Abstract
A high-fat diet (HFD) is a well-known contributing factor in the development of obesity. Most rats fed HFDs become obese. Those that avoid obesity when fed HFDs are considered diet resistant (DR). We performed a microarray screen to identify genes specific to the mesenteric fat of DR rats and revealed high expression of guanylin and guanylyl cyclase C (GC-C) in some subjects. Our histologic studies revealed that the cellular source of guanylin and GC-C is macrophages. Therefore, we developed double-transgenic (Tg) rats overexpressing guanylin and GC-C in macrophages and found that they were resistant to the effects of HFDs. In the mesenteric fat of HFD-fed Tg rats, Fas and perilipin mRNAs were downregulated, and those of genes involved in fatty acid oxidation were upregulated, compared with the levels in HFD-fed wild-type rats. In vitro studies demonstrated that lipid accumulation was markedly inhibited in adipocytes cocultured with macrophages expressing guanylin and GC-C and that this inhibition was reduced after treatment with guanylin- and GC-C-specific siRNAs. Our results suggest that the macrophagic guanylin-GC-C system contributes to the altered expression of genes involved in lipid metabolism, leading to resistance to obesity.
Keywords: diet resistance, lipid metabolism, adipocytes, dietary lipids, obesity
The worldwide incidence of obesity has accelerated during the last decade. Obesity is caused by excessive accumulation of white adipose tissue, often as the result of ingesting calories in excess of daily requirements (1, 2). In investigations of disease outcome, excess adipose tissue is defined by using the body mass index (BMI), which is calculated as weight (kg)/height (m2). A BMI of 25.0–25.9 kg/m2 corresponds to an overweight condition, whereas obesity is defined as a BMI of 30 kg/m2 or greater (3, 4). Adipose depots in mammals are distributed throughout the body and include the fat surrounding the heart and the subcutaneous, retroperitoneal, and mesenteric fat. The amount of mesenteric fat is thought to be correlated most strongly with morbidity rate in obesity (5). Therefore, investigating the system regulating adipocytes in the mesenteric fat may yield insight into target molecules for treating or preventing obesity-related diseases.
Both humans and animals vary in their body-weight responses to high-fat diets (HFDs). When animals are fed HFDs, most of them increase in body weight, with higher levels of adiposity than occur when standard chow is fed. However, a few subjects fed HFDs show less weight gain than do control animals fed standard chow or obesity-prone (diet-induced obesity, DIO) animals. These animals that do not become obese even when fed HFDs are categorized as being “diet resistant” (DR) (6, 7). To investigate the characteristics of genes expressed in the mesenteric fat tissues, we used a DNA microarray to screen gene expression patterns in DR, DIO, and control rats. This screening revealed increases in guanylin and guanylyl cyclase C (GC-C) mRNAs in three of the seven DR rats evaluated. Immunohistochemical data confirmed that both guanylin and GC-C are present in the mesenteric macrophages of DR rats that show high levels of expression of these genes. Because a proinflammatory state induced by the infiltration of macrophages into adipose tissues is strongly associated with obesity progression (8, 9), we hypothesized that the guanylin and GC-C present in the mesenteric macrophages of some DR rats contribute to the regulation of obesity.
Guanylin is an endogenous ligand of GC-C (10, 11). Guanylin and GC-C are present mainly in the intestine and function to maintain homeostasis of body fluid (11–16). Guanylyl cyclases (GC) are a family of enzymes that metabolize GTP to cGMP. The guanylyl cyclases GC-A and GC-B are the principal receptors for natriuretic peptides (atrial natriuretic peptide, ANP; brain natriuretic peptide, BNP; and C-type natriuretic peptide, CNP). Natriuretic peptides are involved in regulating blood pressure and blood volume (17), and several reports indicate that they also modulate lipolysis by activating GC-A (18, 19). Furthermore, natriuretic peptides and cGMP cascades promote the biogenesis of muscle mitochondria and prevent obesity (20). These findings suggest that the guanylin-GC-C system in mesenteric macrophages also contributes to the regulation of adipocytes.
We first investigated whether transgenic (Tg) rats that overexpressed guanylin and GC-C in macrophages show resistance to obesity when fed HFDs. Next, we examined the size of adipocytes; the plasma levels of free fatty acid (FFA), glycerol, and total cholesterol; and the expression of genes involved in adipocyte maturation, droplet formation, and fatty acid oxidation in the mesenteric fat of Tg rats and wild-type (WT) rats. Finally, we investigated the practical contributions of guanylin and GC-C to adipocyte maturation and droplet formation by using a coculture system of mesenteric adipocytes and macrophages that expresses both guanylin and GC-C. We also explored this system by using guanylin- and GC-C-specific siRNAs. Our data demonstrate that the guanylin-GC-C system has important roles in the regulation of genes involved in lipid metabolism in mesenteric fat.
MATERIALS AND METHODS
Microarray screening and histologic analysis
To obtain DR rats, we purchased male Wistar rats and Sprague-Dawley (SD) rats (age, 3 weeks; n = 30−40 each) (Charles River, Shiga, Japan) and fed them an HFD (5.2 kcal/g) containing 60% fat, 20% carbohydrate, and 20% protein (Research Diets, New Brunswick, NJ) for 12 weeks to induce obesity. We also fed male Wistar rats and SD rats (age, 3 weeks; n = 10 each) standard chow (3.4 kcal/g) containing 4.6% fat, 51% carbohydrate, and 25% protein (CE-2; CLEA, Tokyo, Japan) and used them as control rats. This protocol yielded seven DR rats whose body weights were less than the average of the control group. We used an RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany) to extract total RNA from the mesenteric fat tissues of i) DR rats (n = 7), ii) representative DIO rats (n = 7), and iii) control rats (n = 7) that were fed standard chow and randomly selected. For microarray screening, we used mRNA extracted from the mesenteric fat tissues of three DIO, three control, and seven DR rats. Reverse transcription, cDNA synthesis, and production of biotin-labeled cRNA were performed by using 10 μg total RNA as the initial template. The labeled cRNA was purified, fragmented, and hybridized to GeneChip rat microarrays (230 2.0; Affymetrix, Santa Clara, CA). After being washed and stained, the hybridized arrays were scanned with a confocal laser scanner (Hewlett Packard, Palo Alto, CA). The data were analyzed by using GeneSpring GX software (Agilent Technologies, Santa Clara, CA).
This microarray screening revealed a moderate to high increase in the number of guanylin and GC-C mRNA transcripts in three of the seven DR rats evaluated. We therefore confirmed these results through quantitative PCR of mRNA from 7 representative DIO rats, 7 random control rats, and the 7 DR rats. Real-time PCR was conducted by using a LightCycler system (Roche Diagnostics, Mannheim, Germany) and SYBR Premix Ex Taq II Kit (Takara Bio, Otsu, Japan). Primer sequences are listed in supplementary Table II. mRNA levels of target genes were normalized against the levels of glyceraldehyde-3-phosphodehydrogenase (GAPDH).
