Abstract
Apoptosis and autophagy are two evolutionarily conserved processes that maintain homeostasis during stress. Although the two pathways utilize fundamentally distinct machinery, apoptosis and autophagy are highly interconnected and share many key regulators. The crosstalk between apoptosis and autophagy is complex, as autophagy can function to promote cell survival or cell death under various cellular conditions. The molecular mechanisms of crosstalk are beginning to be elucidated and have critical implications for the treatment of various diseases, such as cancer. Sphingolipids are a class of bioactive lipids that mediate many key cellular processes, including apoptosis and autophagy. By targeting several of the shared regulators, sphingolipid metabolites differentially regulate the induction of apoptosis and autophagy. Importantly, individual sphingolipid species appear to “switch” autophagy toward cell survival (e.g., sphingosine-1-phosphate) or cell death (e.g., ceramide, gangliosides). This review assesses the current understanding of sphingolipid-induced apoptosis and autophagy to address how sphingolipids mediate the “switch” between the cell survival and cell death. As sphingolipid metabolism is frequently dysregulated in cancer, sphingolipid-modulating agents, or sphingomimetics, have emerged as a novel chemotherapeutic strategy. Ultimately, a greater understanding of sphingolipid-mediated crosstalk between apoptosis and autophagy may be critical for enhancing the chemotherapeutic efficacy of these agents.
Keywords: ceramide, dihydroceramide, sphingosine, sphingosine-1-phosphate, gangliosides, programmed cell death, switch, cancer therapy
Sphingolipids are membrane lipids that are ubiquitously expressed in all eukaryotic cells. While previously believed to exert only structural roles, sphingolipids are now recognized as signaling molecules within the cell. Nearly two decades ago, pioneering work by Hannun et al. and Kolesnick et al. established the foundation for the bioactive nature of sphingolipids by demonstrating the inhibition of protein kinase C (PKC) by sphingosine and the stimulation of a ceramide-activated protein kinase in response to tumor necrosis factor (TNF)-α, respectively (1, 2). Sphingolipids have since been recognized as critical activators or inhibitors of various protein kinases and phosphatases, receptors, and ion transporters (3). Moreover, sphingolipids have been identified as key regulators of a vast number of cellular processes, including cell growth, adhesion, migration, senescence, apoptosis, and most recently, autophagy (3, 4).
Much of the investigation into sphingolipid signaling has focused on the disparate nature of ceramide and sphingosine-1-phosphate (S1P). Ceramide is typically associated with growth arrest and apoptosis, while S1P promotes cell proliferation and survival. The opposing nature and dynamic balance of intracellular ceramide and S1P, termed the “sphingolipid rheostat,” has been proposed to determine cell fate (5). However, emerging evidence and extensive characterization of the sphingolipid metabolic network suggests that this two-dimensional model does not adequately reflect sphingolipid signaling and function within the cell. In addition to ceramide and S1P, other sphingolipid metabolites, such as sphingosine, dihydroceramide, and gangliosides, have been implicated in the regulation of apoptosis and macroautophagy (hereafter referred to as autophagy). Although autophagy is typically considered to be a cytoprotective mechanism for the suppression of apoptosis, recent evidence indicates that autophagy can also promote cell death (6). Due to the differential regulation of apoptosis and autophagy by sphingolipid metabolites, the sphingolipid network has emerged as a novel molecular switch between the apoptotic and autophagic pathways. This review aims to describe the regulation of apoptosis and autophagy by sphingolipids and begin to position sphingolipid metabolism as a critical regulator of crosstalk between the two cellular pathways.
SPHINGOLIPID METABOLISM
Sphingolipid metabolism is highly interconnected and compartmentalized (3, 7–9). Ceramide, which occupies a central position in the sphingolipid network, can be generated by de novo synthesis, the degradation of complex sphingolipids or the recycling of long chain bases (3, 7–9). Ceramide synthase, which resides in the endoplasmic reticulum (ER), catalyzes the formation of dihydroceramide during de novo synthesis or ceramide during the salvage of sphingolipid bases. Notably, six isoforms of ceramide synthase (CerS1-6; longevity assurance genes 1–6/LASS1–6) have been identified and display varying specificities for fatty acyl-CoA substrate chain lengths (10). Ceramide is the precursor for a number of complex sphingolipids, including glycosphingolipids, sphingomyelin, and ceramide-1-phosphate (3, 7–9). The catabolism of ceramide by ceramidase liberates the sphingolipid base sphingosine, which is readily phosphorylated by sphingosine kinase (SK1 and SK2) to generate S1P. Exit from the sphingolipid network is mediated by S1P lyase (SPL), which irreversibly cleaves S1P to the nonsphingolipid metabolites 2-hexadecenal and phosphoethanolamine.
APOPTOSIS, AUTOPHAGY, AND CROSSTALK
Apoptosis
Programmed cell death (PCD) is essential for proper development and the maintenance of homeostasis (11). Apoptosis is an extensively studied PCD mechanism that is characterized by the activation of a family of cysteine proteases known as caspases. Caspases are activated through extrinsic and intrinsic signaling pathways and mediate the cleavage of a number of cytosolic and nuclear proteins to induce the hallmark morphological features of apoptosis (i.e., nuclear fragmentation and membrane blebbing) (12, 13). Initiation of the extrinsic pathway of apoptosis occurs at the plasma membrane upon the ligation of a death receptor belonging to the TNF receptor superfamily. Multimerization of activated death receptors promotes the assembly of the death-inducing signaling complex (DISC), which serves as a platform for procaspase-8 oligomerization and auto-activation through self-cleavage. In contrast, initiation of the intrinsic pathway occurs in response to intracellular stress signals, such as DNA damage or cytotoxic stress. Activation and oligomerization of the proapoptotic Bcl-2 family proteins Bax and Bak trigger mitochondrial outer membrane permeabilization (MOMP) and the release of apoptogenic factors, such as cytochrome c (cyt c) (Fig. 1). Cytosolic cyt c associates with Apaf-1 and procaspase-9 in a multiprotein complex known as the apoptosome to promote caspase-9 self-activation. Ultimately, the extrinsic and intrinsic pathways converge with the activation of effector caspases (caspase-3, caspase-6, caspase-7), which cleave cytosolic and nuclear substrates to execute the cell death pathway.
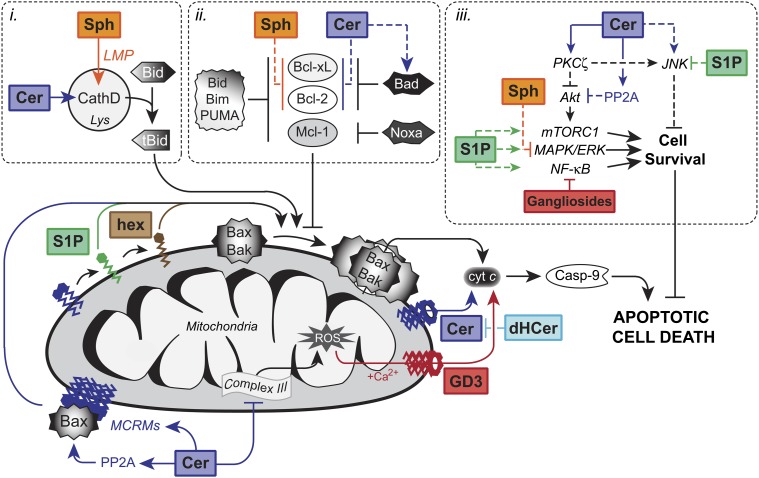
Fig. 1.
A generalized overview of the regulation of apoptosis by sphingolipids. Sphingolipids have direct effects on mitochondrial function. Ceramide (Cer) assembles channels in the outer membrane of mitochondria to promote the release of cytochrome c (cyt c) for caspase-9 activation. Dihydroceramide (dHCer) inhibits ceramide channel formation. Similarly, the ganglioside GD3 permeabilizes mitochondria in a ROS and calcium-dependent manner. Additionally, Cer directly inhibits mitochondrial complex III to generate ROS. Cer promotes Bax activation and recruitment to the mitochondria through the PP2A-dependent dephosphorylation of Bax and formation of mitochondrial ceramide-rich macrodomains (MCRMs). Furthermore, mitochondrial Cer is metabolized to S1P and hexadecenal (hex), which directly activate Bax and Bak, respectively. i) Lysosomal effects. Cer directly binds and activates the lysosomal protease cathepsin D to enhance Bid cleavage and induction of the mitochondrial pathway of apoptosis. At low to moderate concentrations, sphingosine (Sph) becomes protonated and trapped within lysosomes, leading to lysosomal membrane permeabilization (LMP) and cleavage of Bid for the induction of apoptosis. ii) Bcl-2 family. Sph downregulates antiapoptotic Bcl-2 and Bcl-xL to promote apoptosis. Cer activates PP1 and PP2A to regulate the alternative splicing of apoptosis-promoting variants Bcl-xS and Caspase-9 and inhibit the antiapoptotic effects of Bcl-2, respectively. iii) Kinase signaling. Cer directly activates protein kinase C ζ (PKCζ), which mediates the activation of JNK and inhibition of Akt to promote apoptosis. S1P suppresses Cer-mediated activation of JNK and activates pro-survival Akt/mTORC1, MAPK/ERK, and NF-κB signaling pathways through cell surface receptors. Sph-induced apoptosis is characterized by suppression of MAPK/ERK signaling, while gangliosides enhance apoptosis through the inhibition of NF-κB.
Autophagy
Autophagy is a catabolic process in which cytoplasmic components are sequestered in double-membrane vesicles, known as autophagosomes, and delivered to lysosomes for degradation and recycling. Autophagy promotes cell survival during stress, such as hypoxia or nutrient deprivation, through the removal of damaged proteins and/or organelles and the generation of nutrients; however, autophagy can also mediate caspase-dependent and independent cell death (14–17). Autophagosome biogenesis is divided into three steps: initiation, nucleation, and elongation/expansion/closure; each of which is tightly regulated by a specific set of autophagy-related (Atg) proteins (Fig. 2) (18–20). The Unc-51-like kinase 1 (Ulk1; mammalian homolog of Atg1) complex initiates autophagosome biogenesis. Importantly, the Ulk1 complex is negatively regulated by mechanistic target of rapamycin complex 1 (mTORC1), which phosphorylates Ulk1 and the associated protein Atg13 during nutrient-rich conditions to inhibit autophagy (21). Upon nutrient depletion, the cellular energy sensor AMP-activated protein kinase (AMPK) suppresses mTORC1 and directly activates Ulk1 through phosphorylation to initiate autophagy (21). The Beclin 1-containing class III phosphatidylinositide 3-kinase (PI3KC3, Vps34) complex I regulates nucleation of autophagosomal membranes. Beclin 1 (mammalian homolog of Atg6) positively regulates PI3KC3 activity to generate phosphatidylinositol 3-monophosphate (PI3P), which likely mediates the recruitment of additional Atg proteins for autophagosome biogenesis (22). The elongation, expansion, and closure of autophagosomal membranes require the Atg12-Atg5 and microtubule-associated protein 1 light chain 3 (LC3)-phosphatidylethanolamine (PE) ubiquitin-like conjugation systems (23). LC3-II/LC3-PE is the only protein known to be directly associated with the autophagosomal membrane throughout the process of autophagy and thus is a widely used marker of autophagosomes (24). Finally, autophagosome-lysosome fusion delivers autophagic cargo to the lysosome for degradation and recycling.
