Abstract
Cardiac triacylglycerol (TAG) stores buffer the intracellular availability of long chain fatty acid (LCFA) that act as nuclear receptor ligands, substrate for lipotoxic derivatives, and high energy-yield fuel. The kinetic characteristics of TAG turnover and homeostatic mechanisms linking uptake and storage dynamics in hearts have until now remained elusive. This work examines TAG pool dynamics in the intact beating heart, under normal conditions and in response to acute gene expression-induced changes in CD36. Dynamic mode 13C NMR elucidated multiple kinetic processes in 13C-palmitate incorporation into TAG: an initial, saturable exponential component and a slower linear rate. Although previous work indicates the linear component to reflect TAG turnover, we hypothesized the saturable exponential to reflect transport of LCFA across the sarcolemma. Thus, we overexpressed the LCFA transporter CD36 through cardiac-specific adenoviral infection in vivo. Within 72 h, CD36 expression was increased 40% in intact hearts, accelerating the exponential phase relative to PBS-infused hearts. TAG turnover also increased with elevations in adipose triglyceride lipase (ATGL) and a modest increase in diacylglycerol acyltransferase 1 (DGAT1), without a significant expansion of the intracellular lipid pools. The results demonstrate a dynamic system of reciprocal gene regulation that couples saturable LCFA uptake across the sarcolemma to TAG synthesis/lipolysis rates.
Keywords: nuclear magnetic resonance, fatty acids, triglycerides
The specific kinetic characteristics of intramyocellular triacylglycerol (TAG) turnover, involving synthesis and lipolysis in the intact beating heart, have been largely uncharacterized to date, and the mechanisms linking uptake and storage dynamics have until now remained elusive. This work examines the dynamics of the TAG pool in the intact heart, under normal conditions and in response to acute changes in in vivo, transporter protein-mediated lipid uptake, in the absence of developmental adaptations that might otherwise occur in a murine mouse model. Through the use of dynamic mode 13CNMR and endpoint enrichment analysis via LC/MS, we have been able to quantify the turnover of LCFA in and out of the TAG pool and identify distinct exponential and linear characteristics that are associated with regulatory processes of lipid dynamics in the cell (1–3).
TAG is the major source of energy stores within the heart. Traditionally viewed as an inert pool of unmetabolized long chain fatty acids, the newer, emerging realization is that TAG content in the cardiomyocyte is a dynamic metabolic pool that supports the high demand for fatty acid oxidation by the heart, as well as contributes fatty acids that serve as ligands for nuclear receptors and the induction of nuclear transcription factor activity (1, 2, 4, 5). Additionally, the rates of TAG turnover and content serve as neutral buffers implicated in limiting the formation and accumulation of physiological active and potentially toxic acyl intermediates and derivatives (6, 7). TAG turnover responds to the magnitude of lipid pool; TAG turnover increases following high-fat diet (1) in diabetes (3) or in mouse models of increased lipid mobilization, such as the peroxisome proliferator-activated receptor (PPAR)α or peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) overexpressing mice (1, 8). Conversely, TAG turnover is decreased in hearts with decompensated hypertrophy, in which PPAR activity becomes compromised (2, 9). Thus, the endogenous lipid store of the cardiomyocyte can play an essential role in the support and regulation of cellular processes in general and contractile activity in particular (2, 4).
This dynamic characteristic and consequentially active role for TAG has recently been implicated to potentially mediate cardiac dysfunction under disease states (2, 10). However, the mechanisms governing the entry of LCFA into cells in general and the heart specifically are poorly defined, and the consequences of cellular uptake rates of LCFA on TAG dynamics are unknown (11–14). The current study visualizes via 13C NMR spectroscopy and thereby elucidates previously unrealized, multiple kinetic characteristics of the TAG pool in the intact functioning heart that are influenced by cellular uptake of LCFA and the intracellular balance of TAG synthesis and lipolysis. The approach is extended further to elucidate a sarcollemal membrane transport-sensitive component through acute manipulation of the expression of the fatty acid transporter protein CD36 in the functioning heart.
Recently, LCFA transport into freshly isolated cardiac myocytes was demonstrated to occur via two distinct processes: a fast exponential component [time constant (τ) of 1–2 min] modulated by CD36 expression, and a slow linear component sensitive to inhibitors of LCFA metabolism (15). Thus, we hypothesized that such kinetic features of LCFA uptake would be reflected in the NMR-observable dynamics of TAG enrichment with 13C-enriched LCFA. To examine whether LCFA transporter kinetics were discernable in the intact functioning heart via 13C NMR detection of TAG enrichment kinetics and to better understand how LCFA transport might affect TAG turnover, we have used an in vivo model of exogenous gene delivery to overexpress the rat isoform of CD36 in a cardiac-specific manner. Dynamic mode 13C NMR of the intact functioning heart resolved distinct phases of TAG enrichment that can be elucidated as distinct physiological processes and that are consistent with prior observations of LCFA transport across the sarcolemma of isolated cardiomyocytes (15) with an eventual linear rate of TAG enrichment at steady state. The findings support our hypothesis that early and late phase kinetics of the isotopic enrichment of TAG from 13C LCFA can be distinguished and reflect the separate components of LCFA entry into the cardiomyocyte versus TAG turnover and that these separate rate components are tightly coupled and coordinated by reciprocal control of gene expression.
MATERIALS AND METHODS
In vivo exogenous gene delivery and CD36 augmentation
Three days prior to heart excision for biochemical analysis or isolated heart perfusions, adenovirus (∼7 × 1012 viral particles/ml) carrying rat CD36 cDNA (adv.CD36) (Vector Biolabs, Philadelphia, PA) was delivered in vivo to rat hearts (male, Sprague-Dawley, 350–400 g). Hearts were isolated 3 days after viral delivery when protein levels are significantly increased in the absence of inflammation, which is not evident until 5 days after infection (16, 17). This open-chest, cross-clamp technique has been described in detail in our previous reports (17–19). Typically, the efficiency of infection, as measured by the number of individual cardiomyocytes, is 60% using this technique (17). The adenovirus construct contained the cDNA for the rat isoform CD36 (GenBank accession L19658) (20, 21), a generous gift from M. Febbraio, Lerner Research Institute, Cleveland Clinic. Control groups underwent a sham surgical procedure with delivery of a PBS bolus or null adenovirus (adv.null, Vector Biolabs). Adenovirus stocks were amplified in HEK-293 cells. All procedures were approved by the University of Illinois at Chicago Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, revised 2011).
Isolated perfused rat heart
Three days after surgical delivery of the adv.CD36 or PBS, rats hearts were excised and perfused as previously described (2, 22). Hearts were perfused with 116 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 1 mM lactate, 5 mM glucose, and 0.4 mM unlabeled palmitate/albumin complex (3:1 molar ratio). After stabilization and baseline measurements to determine natural abundance of 13C, hearts were supplied buffer of similar content with replacement of the palmitate with [2,4,6,8,10,12,14,16-13C8] palmitate for 40 min. Hearts then were frozen for lipid analysis, 13C endpoint enrichment, and protein expression.
