Garvin M. R., Wade D. P. 2000. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275: 34508–34511 [DOI] [PubMed] [Google Scholar]
- 49.Wang N., Lan D., Chen W., Matsuura F., Tall A. R. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101: 9774–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji Y., Jian B., Wang N., Sun Y., Moya M. L., Phillips M. C., Rothblat G. H., Swaney J. B., Tall A. R. 1997. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 272: 20982–20985 [DOI] [PubMed] [Google Scholar]
- 51.Yancey P. G., de la Llera-Moya M., Swarnakar S., Monzo P., Klein S. M., Connelly M. A., Johnson W. J., Williams D. L., Rothblat G. H. 2000. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 275: 36596–36604 [DOI] [PubMed] [Google Scholar]
- 52.Leon C., Hill J. S., Wasan K. M. 2005. Potential role of acyl-coenzyme A:cholesterol transferase (ACAT) Inhibitors as hypolipidemic and antiatherosclerosis drugs. Pharm. Res. 22: 1578–1588 [DOI] [PubMed] [Google Scholar]
- 53.Oram J. F. 2002. ABCA1 as a new therapeutic target for treating cardiovascular disease. Drug News Perspect. 15: 24–28 [DOI] [PubMed] [Google Scholar]
- 54.Singaraja R. R., Fievet C., Castro G., James E. R., Hennuyer N., Clee S. M., Bissada N., Choy J. C., Fruchart J. C., McManus B. M., et al. 2002. Increased ABCA1 activity protects against atherosclerosis. J. Clin. Invest. 110: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oram J. F., Heinecke J. W. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372 [DOI] [PubMed] [Google Scholar]
- 56.Nagashima M., Watanabe T., Shiraishi Y., Morita R., Terasaki M., Arita S., Hongo S., Sato K., Shichiri M., Miyazaki A., et al. 2010. Chronic infusion of salusin-alpha and -beta exerts opposite effects on atherosclerotic lesion development in apolipoprotein E-deficient mice. Atherosclerosis. 212: 70–77 [DOI] [PubMed] [Google Scholar]
- 57.Xu G., Watanabe T., Iso Y., Koba S., Sakai T., Nagashima M., Arita S., Hongo S., Ota H., Kobayashi Y., et al. 2009. Preventive effects of heregulin-beta1 on macrophage foam cell formation and atherosclerosis. Circ. Res. 105: 500–510 [DOI] [PubMed] [Google Scholar]
- 58.Accad M., Smith S. J., Newland D. L., Sanan D. A., King L. E., Jr, Linton M. F., Fazio S., Farese R. V., Jr 2000. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J. Clin. Invest. 105: 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oram J. F., Lawn R. M. 2001. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 42: 1173–1179 [PubMed] [Google Scholar]
- 60.Yancey P. G., Bortnick A. E., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Rothblat G. H. 2003. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 712–719 [DOI] [PubMed] [Google Scholar]
- 61.Mott S., Yu L., Marcil M., Boucher B., Rondeau C., Genest J., Jr 2000. Decreased cellular cholesterol efflux is a common cause of familial hypoalphalipoproteinemia: role of the ABCA1 gene mutations. Atherosclerosis. 152: 457–468 [DOI] [PubMed] [Google Scholar]
- 62.Calpe-Berdiel L., Rotllan N., Palomer X., Ribas V., Blanco-Vaca F., Escola-Gil J. C. 2005. Direct evidence in vivo of impaired macrophage-specific reverse cholesterol transport in ATP-binding cassette transporter A1-deficient mice. Biochim. Biophys. Acta. 1738: 6–9 [DOI] [PubMed] [Google Scholar]
- 63.Nissen S. E., Tuzcu E. M., Brewer H. B., Sipahi I., Nicholls S. J., Ganz P., Schoenhagen P., Waters D. D., Pepine C. J., Crowe T. D., et al. 2006. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N. Engl. J. Med. 354: 1253–1263 [DOI] [PubMed] [Google Scholar]
- 64.Tiwari R. L., Singh V., Barthwal M. K. 2008. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 28: 483–544 [DOI] [PubMed] [Google Scholar]
- 65.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300 [DOI] [PubMed] [Google Scholar]
- 66.Fazio S., Linton M. 2006. Failure of ACAT inhibition to retard atherosclerosis. N. Engl. J. Med. 354: 1307–1309 [DOI] [PubMed] [Google Scholar]
- 67.Wulff H., Calabresi P. A., Allie R., Yun S., Pennington M., Beeton C., Chandy K. G. 2003. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J. Clin. Invest. 111: 1703–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beeton C., Wulff H., Standifer N. E., Azam P., Mullen K. M., Pennington M. W., Kolski-Andreaco A., Wei E., Grino A., Counts D. R., et al. 2006. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA. 103: 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rangaraju S., Chi V., Pennington M. W., Chandy K. G. 2009. Kv1.3 potassium channels as a therapeutic target in multiple sclerosis. Expert Opin. Ther. Targets. 13: 909–924 [DOI] [PubMed] [Google Scholar]
- 70.Stemme S., Holm J., Hansson G. K. 1992. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler. Thromb. 12: 206–211 [DOI] [PubMed] [Google Scholar]
- 71.Watanabe M., Sangawa A., Sasaki Y., Yamashita M., Tanaka-Shintani M., Shintaku M., Ishikawa Y. 2007. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J. Atheroscler. Thromb. 14: 325–331 [DOI] [PubMed] [Google Scholar]
- 72.Koo G. C., Blake J. T., Talento A., Nguyen M., Lin S., Sirotina A., Shah K., Mulvany K., Hora D., Jr, Cunningham P., et al. 1997. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J. Immunol. 158: 5120–5128 [PubMed] [Google Scholar]
- 73.Keizer R. J., Huitema A. D., Schellens J. H., Beijnen J. H. 2010. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 49: 493–507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.