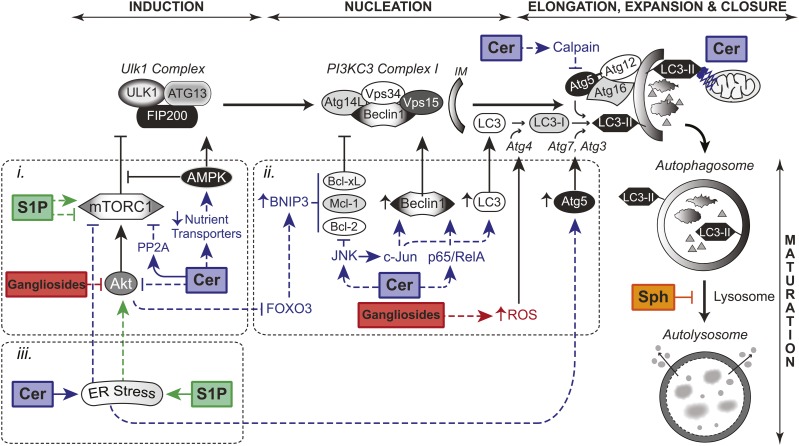
Fig. 2.
A generalized overview of the regulation of autophagy by sphingolipids. Sphingolipids have direct effects on autophagy. Ceramide (Cer) activates calpain, which cleaves Atg5 to generate a protein fragment that promotes apoptosis and suppresses autophagy. Mitochondrial Cer mediates mitophagy (i.e., autophagic degradation of mitochondria) through the direct association of ceramide with LC3-II. Sphingosine (Sph) is likely to suppress autophagosome maturation. i) mTORC1 signaling. Cer suppresses Akt activation to relieve the downstream inhibitory effects of mTORC1 on autophagy. Similarly, the ganglioside-induced autophagy is associated with the suppression of Akt. Acid sphingomyelinase-derived Cer also shuts down mTORC1 activity during amino acid deprivation in a PP2A-dependent manner. Cer-induced autophagy is also associated with the downregulation of nutrient transporter proteins to activate autophagy through AMPK and lead to a bioenergetic crisis for the induction of cell death. S1P differentially regulates mTOR activity. Overexpression of SK1 as well as ligation of the cell surface receptor S1P5 inhibits mTORC1 to induce S1P-mediated autophagy. In contrast, ligation of S1P3 by S1P is reported to activate mTORC1 for the suppression of Cer-induced autophagy. ii) Transcriptional regulation. Cer-mediated inhibition of Akt activates FOXO3 to upregulate BNIP3 expression. BNIP3 liberates Beclin 1 for the induction of autophagy through the competitive binding of Bcl-2, Bcl-xL, and Mcl-1. Cer-mediated activation of JNK and the transcription factor, c-Jun, upregulates Beclin 1 and LC3 expression to promote autophagy. JNK activation also disrupts the inhibitory Beclin 1:Bcl-2 complex through direct phosphorylation of Bcl-2. The upregulation of Beclin 1 by Cer also is mediated by the activation of NF-κB. Additionally, ganglioside-induced autophagy is dependent on the production of ROS, which regulates Atg4 activity. iii). ER stress. The accumulation of Cer and S1P within the ER has been associated with the induction of ER stress leading to autophagy. Interestingly, S1P-mediated ER stress leads to the activation of Akt for cell survival. In contrast, Cer-mediated ER stress has been associated with inhibition of mTORC1 for autophagy induction as well as the upregulation of Atg5 and Beclin 1.
Crosstalk between apoptosis and autophagy
The functional crosstalk between apoptosis and autophagy is complex. Under certain cellular contexts, autophagy functions as a stress-response to suppress apoptosis and promote cell survival. However in other settings, autophagy may serve as a mechanism of caspase-dependent or independent cell death (14–17). Recent investigation into the crosstalk between apoptosis and autophagy has identified many common regulators (6, 25) (Table 1). The Bcl-2 family proteins provide a molecular link between the mitochondrial pathway and autophagy (26). In addition to suppressing apoptosis, the antiapoptotic Bcl-2 proteins (Bcl-2, Bcl-xL, and Mcl-1) bind the BH3 domain of Beclin 1 to suppress PI3KC3 activity and autophagosome nucleation. Furthermore, several key members of the extrinsic pathway, such as caspase-8 and FLICE-like inhibitor proteins (FLIP), negatively regulate autophagy (27, 28). Conversely, accumulation of autophagosomal membranes can serve as a platform for caspase-8 activation through an intracellular death-inducing signaling complex (iDISC) (29–34). Interestingly, several autophagic proteins are also reported to stimulate apoptosis independent of autophagic functions (Table 1). For example, the calpain-mediated cleavage of Atg5 generates a proapoptotic protein fragment that translocates to the mitochondria to initiate apoptosis (35). Similarly, the caspase-mediated cleavage of Beclin 1 suppresses autophagy and activates the mitochondrial pathway (36–38). Finally, apoptosis and autophagy also share several common transcriptional regulators [e.g., nuclear factor-kappa B (NF-κB) and p53] and kinase signaling pathways (e.g., PI3K/PTEN/Akt/mTOR and JNK) that mediate cell fate.
TABLE 1.
Shared regulators of autophagy and apoptosis
| Autophagy |
Apoptosis |
|||
| Positive Regulators | Negative Regulators | Positive Regulators | Negative Regulators | |
| Apoptosis proteins | Bcl-2, Bcl-xL, Mcl-1 | Bcl-2, Bcl-xL, Mcl-1 | ||
| Bad, BNIP3, NIX | Bad, BNIP3, NIX | |||
| Bim | Bim | |||
| FLIPs | FLIPs | |||
| Caspase-8 | Caspase-8 | |||
| Autophagy proteins | Beclin 1 | Caspase-cleaved Beclin 1 | ||
| Atg5 | Atg5, Calpain-cleaved Atg5 | |||
| Atg12 | Atg12, Atg3-Atg12 | |||
| Bif-1 | Bif-1 | |||
| p62 | p62 | p62 | ||
| Transcription factors | NF-κB | NF-κB | NF-κB | |
| p53 | p53 | p53 | ||
| Kinase signaling | PI3K/Akt/mTOR | PI3K/Akt/mTOR | ||
| JNK | JNK | |||
| Sphingolipids | Sphingosine-1- phosphate | Sphingosine-1- phosphate | ||
| Dihydroceramides | Dihydroceramides? | |||
| Ceramides | Ceramides | |||
| Gangliosides | Gangliosides | |||
| Sphingosine? | Sphingosine | |||
SPHINGOLIPIDS IN APOPTOSIS AND AUTOPHAGY
Ceramide
Ceramide was first recognized as a regulator of apoptosis (39) and cellular senescence (40) in the 1990s and has since emerged as a critical mediator of cell death. Notably, ceramide accumulation is observed to occur secondary to the primary effects of radiation treatment and many chemotherapeutics, including but not limited to doxorubicin, etoposide, vincristine, and taxol (41). Interestingly, ceramide clearance or metabolism has been identified as a feature of many drug-resistant cancers (42). As a result, the elevation of ceramide levels through exogenous delivery, stimulation of de novo synthesis, or inhibition of ceramide metabolism has become an attractive chemotherapeutic strategy (41). Although the use of exogenous short-chain ceramide analogs may not necessarily mimic the effects of endogenous long-chain or very long chain ceramides, short-chain analogs are converted to endogenous ceramides within the cell (43, 44). Remarkably despite years of study, a unified mechanism of ceramide-induced apoptosis has yet to be identified; however, ambiguity in ceramide signaling is not all that surprising when one considers the diversity of ceramide species that exist within the cell. Ceramide is a substrate or product of more than 28 distinct enzymes and consists of more than 200 individual species with varying head groups and/or acyl chain lengths (45). Furthermore, the condensation of alanine with palmitoyl CoA by serine palmitoyltransferase (SPT) has been demonstrated to result in the generation of a new class of “ceramides” containing a 1-deoxysphingoid backbone (46). The “many ceramides” paradigm hypothesizes that individual ceramide molecules are generated within distinct biochemical pathways and subcellular compartments to exert unique functions (45). For example, a recent study suggests a proapoptotic role of CerS1-generated C18:0-ceramide and prosurvival function of CerS6-generated C16:0-ceramide (47); however, an additional study has proposed that long-chain ceramides (C16:0, C18:0, C20:0) are antiproliferative, whereas very long chain ceramides (C24:0, C24:1) promote cell proliferation (48). Continued investigation and use of MS/MS lipidomics will greatly aid in the identification of individual ceramide species and their respective biological effects.
While individual ceramide species may differentially regulate apoptotic signaling pathways, studies over the past two decades have implicated the mitochondria as a key site of ceramide-mediated apoptosis (Fig. 1). Early investigation demonstrated direct effects of ceramide on mitochondrial function. In these studies, the addition of C2-ceramide to isolated mitochondria directly inhibited mitochondrial complex III to generate reactive oxygen species (ROS) (49, 50). Later in vitro studies revealed that long- and short-chain ceramides assemble channels in the outer mitochondrial membrane to release mitochondrial proteins, including cyt c (51, 52). Interestingly, while independent of Bax and Bak, ceramide channels are inhibited by antiapoptotic Bcl-2 proteins (53). While the in vivo relevance of these results remains to be demonstrated, mitochondrial ceramide levels are observed to increase during apoptosis in response to diverse stimuli, including CD95/Fas and radiation (54). Furthermore, selective targeting of bacterial SMase to various organelles has revealed that only a mitochondrial pool of ceramide is sufficient to induce apoptosis in MCF7 breast adenocarcinoma cells (55). Consistent with these results, mitochondria-associated membranes have been shown to mediate the transfer of ceramide between the ER and mitochondrial compartments and permeabilize mitochondria in an in vitro system (56).
In addition, ceramide has been reported to induce Bax-dependent apoptosis in several cancers, including glioblastoma (57), breast cancer (58), prostate cancer (59), colon cancer (59), and acute myeloid leukemia (60). Increased mitochondrial ceramide enhances Bax translocation to the mitochondria in both a cell free system as well as MCF7 cells stimulated with TNF-α (58). Furthermore, ceramide and activated Bax directly interact in vitro to synergistically induce outer membrane permeabilization in isolated mitochondria (61). Moreover, Bax association with ceramide-enriched detergent-resistant membrane domains occurs in situ upon ischemia/reperfusion, a well-characterized apoptotic model (62). Further investigation has characterized the assembly of mitochondrial ceramide-rich macrodomains (MCRM) with enhanced association of Bax oligomers upon exposure to radiation (63). Interestingly, ceramide transfer to the mitochondria serves as a substrate for metabolism to S1P and/or hexadecenal, which directly promote Bax and Bak activation, respectively (64). Therefore, local regulation of sphingolipid metabolism within mitochondria appears to have a critical role in apoptosis.
In addition to potential direct lipid effects, C2-ceramide enhances the activation and translocation of Bax through the dephosphorylation of Ser184 (65). Bax dephosphorylation has been suggested to occur through PP2A as PP2A dephosphorylates Ser184 in vitro and interacts with Bax upon ceramide treatment in intact cells (65). Notably, the phosphatases protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) are directly activated by ceramides (66, 67). Additionally, C2-ceramide induces the PP2A-dependent dephosphorylation of Bcl-2 at Ser70 to inhibit its antiapoptotic activity and promote its binding to p53 for the induction of apoptosis (68–70).