13C NMR spectroscopy
13CNMR spectra were acquired from isolated hearts as previously reported (2, 3, 22). Briefly, perfused hearts were positioned in a 20 mm broadband probe in a 9.4-T/89 mm vertical bore, superconducting NMR magnet. Carbon spectra were acquired at 101 MHz with bilevel broadband decoupling. Sequential 13C NMR spectra were collected (2 s interpulse interval) by time, averaging over 1 min intervals, to detect the progressive incorporation of 13C-labeled palmitate into the TAG pool under equilibrium conditions over 40 min. The baseline signal from 13C natural abundance (1.1%) was collected prior to enrichment for digital subtraction from data accumulation during the isotope enrichment phase. The relative rate of incorporation of 13C palmitate into TAG was determined from the increase in 13C signal intensity at 30.5 ppm from the methylene carbons within TAG (22). Absolute 13C enrichment over the entire time course was quantified by endpoint enrichment determination from LC/MS analysis. The resulting isotope enrichment data from each heart were fitted to a two-component kinetic model, which was composed of a monoexponential and a linear fit. The monoexponential component was characterized by the associated time constant (τ). TAG turnover was determined from the slope of the linear fit and was equal to TAG content multiplied by the 13C fractional enrichment of TAG divided by enrichment duration during the linear phase (2, 3).
The fractional contribution of 13C palmitate to mitochondrial acetyl CoA production was determined from the endpoint isotopomer distribution of 13C in the glutamate pool (3, 23). Neutralized acid extracts from heart tissue was lyophilized and reconstituted in 0.5 ml of 2H2O. In vitro 13C and 1H spectra were acquired on a 9.8 or 14.1-T magnet with a 5 mm 13C/1H probe.
Myocardial lipid analysis and endpoint fractional 13C TAG enrichment
Total lipids were extracted from frozen heart tissue, as described previously with some modifications (2, 3, 8). Frozen heart tissue (30–50 mg) was homogenized in 500 μl of PBS, and the protein concentration was determined from a 30 μl aliquot via BCA protein assay (Thermo Scientific). Total lipids were extracted from the remaining homogenate via Folch extraction, and the dried lipids were resuspended in 500 μl of chloroform. TAG, diacylglycerol (DAG), and monoacylglycerol (MAG) were separated from phospholipids by passage through BondElut NH2 solid-phase extraction column (Agilent). The lipid extract was applied to a NH2 column preconditioned with hexane. The column was rinsed with 1 ml of 2 chloroform:1 2-propanol (v:v), and the effluent was collected. The collected effluent was evaporated in a RapidVap (Labconco), and the lipid sample was either resuspended in 500 μl of chloroform for the separation of DAG from TAG and MAG (effluent A) or in 470 μl of methanol and 30 μl of 1 N NaOH (effluent B) for saponification (1 h at 70°C). Following saponification of effluent B, the sample was neutralized (10 μl of 10% formic acid), and the fatty acids were extracted in two washes of 500 μl of hexane. The hexane layers were combined and evaporated, the sample was resuspended in 1 chloroform:1 methanol (v:v) (Optima grade), and the fractional 13C enrichment of the fatty acids was determined by LC/MS. Total TAG concentration was quantified in the total lipid extract and normalized to the protein concentration (Wako Pure Chemical Industries).
DAG was separated from TAG and MAG by applying effluent A to a new NH2 column preconditioned with hexane. The column was rinsed with 5 ml of 1 diethyl ether:10 methylene chloride:89 hexane (v:v), and the effluent containing TAG was discarded. DAG was then released from the column by rinsing the column with 5 ml of 15 ethyl acetate:85 hexane (v:v). The effluent was collected to determine total DAG concentration, whereas MAG was retained on the column and discarded. The collected effluent was evaporated and resuspended in 470 μl of methanol and 30 μl of 1 N KOH and saponified to release glycerol. The free glycerol concentration was determined using a commercially available free glycerol kit (Abcam). The saponified glycerol sample was neutralized (10 μl of 10% formic acid), and following evaporation of the solvent, it was resuspended in 125 μl of the glycerol assay buffer from the glycerol kit. Contamination of TAG and MAG in the DAG effluent was less than 2% using standard mixes of 10 tripalmitin:1 diolein:1 monoarachidonin (molar ratio). By washing the final saponified material with hexane, it was possible to collect the released free fatty acids and confirm adequate separation of the different glycerides via LC/MS.
Total ceramides were isolated from frozen excised unperfused hearts and quantified by precursor-product scans followed by LC-ESI-MS/MS with multiple reaction monitoring based on the method of Sullards et al. (24). Frozen heart tissue (20–30 mg) was homogenized in PBS (10 μl/mg tissue), and then 500 μl of methanol and 250 μl of chloroform were added to 100 μl of tissue homogenate to extract ceramide. Protein concentration was determined in the tissue homogenate by BCA protein assay. One microliter of 0.5 mM C17 ceramide (N-heptadecanoyl-D-erythro-sphingosine) was included as an internal standard to enable quantification. A series of standard curves were generated in which the signal from a fixed concentration of C17 ceramide was measured in the presence of varying concentrations of C14, C16, C18, C18:1, C20, C22, C24, and C24:1 ceramides. The slopes of the relationships were used to account for matrix effects and to determine the concentration of the individual ceramide species in lipid samples based on their relative abundance and the known concentration of the internal standard. All ceramide standards were purchased from Avanti Polar Lipids.
The total long chain fatty acyl CoA concentration was determined as described previously by HPLC (25, 26). The data was normalized to milligrams of protein by converting wet weight to milligrams of protein using the average value determined when isolating DAG. There was 55 mg of protein in 1 g of tissue wet weight.
Measurement of inflammatory cytokines
The levels of interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α were assessed in frozen excised heart tissue using the Bio-Plex Pro rat cytokine assay kit (Bio-Rad). Hearts were excised from rats 72 h after adv.CD36 or adv.null infection or from rats that did not undergo surgical intervention. Cytokine levels were determined in 90 μg of protein isolate.
Protein expression
Protein expression was measured by Western blot in heart tissue using commercially available antibodies. Total cardiac membranes were isolated by the protocol of McCullough et al. (27), and 40 μg of protein (BCA protein assay) was loaded to measure CD36 expression. For CD36 expression, the α1 subunit of the Na+K+ATPase was used as a loading control. Diacylglycerol acyltransferase 1 (DGAT1) (30 μg) and adipose triglyceride lipase (ATGL) (75 μg) expression was measured in whole-tissue lysates, with calsequestrin as a loading control. Intensity of the bands of interest was normalized to the intensity of the loading control, and the relative increase in expression over baseline (PBS) was reported.
Statistical analysis
All data are presented as means ± SE, and differences in means were determined by unpaired t-test when comparing two groups or by one-way ANOVA when comparing three or more groups. Means were statistically different when P < 0.05.