We performed immunohistochemical studies to investigate the cellular sources of guanylin and GC-C. Mesenteric adipose tissues from DR rats with high guanylin and GC-C mRNA expression were fixed in 3.7% formaldehyde at 4°C for 24 h, dehydrated, embedded in paraffin, and sectioned (thickness, 3 μm). The sections were deparaffinated with xylene and microwaved (500 W for 5 min) in 0.01 M sodium citrate buffer (pH 6.0). The sections were treated with 0.3% hydrogen peroxide for 1 h to inactivate endogenous peroxidases and then incubated for 2 days at 4°C with antiguanylin antiserum (Abcam, Cambridge, UK; diluted 1:500), anti-GC-C antiserum (Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:100), or control IgG (Sigma, St. Louis, MO; diluted 1:1000). Western blotting performed with each antiserum showed that they each detected a single protein band of the expected size (supplementary Fig. I-B). The sections were then washed with phosphate buffered saline (PBS; pH 7.4) for 30 min, incubated with biotinylated secondary antibody (diluted 1:250), and incubated with avidin-biotin peroxidase complex (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) for 2 h. The sections were stained with 0.02% 3-3′-diaminobenzidine and 0.05% hydrogen peroxide in Tris buffer (pH 7.6) for 10 min at room temperature. Mesenteric fat sections from DR rats that had been incubated with antiguanylin antiserum or anti-GC-C antiserum subsequently were stained with an anti-macrophage marker (CD68; Serotec Ltd., Oxford, UK; diluted 1:100) and then 3,3′,5,5′-tetramethylbenzidine. The sections obtained from control and DIO rats were stained with antiguanylin, anti-GC-C, or anti-CD68 antiserum. All procedures were performed in accordance with the Japanese Physiological Society's guidelines for animal care. This protocol was approved by the Ethics Review Committee for Animal Experimentation of the Faculty of Medicine, University of Miyazaki.
Bacterial artificial chromosome constructs
Guanylin and GC-C immunoreactivities were present in macrophages in the mesenteric fat of DR rats with high-level expression of guanylin and GC-C. To generate guanylin-GC-C double-Tg rats that overexpressed guanylin and GC-C in macrophages, we used the SR-A promoter. A rat macrophage bacterial artificial chromosome (BAC) clone (CH230-56C20) containing SR-1 (the rat ortholog of the human SR-A gene, i.e., rat SR-A) and a human BAC clone (RP11-627G14) containing the guanylyl cyclase activator 2A (guanylin) gene were obtained from the BACPAC Resources Center at the Children's Hospital Oakland Research Institute (Oakland, CA). A human guanylyl cyclase 2C (GC-C) cDNA clone, MGC: 168157 (IMAGE: 9020534), was purchased from DNAFORM (Kanagawa, Japan). Chimeric SR-A-guanylin and SR-A-GC-C BAC transgenic constructs harboring the human guanylin gene and human GC-C cDNA were generated by BAC recombineering. To this end, we used a Red/ET Counter Selection BAC Modification Kit (Gene Bridges, Heidelberg, Germany) to separately replace the SR-A coding sequence with the human guanylin genomic sequence and the human GC-C open reading frame (21). BAC modifications were verified by DNA sequencing. BAC transgenic constructs were purified independently for microinjection by using a previously described procedure (22), with slight modification. BAC transgenic constructs were extracted from 250 ml of Escherichia coli (E. coli) culture by using a Nucleobond Plasmid Purification kit (Macherey-Nagel, Diiren, Germany). Aliquots (10 μg each) of each BAC transgenic construct were linearized overnight with PI-SceI endonuclease (New England Biolabs, Beverly, MA). For each construct, the linearized BAC DNA fragments were separated by means of pulsed-field gel electrophoresis (PFGE) and extracted by using electroelution. After dialysis against buffer containing 10 mM Tris-HCl and 0.1 mM EDTA, aliquots of each DNA solution underwent PFGE for size and quality control. Each BAC DNA solution was diluted to 1 ng/μl, and the two diluted solutions were mixed in equal volumes. The aliquots of the mixed BAC DNA solution were stored at 4°C until microinjection.
Guanylin-GC-C BAC transgenic rats
Rats transgenic for both guanylin and GC-C were generated by pronuclear injection of embryos from SD rats (Charles River). Transgenic founders carrying the BAC transgenic construct were assessed by Southern blotting. PstI- or DraI-digested DNA prepared from tail samples was hybridized with the [32P]labeled fragment of guanylin or the GC-C fragment, respectively. This Southern analysis revealed that 12 of the 102 founders contained both transgenes; additional founders had either single transgene. There were no obvious gross phenotypic differences between the transgene-positive and transgene-negative littermates. Guanylin and GC-C transgenic founders were bred with SD rats. The protein levels of guanylin and GC-C in the mesenteric fat tissues of male Tg and WT progeny were examined by using Western blotting.
Western blotting
Cells or tissues were harvested in ProteoJET mammalian cell lysis reagent (Fermentas Life Sciences, Ontario, Canada) or CelLytec MT mammalian tissue lysis-extraction reagent (Sigma) with proteinase inhibitor cocktail (Roche), and protein concentrations were determined with a BCA kit (Pierce, Rockford, IL). Each lane of an 8% or 15% Tris-glycine SDS-PAGE gel contained 30 μg total protein. After transfer onto polyvinylidene difluoride membrane (Millipore, Tokyo, Japan), blots were incubated overnight at 4°C with the following primary antibodies: anti-guanylin (Abcam), 1:1000; anti-GC-C (Santa Cruz), 1:1000; anti-β-actin (Cell Signaling Technology, Beverly, MA), 1:2000; and anti-CD68 (Serotec), 1:1000. Horseradish peroxidase-conjugated secondary antibodies were applied, and immunoblot signals were detected by using the ECL Plus Western blot detection kit (Amersham Biosciences, Piscataway, NJ).
Animals, diets, and experimental procedures
Tg and WT rats (age, 6 weeks; sex, male; n = 10 each) were housed individually in plastic cages in a room at constant temperature and under a 12:12 h light:dark cycle (lights on, 08:00 to 20:00). They were allowed ad libitum access to food and water throughout the study. All rats received standard laboratory chow until they were 6 weeks old, at which time WT and Tg rats (n = 5 each) were randomly selected to receive either standard chow (3.4 kcal/g) containing 4.6% fat, 51% carbohydrate, and 25% protein (CE-2; CLEA) or an HFD (5.2 kcal/g) containing 60% fat, 20% carbohydrate, and 20% protein (Research Diets). Body weight and food intake were monitored once weekly until the rats were 21 weeks old.
Weight and histologic analysis of mesenteric fat in Tg rats
At the age of 21 weeks, Tg and WT rats were fasted for 16 h and then anesthetized with 50 mg/kg pentobarbital intraperitoneally. The mesenteric fat was dissected and weighed. Some of the fat was fixed for 24 h at 4°C in 3.7% formaldehyde, dehydrated, embedded in paraffin, and sectioned (thickness, 3 μm). The sections were deparaffinated in xylene and stained with hematoxylin and eosin so that the size of adipocytes could be evaluated. The diameter of adipocytes was quantified by measuring two randomly selected visual fields in two different sections from each of three HFD Tg and WT rats.
Plasma analysis
Blood samples were obtained from Tg and WT rats after overnight fasting. Plasma FFA levels were measured by SRL Co., Ltd. (Tokyo, Japan). Plasma levels of glycerol and total cholesterol were measured with the LipoSEARCH system (Skylight Biotech, Akita, Japan) (23). Plasma insulin levels were measured with ELISA kits (Morinaga, Kanagawa, Japan). Blood glucose was measured by using the glucose oxidase method (Ascensia Breeze 2, Bayer Medical, Leverkusen, Germany).