Furthermore, ceramide-mediated apoptosis is regulated by transcriptional mechanisms. Exogenous ceramide and the stimulation of de novo ceramide synthesis dynamically regulate the splicing of Bcl-x and caspase-9 in human lung carcinoma A549 cells prior to the induction of apoptosis (71). Specifically, ceramide upregulates the apoptosis-promoting variants Bcl-xS and caspase-9, while correspondingly downregulating the antiapoptotic variants Bcl-xL and caspase-9b (71–73). As this effect was blocked by the PP1 and PP2A inhibitor calyculin A but not by okadaic acid, a PP2A-specific inhibitor, ceramide-induced alternative splicing is suggested to occur through a PP1-dependent mechanism (71). Moreover, serine/arginine-rich (SR) proteins, which are known modulators of mRNA splicing, have been identified as PP1 substrates (74), thus providing a potential mechanism for the alterative splicing of Bcl-x and caspase-9 in response to ceramide.
PKCζ and the lysosomal protease cathepsin D are additional direct effectors of ceramide that have been implicated in ceramide-mediated growth arrest and apoptosis. Ceramide directly activates PKCζ in vitro and in intact cells (75–77). Moreover, C6-ceramide treatment of A7r5 smooth muscle cells stimulates the recruitment of PKCζ to structured lipid microdomains (“lipid rafts”) where it becomes activated to suppress Akt3 phosphorylation (Ser34) and cell proliferation (76, 78). Furthermore, PKCζ has been linked to the activation of the stress-activated protein kinase JNK (75, 77). PKCζ also functions to promote cell survival through the inhibition of acid SMase in response to UV-C irradiation (79) and the phosphorylation of Bax (80). Cathepsin D is a lysosomal aspartyl protease implicated in the induction of apoptosis. Short- and long-chain ceramides directly bind cathepsin D to promote its proteolytic maturation and activation in vitro and in vivo (81). Upon stimulation with TNF-α/cycloheximide or gemcitabine, the acid SMase-derived accumulation of endosomal and lysosomal ceramide enhances cathepsin D-mediated cleavage of the BH3-only protein Bid to activate the mitochondrial pathway of apoptosis (82, 83). Therefore, the direct effectors, PKCζ and cathepsin D, also regulate the induction of apoptosis in response to ceramide.
In addition to apoptosis, ceramide has more recently been implicated in the induction of autophagy (84–94) (Fig. 2). As autophagy has dual functions in cell survival and cell death, defining a role of ceramide-induced autophagy has critical implications for enhancing the efficacy of ceramide-generating chemotherapeutics and sphingomimetics. Endogenous ceramide species are critical for the induction of autophagy, as inhibition of CerS by fumonisin B1 or SPT by myriocin completely suppresses autophagy induced in response to short-chain ceramides and tamoxifen, respectively (85, 88). Consistently, de novo synthesis is reported to be essential for the induction of autophagy in activated RAW264.7 cells (90) as well as in Saccharomyces cerevisiae (95). However, the mechanism and function of ceramide-induced autophagy remains unclear.
Ceramide is a well-established suppressor of Akt (96); thus inactivation of mTOR signaling downstream of Akt can be hypothesized as a mechanism by which ceramide induces autophagy. Indeed, the accumulation of ceramide upon exposure to exogenous C2-ceramide, tamoxifen, or the glucosylceramide synthase inhibitor D,L-Threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) suppresses Akt activity to stimulate autophagy in human colon cancer and breast cancer cell lines (85). Additionally, recent work has shown that amino acid deprivation enhances ceramide levels to lead to the PP1/PP2A-dependent inactivation of mTOR for the induction of autophagy (94). Interestingly, acid SMase rather than de novo synthesis or the sphingosine salvage pathway was responsible for the increase in ceramide levels and induction of autophagy during amino acid depletion; however, the mechanism of acid SMase activation under these conditions remains unclear (94). In contrast, ceramide induces starvation under nutrient-rich conditions through the suppression of nutrient transporter protein expression in murine prolymphocytic FL5.12 cells, resulting in the AMPK-mediated induction of autophagy, bioenergetic crisis, and cell death (86).
Disruption of the inhibitory Beclin 1:Bcl-2 complex has also emerged as a common mechanistic theme in ceramide-induced autophagy. Exogenous C2-ceramide, tamoxifen, and PDMP enhance the expression of Beclin 1 to promote autophagy (85). Increased expression of Beclin 1 is a contributing factor in the induction of lethal autophagy in human leukemia cell lines treated with arsenic trioxide, an agent that stimulates de novo ceramide synthesis and inhibits ceramide metabolism to glucosylceramide (97, 98). Ceramide is a well-established activator of the stress-activated kinase JNK (99, 100), and the JNK-mediated activation of the transcription factor c-Jun enhances Beclin 1 expression (89). Additionally, c-Jun positively regulates the transcription of LC3 to increase the autophagic process in response to ceramide (91). Furthermore, activation of the NF-κB subunit p65/RelA is reported to mediate Beclin 1 upregulation during ceramide treatment (87). In addition to altering the balance between Beclin 1 and Bcl-2 protein levels, endogenous ceramide liberates Beclin 1 for autophagy induction through the JNK-mediated phosphorylation of Bcl-2 (88). Notably, Bcl-2 has emerged a critical regulator of ceramide-mediated crosstalk between autophagy and apoptosis. Specifically, the intracellular localization of Bcl-2 is a key factor in mediating the crosstalk, as the ceramide-dependent dephosphorylation of mitochondrial Bcl-2 by PP2A promotes apoptosis while the JNK-dependent phosphorylation of ER-targeted Bcl-2 enhances ceramide-induced autophagy.
In addition to the upregulation of Beclin 1, enhanced expression of the mitochondrial BH3-only protein BNIP3 promotes Beclin 1 dissociation from the inhibitory complex through the competitive binding of antiapoptotic Bcl-2 proteins (101). C2-ceramide was demonstrated to enhance BNIP3 expression in several malignant glioma cell lines, leading to autophagic cell death (84). Similarly, treatment of malignant glioma cells with arsenic trioxide upregulated BNIP3 to induce caspase-independent cell death (102, 103). Activation of Forkhead box protein O3 (FOXO3), a transcription factor negatively regulated by Akt, mediates the upregulation of BNIP3 in response to ceramide (104). Interestingly, the BNIP3 homolog BNIP3L/NIX is an important regulator of mitophagy, a process for selective degradation of mitochondria by autophagy. As ceramide is a well-established inducer of mitochondrial damage, the induction of mitophagy may contribute to increased autophagic activity in response to ceramide. Indeed, a recent report has demonstrated that mitochondrial ceramide selectively targets mitochondria to LC3-II-containing autophagolysosomes through direct interaction between ceramide and LC3-II (93). In further support of this model, mitochondrially targeted C6-ceramide preferentially induces mitochondrial damage and protective autophagy in comparison to untargeted C6-ceramide (105).
Ceramide-mediated autophagy is also attributed to the induction of ER stress. Downregulation of ER-localized CerS2, which has a preference for very long chain acyl groups (C22–C26), results in the significant accumulation of long-chain ceramides (C14-Cer and C16-Cer) and the induction of ER stress-dependent autophagy to protect the cell from cell death (106). In contrast, stimulation of de novo ceramide synthesis by the cannabinoid tetrahydrocannabinol (THC) induces the ER stress-mediated inhibition of mTORC1 for autophagosome nucleation (107). Interestingly, autophagy under these conditions served to promote mitochondrial apoptosis, thereby demonstrating a switch between ceramide-mediated autophagy and apoptosis (107). Likewise, melanoma differentiation-associated gene 7 (mda-7)/interleukin-24 (IL-24) initiates autophagy through the ER stress and eukaryotic translation initiation factor 2α kinase (PERK)-dependent stimulation of de novo ceramide synthesis and upregulation of Beclin 1 and Atg5 (108, 109). In a manner similar to THC, mda-7/IL-24 switched autophagy to apoptosis through the calpain-mediated cleavage of Atg5 (109). Consistently, the accumulation of endogenous ceramide in response to doxorubicin or exogenous C6-ceramide activates calpain to promote Atg5 cleavage and the induction of apoptosis (110). However, activation of calpain in response to ceramide also is reported to stimulate NF-κB survival signaling (111); thus, ceramide-mediated activation of calpain likely tightly regulates cell fate.
Further investigation will clarify the function of ceramide-induced autophagy and allow for the identification of appropriate autophagy modulation (i.e., inhibition or stimulation) during ceramide treatment. In light of recent studies demonstrating the chain length-specific roles of ceramide, it has recently been revealed that CerS5 and C14:0 ceramide are required for induction of autophagy leading to hypertrophy in cardiomyocytes (92). In addition to highlighting a pathogenic function of ceramide-induced autophagy, the study suggests that chain length-specific ceramides may differentially regulate autophagy. Additional MS/MS lipidomics will greatly aid in discerning the role of individual ceramide species in the regulation of apoptosis, autophagy, and crosstalk.
Sphingosine
The bioactive nature of sphingosine was first demonstrated in 1986 when the lipid was shown to inhibit PKC, a key survival signaling kinase (1). As with ceramide, sphingosine has become associated with the induction of apoptosis (Fig. 1). However, due to the metabolic interconversion of the two lipids, identification of sphingolipid-specific apoptotic signaling has been challenging. Although sphingosine has been observed to induce apoptosis independent of the salvage pathway (112–115), it has also been reported to occur in a CerS-dependent manner (116). Therefore, the significance of sphingosine metabolism in the induction of apoptosis may be cell type- and/or stimulus-dependent.
Despite these limitations, differential mechanisms of apoptosis induction have been identified for ceramide and sphingosine. Ceramide and sphingosine are opposing regulators of the ERK and SAPK cascades (100). While ceramide-induced apoptosis has been associated with the strong activation of JNK and weak inhibition of ERK in U937 human monoblastic leukemia cells, apoptosis in response to sphingosine was accompanied by the weak activation of JNK and strong inhibition of MAPK/ERK (117). Furthermore, while the pan-caspase inhibitor z-VAD-fmk rescues cells from apoptosis in response to ceramide and sphingosine, the granzyme B inhibitor z-AAD-fmk and caspase-8 inhibitor z-IETD-fmk protect against only sphingosine-mediated cell death (118). Furthermore, sphingosine has been shown to assemble channels in the outer mitochondrial membrane of isolated mitochondria; however, the channels exhibit a significantly shorter half-life than ceramide channels and are too small to permit the release to apoptogenic proteins (119). Therefore, there appear to be fundamental differences in ceramide- and sphingosine-induced apoptosis.
Sphingosine has also been shown to regulate the expression of antiapoptotic Bcl-2 proteins to modulate apoptosis. Exogenous sphingosine downregulates Bcl-2 to promote apoptosis in HL-60 human AML cells (120). Moreover, in DU-145 prostate cancer cells, sphingosine suppresses Bcl-xL expression to enhance apoptosis (121). However, the mechanism(s) responsible for these effects remain to be characterized. Interestingly, depletion of S1P lyase and the resulting accumulation of S1P have been shown to upregulate the expression of antiapoptotic Bcl-2 proteins to promote chemoresistance and oncogenesis (122). Therefore, it is possible that sphingosine accumulation disrupts the sphingosine/S1P balance to alter Bcl-2 and/or Bcl-xL expression. In addition, sphingosine has been shown to regulate sphingosine-dependent protein kinase-1 (SDK1), a truncated protein containing the kinase domain of PKCδ (123). SDK1 is directly activated by sphingosine and phosphorylates isoforms of 14-3-3 on its dimer interface to presumably disrupt 14-3-3 dimerization (123, 124). Disruption in the conformation and/or dimerization of the chaperone protein is hypothesized to suppress binding of kinases involved in signal transduction (124).