RESULTS
Adenovirus delivery does not alter the concentration of proinflammatory intramyocardial cytokines
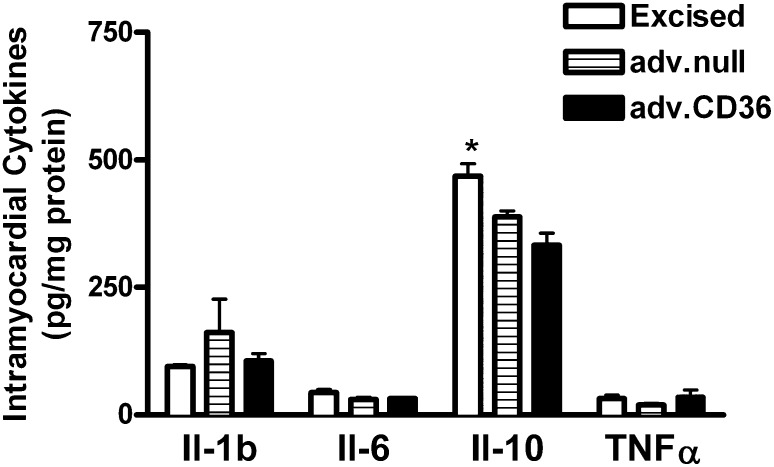
The delivery of adenovirus to rat hearts in vivo did not lead to a significant increase in cytokine levels in excised hearts at 72 h postdelivery (Fig. 1). These results are consistent with other reports on the time course of inflammation induce by adenovirus in vivo in other organ systems (28). There was a significant decrease in IL-10 levels following adenoviral delivery.
Fig. 1.
Intramyocardial cytokine levels. The average level of cytokines measured in frozen heart tissue excised from rats 72 h after adenovirus (adv.null or adv.CD36) or from rats not undergoing a surgical procedure (excised). Data are presented as means ± SE. n = 3 for excised and adv.CD36, n = 5 for adv.null; *P < 0.05 versus hearts receiving adenovirus.
CD36 overexpression through in vivo adenoviral infection
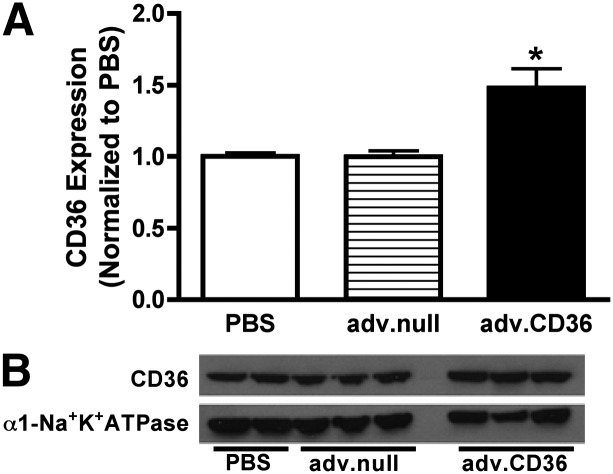
We chose an acute model of CD36 overexpression in the rat to investigate the relationship between LCFA uptake and TAG dynamics. Previously published work has demonstrated that CD36 expression modifies the exponential function describing LCFA transport across the sarcolemma in isolated cardiac myocytes (15). The model for exogenous gene delivery has been described in detail elsewhere and has been used to induce in vivo cardiac-specific overexpression of proteins through the injection of adenoviral vectors into the aortic root (17, 18, 29). As an acute model of gene manipulation, the approach is not complicated by developmental adaptations or changes induced in response to chronic gene manipulation. At 72 h postdelivery of vector containing cDNA for rat CD36 (adv.CD36) or sham infusion with PBS, hearts were isolated from rats and perfused for metabolic rate measurements by 13C NMR. Rate pressure product as an index of cardiac work was similar between the CD36 and sham groups (35,791 ± 2,149 mmHg × beats/min sham, n = 5, versus 34,489 ± 2,689 mmHg × beats/min adv.CD36, n = 7), and therefore not a variable in the analysis of intramyocellular lipid dynamics. Content of the sarcolemmal protein CD36 increased by ∼40% (relative to Na+/K+-ATPase) over shams, as measured in total membrane isolates (Fig. 2). No effect of adenovirus alone (adv.null) on CD36 expression was evident.
Fig. 2.
Induced in vivo CD36 expression. Bar represents the average expression from 8 sham (PBS), 3 adv.null, and 10 adv.CD36 hearts measured 3 days after surgery. Representative blots presented below the average data were taken from the same gel. Data are presented as means ± SE; *P < 0.05 versus sham.
Dynamic 13C NMR data reveals distinct kinetic features of fatty acid storage in the heart
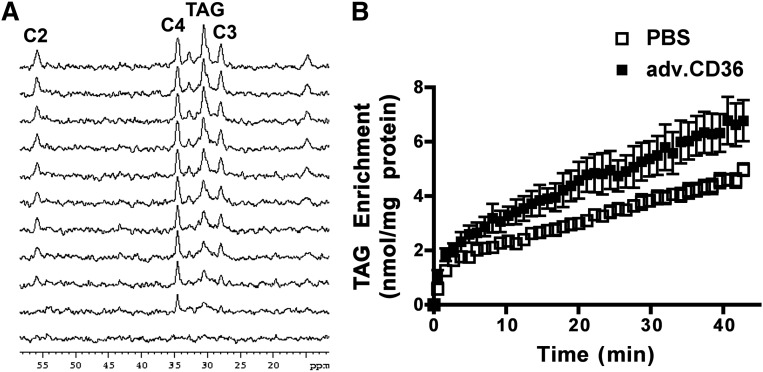
From sequential proton-decoupled 13C NMR spectra (1 min time resolution), incorporation of 13C enriched palmitate into the steady-state TAG pool (30.5 ppm) was monitored (Fig. 3A), which in tandem with quantitative endpoint LC/MS enabled detailed analysis of palmitate uptake and TAG turnover (2, 3, 22). The time course for enrichment of the TAG pool in the intact heart supplied with [2,4,6,8,-10,12,14,16-13C8]palmitate (0.4 mM) in the presence of glucose (5 mM) is plotted in Fig. 3. At the end of the 40 min perfusion, ∼15% of the TAG pool was 13C-enriched (14.7 ± 0.8, n = 5 sham versus 17.1 ± 0.03, n = 7 adv.CD36).
Fig. 3.
Incorporation of 13C-palmitate into intramyocardial TAG. (A) Representative 13C spectra from a control intact perfused heart obtained sequentially (1 min acquisition, from bottom). For simplicity of presentation, only every fourth trace is presented. (B) 13C enrichment of the TAG pool as a function of time was determined from signal intensity of the methylene at 30.5 ppm. Data are presented as means ± SE. n = 5 for sham and adv.CD36.
Dynamic data sets of 13C NMR spectra elucidated two distinct kinetic features of TAG enrichment (Fig. 3): an initial enrichment phase characterized by a single, saturable exponential function (highlighted in Fig. 4A), and a slower linear phase (2, 3). The findings presented below link these two kinetic features to the dynamic processes of LCFA transport/uptake into the cardiomyocyte and turnover within the intramyocellular TAG pool. Of course, it is important to remember that although these separate kinetics are detected by isotope enrichment as separate temporal events, the actual events associated with the exponential (LCFA transport and uptake) and linear (TAG turnover) components coincide, these dynamic processes coincide, and the kinetic features are superimposed. Thus, the introduction of isotopically enriched LCFA enables the temporal relationship of these kinetics to be elucidated through detection of the initial introduction of the 13C palmitate to transport into the cardiomyocyte followed by incorporation into the TAG pool.