Adipocyte differentiation, droplet formation, and fatty acid oxidation in the mesenteric fat
Guanylin-GC-C double-Tg rats and WT rats (n = 5 each) were euthanized at 21 weeks of age, and total RNA was extracted from the mesenteric fat tissues with an RNeasy Lipid Tissue Mini Kit (Qiagen). The numbers of mRNA transcripts corresponding to genes involved in adipocyte maturation, droplet formation, and fatty acid oxidation in mesenteric fat were measured by quantitative PCR. Real-time PCR was conducted by using the LightCycler system described earlier; primer sequences are listed in supplementary Table II. In addition, mRNAs that varied in expression in the mesenteric fat between HFD Tg rats and WT rats were examined in other adipose tissues, namely subcutaneous and epididymal fat and brown adipose tissue. The mRNA levels of target genes were normalized against those of GAPDH.
Histologic analysis and triacylglycerol content of livers of Tg rats
The livers of HFD Tg and HFD WT rats were removed for gross examination and sectioned for hematoxylin and eosin staining, as described earlier. For assay of the hepatic triacylglycerol contents, the lipids from 25 mg tissue were extracted in 1 ml of chloroform:methanol [2:1 (v/v)] mixture as described (24), and triacylglycerol was quantified with a triglyceride E kit (Wako Chemicals, Tokyo, Japan).
Expression of guanylin and GC-C mRNA in peritoneal macrophages
To isolate peritoneal macrophages, WT and Tg rats were injected intraperitoneally with 5 ml of 4% thioglycollate. Exudated cells were collected by washing the peritoneal cavity with 0.9% saline four days after injection. These cells were incubated for 3 h at 37°C in a humidified 5% CO2 incubator, and adherent cells were used as peritoneal macrophages (25). We extracted mRNA from these peritoneal macrophages and examined the mRNA expression of guanylin and GC-C by using quantitative PCR as described earlier; the mRNA levels of target genes were normalized against those of the ribosomal protein 36B4 mRNA.
Coculture of primary mesenteric adipocytes and macrophages
Adipocytes were cultured in a commercially available primary culture system (Visceral Adipocyte Culture Kit VACH2; Primary Cell Co., Ltd., Hokkaido, Japan). The culture system included the stromal vascular cell fraction from the mesenteric fat tissue of SD rats and a differentiation medium that was based on DMEM and F12; this medium did not include indomethacin, dexamethasone, or peroxisome proliferator-activated receptor γ (PPAR-γ) agonist (26). Coculture was performed by adding peritoneal macrophages (1 × 105 cells/well) of Tg or WT rats to wells containing undifferentiated primary adipocytes (1 × 105 cells/well; 24-well plate). The cells were incubated at 37°C in a humidified 5% CO2 incubator for 8 days to induce differentiation. The culture medium was replaced with fresh medium every 2 days. After the 8 day incubation period, the total mixed-cell population was harvested and total RNA was extracted with an RNeasy Micro kit (Qiagen).
Transfection of macrophages and coculture with primary mesenteric adipocytes
NR8383 cells, an alveolar macrophage cell line that expresses both guanylin and GC-C (27), were cultured in F12 medium supplemented with 10% fetal bovine serum. By using Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's instructions, cells were transfected with a mixture of siRNAs specific for guanylin and GC-C (Stealth siRNAs; Invitrogen) or with negative control siRNAs; 25 nM of each oligonucleotide was transfected. At 24 h after transfection, undifferentiated primary adipocytes (1 × 105 cells/well; 24-well plate) were cocultured with transfected cells (1 × 105 cells/well). The cells were incubated at 37°C in a humidified 5% CO2 incubator for 6 days to induce differentiation. The culture medium was replaced with fresh medium every 2 days. After the 6 day incubation period, the total mixed-cell population was harvested and total RNA was extracted with an RNeasy Micro kit (Qiagen).
Oil Red O staining
Oil Red O staining was performed with a Lipid Assay kit (Primary Cell Co., Ltd.). Briefly, cells were washed with PBS and fixed with 10% neutral buffered formalin solution overnight at room temperature. The cells were washed again with PBS and stained for 15 min in freshly prepared Oil Red O solution (Oil Red O stock solution:H2O, 3:2 [v/v]). The stain was removed, and the cells were washed three times with water and then photographed.
Adipocyte differentiation and droplet formation in a coculture system
The expression of genes involved in adipocyte differentiation and maturation in the cultured adipocytes was evaluated by using the LightCycler system as described earlier; the PCR oligonucleotide primers are listed in supplementary Table II. mRNA levels of target genes were normalized against those of the ribosomal protein 36B4 mRNA.
Statistical analysis
We compared groups of data by using Student t-tests or ANOVA (ANOVA) with post hoc Tukey-Kramer tests (IBM SPSS Statistics 20.0, Armonk, NY). P values less than 0.05 were considered significant (two-tailed tests). Data are reported as means ± SEM.
RESULTS
Expression of guanylin and GC-C in the mesenteric fat of DR rats
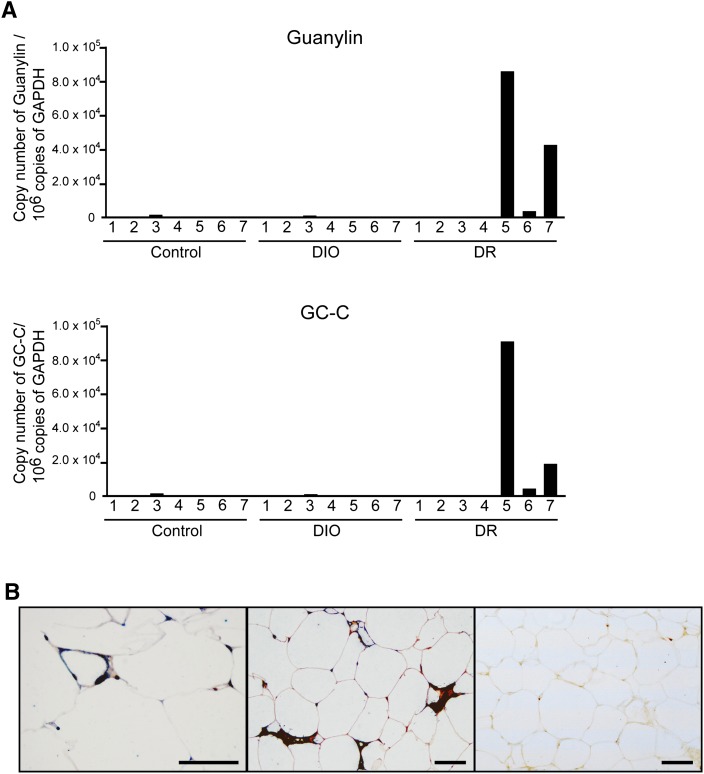
Microarray screening revealed unique increases in the mRNAs of guanylin and its receptor GC-C in the mesenteric fat of three of the seven DR rats evaluated. These results were verified by quantitative PCR (Fig. 1A). Microarray screening also revealed many other genes that were overexpressed in the mesenteric fat of DR rats with high expression of guanylin and GC-C (supplementary Table I). We focused here on high expression of both guanylin and GC-C, because guanylin is a specific ligand for GC-C. In addition, histologic data indicated that most guanylin- or GC-C-immunoreactive cells in the mesenteric fat tissues of DR rats with high expression of guanylin and GC-C colocalized with CD68-immunoreactive cells (Fig. 1B); a negative control study performed by using IgG did not show any immunoreactivity (Fig. 1B). No immunoreactivity for guanylin or GC-C was detected in the mesenteric adipose tissues from DIO and control rats (supplementary Fig. I-A).
Fig. 1.