Alternatively, sphingosine-induced apoptosis has been reported to initiate at the lysosome. At low to moderate doses, sphingosine becomes protonated within the acidic environment of the lysosome to result in the dose-dependent rupture of the lysosomal membrane and release of lysosomal proteases, such as cathepsin D, which cleave Bid to activate the mitochondrial pathway (125). Notably, the induction of autophagy in response to sphingosine has not been reported. However, due to the effects on lysosomal function, we hypothesize that the lysosomal accumulation of sphingosine will block autophagic flux (Fig. 2). Interestingly, inhibition of SK by the pan-SK inhibitor SKI-I has been demonstrated to result in iDISC-dependent cell death (30). Although the role of sphingolipids in iDISC formation is unclear, it is tempting to speculate that sphingosine accumulation and lysosomal dysfunction may disrupt autophagic flux to mediate the accumulation of autophagosomal membranes for iDISC-dependent cell death. Moreover, due to the reported effects of sphingosine on Bcl-2 and Bcl-xL expression, it is possible that sphingosine may regulate the PI3KC3 complex I. Further study and the use of MS/MS lipidomics will aid in the identification of sphingosine-specific effects on the apoptotic and autophagic pathways.
Sphingosine-1-phosphate
Sphingosine-1-phosphate was identified as a promitogenic, lipid-derived second messenger in 1993 (126). A short time later, S1P was shown to have antiapoptotic properties (Fig. 1) as the lipid antagonized ceramide-mediated apoptosis through the activation of ERK and suppression of ceramide-induced JNK activation (5), thereby establishing the foundation for the “sphingolipid rheostat.” Further characterization of S1P-mediated cellular effects has revealed that SK1 and SK2 isoforms differentially regulate cell fate. While SK1 activity is associated with mitogenic and antiapoptotic effects, overexpression of SK2 has been shown to promote cell death (127–129). Furthermore, SK1 is an oncogene that is upregulated in many cancers and associated with drug resistance, whereas expression of SK2 sensitizes cells to chemotherapeutic agents (130). The differential cellular effects of SK1 and SK2 are attributed to differences in ceramide production. SK2 is proposed to act in conjunction with S1P phosphatase to phosphorylate/dephosphorylate sphingosine for ceramide production via the salvage pathway, while cytosolic SK1 generates S1P that may suppress ceramide generation (44, 131).
Signaling by S1P occurs through intracellular and extracellular mechanisms (132). S1P is a ligand of five G-protein coupled receptors, S1P1–S1P5, which are coupled to various signaling pathways, such as Akt/mTOR (133), NF-κB (134), and MAPK (135). S1P also has intracellular functions. For example, S1P activates calcium channels in a pertussis toxin-independent manner to mobilize calcium stores (136). Notably, the SK2-mediated generation of nuclear S1P inhibits the activity of histone deacetylase 1 and 2 (HDAC1 and HDAC2) to regulate gene expression (137). This work identified S1P as the first nuclear lipid associated with the epigenetic regulation of gene expression and exposes nuclear sphingolipid metabolism as an intriguing area of study for the regulation of autophagy and apoptosis. Interestingly, a recent study has linked enhanced nuclear S1P in SPP1 deficient fibroblasts with reduced HDAC activity and dysregulation of calcium homeostasis (138). Furthermore as previously noted, the generation of mitochondrial S1P directly activates Bak to promote the release of cytochrome c; therefore, further stressing the importance of subcellular sphingolipid pools in regulating cell fate (64).
Consistent with the generally protective role of S1P, S1P-induced autophagy (Fig. 2) appears to function as a survival mechanism. Notably, SK activity is stimulated in response to nutrient deprivation and mediates the induction of autophagy for the survival of human breast cancer MCF-7 cells during starvation (139). In agreement with these results, enhanced SK activity has previously been reported during starvation in Saccharomyces cerevisiae, thus suggesting a general role for SK in stress response (140). Furthermore, overexpression of SK1 induces autophagy under nutrient-rich conditions through the inhibition of mTOR (139). Importantly, SK1(S1P)-induced autophagy occurs independently of ceramide synthase and the class I PI3K signaling arm (139).
In contrast to the overexpression of cytosolic SK1, depletion of ER-localized SPP1 induces the accumulation of S1P and the induction of autophagy in an ER stress-dependent manner (141). Notably, the induction of ER stress-mediated autophagy did not stimulate cell death but, rather, led to the PERK-dependent activation of Akt to promote cell survival (141). Interestingly, doxorubicin treatment dramatically reduces the autophagic activity of SPP1-depleted cells and sensitizes the cells to apoptosis; but surprisingly, apoptosis occurred in an autophagy-dependent manner (110). The authors concluded that doxorubicin enhances de novo ceramide synthesis to suppress Akt and stimulate the calpain-mediated cleavage of Atg5, thus effectively switching protective autophagy in SPP1-depleted cells to apoptosis (110). It is interesting to note that although the depletion of ER-localized SPL enhanced autophagy in this system, the cells were not sensitized to doxorubicin (110); thus SPP1 and SPL, which mediate the salvage pathway and irreversible cleavage of S1P, respectively, appear to regulate different pools of S1P.
It is evident that, as with many other sphingolipid metabolites, the intracellular localization of S1P appears to be an important determinant of S1P-induced autophagy. Likewise, the intrinsic and extrinsic regulation of autophagy by S1P is distinct. Exogenous S1P induces autophagy in human prostate cancer cells under serum-free conditions through the S1P5-dependent inhibition of mTOR (142). In contrast, the binding of exogenous S1P to S1P3 activates mTOR to suppress ceramide-mediated autophagy and rescue cells from autophagy-associated cell death (94). Thus, in addition to inducing autophagy, S1P functions to counter the effects of ceramide in the sphingolipid rheostat.
Additionally, autophagy has been described in response to the pan-SK inhibitors SKI-I, dimethylsphingosine, and SKI-2, as well as the SK2-specific inhibitor ABC294640 (30, 143). Notably, SKI-I has been demonstrated to stimulate the autophagy-dependent activation of caspase-8 and initiation of the caspase cascade via iDISC formation (30). In contrast, dimethylsphingosine, SKI-2, and ABC294640 induced autophagic cell death that was associated with a decrease in Akt activity and upregulation of Beclin 1 (85, 143), mechanisms similar to that described by ceramide. Furthermore, the water-soluble sphingosine analog FTY720 has been shown to induce caspase-independent cell death through the downregulation of nutrient transporters and induction of autophagy (144, 145). However, treatment with FTY720, which is reported to inhibit S1P lysase (146), SK1 (147), and CerS (148, 149), induced such effects independent of ceramide production and S1P receptors (145), suggesting a mechanism that is distinct from ceramide and the SK inhibitors described above.
Further study is required to characterize the effects of SK1 and SK2 in the induction of autophagy and regulation of cell survival or cell death. Moreover, additional characterization of S1P-mediated autophagy will confirm whether this pathway functions solely as a protective mechanism for cell survival or whether S1P can also initiate autophagic cell death. The induction of exclusively cytoprotective autophagy in response to S1P would be highly interesting given the association of SK1 with chemoresistance (150).
Dihydroceramide
The ceramide precursor, dihydroceramide, contains the sphingoid base sphinganine rather than sphingosine. Dihydroceramide has historically been considered to be a biologically inactive lipid (151); however, recent studies have proposed a bioactive role for the lipid. For example, accumulation of dihydroceramides has been implicated in the induction of cell death in response to the anticancer agents fenretinide (152) and γ-tocotrienol (153) in several cancer cell lines. In contrast, short and long chain dihydroceramides have also been suggested to exhibit antiapoptotic effects (Fig. 1), as the lipids are able to inhibit ceramide channel assembly in isolated mitochondria (154). Moreover, the ER-localized enzyme dihydroceramide Δ4-desaturase 1 (DES1), which metabolizes dihydroceramide to proapoptotic ceramide, requires myristoylation on its N-terminus for full activity (155). Interestingly, the N-myristolyation of DES1 has been suggested to target a portion of the enzyme to the mitochondria in COS-7 cells to mediate the apoptotic effects of myristic acid (156). It is therefore tempting to speculate that the metabolism of mitochondrial dihydroceramide and removal of an antiapoptotic influence may work in conjunction with the local accumulation of ceramide to stimulate apoptosis. In addition, the accumulation of dihydroceramide has been reported to enhance cell survival and suppress cell proliferation during hypoxia (157). Interestingly, dihydroceramide is proposed to promote cell survival during hypoxia though the induction of autophagy while also serving as a lipid reserve for the rapid production of ceramide for cellular damage upon reperfusion (157). If this hypothesis is validated, dihydroceramide would serve as a unique regulator of cell fate to “switch” cytoprotective autophagy to ceramide-mediated apoptosis in response to stress. Currently, several studies have implicated dihydroceramide in the regulation of autophagy (153, 158–160). While the studies demonstrate the induction of autophagy in response to exogenous C2-dihydroceramide or the accumulation of endogenous dihydroceramide by resveratrol, γ-tocotrienol, and the dihydroceramide desaturase inhibitor XM462, the signaling pathways responsible for the induction remain undefined. Further investigation of dihydroceramide is warranted to confirm what role, if any, the lipid plays in regulating cell fate.
Gangliosides
Gangliosides, complex glycosphingolipids that are synthesized in the Golgi complex, are characterized by a lactoyslceramide backbone and the addition of one, two, or three sailic acid residues (GM, GD, and GT, respectively). Like ceramide, gangliosides are involved in apoptosis (Fig. 1) and autophagy (Fig. 2). The ganglioside GD3 has been observed to directly permeabilize mitochondria in vitro as well as induce mitochondrial permeability, cyt c release, and caspase activation in intact cells (161–163). Mitochondrial permeabilization is inhibited in the presence of antioxidants and cyclosporin A, thereby indicating a role for ROS production and Ca2+ in GD3-mediated mitochondrial effects (161, 164). Interestingly, ceramide-mediated apoptosis has been shown to require GD3 synthesis, and in fact, apoptosis can be induced upon the transient overexpression of GD3 synthase (165). Additional studies have demonstrated the mitochondrial accumulation of GD3 in several cell lines in response to C2-ceramide or TNF-α treatment, suggesting that GD3 may contribute to the apoptotic pathway in intact cells (166–168). GD3 is normally localized to the plasma membrane and endosomal/Golgi network; however, upon stimulation, such as TNF-α treatment, GD3 rapidly redistributes from the plasma membrane to the endosomal compartment where it colocalizes with Rab5-positive early endosomes and Rab7-positive late endosomes (167). Although the details of GD3 transport remain unclear, transport to the mitochondria is likely mediated by actin cytoskeleton vesicular trafficking, as GD3 is observed to colocalize with the actin cytoskeleton protein ezrin (169). In addition to direct mitochondrial effects, the gangliosides GD1a, GM1, and GD3 have been shown to suppress NF-κB translocation to the nucleus, thereby blunting the transcription of cytoprotective genes and promoting apoptosis (170, 171).
On the contrary, ganglioside-induced activation of NF-κB has been shown to promote autophagic cell death in astrocytes in a context-dependent manner, as this effect was not observed in Ewing sarcoma cells regardless of NF- κB activation (172). Furthermore, ganglioside-induced autophagic cell death has been shown to be dependent on the generation of ROS, inhibition of Akt/mTOR, activation of ERK, and formation of “lipid rafts” (172, 173). The generation of ROS during starvation is essential for autophagosome formation through the redox regulation of Atg4 (174). However, a defined role for “lipid rafts” or individual gangliosides in autophagy is unclear. Moreover, early work showed that glycosphingolipids are substrates for the autophagic pathway in HT-29 human colon adenocarcinoma cells (175). Interestingly, multidrug resistance has been associated with the accumulation of glycosylceramides, a precursor of more complex glycosphingolipids, such as gangliosides (176). Because autophagy serves as a key cytoprotective mechanism to limit drug-induced apoptosis, future study into the role of glucosylceramide/glycosphingolipids and autophagy may provide additional explanation for the association between (glyco)sphingolipid metabolism and drug resistance.