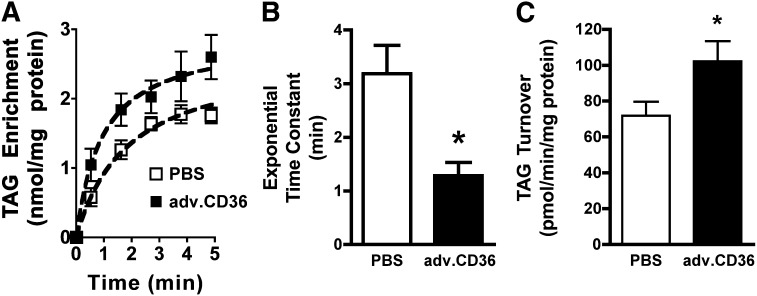
Fig. 4.
TAG dynamics. (A) Initial exponential phase of TAG enrichment showing the mean data as well as an exponential fit of the saturable component of TAG enrichment. (B) Time constant (τ) of exponential enrichment phase of TAG. (C) Rate of TAG turnover. For all panels, means ± SE, n = 5 for sham and adv.CD36, *P < 0.05.
The time constant (τ) of the initial exponential enrichment of TAG among sham hearts was τ = 3.18 ± 0.53 min (n = 5), similar to the exponential transport of LCFA transport into isolated cardiac myocytes (15). CD36 overexpression dramatically accelerated this exponential phase of the observed TAG enrichment with 13C palmitate, reducing the time constant by 60% compared with shams (P < 0.05, Fig. 3B). The responses of the saturable exponential component to increased CD36 content indicate that the first phase of TAG enrichment is defined by LCFA transport across the sarcolemma in the intact functioning heart, which is consistent with studies showing a slowed exponential uptake of LCFA into isolated cells that are transporter-deficient from the CD36 null mice (15).
TAG turnover and LCFA uptake are dynamically coupled
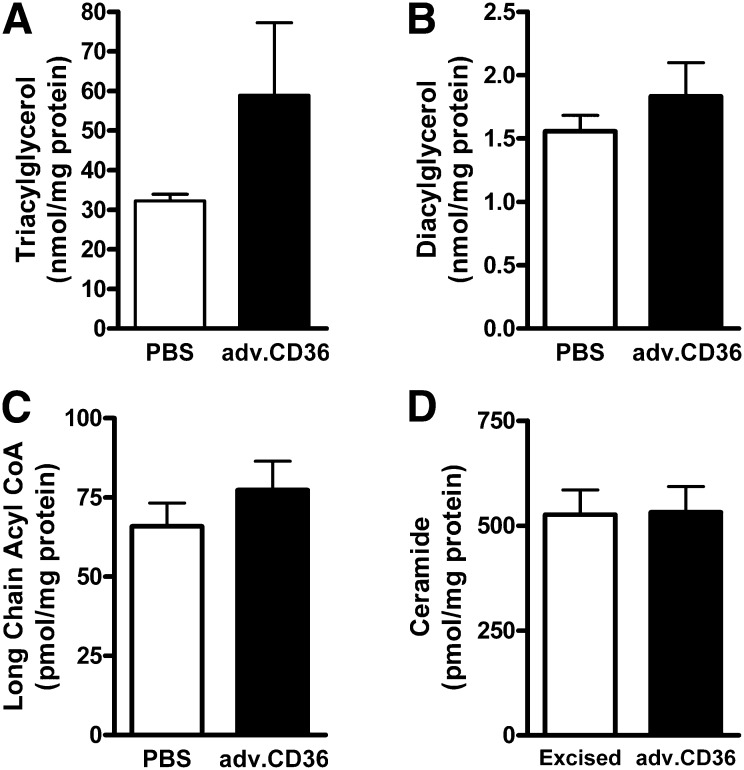
As previously noted for steady-state conditions of TAG content, the linear enrichment of TAG with 13C represents the TAG turnover rate (2, 30). At steady state, the on-rate (synthesis) for 13C palmitate into the TAG pool is equal to the off-rate (lipolysis) (1–3). Surprisingly, accelerating the uptake phase of TAG enrichment by acute CD36 overexpression also induced a more dynamic TAG pool. Although not as pronounced as the response of the saturable exponential component to elevated CD36, TAG turnover was increased by 30% (P < 0.05) in CD36 hearts over shams (Fig. 4C). Despite a trend toward increased TAG content in adv.CD36-infected hearts versus PBS, these differences did not reach statistical significance (P = 0.13, Fig. 5A), largely as a consequence of increased variability in the treated group. One heart, in particular, contributed to that variability: it displayed the highest induction of CD36 (2-fold), it had the fastest exponential rate (τ = 0.6 min), and it had the fastest rate of TAG turnover (136 pmol/min/mg protein). The TAG content in this heart was 164 nmol/mg protein. An increase in CD36 expression did not alter myocardial concentrations of long chain fatty acyl CoA, DAG, or ceramide (Fig. 5B–D). Overall, the results are consistent with an increase in TAG turnover independent of an expansion of the intramyocardial lipid pool.
Fig. 5.
CD36 effect on intramyocardial lipid pool concentrations. The concentration of TAG (A), DAG (B), and long chain fatty acyl CoA (C) measured in frozen heart tissue from isolated perfused hearts (for A, n = 5 for PBS, n = 7 adv.CD36; for B and C, n = 5 PBS, n = 6 adv.CD36) The concentration of ceramide (D) was measured in unperfused hearts (n = 3 for both). Data are means ± SE.
Reciprocal gene expression couples LCFA uptake with TAG synthesis/lipolysis
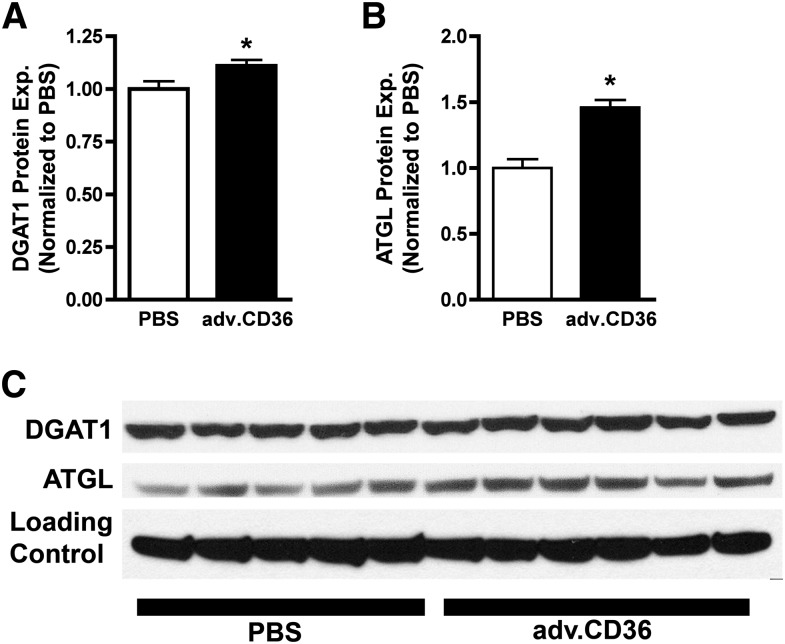
Because TAG turnover responded to increased palmitate transport due to CD36 overexpression, the activity of TAG synthases and lipases is clearly influenced by increased LCFA transport into the cardiomyocyte. Surprisingly, acute overexpression of CD36 also induced elevated content of the key synthase DGAT1, which catalyzes the final step in TAG synthesis, and the key lipase ATGL, which catalyzes the first step in TAG lipolysis and is believed to be the rate-limiting TAG lipase in heart (31). In response to CD36 overexpression and consequential increases in LCFA uptake, the protein content of DGAT1 was elevated by 10% and ATGL was elevated by 46% (Fig. 6), indicating that these enzymes are reciprocally regulated by LCFA uptake over a relatively short period of time.