GC-C mRNA expression in mesenteric fat tissue. (A) Quantitative PCR for guanylin or GC-C-mRNA. Cont, control rats fed standard chow; DIO, rats with HFD-induced obesity; DR, rats with resistance to an HFD. (B) Double immunostaining of mesenteric fat with anti-guanylin or anti-GC-C plus anti-CD68 antibodies in DR rats. Left panel, guanylin (brown) and CD68 (blue); middle panel, GC-C (brown) and CD68 (blue); right panel, control IgG. Scale bar, 50 μm.
Generation of guanylin-GC-C double-Tg rats
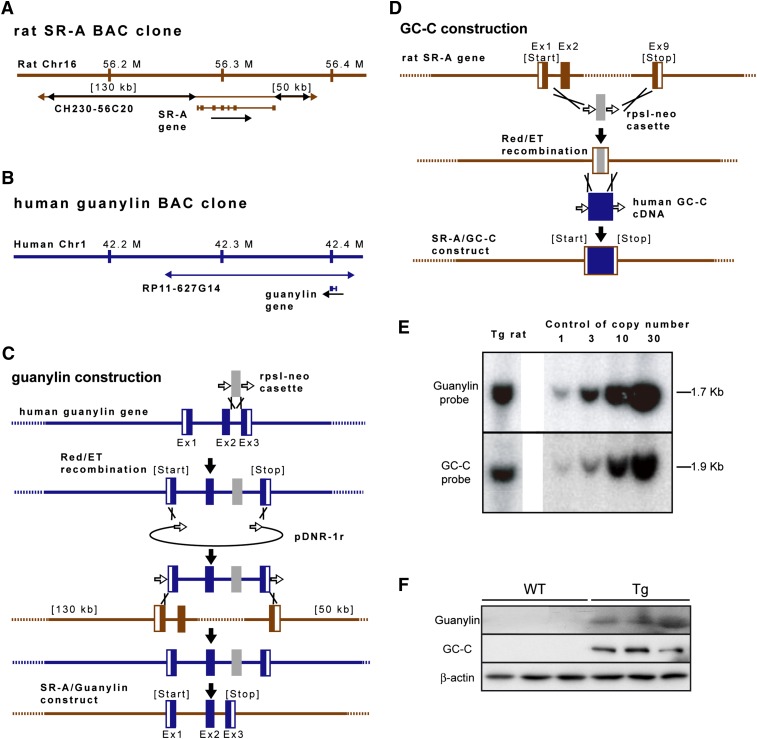
To investigate whether overexpression of guanylin and GC-C in macrophages contributes to anti-obesity, we generated guanylin-GC-C double-Tg rat lines to drive coexpression of the human guanylin and GC-C genes. We identified a rat SR-A BAC clone that included 130 kb of the 5′ flanking region, the SR-A gene itself, and 50 kb of the 3′ flanking region (Fig. 2A). By using BAC recombineering, we precisely removed the entire genomic sequence of the SR-A gene (i.e., start to stop) from the rat BAC clone and replaced it with either the human guanylin genomic sequence (2 kb sequence from a human BAC clone; Fig. 2B, C) or the human GC-C cDNA sequence (Fig. 2D); because the human GC-C gene is large (89 kb), we used cDNA rather than the genomic sequence for the GC-C construct. The integrity of each chimeric BAC transgenic construct was confirmed by sequence analysis (data not shown). After BAC modification, each linearized BAC transgenic construct was independently purified. Both constructs then were combined in equal amounts and injected into pronucleus-stage rat embryos to establish transgenic lines. Five founders transmitted the BAC transgenic constructs to F1 pups. A line that harbored 10 copies of each BAC transgenic construct was used for subsequent experiments (Fig. 2E). The mesenteric fat tissues of male Tg rats, but not male WT rats, showed protein expression of guanylin and GC-C (Fig. 2F).
Fig. 2.
Generation of guanylin-GC-C BAC transgenic (Tg) rats. Structures of the (A) rat SR-A (CH230-56C20) and (B) human guanylin (RP11-627G14) BAC clones. Each BAC clone contains the full-length coding sequence and the 5- and 3-flanking sequences. (C, D) Schematic diagram of transgene construction. By using Red/ET recombination, the coding region of the rat SR-A gene was replaced with either (C) the entire human guanylin gene or (D) the human GC-C cDNA fragment. Ex, exon. (E) Southern blot analysis of guanylin-GC-C double-Tg lines. Hybridization with a radioactive probe showed that the BAC-transduced human guanylin and GC-C sequences were detected as a 1.7 kb PstI fragment and a 1.9 kb Dra I fragment, respectively. The copy number of each integrated transgene was determined through comparison with the intensity of a control signal (right panel). (F) Western blot analysis of the protein levels of guanylin and GC-C in the mesenteric fat tissues of male Tg and WT rats fed standard chow. β-actin was used as a loading control.
Resistance of guanylin-GC-C Tg rats to an HFD
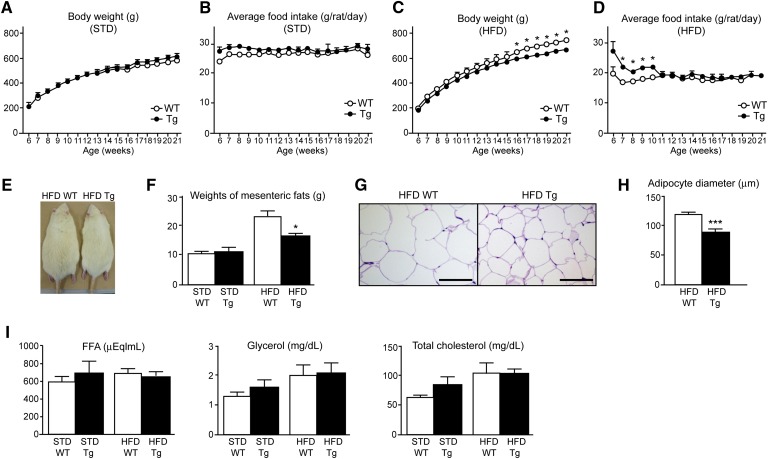
To elucidate the roles of guanylin and GC-C in resistance to an HFD in vivo, we assessed the body weight, food intake, and weight and adipocyte size of the mesenteric fat in Tg and WT rats fed standard chow or an HFD. We also investigated the plasma levels of FFA, glycerol, and total cholesterol in Tg and WT rats. There were no significant differences in body weight and food intake between Tg and WT rats that received standard chow (Fig. 3A, B). In contrast, after 10 weeks of an HFD (i.e., by 16 weeks of age), Tg rats exposed to the HFD (HFD Tg rats) weighed significantly less (P < 0.05) than did WT rats fed the same diet (HFD WT rats) (Fig. 3C). Although HFD Tg rats consumed more (P < 0.05) food than did HFD WT rats during weeks 1 through 4 of HFD feeding (i.e., 10 weeks of age), food intake did not differ between these two groups thereafter (Fig. 3D). In addition, the lower body weights of HFD Tg rats were visually apparent (Fig. 3E). Whereas the weight of the mesenteric fat did not differ between Tg and WT rats that were fed standard chow, that of HFD Tg rats was less (P < 0.05) than that of HFD WT rats (Fig. 3F), and the adipocytes were significantly smaller in HFD Tg rats than in HFD WT rats (Fig. 3G, H). Plasma levels of FFA, glycerol, and total cholesterol did not differ between Tg and WT rats on the same diet (Fig. 3I).
Fig. 3.