SUMMARY AND FUTURE PERSPECTIVES
The crosstalk between apoptosis and autophagy is complex, as autophagy has paradoxical functions within the cell. The induction of autophagy can function as i) a cell survival mechanism, ii) a mechanism of caspase-independent (autophagic) cell death, or iii) a mechanism of caspase-dependent (iDISC-mediated) cell death. We propose that outcome of autophagy induction depends on the balance of autophagosomal membrane formation (autophagosome initiation) and autophagic degradation (maturation). If autophagosomes readily undergo maturation and degradation, autophagy functions as a cell survival mechanism to rid the cell of damaged proteins and/or organelles and provide protection from cell death. However, if autophagosome maturation occurs in excess, extensive degradation of cytosolic components will cause the cell to succumb to caspase-independent, autophagic cell death. In contrast, an accumulation of unsealed autophagosomal membranes or immature autophagosomes functions to promote caspase-dependent cell death through the stabilization of iDISC and activation of caspase-8. In this respect, any disruption in autophagic flux may greatly affect cell fate. Furthermore, this model emphasizes the importance of examining autophagic flux by cotreatment with lysosomal inhibitors or utilization of tandem-fluorescent LC3 (RFP-GFP-LC3) when analyzing autophagy in response to sphingolipids (24).
As discussed, sphingolipids are critical regulators of apoptosis and autophagy. Although more investigation is required, particularly regarding the regulation of autophagy by sphingolipids, some initial conclusions can be drawn from the current literature. Although ceramide is a well-established inducer of apoptosis via activation of the mitochondrial pathway, more recent studies have implicated ceramide in the induction of autophagy. Ceramide has been demonstrated to initiate autophagy via the activation of JNK (88, 89), upregulation of Beclin 1 (85, 87, 89, 97, 98) or BNIP3 (84, 102, 103), inhibition of Akt (85), and the downregulation of nutrient transporter proteins (86). Furthermore, mitochondrial ceramide appears to directly interact with autophagosomal membrane-bound LC3-II to mediate the recruitment of damaged mitochondria to lysosomes for degradation (termed “mitophagy”). Given the direct effects of ceramide, GD3, S1P, and hexadecenal on mitochondrial function, further investigation of the mechanism and regulation of mitochondrial sphingolipid metabolism is warranted.
In addition, a biophysical role for sphingolipids in autophagy is unknown. Notably, although not discussed in this review, the biophysical properties of ceramide allow for the induction of membrane curvature, membrane fusion, and the formation of lipid rafts (reviewed in Ref. 177), all or some of which may mediate autophagosome biogenesis and/or maturation. In support of this hypothesis, a recent study demonstrated that the inhibition of sphingolipid synthesis in Saccharomyces cerevisiae suppressed autophagosome biogenesis through a mechanism that was both independent of the Atg12-Atg5 and Atg8-PE conjugation systems as well as the formation of preautophagosomal structures (PAS), suggesting that sphingolipids play a critical role in the elongation, expansion, or closure of autophagosomal membranes (95). In addition, de novo ceramide synthesis is essential for autophagy following macrophage activation, and ceramide is observed to associate with autophagosomes in these cells (90). The isolation and lipidomic analysis of purified autophagosomes would enhance our understanding of sphingolipids in autophagy and reveal which sphingolipid metabolites regulate autophagosome formation.
Interestingly, while the current literature is limited, sphingolipid metabolites appear to differentially regulate the function of autophagy (i.e., cytoprotective versus cytotoxic), suggesting that sphingolipids may regulate the balance between autophagosome formation and maturation. Consistent with its mitogenic effects, S1P-dependent autophagy has thus far only been associated with cell survival (139, 141, 142). However, given the contrasting functions of SK1 and SK2 in regulating cell survival, future investigation into the differential effects of SK1 and SK2 on autophagy induction and function would be intriguing. In contrast to S1P, the current literature supports the notion that ceramide-induced autophagy functions to promote cell death, either through the induction of autophagic cell death (84, 88, 97, 98, 102, 103) or by “switching” off autophagy and inducing apoptosis through the calpain-mediated cleavage of Atg5 (109, 110) and/or iDISC formation (30). Consistent with the “many ceramides” hypothesis and recent reports demonstrating the chain-length-specific properties of ceramides in the induction of apoptosis, it is likely that individual ceramide species may differentially regulate autophagy.
In a similar manner to ceramide, ganglioside species have been reported to induce autophagic cell death (172, 173); however, further investigation is required to determine whether this occurs through mechanisms that are similar to or distinct from that of ceramide. Furthermore, we have hypothesized that the accumulation of sphingosine in response to SK inhibition may promote iDISC formation as a result of lysosomal dysfunction to mediate the “switch” between autophagy and apoptosis. Interestingly, the accumulation of dihydroceramides during hypoxia is associated with the induction of cytoprotective autophagy and is hypothesized to function as a “reserve” for ceramide generation upon reperfusion (157). Given this hypothesis, it is intriguing to suggest that the accumulation of dihydroceramide serves as an additional “switch” to regulate cell fate; however, the biological activity of dihydroceramide is still controversial and remains unclear. All in all, further investigation is needed to examine the role of sphingolipid species in regulating autophagy induction as well as the balance between autophagosome initiation and maturation.
The association of sphingolipids with autophagy has exposed yet another critical function of the bioactive lipids. Furthermore, recent progress in elucidating the crosstalk between apoptosis and autophagy has shed light on new mechanisms by which sphingolipids may regulate these processes and control cell fate. The ability to regulate the crosstalk between apoptosis and autophagy has significant implications for many diseases. For example, CerS5-mediated autophagy has recently been implicated in lipotoxic cardiomyopathy and hypertrophy (92). Furthermore, several sphingolipid storage disorders (e.g., Sandhoff disease, Niemann-Pick disease type C) show signs of dysregulated autophagy (178, 179). Moreover, dysregulated sphingolipid metabolism occurs in numerous cancers and has been shown to contribute to cancer progression as well as chemoresistance (4). Interestingly, sphingolipid metabolism is an exploitable target for the generation of novel chemotherapeutics and has been validated through the development of several sphingomimetics (4, 41, 180). Although many studies have shown that sphingolipid modulation can promote cancer cell death in vitro and the suppression of tumor growth in vivo, sphingolipid-induced autophagy is not clearly understood. Further investigation regarding sphingolipid-induced autophagy as well as sphingolipid-mediated crosstalk between apoptosis and autophagy will help understand how best to modulate autophagy in combination with these agents. Specifically, the identification of sphingolipid-mediated mechanisms by which cytoprotective autophagy can be shifted to cell death may provide a novel strategy to enhance the cytotoxic efficacy of sphingomimetics and other chemotherapeutics.
Footnotes
Abbreviations:
- Atg
- autophagy-related
- CerS
- ceramide synthase
- cyt c
- cytochrome c
- ER
- endoplasmic reticulum
- GCS
- glucosylceramide synthase
- iDISC
- intracellular death-inducing signaling complex
- LC3
- microtubule-associated protein 1 light chain 3
- NF-κB
- nuclear factor kappa B
- PKC
- protein kinase C
- S1P
- sphingosine-1-phosphate
- SK
- sphingosine kinase
- TNF
- tumor necrosis factor
This work is supported by National Cancer Institute Grants CA-82197, CA-129682, and CA-171501, and by the Lois High Berstler Endowment Fund. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. 1986. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J. Biol. Chem. 261: 12604–12609 [PubMed] [Google Scholar]
- 2.Dressler K. A., Mathias S., Kolesnick R. N. 1992. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 255: 1715–1718 [DOI] [PubMed] [Google Scholar]
- 3.Hannun Y. A., Obeid L. M. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150 [DOI] [PubMed] [Google Scholar]
- 4.Ryland L. K., Fox T. E., Liu X., Loughran T. P., Kester M. 2011. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol. Ther. 11: 138–149 [DOI] [PubMed] [Google Scholar]
- 5.Cuvillier O., Pirianov G., Kleuser B., Vanek P. G., Coso O. A., Gutkind J. S., Spiegel S. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 381: 800–803 [DOI] [PubMed] [Google Scholar]
- 6.Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. 2007. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8: 741–752 [DOI] [PubMed] [Google Scholar]
- 7.Gault C. R., Obeid L. M., Hannun Y. A. 2010. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 688: 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartke N., Hannun Y. A. 2009. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 50: S91–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrill A. H., Jr 2011. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111: 6387–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullen T. D., Hannun Y. A., Obeid L. M. 2012. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 441: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson D. A., White E. 2004. Exploiting different ways to die. Genes Dev. 18: 1223–1226 [DOI] [PubMed] [Google Scholar]
- 12.Fulda S., Debatin K. M. 2006. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 25: 4798–4811 [DOI] [PubMed] [Google Scholar]
- 13.Li J., Yuan J. 2008. Caspases in apoptosis and beyond. Oncogene. 27: 6194–6206 [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G., Marino G., Levine B. 2010. Autophagy and the integrated stress response. Mol. Cell. 40: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. 2008. Autophagy fights disease through cellular self-digestion. Nature. 451: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu S., Kanaseki T., Mizushima N., Mizuta T., Arakawa-Kobayashi S., Thompson C. B., Tsujimoto Y. 2004. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 6: 1221–1228 [DOI] [PubMed] [Google Scholar]
- 17.Yu L., Alva A., Su H., Dutt P., Freundt E., Welsh S., Baehrecke E. H., Lenardo M. J. 2004. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 304: 1500–1502 [DOI] [PubMed] [Google Scholar]
- 18.Mizushima N., Yoshimori T., Ohsumi Y. 2011. The role of atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27: 107–132 [DOI] [PubMed] [Google Scholar]
- 19.Yang Z., Klionsky D. J. 2009. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 335: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrpour M., Esclatine A., Beau I., Codogno P. 2010. Overview of macroautophagy regulation in mammalian cells. Cell Res. 20: 748–762 [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N. 2010. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 22: 132–139 [DOI] [PubMed] [Google Scholar]