Fig. 6.
CD36 effect on DGAT1 and ATGL expression levels. The protein expression of key TAG synthase DGAT1 (A) and TAG lipase ATGL (B) with the actual blot images (C). Calsequestrin was used as a loading control. For both figures, means ± SE, n = 5 for sham and n = 6 for adv.CD36, *P < 0.05.
Fractional contribution of exogenous LCFA to acetyl CoA formation
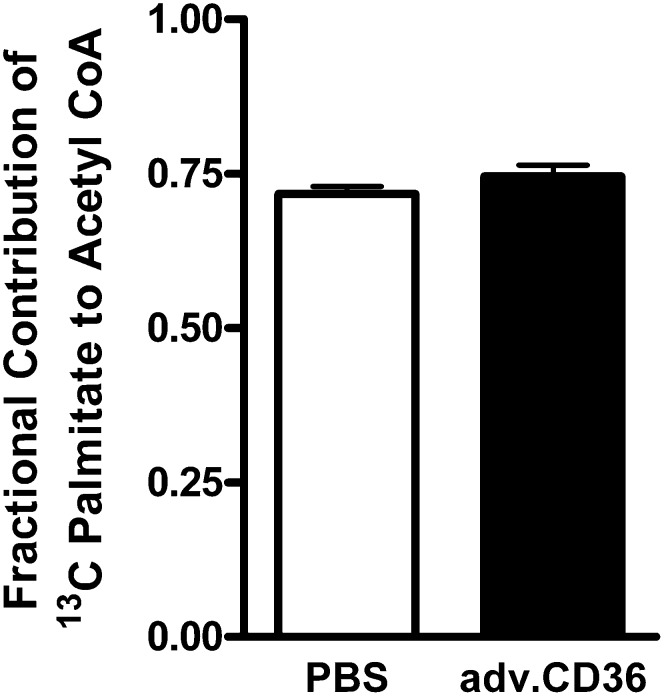
Increasing CD36 content induced no effects on the relative contribution of the exogenous 13C palmitate to β-oxidation and the subsequent production of acetyl CoA for oxidative energy production in the mitochondria. As shown in Fig. 7, in vitro 13C NMR of acid extracts from myocardial samples demonstrated that the palmitate contribution to β-oxidation was similar between CD36 hearts and shams, and therefore not surprisingly, the balance of exogenous LCFA oxidation to mitochondrial ATP production versus other fuel sources, such as glucose, remained unperturbed at baseline levels of cardiac work in each group.
Fig. 7.
Palmitate contribution to mitochondrial oxidation. The fractional contribution of exogenous 13C-palmitate to mitochondrial acetyl CoA formation was not altered by adv.CD36 administration. Data are means ± SE, n = 5 for sham and n = 7 for adv.CD36.
DISCUSSION
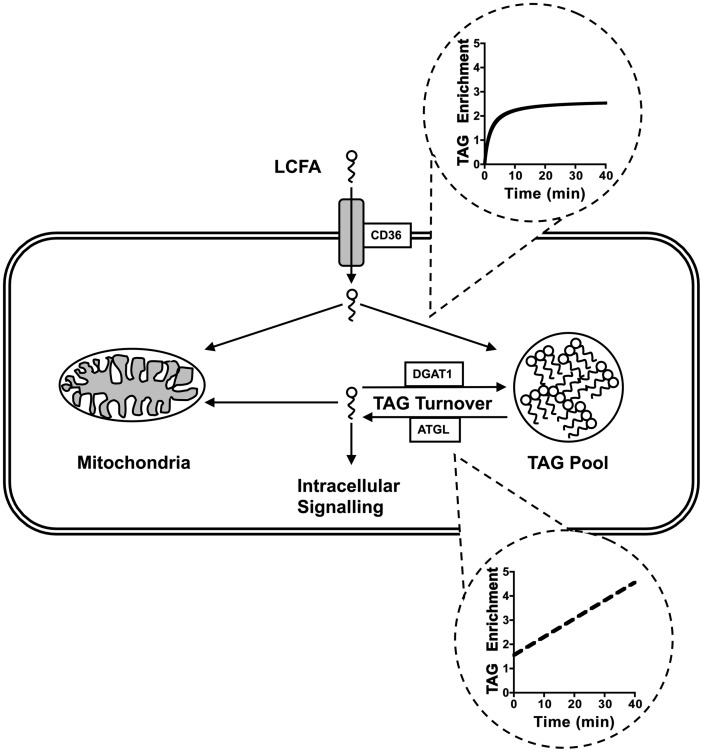
The present study provides a detailed analysis of the relationship between LCFA uptake across the sarcolemma via protein-mediated transport and incorporation into the TAG pool. Dynamic mode 13C NMR of the intact functioning heart resolved distinct phases of TAG enrichment that could be elucidated as distinct physiological processes, which are consistent with prior observations of LCFA transport across the sarcolemma of isolated cardiomyocytes (15). The results demonstrate that entry of LCFA into the heart is tightly coupled to both TAG synthesis and lipolysis, as the overexpression of CD36 increases the turnover rate of LCFA through the TAG pool. Importantly, the primary effect of the acute increase in CD36 was not simply an expansion of the neutral lipid pool but, rather, the activation of overall TAG turnover. The reciprocal regulation of LCFA uptake and TAG turnover extends to changes in enzyme expression downstream of LCFA transport. The data are consistent with a model of reciprocal regulation between LCFA uptake, TAG synthesis, and TAG lipolysis that works to buffer the intracellular availability of LCFA while preventing the accumulation of potentially toxic fatty acid metabolites (Fig. 8).
Fig. 8.
Working model of the relationship between LCFA uptake and TAG dynamics. The two phases of extracellular LCFA enrichment into the TAG pool, as measured by dynamic mode NMR, are separated into their unique kinetic components, and the role these components play in LCFA metabolism within the cardiac myocyte is depicted. The interplay between CD36, DGAT1, and ATGL in controlling TAG turnover is presented. The model helps to define how reciprocal regulation of the expression of these three proteins can control intracellular signaling by buffering the intracellular concentration of LCFA and their metabolites.