Parameters of energy metabolism in guanylin-GC-C double-Tg rats. (A‑D) Body weight (A, C) and food intake (B, D) of WT and Tg rats that were fed standard chow (STD; A, B) or an HFD (C, D). (E) Macroscopic appearance of 21-week-old HFD WT and HFD Tg rats. (F) Weight of the mesenteric fat of WT and Tg rats fed an STD or an HFD. (G) Hematoxylin-eosin staining of sections of mesenteric fat. Scale bar, 100 μm. (H) Diameters of adipocytes from HFD Tg and WT rats. Data are presented as means ± SEM (n = 3). ***P < 0.001 versus WT rats. (I) Plasma concentrations of free fatty acid (FFA), glycerol, and total cholesterol. All data are presented as means ± SEM (n = 4 or 5). *P < 0.05 compared with value for WT rats on the same diet.
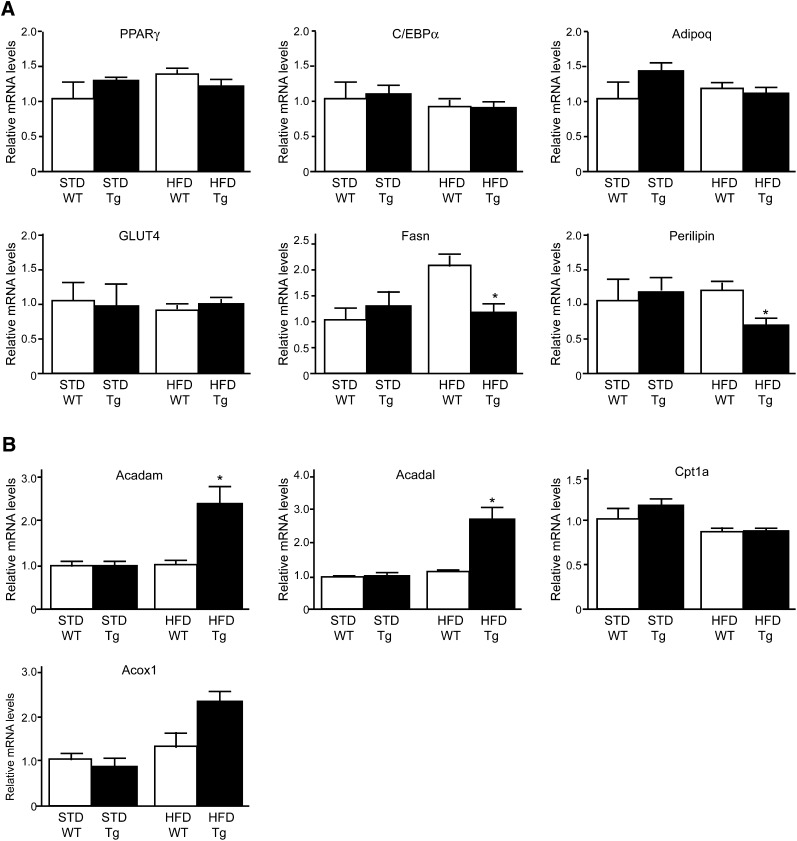
Downregulation of mRNA expression of genes involved in droplet formation factors in Tg rats fed HFD
The mRNA levels of mature adipocyte markers, including PPARγ, CCAAT-enhancer-binding protein α (C/EBPα), adiponectin, and glucose transporter 4 (GLUT4), did not differ between HFD Tg and HFD WT rats or between Tg and WT rats fed standard chow. In contrast, mRNA levels of Fas and perilipin, which are important elements involved in lipid droplet formation (28, 29), were significantly (P < 0.05) lower in HFD Tg rats than in HFD WT rats (Fig. 4A) but did not differ between Tg and WT rats fed standard chow (Fig. 4A). In contrast, there were no significant differences in Fas or perilipin mRNA levels in the subcutaneous and epididymal fat and brown adipose tissue between HFD Tg and WT rats (supplementary Fig. II-A). Furthermore, guanylin and GC-C mRNA levels in the subcutaneous and epididymal fat and brown adipose tissue of HFD Tg rats were markedly lower than those in the mesenteric fat of HFD Tg rats (supplementary Fig. II-B). These results suggest that guanylin-GC-C Tg rats acquire a function that regulates genes involved in lipid droplet formation, mainly in the mesenteric fat, particularly when fed an HFD.
Fig. 4.
Gene expression in the mesenteric fat of WT and guanylin-GC-C double-Tg rats. (A, B) Quantitative RT-PCR analysis of mRNA transcripts of genes associated with (A) adipocyte maturation and droplet formation and (B) fatty acid oxidation in mesenteric adipose tissue. Data were normalized to the GAPDH mRNA levels and are presented as means ± SEM (n = 4 or 5). *P < 0.05 compared with value for WT rats on the same diet. Acadam, acyl-CoA dehydrogenase C4 to C12 straight-chain; Acadl, acyl-CoA dehydrogenase long chain; Acox1, acyl-CoA oxidase 1 palmitoyl; Adipoq, adiponectin; C/EBPα, CCAAT-enhancer-binding protein α; Cpt1a, carnitine palmitoyltransferase 1a; GLUT4, glucose transporter 4; PPARγ, peroxisome proliferator-activated receptor γ.
Upregulation of mRNA expression of genes involved in fatty acid oxidation in the mesenteric fat of HFD Tg rats
We then examined the mRNA levels of enzymes involved in fatty acid oxidation in the mesenteric fat. mRNA levels of carnitine palmitoyltransferase 1a and acyl-CoA oxidase 1 did not differ between Tg rats and WT rats, whereas those of acyl-CoA dehydrogenase (Acad) mRNA were significantly (P < 0.05) greater in HFD Tg rats than in the other groups (Fig. 4B). As with the mRNA expression of genes involved in droplet formation process, the expression of Acadm and Acadl mRNAs in the subcutaneous and epididymal fat and brown adipose tissue did not differ between HFD Tg and WT rats (supplementary Fig. II-A).
Expression of guanylin and GC-C mRNAs in peritoneal macrophages
The macrophage marker F4/80 was expressed in the peritoneal macrophages of both Tg and WT rats (supplementary Fig. III). Both guanylin and GC-C mRNAs were expressed in the peritoneal macrophages, mesenteric fat tissues, and stromal vascular cell fraction of Tg rats but not WT rats (supplementary Fig. III).
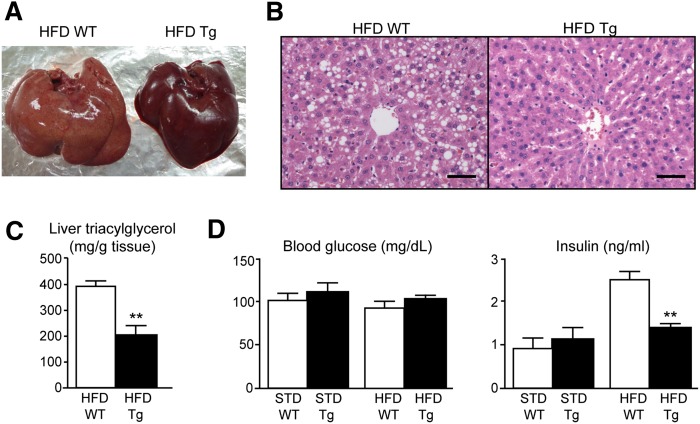
Triacylglycerol accumulation in the liver and plasma glucose and insulin levels
Lipid accumulation in the livers of HFD Tg rats was notably mild compared with that in HFD WT rats (Fig. 5A, B). Accordingly, the hepatic triacylglycerol content was significantly (P < 0.05) lower in HFD Tg rats than in HFD WT rats (Fig. 5C). There were no significant differences in blood glucose levels between Tg and WT rats, whereas plasma insulin levels were significantly lower in HFD Tg rats than in HFD WT rats (Fig. 5D).