- 22.Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T. 2008. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182: 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng J., Klionsky D. J. 2008. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 9: 859–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., et al. 2008. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 4: 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giansanti V., Torriglia A., Scovassi A. I. 2011. Conversation between apoptosis and autophagy: “Is it your turn or mine? Apoptosis. 16: 321–333 [DOI] [PubMed] [Google Scholar]
- 26.Levine B., Sinha S., Kroemer G. 2008. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 4: 600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oral O., Oz-Arslan D., Itah Z., Naghavi A., Deveci R., Karacali S., Gozuacik D. 2012. Cleavage of Atg3 protein by caspase-8 regulates autophagy during receptor-activated cell death. Apoptosis. 17: 810–820 [DOI] [PubMed] [Google Scholar]
- 28.Lee J. S., Li Q. L., Lee J. Y., Lee S. H., Jeong J. H., Lee H. R., Chang H., Zhou F. C., Gao S. J., Liang C. Y., et al. 2009. FLIP-mediated autophagy regulation in cell death control. Nat. Cell Biol. 11: 1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyo J. O., Jang M. H., Kwon Y. K., Lee H. J., Jun J. I. L., Woo H. N., Cho D. H., Choi B., Lee H., Kim J. H., et al. 2005. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J. Biol. Chem. 280: 20722–20729 [DOI] [PubMed] [Google Scholar]
- 30.Young M. M., Takahashi Y., Khan O., Park S., Hori T., Yun J., Sharma A. K., Amin S., Hu C-D., Zhang J., et al. 2012. Autophagosomal membrane serves as a platform for an intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J. Biol. Chem. 287: 12455–12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell B. D., Leverrier S., Weist B. M., Newton R. H., Arechiga A. F., Luhrs K. A., Morrissette N. S., Walsh C. M. 2008. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc. Natl. Acad. Sci. USA. 105: 16677–16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laussmann M. A., Passante E., Dussmann H., Rauen J. A., Wurstle M. L., Delgado M. E., Devocelle M., Prehn J. H. M., Rehm M. 2011. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 18: 1584–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang H., White E. J., Rios-Vicil C. I., Xu J., Gomez-Manzano C., Fueyo J. 2011. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J. Virol. 85: 4720–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan J-A., Ullman E., Dou Z., Zong W-X. 2011. Inhibition of protein degradation induces apoptosis through a microtubule-associated protein 1 light chain 3-mediated activation of caspase-8 at intracellular membranes. Mol. Cell Biol. 31: 3158–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yousefi S., Perozzo R., Schmid I., Ziemiecki A., Schaffner T., Scapozza L., Brunner T., Simon H. U. 2006. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 8: 1124–1132 [DOI] [PubMed] [Google Scholar]
- 36.Wirawan E., Vande Walle L., Kersse K., Cornelis S., Claerhout S., Vanoverberghe I., Roelandt R., De Rycke R., Verspurten J., Declercq W., et al. 2010. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 1: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo S., Rubinsztein D. C. 2010. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 17: 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho D. H., Jo Y. K., Hwang J. J., Lee Y. M., Roh S. A., Kim J. C. 2009. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 274: 95–100 [DOI] [PubMed] [Google Scholar]
- 39.Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. 1993. Programmed cell death induced by ceramide. Science. 259: 1769–1771 [DOI] [PubMed] [Google Scholar]
- 40.Venable M. E., Lee J. Y., Smyth M. J., Bielawska A., Obeid L. M. 1995. Role of ceramide in cellular senescence. J. Biol. Chem. 270: 30701–30708 [DOI] [PubMed] [Google Scholar]
- 41.Barth B. M., Cabot M. C., Kester M. 2011. Ceramide-based therapeutics for the treatment of cancer. Anticancer. Agents Med. Chem. 11: 911–919 [DOI] [PubMed] [Google Scholar]
- 42.Senchenkov A., Litvak D. A., Cabot M. C. 2001. Targeting ceramide metabolism–a strategy for overcoming drug resistance. J. Natl. Cancer Inst. 93: 347–357 [DOI] [PubMed] [Google Scholar]
- 43.Ogretmen B., Pettus B. J., Rossi M. J., Wood R., Usta J., Szulc Z., Bielawska A., Obeid L. M., Hannun Y. A. 2002. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 277: 12960–12969 [DOI] [PubMed] [Google Scholar]
- 44.Le Stunff H., Giussani P., Maceyka M., Lepine S., Milstien S., Spiegel S. 2007. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J. Biol. Chem. 282: 34372–34380 [DOI] [PubMed] [Google Scholar]
- 45.Hannun Y. A., Obeid L. M. 2011. Many ceramides. J. Biol. Chem. 286: 27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zitomer N. C., Mitchell T., Voss K. A., Bondy G. S., Pruett S. T., Garnier-Amblard E. C., Liebeskind L. S., Park H., Wang E., Sullards M. C., et al. 2009. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 284: 4786–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senkal C. E., Ponnusamy S., Bielawski J., Hannun Y. A., Ogretmen B. 2010. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 24: 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann D., Lucks J., Fuchs S., Schiffmann S., Schreiber Y., Ferreiros N., Merkens J., Marschalek R., Geisslinger G., Grosch S. 2012. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int. J. Biochem. Cell Biol. 44: 620–628 [DOI] [PubMed] [Google Scholar]
- 49.Gudz T. I., Tserng K. Y., Hoppel C. L. 1997. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 272: 24154–24158 [DOI] [PubMed] [Google Scholar]
- 50.García-Ruiz C., Colell A., Mari M., Morales A., Fernandez-Checa J. C. 1997. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 272: 11369–11377 [DOI] [PubMed] [Google Scholar]
- 51.Siskind L. J., Colombini M. 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 275: 38640–38644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siskind L. J., Kolesnick R. N., Colombini M. 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277: 26796–26803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siskind L. J., Feinstein L., Yu T. X., Davis J. S., Jones D., Choi J., Zuckerman J. E., Tan W. Z., Hill R. B., Hardwick J. M., et al. 2008. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J. Biol. Chem. 283: 6622–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsko C. M., Hunter O. C., Rabinowich H., Lotze M. T., Amoscato A. A. 2001. Mitochondrial lipid alterations during Fas- and radiation-induced apoptosis. Biochem. Biophys. Res. Commun. 287: 1112–1120 [DOI] [PubMed] [Google Scholar]
- 55.Birbes H., El Bawab S., Hannun Y. A., Obeid L. M. 2001. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 15: 2669–2679 [DOI] [PubMed] [Google Scholar]
- 56.Stiban J., Caputo L., Colombini M. 2008. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J. Lipid Res. 49: 625–634 [DOI] [PubMed] [Google Scholar]
- 57.Sawada M., Nakashima S., Banno Y., Yamakawa H., Takenaka K., Shinoda J., Nishimura Y., Sakai N., Nozawa Y. 2000. Influence of Bax or Bcl-2 overexpression on the ceramide-dependent apoptotic pathway in glioma cells. Oncogene. 19: 3508–3520 [DOI] [PubMed] [Google Scholar]
- 58.Birbes H., Luberto C., Hsu Y-T., El Bawab S., Hannun Y. A., Obeid L. M. 2005. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem. J. 386: 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Haefen C., Wieder T., Gillissen B., Starck L., Graupner V., Dorken B., Daniel P. T. 2002. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 21: 4009–4019 [DOI] [PubMed] [Google Scholar]
- 60.Kim H. J., Mun J. Y., Chun Y. J., Choi K. H., Kim M. Y. 2001. Bax-dependent apoptosis induced by ceramide in HL-60 cells. FEBS Lett. 505: 264–268 [DOI] [PubMed] [Google Scholar]
- 61.Ganesan V., Perera M. N., Colombini D., Datskovskiy D., Chadha K., Colombini M. 2010. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 15: 553–562 [DOI] [PubMed] [Google Scholar]
- 62.Martínez-Abundis E., Correa F., Pavón N., Zazueta C. 2009. Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. FEBS J. 276: 5579–5588 [DOI] [PubMed] [Google Scholar]
- 63.Lee H., Rotolo J. A., Mesicek J., Penate-Medina T., Rimner A., Liao W. C., Yin X. L., Ragupathi G., Ehleiter D., Gulbins E., et al. 2011. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE. 6: e19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chipuk J. E., McStay G. P., Bharti A., Kuwana T., Clarke C. J., Siskind L. J., Obeid L. M., Green D. R. 2012. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 148: 988–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xin M., Deng X. 2006. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J. Biol. Chem. 281: 18859–18867 [DOI] [PubMed] [Google Scholar]
- 66.Dobrowsky R. T., Hannun Y. A. 1992. Ceramide stimulates a cytosolic protein phosphatase. J. Biol. Chem. 267: 5048–5051 [PubMed] [Google Scholar]
- 67.Chalfant C. E., Kishikawa K., Mumby M. C., Kamibayashi C., Bielawska A., Hannun Y. A. 1999. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J. Biol. Chem. 274: 20313–20317 [DOI] [PubMed] [Google Scholar]
- 68.Ruvolo P. P., Deng X., Ito T., Carr B. K., May W. S. 1999. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J. Biol. Chem. 274: 20296–20300 [DOI] [PubMed] [Google Scholar]
- 69.Ruvolo P. P., Clark W., Mumby M., Gao F., May W. S. 2002. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J. Biol. Chem. 277: 22847–22852 [DOI] [PubMed] [Google Scholar]
- 70.Deng X., Gao F., May W. S. 2009. Protein phosphatase 2A inactivates Bcl2’s antiapoptotic function by dephosphorylation and up-regulation of Bcl2-p53 binding. Blood. 113: 422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chalfant C. E., Rathman K., Pinkerman R. L., Wood R. E., Obeid L. M., Ogretmen B., Hannun Y. A. 2002. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J. Biol. Chem. 277: 12587–12595 [DOI] [PubMed] [Google Scholar]
- 72.Minn A. J., Boise L. H., Thompson C. B. 1996. Bcl-x(S) anatagonizes the protective effects of Bcl-x(L). J. Biol. Chem. 271: 6306–6312 [DOI] [PubMed] [Google Scholar]
- 73.Srinivasula S. M., Ahmad M., Guo Y., Zhan Y., Lazebnik Y., Fernandes-Alnemri T., Alnemri E. S. 1999. Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis. Cancer Res. 59: 999–1002 [PubMed] [Google Scholar]
- 74.Chalfant C. E., Ogretmen B., Galadari S., Kroesen B. J., Pettus B. J., Hannun Y. A. 2001. FAS activation induces dephosphorylation of SR proteins; dependence on the de novo generation of ceramide and activation of protein phosphatase 1. J. Biol. Chem. 276: 44848–44855 [DOI] [PubMed] [Google Scholar]
- 75.Bourbon N. A., Yun J., Kester M. 2000. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J. Biol. Chem. 275: 35617–35623 [DOI] [PubMed] [Google Scholar]
- 76.Bourbon N. A., Sandirasegarane L., Kester M. 2002. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem. 277: 3286–3292 [DOI] [PubMed] [Google Scholar]
- 77.Wang Y. M., Seibenhener M. L., Vandenplas M. L., Wooten M. W. 1999. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NF-kappaB/JNK kinase and cell survival. J. Neurosci. Res. 55: 293–302 [DOI] [PubMed] [Google Scholar]
- 78.Fox T. E., Houck K. L., O'Neill S. M., Nagarajan M., Stover T. C., Pomianowski P. T., Unal O., Yun J. K., Naides S. J., Kester M. 2007. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J. Biol. Chem. 282: 12450–12457 [DOI] [PubMed] [Google Scholar]