The findings reported above elucidate two phases of LCFA uptake into the TAG pool of the intact heart, each associated with a specific process: an initial saturable exponential component, which is consistent with a receptor-mediated process and shown here to be dependent on the protein-mediated transport activity at the sarcolemma, and a slower linear phase, which is determined by the rate of intracellular metabolism. By detecting the progressive incorporation of 13C-enriched LCFA into TAG in each heart, we were able to define these two components of TAG enrichment in the intact heart as temporally distinct. From initial observations of isotope enrichment rates with 13C NMR and the relative similarity between the initial exponential phase and the exponential component describing LCFA transport (15), we hypothesized that these temporal events are defined by LCFA transport and TAG turnover, respectively, which are now confirmed by the response to acute induction of CD36 expression. These two processes are continually active and occurring simultaneously, but detecting the incorporation of 13C palmitate into TAG in real-time elucidated these two distinct phases and enabled quantitative analysis.
The initial phase of TAG enrichment is a saturable process that is described by an exponential time course, and overexpression of CD36 led to an increase in the rate of the exponential for this initial phase. In isolated cardiac myocytes, the rate of the exponential function describing LCFA transport into the cell is significantly slowed in CD36 null mice, further supporting our finding that the saturable exponential function is linked to CD36 activity (15). Thus, the initial phase of TAG enrichment is defined by protein-mediated LCFA transport across the sarcolemma. An alternative hypothesis could be that the change in the exponential function is due to changes in the intracellular TAG content. Although it is likely that an increase in TAG content would increase the total flux of LCFA into the heart as intracellular lipid appears to be the driving force for LCFA entry (15), this would not be sufficient to explain a change in the exponential time constant. The exponential time constant describes LCFA entry in a concentration-independent manner. The overexpression of CD36 fundamentally alters the coupling of LCFA uptake and esterification beyond a mechanism that can be explained by simple mass action.
The acute overexpression of CD36 also induced increased TAG turnover in the whole heart that was linked to increased expression of the key synthase and lipase proteins DGAT1 and ATGL. We did not, however, see an increase in DAG concentration. A change in DAG concentration is not consistently seen in mouse models of DGAT1 manipulation (7, 32, 33), and the difference in the relative increases in ATGL and DGAT1 in our study likely prevents any buildup of DAG. The responsive changes in enzyme expression could be the result of a direct effect of CD36 overexpression or a response to the increased uptake of LCFA and subsequent ligand action for nuclear receptor activation (34, 35). Reciprocal regulation among DGAT1, ATGL, and CD36 has been demonstrated in some animal models. Gene expression of CD36 is increased in the heart of the DGAT1 overexpressing mouse and decreased in the DGAT1 null mouse (7, 32). Acute treatment of mice with a pharmacological inhibitor of DGAT1 led to a significant decrease in the cardiac expression of CD36 and ATGL after just 8 h (32). The relationship among DGAT1, ATGL, and the expression of key enzymes in the lipid uptake pathway extends beyond CD36, as LPL is also influenced by changes in DGAT1 expression (7, 32).
LCFA or its metabolites could induce the protein expression of DGAT1 and ATGL directly or through a secondary induction of transcription factors. Feeding a high-fat diet increases the cardiac expression of DGAT1 and ATGL, an intervention that is associated with increased lipid uptake (1, 32, 36). How LCFA or their metabolites could induce DGAT1 and ATGL expression has not been determined; however, one potential mechanism is through an increase in activity of the transcription factor PPARα. LCFA and its metabolites serve as endogenous ligands for PPARα activation, with many of the genes responsible for the utilization and storage of LCFA regulated by PPARα activity (35). DGAT1 expression is increased in hearts from PPARα overexpressing mice (1) and a PPAR binding site has been identified within the DGAT1 promoter (37), but the ability of PPARα to directly regulate DGAT1 expression remains unknown. ATGL expression has been shown to affect PPARα expression/activity, but the reverse process of PPARα regulation of ATGL expression has not been shown.
As a significant source of ATP production, LCFA released via lipolysis of the TAG pool contributes to β-oxidation and mediates intracellular signaling, either directly or as a precursor for other lipid ligands (1, 4, 5). Such LCFA can activate the PPARα signaling cascade, and this nuclear hormone receptor induces target gene expression for LCFA metabolism (35). Similar to support of the oxidative metabolism, LCFA originating from the TAG pool appears to be a significant source of PPARα ligands within the myocardium (4). This suggests that incoming LCFA first needs to be esterified to efficiently activate PPARα, further highlighting the importance of understanding LCFA cycling through the TAG pool.
CD36 influences the turnover rate of LCFA within the TAG pool, as shown by the present study, enabling either direct or indirect CD36-induced release of esterified LCFA via ATGL activity. The heart has a high metabolic demand with limited energetic stores and is therefore dependent on the coronary circulation for provision of substrates. The metabolic machinery within the cardiomyocyte must therefore adjust to circulating substrate concentrations. Our findings demonstrate that entry of LCFA via CD36 is tightly coupled to TAG esterification and turnover, as we did not see an expansion of the lipid pools upstream of TAG, namely, long chain fatty acyl CoA and DAG. Despite the extra steps that now are evident between LCFA transport and PPARα activation, the heart remains capable of regulating intracellular metabolism based on the extracellular lipid concentrations. In both this study of acute in vivo CD36 expression and metabolic rates in the intact rat heart and in previous studies of DGAT1 manipulation, the activity and expression of CD36, ATGL, and DGAT1 are coordinated to ensure an appropriate rate of TAG turnover for the level of LCFA uptake and vice versa (7, 32, 38). This reciprocal regulation would allow for an appropriate level of PPARα activation for the level of LCFA uptake and/or an appropriate level of LCFA uptake for the rate of TAG turnover.
TAG turnover rate is increasingly realized to be of the utmost importance to maintaining normal cellular metabolism rather than the static size of the TAG pool (2, 3, 38, 39). A careful balance must be maintained between buffering the potentially toxic or physiologically active acyl derivatives while ensuring an adequate supply of both energy sources and signaling ligands (4, 7, 32, 40). Mere increases or decreases in TAG content alone cannot indicate any relative level of “lipotoxicity” that is presented by association to pool size. The changes in intracellular lipid dynamics we have indentified in the present study would be unseen by looking at static pool sizes alone. To accurately assess the contribution of TAG to cardiac health, a comprehensive understanding of TAG dynamics and associated processes of LCFA uptake into the cardiomyocyte are necessary. A clear example of this need to better comprehend TAG dynamics is a comparison of chronic models of altered DGAT1 expression, the DGAT1 overexpressing mouse versus the DGAT1 null mouse (7, 32). Both models show a reduction in myocardial ceramide relative to wild-type; however, the DGAT1 overexpressing mouse displays increased myocardial TAG, whereas the DGAT1 null mouse displays reduced TAG.
We saw no effect of an acute increase in CD36 expression on the relative contribution of exogenous 13C-enriched LCFA to acetyl CoA formation, despite significant increases in TAG turnover. Other work on animal models of diabetes/obesity suggests a correlation between increased CD36 expression and fatty acid oxidation. In these models, however, there appears to be a difference between hearts from insulin-resistant obese animals and overtly diabetic animals, despite similarly elevated CD36 content (41–44). Such changes in the relative utilization of LCFA in models of diabetes and obesity may prove secondary to chronic changes in substrate provision and elevated LCFA uptake instead of being direct effects of increased CD36 expression.