Fig. 5.
(A) Macroscopic appearance of the liver. (B) Hematoxylin-eosin staining of liver sections. Scale bar, 50 μm. (C) Triacylglycerol content of the livers of Tg and WT rats. (D) Blood glucose and plasma insulin levels in Tg and WT rats. Data are presented as means ± SEM (n = 4 or 5). **P < 0.01 compared with value for WT rats on the same diet.
Role of guanylin-GC-C system in inhibition of droplet formation in primary cultured mesenteric fat cells
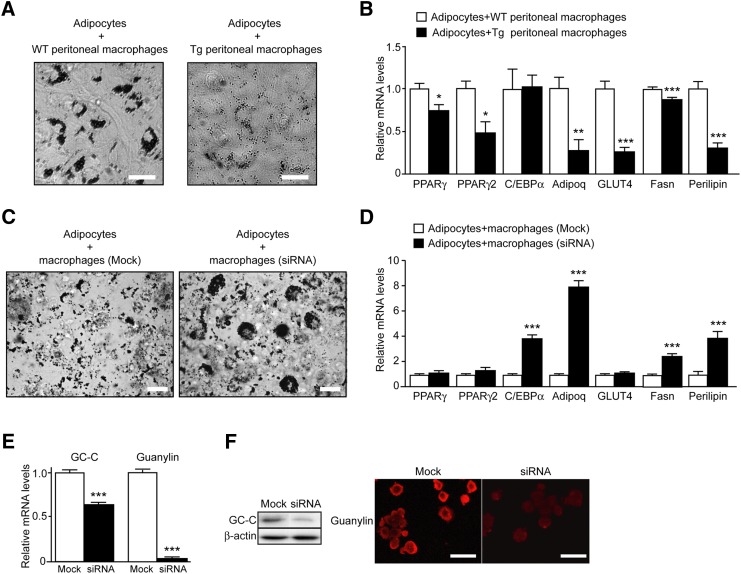
To elucidate whether the guanylin-GC-C system is involved in regulating the accumulation of lipid droplets, we examined lipid accumulation in a coculture system comprising adipocytes and peritoneal macrophages obtained from Tg and WT rats. Eight days after inducing adipocyte differentiation in these cells, we stained them with Oil Red O and showed that the adipocytes cocultured with macrophages of Tg rats had less lipid droplet accumulation than did adipocytes cultured with those of WT rats (Fig. 6A). The mRNA levels of PPARγ, PPARγ2, adiponectin, GLUT4, Fas, and perilipin were significantly (P < 0.05) lower in adipocytes cocultured with peritoneal macrophages of Tg rats compared with those of WT rats (Fig. 6B).
Fig. 6.
Coculture of primary adipocytes with guanylin- and GC-C-expressing macrophages. (A) Lipid accumulation in adipocytes cocultured with peritoneal macrophages of WT and Tg rats. Neutral lipids were stained with Oil Red O on day 8 after the induction of adipogenesis. Scale bar, 20 μm. (B) Quantitative PCR of genes involved in adipocyte maturation and droplet formation. Data were normalized against the mRNA levels of ribosomal protein 36B4 and are expressed as fold induction relative to that of adipocytes with peritoneal macrophage of WT rats. Data are presented as means ± SEM (n = 16). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the value for adipocytes cocultured with peritoneal macrophages of WT rats. (C) Lipid accumulation in adipocytes cocultured with nontargeting control mock-transfected macrophages or cocultured with macrophages transfected with guanylin and GC-C siRNAs. Neutral lipids were stained with Oil Red O six days after the induction of adipogenesis. Scale bar, 20 μm. (D) Quantitative PCR of genes involved in adipocyte maturation and droplet formation. Data were normalized against the amount of 36B4 mRNA and were expressed as fold induction relative to that of adipocytes cocultured with mock-transfected macrophages. Data are presented as means ± SEM (n = 5). ***P < 0.001 compared with the value for adipocytes cocultured with mock-transfected macrophages. (E) mRNA levels of GC-C and guanylin in macrophages 24 h after their transfection with specific siRNAs against GC-C or guanylin or mock siRNA. ***P < 0.001 compared with value for mock-transfected macrophages (n = 7). (F) Western blot analysis of GC-C and immnunohistochemical analysis of guanylin in macrophages 6 days after transfection with GC-C, guanylin, or mock siRNA. β-actin was used as a loading control. For immunohistochemical analysis, cultured cells were fixed with paraformaldehyde and stained with anti-guanylin antibody and Alexa-594-conjugated anti-rabbit IgG. Scale bar, 50 μm.
We further investigated the link between guanylin-GC-C expression and lipid droplet accumulation by using siRNAs specific to guanylin and GC-C. Guanylin and GC-C mRNA levels in NR8383 cells were downregulated after transfection with guanylin- and GC-C-specific siRNAs (Fig. 6E). Western blotting and immunohistochemistry revealed decreases in the protein levels of GC-C and guanylin, respectively, in NR8383 cells after transfection with each siRNA (Fig. 6F). When adipocytes and NR8383 cells were cocultured for 6 days in the presence of guanylin- and GC-C-specific siRNAs, the inhibition of lipid accumulation in cocultured adipocytes was reduced (Fig. 6C). Guanylin- and GC-C-specific siRNAs partially increased the mRNA expression levels of C/EBPα, adiponectin, Fas, and perilipin mRNA expression in cocultured samples; in contrast, these siRNAs had little effect on the mRNA levels of PPARγ, PPARγ2, and GLUT4 (Fig. 6D).
DISCUSSION
We revealed high-level expression of guanylin and GC-C in the mesenteric macrophages of some DR rats. We therefore suspect that the guanylin-GC-C system in mesenteric macrophages is involved in the regulation of lipid metabolism, leading to a phenotype of diet resistance. We detected high-level expression of guanylin and GC-C in three of seven DR rats whose weights were low compared with those of control and DIO rats. In other words, not all DR rats overexpress guanylin and GC-C in their mesenteric fat tissues. In fact, gene expression related to Wnt signaling, vascularization, tissue remodeling, caveolae, and cytoskeletal structure also differs among DR mice (30, 31), indicating that multiple diverse molecules may contribute to the phenotype of these animals. Our microarray screening revealed altered expression of many genes in the mesenteric fat of DR rats showing high expression of guanylin and GC-C. We took into account the fact that guanylin is a peptide ligand of GC-C and considered that the guanylin-GC-C system may play a role in the induction of the DR phenotype. To date, we have not found high-level expression of guanylin and GC-C in the mesenteric fat of rats fed standard chow, suggesting that an HFD may induce the expression of these factors in mesenteric macrophages to ultimately suppress lipid accumulation in some subjects. Although we used outbred rat strains here, even homogenous inbred mouse strains, such as C57BL/6J, show a wide distribution in body weight gain after being fed an HFD (30, 31). These findings suggest that multiple factors, including unstable stochastic systems and epigenetic modifications (30), affect susceptibility to an HFD.