- 79.Charruyer A., Jean C., Colomba A., Jaffrezou J. P., Quillet-Mary A., Laurent G., Bezombes C. 2007. PKCzeta protects against UV-C-induced apoptosis by inhibiting acid sphingomyelinase-dependent ceramide production. Biochem. J. 405: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xin M., Gao F., May W. S., Flagg T., Deng X. 2007. Protein kinase C zeta abrogates the proapoptotic function of Bax through phosphorylation. J. Biol. Chem. 282: 21268–21277 [DOI] [PubMed] [Google Scholar]
- 81.Heinrich M., Wickel M., Schneider-Brachert W., Sandberg C., Gahr J., Schwandner R., Weber T., Saftig P., Peters C., Brunner J., et al. 1999. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 18: 5252–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinrich M., Neumeyer J., Jakob M., Hallas C., Tchikov V., Winoto-Morbach S., Wickel M., Schneider-Brachert W., Trauzold A., Hethke A., et al. 2004. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 11: 550–563 [DOI] [PubMed] [Google Scholar]
- 83.Dumitru C. A., Sandalcioglu I. E., Wagner M., Weller M., Gulbins E. 2009. Lysosomal ceramide mediates gemcitabine-induced death of glioma cells. J. Mol. Med. (Berl.) 87: 1123–1132 [DOI] [PubMed] [Google Scholar]
- 84.Daido S., Kanzawa T., Yamamoto A., Takeuchi H., Kondo Y., Kondo S. 2004. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 64: 4286–4293 [DOI] [PubMed] [Google Scholar]
- 85.Scarlatti F., Bauvy C., Ventruti A., Sala G., Cluzeaud F., Vandewalle A., Ghidoni R., Codogno P. 2004. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J. Biol. Chem. 279: 18384–18391 [DOI] [PubMed] [Google Scholar]
- 86.Guenther G. G., Peralta E. R., Rosales K. R., Wong S. Y., Siskind L. J., Edinger A. L. 2008. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl. Acad. Sci. USA. 105: 17402–17407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Copetti T., Bertoli C., Dalla E., Demarchi F., Schneider C. 2009. p65/RelA modulates BECN1 transcription and autophagy. Mol. Cell. Biol. 29: 2594–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pattingre S., Bauvy C., Carpentier S., Levade T., Levine B., Codogno P. 2009. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J. Biol. Chem. 284: 2719–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D. D., Wang L. L., Deng R., Tang J., Shen Y., Guo J. F., Wang Y., Xia L. P., Feng G. K., Liu Q. Q., et al. 2009. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 28: 886–898 [DOI] [PubMed] [Google Scholar]
- 90.Sims K., Haynes C. A., Kelly S., Allegood J. C., Wang E., Momin A., Leipelt M., Reichart D., Glass C. K., Sullards M. C., et al. 2010. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 285: 38568–38579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun T., Li D., Wang L., Xia L., Ma J., Guan Z., Feng G., Zhu X. 2011. c-Jun NH2-terminal kinase activation is essential for up-regulation of LC3 during ceramide-induced autophagy in human nasopharyngeal carcinoma cells. J. Transl. Med. 9: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russo S. B., Baicu C. F., Van Laer A., Geng T., Kasiganesan H., Zile M. R., Cowart L. A. 2012. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122: 3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sentelle R. D., Senkal C. E., Jiang W., Ponnusamy S., Gencer S., Panneer Selvam S., Ramshesh V. K., Peterson Y. K., Lemasters J. J., Szulc Z. M., et al. 2012. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 8: 831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taniguchi M., Kitatani K., Kondo T., Hashimoto-Nishimura M., Asano S., Hayashi A., Mitsutake S., Igarashi Y., Umehara H., Takeya H., et al. Regulation of autophagy and its associated cell death by sphingolipid rheostat: reciprocal role of ceramide and sphingosine-1-phosphate in the mTOR pathway. J. Biol. Chem. Epub ahead of print. October 3, 2012; doi:10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamagata M., Obara K., Kihara A. 2011. Sphingolipid synthesis is involved in autophagy in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 410: 786–791 [DOI] [PubMed] [Google Scholar]
- 96.Zhou H., Summers S. A., Birnbaum M. J., Pittman R. N. 1998. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 273: 16568–16575 [DOI] [PubMed] [Google Scholar]
- 97.Dbaibo G., Kfoury Y., Darwiche N., Panjarian S., Kozhaya L., Nasr R., Abdallah M., Hermine O., El-Sabban M., de The H., et al. 2007. Arsenic trioxide induces accumulation of cytotoxic levels of ceramide in acute promyelocytic leukemia and adult T-cell leukemia/lymphoma cells through de novo ceramide synthesis and inhibition of glucosylceramide synthase activity. Haematologica. 92: 753–762 [DOI] [PubMed] [Google Scholar]
- 98.Qian W., Liu J., Jin J., Ni W., Xu W. 2007. Arsenic trioxide induces not only apuboptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk. Res. 31: 329–339 [DOI] [PubMed] [Google Scholar]
- 99.Westwick J. K., Bielawska A. E., Dbaibo G., Hannun Y. A., Brenner D. A. 1995. Ceramide activates the stress-activated protein kinases. J. Biol. Chem. 270: 22689–22692 [DOI] [PubMed] [Google Scholar]
- 100.Coroneos E., Wang Y., Panuska J. R., Templeton D. J., Kester M. 1996. Sphingolipid metabolites differentially regulate extracellular signal-regulated kinase and stress-activated protein kinase cascades. Biochem. J. 316: 13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maiuri M. C., Le Toumelin G., Criollo A., Rain J. C., Gautier F., Juin P., Tasdemir E., Pierron G., Troulinaki K., Tavernarakis N., et al. 2007. Functional and physical interaction between Bcl-X-L and a BH3-like domain in Beclin-1. EMBO J. 26: 2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanzawa T., Kondo Y., Ito H., Kondo S., Germano I. 2003. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 63: 2103–2108 [PubMed] [Google Scholar]
- 103.Kanzawa T., Zhang L., Xiao L. C., Germano I. M., Kondo Y., Kondo S. 2005. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 24: 980–991 [DOI] [PubMed] [Google Scholar]
- 104.Mammucari C., Milan G., Romanello V., Masiero E., Rudolf R., Del Piccolo P., Burden S. J., Di Lisi R., Sandri C., Zhao J., et al. 2007. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6: 458–471 [DOI] [PubMed] [Google Scholar]
- 105.Hou Q., Jin J. F., Zhou H., Novgorodov S. A., Bielawska A., Szulc Z. M., Hannun Y. A., Obeid L. M., Hsu Y. T. 2011. Mitochondrially targeted ceramides preferentially promote autophagy, retard cell growth, and induce apoptosis. J. Lipid Res. 52: 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spassieva S. D., Mullen T. D., Townsend D. M., Obeid L. M. 2009. Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem. J. 424: 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salazar M., Carracedo A., Salanueva I. J., Hernandez-Tiedra S., Lorente M., Egia A., Vazquez P., Blazquez C., Torres S., Garcia S., et al. 2009. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119: 1359–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yacoub A., Hamed H. A., Allegood J., Mitchell C., Spiegel S., Lesniak M. S., Ogretmen B., Dash R., Sarkar D., Broaddus W. C., et al. 2010. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 70: 1120–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhutia S. K., Dash R., Das S. K., Azab B., Su Z. Z., Lee S. G., Grant S., Yacoub A., Dent P., Curiel D. T., et al. 2010. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 70: 3667–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lépine S., Allegood J. C., Edmonds Y., Milstien S., Spiegel S. 2011. Autophagy induced by deficiency of sphingosine-1-phosphate phosphohydrolase 1 is switched to apoptosis by calpain-mediated Atg5 cleavage. J. Biol. Chem. 286: 44380–44390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demarchi F., Bertoli C., Greer P. A., Schneider C. 2005. Ceramide triggers an NF-kappaB-dependent survival pathway through calpain. Cell Death Differ. 12: 512–522 [DOI] [PubMed] [Google Scholar]
- 112.Cuvillier O., Edsall L., Spiegel S. 2000. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J. Biol. Chem. 275: 15691–15700 [DOI] [PubMed] [Google Scholar]
- 113.Isogai C., Murate T., Tamiya-Koizumi K., Yoshida S., Ito T., Nagai H., Kinoshita T., Kagami Y., Hotta T., Hamaguchi M., et al. 1998. Analysis of bax protein in sphingosine-induced apoptosis in the human leukemic cell line TF1 and its bcl-2 transfectants. Exp. Hematol. 26: 1118–1125 [PubMed] [Google Scholar]
- 114.Sweeney E. A., Sakakura C., Shirahama T., Masamune A., Ohta H., Hakomori S., Igarashi Y. 1996. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int. J. Cancer. 66: 358–366 [DOI] [PubMed] [Google Scholar]
- 115.Phillips D. C., Martin S., Doyle B. T., Houghton J. A. 2007. Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res. 67: 756–764 [DOI] [PubMed] [Google Scholar]
- 116.Cuvillier O., Nava V. E., Murthy S. K., Edsall L. C., Levade T., Milstien S., Spiegel S. 2001. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death Differ. 8: 162–171 [DOI] [PubMed] [Google Scholar]
- 117.Jarvis W. D., Fornari F. A., Jr, Auer K. L., Freemerman A. J., Szabo E., Birrer M. J., Johnson C. R., Barbour S. E., Dent P., Grant S. 1997. Coordinate regulation of stress- and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol. Pharmacol. 52: 935–947 [DOI] [PubMed] [Google Scholar]
- 118.Sweeney E. A., Inokuchi J., Igarashi Y. 1998. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 425: 61–65 [DOI] [PubMed] [Google Scholar]
- 119.Siskind L. J., Fluss S., Bui M., Colombini M. 2005. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J. Bioenerg. Biomembr. 37: 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakakura C., Sweeney E. A., Shirahama T., Hakomori S., Igarashi Y. 1996. Suppression of bcl-2 gene expression by sphingosine in the apoptosis of human leukemic HL-60 cells during phorbol ester-induced terminal differentiation. FEBS Lett. 379: 177–180 [DOI] [PubMed] [Google Scholar]
- 121.Shirahama T., Sakakura C., Sweeney E. A., Ozawa M., Takemoto M., Nishiyama K., Ohi Y., Igarashi Y. 1997. Sphingosine induces apoptosis in androgen-independent human prostatic carcinoma DU-145 cells by suppression of bcl-X(L) gene expression. FEBS Lett. 407: 97–100 [DOI] [PubMed] [Google Scholar]
- 122.Colié S., Van Veldhoven P. P., Kedjouar B., Bedia C., Albinet V., Sorli S. C., Garcia V., Djavaheri-Mergny M., Bauvy C., Codogno P., et al. 2009. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 69: 9346–9353 [DOI] [PubMed] [Google Scholar]
- 123.Hamaguchi A., Suzuki E., Murayama K., Fujimura T., Hikita T., Iwabuchi K., Handa K., Withers D. A., Masters S. C., Fu H., et al. 2003. Sphingosine-dependent protein kinase-1, directed to 14-3-3, is identified as the kinase domain of protein kinase C delta. J. Biol. Chem. 278: 41557–41565 [DOI] [PubMed] [Google Scholar]
- 124.Megidish T., Cooper J., Zhang L. X., Fu H. A., Hakomori S. 1998. A novel sphingosine-dependent protein kinase (SDK1) specifically phosphorylates certain isoforms of 14-3-3 protein. J. Biol. Chem. 273: 21834–21845 [DOI] [PubMed] [Google Scholar]
- 125.Kagedal K., Zhao M., Svensson I., Brunk U. T. 2001. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olivera A., Spiegel S. 1993. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 365: 557–560 [DOI] [PubMed] [Google Scholar]
- 127.Okada T., Ding G., Sonoda H., Kajimoto T., Haga Y., Khosrowbeygi A., Gao S., Miwa N., Jahangeer S., Nakamura S. 2005. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 280: 36318–36325 [DOI] [PubMed] [Google Scholar]