The decline in TAG turnover in the failing heart has been previously unappreciated; however, turnover of LCFA through the TAG pool is uncoupled from energetic demand in the failing heart (2, 10). The reduction in LCFA cycling through the TAG pool limits the ability of the hypertrophic heart to respond to increases in workload (2). The decline in LCFA turnover in the failing heart may relate to changes in CD36 expression, as we have shown in the present study that CD36 expression increases TAG turnover. CD36 null mice on a regular diet develop cardiac hypertrophy (45), as do mice chronically treated with a putative CD36 inhibitor (46). The level of CD36 expression in the failing heart is not well characterized. The transition in substrate utilization away from LCFA is mirrored by changes in substrate transporter expression in human studies (47), and a similar relationship was found in failing rat hearts following myocardial infarction (48). The spontaneously hypertensive rat (SHR) continues to express CD36; however, there are mutations in the gene and the protein undergoes less posttranslational modification (39). The result is less targeting of CD36 to the sarcolemma. The disruption in the normal CD36 expression pattern appears to be directly related to the development of heart failure in this model and is associated with an apparent reduction in TAG turnover.
The importance of TAG turnover to myocardial signaling suggests that increasing the turnover of the TAG pool could have beneficial effects on cardiac function independent of LCFA utilization. This concept was recently demonstrated in the ATGL overexpressing mouse, in which protection from pressure overload-induced heart failure was conferred without an increase in LCFA oxidation, possibly due to a maintenance of normal PPARα activity (26). Interestingly, in so-called physiological or exercise-induced cardiac hypertrophy, there is an increase in CD36 expression levels (49). Exercise-induced hypertrophy diverges significantly from pathological hypertrophy, in that there is actually an increase in fatty acid metabolism (50). The response of the exercised heart appears to be to increase TAG turnover (7).
In summary, we have elucidated the dynamic coupling between the kinetics of LCFA transport across the sarcolemma and TAG pool turnover rates within the intact functioning rat heart following acute expression of an exogenous gene for the transporter protein CD36. 13C NMR elucidated a discernable transport component in the 13C-enrichment kinetics of TAG that was characterized by a saturable exponential phase. This initial exponential rate of 13C palmitate incorporation into the TAG pool provides unique insight into issues of sarcolemmal transport behavior and stands to offer valuable new insight for future studies of lipid dynamics in the pathogenesis of an array of metabolically linked cardiomyopathies. Importantly, manipulating uptake rates of LCFA into the cardiac myocyte induced downstream changes in key enzymes involved in TAG turnover, which also served to maintain consistent levels of acyl intermediates. We present a model (Fig. 8) of reciprocal regulation among LCFA uptake, TAG synthesis, and TAG lipolysis that serves to ensure tight control in potentially toxic or physiologically active metabolites, such as DAG and ceramides, while maintaining adequate supply of high-yield energy substrates.
Acknowledgments
The authors thank Dr. Maria Febbraio, Lerner Research Institute, Cleveland Clinic, for supplying cDNA for the rat isoform of CD36 that enabled our construction of the vector.
Footnotes
Abbreviations:
- ATGL
- adipose triglyceride lipase
- DAG
- diacylglycerol
- DGAT1
- diacylglycerol acyltransferase 1
- IL
- interleukin
- LCFA
- long chain fatty acid
- MAG
- monoacylglycerol
- PGC-1α
- peroxisome proliferator-activated receptor-γ coactivator 1α
- PPAR
- peroxisome proliferator-activated receptor
- TAG
- triacylglycerol
- TNF
- tumor necrosis factor
- τ
- exponential time constant
This work was supported by National Institutes of Health Grants R37-HL-49244 and R01-HL-2702 and by Canadian Institutes of Health Research Grant MOP-67053. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Banke N. H., Wende A. R., Leone T. C., O'Donnell J. M., Abel E. D., Kelly D. P., Lewandowski E. D. 2010. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ. Res. 107: 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Donnell J. M., Fields A. D., Sorokina N., Lewandowski E. D. 2008. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J. Mol. Cell. Cardiol. 44: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell J. M., Zampino M., Alpert N. M., Fasano M. J., Geenen D. L., Lewandowski E. D. 2006. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am. J. Physiol. Endocrinol. Metab. 290: E448–E455 [DOI] [PubMed] [Google Scholar]
- 4.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., et al. 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17: 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saddik M., Lopaschuk G. D. 1991. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J. Biol. Chem. 266: 8162–8170 [PubMed] [Google Scholar]
- 6.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr, Ory D. S., Schaffer J. E. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L., Shi X., Bharadwaj K. G., Ikeda S., Yamashita H., Yagyu H., Schaffer J. E., Yu Y. H., Goldberg I. J. 2009. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J. Biol. Chem. 284: 36312–36323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman J. J., Boudina S., Banke N. H., Sambandam N., Han X., Young D. M., Leone T. C., Gross R. W., Lewandowski E. D., Abel E. D., et al. 2008. The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am. J. Physiol. Heart Circ. Physiol. 295: H185–H196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huss J. M., Kelly D. P. 2005. Mitochondrial energy metabolism in heart failure: a question of balance. J. Clin. Invest. 115: 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pound K. M., Sorokina N., Ballal K., Berkich D. A., Fasano M., Lanoue K. F., Taegtmeyer H., O'Donnell J. M., Lewandowski E. D. 2009. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ. Res. 104: 805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampf J. P., Kleinfeld A. M. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda). 22: 7–14 [DOI] [PubMed] [Google Scholar]
- 12.Bonen A., Chabowski A., Luiken J. J., Glatz J. F. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda). 22: 15–29 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton J. A. 2007. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 77: 355–361 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg I. J., Eckel R. H., Abumrad N. A. 2009. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 50(Suppl.): S86–S90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carley A. N., Kleinfeld A. M. 2011. Fatty acid (FFA) transport in cardiomyocytes revealed by imaging unbound FFA is mediated by an FFA pump modulated by the CD36 protein. J. Biol. Chem. 286: 4589–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda Y., Gu Y., Iwanaga Y., Hoshijima M., Oh S. S., Giordano F. J., Chen J., Nigro V., Peterson K. L., Chien K. R., et al. 2002. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation. 105: 502–508 [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell J. M., Lewandowski E. D. 2005. Efficient, cardiac-specific adenoviral gene transfer in rat heart by isolated retrograde perfusion in vivo. Gene Ther. 12: 958–964 [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell J. M., Fields A., Xu X., Chowdhury S. A., Geenen D. L., Bi J. 2008. Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo. Am. J. Physiol. Heart Circ. Physiol. 295: H2483–H2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Donnell J. M., Pound K., Xu X., Lewandowski E. D. 2009. SERCA1 expression enhances the metabolic efficiency of improved contractility in post-ischemic heart. J. Mol. Cell. Cardiol. 47: 614–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668 [PubMed] [Google Scholar]