GC-C, which is located in the intestinal mucosae, was first identified as the target for heat-stable enterotoxins, namely 15- to 30-amino-acid peptides produced by E. coli, Yersinia enterocolitica, and Vibrio cholerae. Heat-stable enterotoxins cause acute diarrhea by inducing Cl– secretion and by inhibiting Na+ and H2O absorption (32–34). Guanylin and uroguanylin were identified in the gastrointestinal tract as endogenous ligands for GC-C (11–16) and have since been thought to help maintain fluid and salt homeostasis in a paracrine manner. GCs or their ligands, or both cascades, have recently been shown to contribute to energy metabolism (20, 35–38). When fed an HFD, rats transgenic for BNP are protected from obesity and insulin resistance, and GC-A knockout mice have diet-induced obesity and glucose intolerance (20). In addition, CNP may play a role in regulating food intake and energy metabolism (35). However, the relationship between the guanylin-GC-C system and adipocyte regulation had not been investigated previously.
To investigate the effects of guanylin and GC-C overexpression on lipid accumulation in vivo, we generated guanylin-GC-C double-Tg rats. The body weights of HFD Tg rats fell below those of HFD WT rats, even though energy intake was similar in HFD Tg and HFD WT rats. In addition, the mesenteric adipocytes were smaller in HFD Tg rats than in HFD WT rats. Accordingly, HFD Tg rats did not show hyperinsulinemia. These findings suggest that Tg rats are resistant to an HFD and thus escape insulin resistance. Tg rats had no diarrhea throughout the study, and the amount of feces and the fecal triglyceride and FFA contents did not differ between HFD Tg and HFD WT rats (data not shown). Our data demonstrate that lipid accumulation clearly was inhibited in the mesenteric fat of HFD Tg rats. Furthermore, mRNA expression of genes involved in droplet formation was decreased in the mesenteric fat, but not in the subcutaneous or epididymal fat tissues and brown adipose tissues, of HFD Tg rats. mRNA expression of genes involved in fatty acid oxidation in the mesenteric fat but not in the subcutaneous or epididymal fat tissues and brown adipose tissues was higher in these rats than in HFD WT rats. These findings may reflect the high levels of expression of guanylin and GC-C in the mesenteric fat tissues of HFD Tg rats (supplementary Fig. II-B). On the other hand, in the mesenteric fat of DR rats with high-level expression of guanylin and GC-C, Fas mRNA expression was downregulated, and perilipin mRNA showed a trend toward downregulation compared with the levels of mRNA expression in control, DIO, and DR rats without guanylin and GC-C expression (data not shown). In contrast, the mRNA expression of enzymes involved in fatty acid oxidation in the mesenteric fat of DR rats with high-level expression of guanylin and GC-C did not differ from that in control, DIO, and DR rats without guanylin and GC-C (data not shown). Although here we declined to investigate the reasons underlying this discrepancy between Tg rats and DR rats with high-level expression of guanylin and GC-C, it may reflect differences in the duration of HFD feeding or the expression levels of guanylin, GC-C, or both, in the mesenteric macrophages.
In this study, we also showed that lipid accumulation in the livers of HFD Tg rats was quite mild compared with that of HFD WT rats. Considering the high levels of expression of guanylin and GC-C in the livers of HFD Tg rats (data not shown), these data suggest that the guanylin-GC-C system regulates lipid accumulation in other organs in addition to the mesenteric fat. To address how guanylin-GC-C system functions for inhibition of lipid accumulation, further investigations would be required.
Recently, apoptosis inhibitor of macrophages (AIM) protein was shown to be transported from macrophages to adipocytes via CD36, thereby decreasing the activity of Fas (39). This process inhibits droplet formation and subsequently stimulates FFA efflux from adipocytes (39). Our data similarly showed that the mRNA levels of Fas and perilipin in the mesenteric fat were lower in HFD Tg rats than in HFD WT rats. These findings suggest that plasma FFA and glycerol levels increase after excess release of lipids from the mesenteric fat of HFD Tg rats. However, plasma levels of FFA and glycerol did not differ between HFD Tg and HFD WT rats. These data indicate that guanylin-GC-C double-Tg rats have a strong ability to catabolize excess lipids and prevent their accumulation. Additional studies to elucidate the functional and mechanistic characteristics of the macrophages expressing guanylin and GC-C likely would yield insight into the unique metabolism of Tg rats.
To investigate the practical contribution of the guanylin-GC-C system to lipid accumulation, we used a coculture system comprising mesenteric fat cells and peritoneal macrophages obtained from Tg and WT rats. Using this coculture system, we showed that the mRNA levels of PPARγ, PPARγ2, adiponectin, GLUT4, Fas, and perilipin were lower in cocultured adipocytes with macrophages of Tg rats than in adipocytes cultured with those of WT rats. In addition, adding guanylin- and GC-C-specific siRNAs to the coculture system partially restored lipid accumulation and marker mRNA expression. In contrast, in vivo experiments indicated that the mRNA expression of PPARγ, adiponectin, and GLUT4 in the mesenteric fat did not differ between HFD Tg and HFD WT rats. Although the inconsistency in mRNA expression in vivo compared with in vitro is difficult to explain, the results may depend on the ratio of macrophages to adipocytes in the coculture system. Taken together, our in vitro study demonstrates that the guanylin-GC-C system may be associated with lipid droplet accumulation and the expression of genes involved in the process of droplet formation and that various factors associated with this system may help to regulate these processes in mesenteric fat. Therefore, this coculture system may be a useful tool for identifying new factors that are involved in lipid accumulation.
We showed here that the guanylin-GC-C system in macrophages likely contributes to the inhibition of lipid accumulation and that this system may be associated with factors involved in inducing diet resistance. Our data indicate that the guanylin-GC-C system decreases the expression of genes involved in lipid droplet accumulation and increases that of other genes involved in fatty acid oxidation in mesenteric fat, leading to the hypothesis that guanylin and GC-C system functions as a regulatory system for lipid metabolism. We also have revealed the expression of guanylin and GC-C mRNA in the liver and peritoneal macrophages of Tg rats, suggesting that the guanylin-GC-C system has important functions in tissues additional to the mesenteric fat. Furthermore, our in vitro data suggest that various factors, including humoral factors, may play an important role in the inhibition of lipid accumulation. To identify the mechanisms or factors involved in regulating lipid accumulation, we are seeking key molecules that are upstream or downstream of the guanylin-GC-C system. We anticipate that elucidating the mechanism that inhibits excess lipid accumulation via the guanylin-GC-C system will help to identify target molecules that can be used to prevent or treat obesity and its related diseases.
Supplementary Material
Acknowledgments
The authors thank A. Miyashita, Y. Aiboshi, and T. Miyanaga (University of Miyazaki, Miyazaki, Japan) for their technical assistance; A. Shiota (Phoenix Bio, Utsunomiya, Japan) for technical help with the BAC transgenesis; H. Tsuda (Nagoya City University) for technical help with isolation of peritoneal macrophages; and S. Kajimura (University of California, San Francisco), K. Grove (Oregon Health & Science University), and N. Murakami (University of Miyazaki) for their helpful discussions.
Footnotes
Abbreviations:
- Acad
- acyl-CoA dehydrogenase
- BAC
- bacterial artificial chromosome
- DIO
- diet-induced obesity
- DR
- diet resistant
- GC-C
- guanylyl cyclase C
- HFD
- high-fat diet
- PPAR
- peroxisome proliferator-activated receptor
- Tg
- transgenic
- WT
- wild-type
This work was supported in part by grants-in-aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Program for Promotion of Basic Research Activities for Innovative Bioscience (PROBRAIN); the Mitsubishi Foundation; the Naito Foundation; and the Takeda Science Foundation.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and two tables.