- 128.Liu H., Toman R. E., Goparaju S. K., Maceyka M., Nava V. E., Sankala H., Payne S. G., Bektas M., Ishii I., Chun J., et al. 2003. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 278: 40330–40336 [DOI] [PubMed] [Google Scholar]
- 129.Igarashi N., Okada T., Hayashi S., Fujita T., Jahangeer S., Nakamura S. 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278: 46832–46839 [DOI] [PubMed] [Google Scholar]
- 130.Min J., Mesika A., Sivaguru M., Van Veldhoven P. P., Alexander H., Futerman A. H., Alexanderl S. 2007. (Dihydro)ceramide synthase 1-regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol. Cancer Res. 5: 801–812 [DOI] [PubMed] [Google Scholar]
- 131.Maceyka M., Sankala H., Hait N. C., Le Stunff H., Liu H., Toman R., Collier C., Zhang M., Satin L. S., Merrill A. H., Jr, et al. 2005. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 280: 37118–37129 [DOI] [PubMed] [Google Scholar]
- 132.Pyne N. J., Pyne S. 2010. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 10: 489–503 [DOI] [PubMed] [Google Scholar]
- 133.Igarashi J., Bernier S. G., Michel T. 2001. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 276: 12420–12426 [DOI] [PubMed] [Google Scholar]
- 134.Siehler S., Wang Y., Fan X., Windh R. T., Manning D. R. 2001. Sphingosine 1-phosphate activates nuclear factor-kappa B through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J. Biol. Chem. 276: 48733–48739 [DOI] [PubMed] [Google Scholar]
- 135.Zondag G. C. M., Postma F. R., Van Etten I., Verlaan I., Moolenaar W. H. 1998. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem. J. 330: 605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meyer zu Heringdorf D., Liliom K., Schaefer M., Danneberg K., Jaggar J. H., Tigyi G., Jakobs K. H. 2003. Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of G-protein-coupled receptors. FEBS Lett. 554: 443–449 [DOI] [PubMed] [Google Scholar]
- 137.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., et al. 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 325: 1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ihlefeld K., Claas R. F., Koch A., Pfeilschifter J. M., Meyer zu Heringdorf D. 2012. Evidence for a link between histone deacetylation and Ca2+ homoeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts. Biochem. J. 447: 457–464 [DOI] [PubMed] [Google Scholar]
- 139.Lavieu G., Scarlatti F., Sala G., Carpentier S., Levade T., Ghidoni R., Botti J., Codogno P. 2006. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J. Biol. Chem. 281: 8518–8527 [DOI] [PubMed] [Google Scholar]
- 140.Lanterman M. M., Saba J. D. 1998. Characterization of sphingosine kinase (SK) activity in Saccharomyces cerevisiae and isolation of SK-deficient mutants. Biochem. J. 332: 525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lépine S., Allegood J. C., Park M., Dent P., Milstien S., Spiegel S. 2011. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ. 18: 350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang C. L., Ho M. C., Lee P. H., Hsu C. Y., Huang W. P., Lee H. 2009. S1P(5) is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am. J. Physiol. Cell Physiol. 297: C451–C458 [DOI] [PubMed] [Google Scholar]
- 143.Beljanski V., Knaak C., Smith C. D. 2010. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J. Pharmacol. Exp. Ther. 333: 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wallington-Beddoe C. T., Hewson J., Bradstock K. F., Bendall L. J. 2011. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy. 7: 707–715 [DOI] [PubMed] [Google Scholar]
- 145.Romero Rosales K., Singh G., Wu K., Chen J., Janes M. R., Lilly M. B., Peralta E. R., Siskind L. J., Bennett M. J., Fruman D. A., et al. 2011. Sphingolipid-based drugs selectively kill cancer cells by down-regulating nutrient transporter proteins. Biochem. J. 439: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bandhuvula P., Tam Y. Y., Oskouian B., Saba J. D. 2005. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J. Biol. Chem. 280: 33697–33700 [DOI] [PubMed] [Google Scholar]
- 147.Vessey D. A., Kelley M., Zhang J., Li L., Tao R., Karliner J. S. 2007. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J. Biochem. Mol. Toxicol. 21: 273–279 [DOI] [PubMed] [Google Scholar]
- 148.Lahiri S., Park H., Laviad E. L., Lu X. Q., Bittman R., Futerman A. H. 2009. Ceramide synthesis is modulated by the sphingosine analog fty720 via a mixture of uncompetitive and noncompetitive inhibition in an acyl-CoA chain length-dependent manner. J. Biol. Chem. 284: 16090–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berdyshev E. V., Gorshkova I., Skobeleva A., Bittman R., Lu X. Q., Dudek S. M., Mirzapoiazova T., Garcia J. G. N., Natarajan V. 2009. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J. Biol. Chem. 284: 5467–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bonhoure E., Pchejetski D., Aouali N., Morjani H., Levade T., Kohama T., Cuvillier O. 2006. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 20: 95–102 [DOI] [PubMed] [Google Scholar]
- 151.Bielawska A., Crane H. M., Liotta D., Obeid L. M., Hannun Y. A. 1993. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J. Biol. Chem. 268: 26226–26232 [PubMed] [Google Scholar]
- 152.Wang H., Maurer B. J., Liu Y. Y., Wang E., Allegood J. C., Kelly S., Symolon H., Liu Y., Merrill A. H., Jr, Gouaze-Andersson V., et al. 2008. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol. Cancer Ther. 7: 2967–2976 [DOI] [PubMed] [Google Scholar]
- 153.Jiang Q., Rao X., Kim C. Y., Freiser H., Zhang Q., Jiang Z., Li G. 2012. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int. J. Cancer. 130: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stiban J., Fistere D., Colombini M. 2006. Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis. 11: 773–780 [DOI] [PubMed] [Google Scholar]
- 155.Beauchamp E., Goenaga D., Le Bloc'h J., Catheline D., Legrand P., Rioux V. 2007. Myristic acid increases the activity of dihydroceramide Delta4-desaturase 1 through its N-terminal myristoylation. Biochimie. 89: 1553–1561 [DOI] [PubMed] [Google Scholar]
- 156.Beauchamp E., Tekpli X., Marteil G., Lagadic-Gossmann D., Legrand P., Rioux V. 2009. N-Myristoylation targets dihydroceramide Delta4-desaturase 1 to mitochondria: partial involvement in the apoptotic effect of myristic acid. Biochimie. 91: 1411–1419 [DOI] [PubMed] [Google Scholar]
- 157.Devlin C. M., Lahm T., Hubbard W. C., Van Demark M., Wang K. C., Wu X., Bielawska A., Obeid L. M., Ivan M., Petrache I. 2011. A dihydroceramide-based response to hypoxia. J. Biol. Chem. 286: 38069–38078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Signorelli P., Munoz-Olaya J. M., Gagliostro V., Casas J., Ghidoni R., Fabrias G. 2009. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 282: 238–243 [DOI] [PubMed] [Google Scholar]
- 159.Puissant A., Robert G., Fenouille N., Luciano F., Cassuto J. P., Raynaud S., Auberger P. 2010. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 70: 1042–1052 [DOI] [PubMed] [Google Scholar]
- 160.Gagliostro V., Casas J., Caretti A., Abad J. L., Tagliavacca L., Ghidoni R., Fabrias G., Signorelli P. 2012. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 44: 2135–2143 [DOI] [PubMed] [Google Scholar]
- 161.Scorrano L., Petronilli V., Di Lisa F., Bernardi P. 1999. Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore. J. Biol. Chem. 274: 22581–22585 [DOI] [PubMed] [Google Scholar]
- 162.Kristal B. S., Brown A. M. 1999. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J. Biol. Chem. 274: 23169–23175 [DOI] [PubMed] [Google Scholar]
- 163.García-Ruiz C., Colell A., Paris R., Fernandez-Checa J. C. 2000. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 14: 847–858 [DOI] [PubMed] [Google Scholar]
- 164.Colell A., Garcia-Ruiz C., Mari M., Fernandez-Checa J. C. 2004. Mitochondrial permeability transition induced by reactive oxygen species is independent of cholesterol-regulated membrane fluidity. FEBS Lett. 560: 63–68 [DOI] [PubMed] [Google Scholar]
- 165.De Maria R., Lenti L., Malisan F., d'Agostino F., Tomassini B., Zeuner A., Rippo M. R., Testi R. 1997. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science. 277: 1652–1655 [DOI] [PubMed] [Google Scholar]
- 166.Rippo M. R., Malisan F., Ravagnan L., Tomassini B., Condo I., Costantini P., Susin S. A., Rufini A., Todaro M., Kroemer G., et al. 2000. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J. 14: 2047–2054 [DOI] [PubMed] [Google Scholar]
- 167.García-Ruiz C., Colell A., Morales A., Calvo M., Enrich C., Fernandez-Checa J. C. 2002. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J. Biol. Chem. 277: 36443–36448 [DOI] [PubMed] [Google Scholar]
- 168.Colell A., Morales A., Fernandez-Checa J. C., Garcia-Ruiz C. 2002. Ceramide generated by acidic sphingomyelinase contributes to tumor necrosis factor-alpha-mediated apoptosis in human colon HT-29 cells through glycosphingolipids formation. Possible role of ganglioside GD3. FEBS Lett. 526: 135–141 [DOI] [PubMed] [Google Scholar]
- 169.Giammarioli A. M., Garofalo T., Sorice M., Misasi R., Gambardella L., Gradini R., Fais S., Pavan A., Malorni W. 2001. GD3 glycosphingolipid contributes to Fas-mediated apoptosis via association with ezrin cytoskeletal protein. FEBS Lett. 506: 45–50 [DOI] [PubMed] [Google Scholar]
- 170.Uzzo R. G., Rayman P., Kolenko V., Clark P. E., Cathcart M. K., Bloom T., Novick A. C., Bukowski R. M., Hamilton T., Finke J. H. 1999. Renal cell carcinoma-derived gangliosides suppress nuclear factor-kappaB activation in T cells. J. Clin. Invest. 104: 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Colell A., Garcia-Ruiz C., Roman J., Ballesta A., Fernandez-Checa J. C. 2001. Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kappa B-dependent survival pathway. FASEB J. 15: 1068–1070 [DOI] [PubMed] [Google Scholar]
- 172.Hwang J., Lee H-J., Lee W-H., Suk K. 2010. NF-[kappa]B as a common signaling pathway in ganglioside-induced autophagic cell death and activation of astrocytes. J. Neuroimmunol. 226: 66–72 [DOI] [PubMed] [Google Scholar]
- 173.Hwang J., Lee S., Lee J. T., Kwon T. K., Kim D. R., Kim H., Park H. C., Suk K. 2010. Gangliosides induce autophagic cell death in astrocytes. Br. J. Pharmacol. 159: 586–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. 2007. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 26: 1749–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ghidoni R., Houri J-J., Giuliani A., Ogier-Denis E., Parolari E., Botti S., Bauvy C., Codogno P. 1996. The metabolism of sphingo(glyco)lipids is correlated with the differentiation-dependent autophagic pathway in HT-29 cells. Eur. J. Biochem. 237: 454–459 [DOI] [PubMed] [Google Scholar]
- 176.Lavie Y., Cao H., Bursten S. L., Giuliano A. E., Cabot M. C. 1996. Accumulation of glucosylceramides in multidrug-resistant cancer cells. J. Biol. Chem. 271: 19530–19536 [DOI] [PubMed] [Google Scholar]
- 177.Goñi F. M., Alonso A. 2006. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim. Biophys. Acta. 1758: 1902–1921 [DOI] [PubMed] [Google Scholar]
- 178.Ko D. C., Milenkovic L., Beier S. M., Manuel H., Buchanan J., Scott M. P. 2005. Cell-autonomous death of cerebellar purkinje neurons with autophagy in Niemann-Pick type C disease. PLoS Genet. 1: 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pacheco C. D., Kunkel R., Lieberman A. P. 2007. Autophagy in Niemann-Pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum. Mol. Genet. 16: 1495–1503 [DOI] [PubMed] [Google Scholar]
- 180.Kester M., Kolesnick R. 2003. Sphingolipids as therapeutics. Pharmacol. Res. 47: 365–371 [DOI] [PubMed] [Google Scholar]