- 21.Ibrahimi A., Sfeir Z., Magharaie H., Amri E. Z., Grimaldi P., Abumrad N. A. 1996. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc. Natl. Acad. Sci. USA. 93: 2646–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell J. M., Alpert N. M., White L. T., Lewandowski E. D. 2002. Coupling of mitochondrial fatty acid uptake to oxidative flux in the intact heart. Biophys. J. 82: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewandowski E. D., Doumen C., White L. T., LaNoue K. F., Damico L. A., Yu X. 1996. Multiplet structure of 13C NMR signal from glutamate and direct detection of tricarboxylic acid (TCA) cycle intermediates. Magn. Reson. Med. 35: 149–154 [DOI] [PubMed] [Google Scholar]
- 24.Sullards M. C., Allegood J. C., Kelly S., Wang E., Haynes C. A., Park H., Chen Y., Merrill A. H., Jr 2007. Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: “inside-out” sphingolipidomics. Methods Enzymol. 432: 83–115 [DOI] [PubMed] [Google Scholar]
- 25.Koonen D. P., Febbraio M., Bonnet S., Nagendran J., Young M. E., Michelakis E. D., Dyck J. R. 2007. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 116: 2139–2147 [DOI] [PubMed] [Google Scholar]
- 26.Kienesberger P. C., Pulinilkunnil T., Sung M. M., Nagendran J., Haemmerle G., Kershaw E. E., Young M. E., Light P. E., Oudit G. Y., Zechner R., et al. 2012. Myocardial ATGL overexpression decreases the reliance on fatty acid oxidation and protects against pressure overload-induced cardiac dysfunction. Mol. Cell. Biol. 32: 740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullagh K. J., Poole R. C., Halestrap A. P., Tipton K. F., O'Brien M., Bonen A. 1997. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. Am. J. Physiol. 273: E239–E246 [DOI] [PubMed] [Google Scholar]
- 28.Liu Q., Zaiss A. K., Colarusso P., Patel K., Haljan G., Wickham T. J., Muruve D. A. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14: 627–643 [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell J., Fields A., Xu X., Lewandowski E. 2007. Metabolic and functional response of failing heart to adrenergic stimulation following SERCA1 overexpression. J. Mol. Cell. Cardiol. 42: S132–S133 [Google Scholar]
- 30.Banke N. H., Yan L., Pound K. M., Dhar S., Reinhardt H., De Lorenzo M. S., Vatner S. F., Lewandowski E. D. 2012. Sexual dimorphism in cardiac triacylglyceride dynamics in mice on long term caloric restriction. J. Mol. Cell. Cardiol. 52: 733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737 [DOI] [PubMed] [Google Scholar]
- 32.Liu L., Yu S., Khan R. S., Ables G. P., Bharadwaj K. G., Hu Y., Huggins L. A., Eriksson J. W., Buckett L. K., Turnbull A. V., et al. 2011. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J. Lipid Res. 52: 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L., Yu S., Khan R. S., Homma S., Schulze P. C., Blaner W. S., Yin Y., Goldberg I. J. 2012. Diacylglycerol acyl transferase 1 overexpression detoxifies cardiac lipids in PPARgamma transgenic mice. J. Lipid Res. 53: 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravarthy M. V., Lodhi I. J., Yin L., Malapaka R. R., Xu H. E., Turk J., Semenkovich C. F. 2009. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 138: 476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madrazo J. A., Kelly D. P. 2008. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J. Mol. Cell. Cardiol. 44: 968–975 [DOI] [PubMed] [Google Scholar]
- 36.Ouwens D. M., Diamant M., Fodor M., Habets D. D., Pelsers M. M., El Hasnaoui M., Dang Z. C., van den Brom C. E., Vlasblom R., Rietdijk A., et al. 2007. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 50: 1938–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig E. H., Mahley R. W., Palaoglu E., Ozbayrakci S., Balestra M. E., Borecki I. B., Innerarity T. L., Farese R. V., Jr 2002. DGAT1 promoter polymorphism associated with alterations in body mass index, high density lipoprotein levels and blood pressure in Turkish women. Clin. Genet. 62: 68–73 [DOI] [PubMed] [Google Scholar]
- 38.Bastie C. C., Hajri T., Drover V. A., Grimaldi P. A., Abumrad N. A. 2004. CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes. 53: 2209–2216 [DOI] [PubMed] [Google Scholar]
- 39.Lauzier B., Merlen C., Vaillant F., McDuff J., Bouchard B., Beguin P. C., Dolinsky V. W., Foisy S., Villeneuve L. R., Labarthe F., et al. 2011. Post-translational modifications, a key process in CD36 function: lessons from the spontaneously hypertensive rat heart. J. Mol. Cell. Cardiol. 51: 99–108 [DOI] [PubMed] [Google Scholar]
- 40.Augustus A. S., Buchanan J., Park T. S., Hirata K., Noh H. L., Sun J., Homma S., D'Armiento J., Abel E. D., Goldberg I. J. 2006. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J. Biol. Chem. 281: 8716–8723 [DOI] [PubMed] [Google Scholar]
- 41.Coort S. L., Hasselbaink D. M., Koonen D. P., Willems J., Coumans W. A., Chabowski A., van der Vusse G. J., Bonen A., Glatz J. F., Luiken J. J. 2004. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes. 53: 1655–1663 [DOI] [PubMed] [Google Scholar]
- 42.Holloway G. P., Snook L. A., Harris R. J., Glatz J. F., Luiken J. J., Bonen A. 2011. In obese Zucker rats, lipids accumulate in the heart despite normal mitochondrial content, morphology and long-chain fatty acid oxidation. J. Physiol. 589: 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P., Lloyd S. G., Zeng H., Bonen A., Chatham J. C. 2005. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am. J. Physiol. Heart Circ. Physiol. 288: H2102–H2110 [DOI] [PubMed] [Google Scholar]
- 44.Burgmaier M., Sen S., Philip F., Wilson C. R., Miller C. C., 3rd, Young M. E., Taegtmeyer H. 2010. Metabolic adaptation follows contractile dysfunction in the heart of obese Zucker rats fed a high-fat “Western” diet. Obesity (Silver Spring). 18: 1895–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irie H., Krukenkamp I. B., Brinkmann J. F., Gaudette G. R., Saltman A. E., Jou W., Glatz J. F., Abumrad N. A., Ibrahimi A. 2003. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc. Natl. Acad. Sci. USA. 100: 6819–6824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusaka Y., Tanaka T., Okamoto F., Terasaki F., Matsunaga Y., Miyazaki H., Kawamura K. 1995. Effect of sulfo-N-succinimidyl palmitate on the rat heart: myocardial long-chain fatty acid uptake and cardiac hypertrophy. J. Mol. Cell. Cardiol. 27: 1605–1612 [DOI] [PubMed] [Google Scholar]
- 47.Heather L. C., Howell N. J., Emmanuel Y., Cole M. A., Frenneaux M. P., Pagano D., Clarke K. 2011. Changes in cardiac substrate transporters and metabolic proteins mirror the metabolic shift in patients with aortic stenosis. PLoS ONE. 6: e26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heather L. C., Cole M. A., Lygate C. A., Evans R. D., Stuckey D. J., Murray A. J., Neubauer S., Clarke K. 2006. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovasc. Res. 72: 430–437 [DOI] [PubMed] [Google Scholar]
- 49.Strøm C. C., Aplin M., Ploug T., Christoffersen T. E., Langfort J., Viese M., Galbo H., Haunso S., Sheikh S. P. 2005. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 272: 2684–2695 [DOI] [PubMed] [Google Scholar]
- 50.Dorn G. W., 2nd 2007. The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 49: 962–970 [DOI] [PubMed] [Google Scholar]