REFERENCES
- 1.Hedley A. A., Ogden C. L., Johnson C. L., Carroll M. D., Curtin L. R., Flegal K. M. 2004. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 291: 2847–2850 [DOI] [PubMed] [Google Scholar]
- 2.Bray G. A., Bellanger T. 2006. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 29: 109–117 [DOI] [PubMed] [Google Scholar]
- 3.Jones P. H. 2010. Management of obesity in the prevention of cardiovascular disease. Methodist Debakey Cardiovasc J. 6: 33–36 [DOI] [PubMed] [Google Scholar]
- 4.James W. P., Rigby N., Leach R. 2006. Obesity and the metabolic syndrome: the stress on society. Ann. N. Y. Acad. Sci. 1083: 1–10 [DOI] [PubMed] [Google Scholar]
- 5.Rosen E. D., Spiegelman B. M. 2006. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 444: 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X. F., Han M., Storlien L. H. 2003. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Brain Res. Mol. Brain Res. 115: 21–28 [DOI] [PubMed] [Google Scholar]
- 7.Levin B. E., Keesey R. E. 1998. Defense of differing body weight set points in diet-induced obese and resistant rats. Am. J. Physiol. 274: R412–R419 [DOI] [PubMed] [Google Scholar]
- 8.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Jr. Ferrante A. W. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867 [DOI] [PubMed] [Google Scholar]
- 10.Lucas K. A., Pitari G. M., Kazerounian S., Ruiz-Stewart I., Park J., Schulz S., Chepenik K. P., Waldman S. A. 2000. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 52: 375–414 [PubMed] [Google Scholar]
- 11.Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. 1992. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA. 89: 947–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiegand R. C., Kato J., Huang M. D., Fok K. F., Kachur J. F., Currie M. G. 1992. Human guanylin: cDNA isolation, structure, and activity. FEBS Lett. 311: 150–154 [DOI] [PubMed] [Google Scholar]
- 13.Schulz S., Chrisman T. D., Garbers D. L. 1992. Cloning and expression of guanylin. Its existence in various mammalian tissues. J. Biol. Chem. 267: 16019–16021 [PubMed] [Google Scholar]
- 14.de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. 1992. Precursor structure, expression, and tissue distribution of human guanylin. Proc. Natl. Acad. Sci. USA. 89: 9089–9093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamra F. K., Forte L. R., Eber S. L., Pidhorodeckyj N. V., Krause W. J., Freeman R. H., Chin D. T., Tompkins J. A., Fok K. F. 1993. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA. 90: 10464–10468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kita T., Smith C. E., Fok K. F., Duffin K. L., Moore W. M., Karabatsos P. J., Kachur J. F., Hamra F. K. 1994. Characterization of human uroguanylin: a member of the guanylin peptide family. Am. J. Physiol. 266: F342–F348 [DOI] [PubMed] [Google Scholar]
- 17.Potter L. R., Abbey-Hosch S., Dickey D. M. 2006. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 27: 47–72 [DOI] [PubMed] [Google Scholar]
- 18.Sengenès C., Berlan M., De Glisezinski I., Lafontan M., Galitzky J. 2000. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 14: 1345–1351 [PubMed] [Google Scholar]
- 19.Sengenès C., Zakaroff-Girard A., Moulin A., Berlan M., Bouloumié A., Lafontan M., Galitzky J. 2002. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283: R257–R265 [DOI] [PubMed] [Google Scholar]
- 20.Miyashita K., Itoh H., Tsujimoto H., Tamura N., Fukunaga Y., Sone M., Yamahara K., Taura D., Inuzuka M., Sonoyama T., et al. 2009. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 58: 2880–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muyrers J. P., Zhang Y., Benes V., Testa G., Ansorge W., Stewart A. F. 2000. Point mutation of bacterial artificial chromosomes by ET recombination. EMBO Rep. 1: 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe K., Hazama M., Katoh H., Yamamura K., Suzuki M. 2004. Establishment of an efficient BAC transgenesis protocol and its application to functional characterization of the mouse Brachyury locus. Exp. Anim. 53: 311–320 [DOI] [PubMed] [Google Scholar]
- 23.Usui S., Hara Y., Hosaki S., Okazaki M. 2002. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 43: 805–814 [PubMed] [Google Scholar]
- 24.Neschen S., Moore I., Regittnig W., Yu C. L., Wang Y., Pypaert M., Petersen K. F., Shulman G. I. 2002. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am. J. Physiol. Endocrinol. Metab. 282: E395–E401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumitru C. D., Ceci J. D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J. H., Patriotis C., Jenkins N. A., Copeland N. G., Kollias G., et al. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 103: 1071–1083 [DOI] [PubMed] [Google Scholar]
- 26.Shimizu K., Sakai M., Ando M., Chiji H., Kawada T., Mineo H., Taira T. 2006. Newly developed primary culture of rat visceral adipocytes and their in vitro characteristics. Cell Biol. Int. 30: 381–388 [DOI] [PubMed] [Google Scholar]
- 27.Helmke R. J., Boyd R. L., German V. F., Mangos J. A. 1987. From growth factor dependence to growth factor responsiveness: the genesis of an alveolar macrophage cell line. In Vitro Cell. Dev. Biol. 23: 567–574 [DOI] [PubMed] [Google Scholar]
- 28.Ducharme N. A., Bickel P. E. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949 [DOI] [PubMed] [Google Scholar]
- 29.Puri V., Czech M. P. 2008. Lipid droplets: FSP27 knockout enhances their sizzle. J. Clin. Invest. 118: 2693–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koza R. A., Nikonova L., Hogan J., Rim J. S., Mendoza T., Faulk C., Skaf J., Kozak L. P. 2006. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozak L. P., Newman S., Chao P. M., Mendoza T., Koza R. A. 2010. The early nutritional environment of mice determines the capacity for adipose tissue expansion by modulating genes of caveolae structure. PLoS ONE. 5: e11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field M., Jr. Graf L. H., Laird W. J., Smith P. L. 1978. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc. Natl. Acad. Sci. USA. 75: 2800–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navaneethan U., Giannella R. A. 2008. Mechanisms of infectious diarrhea. Nat. Clin. Pract. Gastroenterol. Hepatol. 5: 637–647 [DOI] [PubMed] [Google Scholar]
- 34.Okoh A. I., Osode A. N. 2008. Enterotoxigenic Escherichia coli (ETEC): a recurring decimal in infants’ and travelers’ diarrhea. Rev. Environ. Health. 23: 135–148 [DOI] [PubMed] [Google Scholar]
- 35.Inuzuka M., Tamura N., Yamada N., Katsuura G., Oyamada N., Taura D., Sonoyama T., Fukunaga Y., Ohinata K., Sone M., et al. 2010. C-type natriuretic peptide as a new regulator of food intake and energy expenditure. Endocrinology. 151: 3633–3642 [DOI] [PubMed] [Google Scholar]
- 36.Ropero A. B., Soriano S., Tudurí E., Marroquí L., Téllez N., Gassner B., Juan-Picó P., Montanya E., Quesada I., Kuhn M., et al. 2010. The atrial natriuretic peptide and guanylyl cyclase-A system modulates pancreatic beta-cell function. Endocrinology. 151: 3665–3674 [DOI] [PubMed] [Google Scholar]
- 37.Lafontan M., Moro C., Berlan M., Crampes F., Sengenes C., Galitzky J. 2008. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 19: 130–137 [DOI] [PubMed] [Google Scholar]
- 38.Moro C., Smith S. R. 2009. Natriuretic peptides: new players in energy homeostasis. Diabetes. 58: 2726–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurokawa J., Arai S., Nakashima K., Nagano H., Nishijima A., Miyata K., Ose R., Mori M., Kubota N., Kadowaki T., et al. 2010. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 11: 479–492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.