Summary
Flow-diverting stents (Silk and PED) have radically changed the approach to intracranial aneurysm treatment from the use of endosaccular materials to use of an extraaneurysmal endoluminal device.
However, much debate surrounds the most appropriate indications for the use of FD stents and the problems raised by several possible complications.
We analysed our technical difficulties and the early (less than ten days after treatment) and late complications encountered in 30 aneurysms treated comprising 13 giant lesions, 12 large, five with maximum diameters <10 mm and one blister-like aneurysm.
In our experience the primary indications for the use of FD stents can be the symptomatic intracavernous giant aneurysms. Although the extracavernous carotid siphon aneurysms have major risk of bleeding, FD stents are indicated clearly explaining the risks to the patient in case of severe mass effect. There is a very complex assessment for aneurysms of the vertebrobasilar circulation.
Key words: brain aneurysms, flow diverting stent, complications, indications
Introduction
Flow-diverting (FD) stents were made available in late 2007. The new devices are designed to reduce inflow in the aneurysmal sac thereby leading to spontaneous thrombosis without occluding the vessels covered by the stent.
FD stents have fundamentally changed the approach to intracranial aneurysm treatment from the use of endosaccular materials released by a variety of techniques (coiling, remodelling, stent assisted coiling) to use of an extra-aneurysmal endoluminal device. The first literature reports on single and multiple centre trials on the use of FD stents to treat wide-neck (large and giant) and/or fusiform aneurysms are promising 4,6,8,9,11,12,18-21,29. Good results have also been published recently on the use of FD stents to treat very small aneurysms (<5 mm) 17.
However, much debate surrounds the most appropriate indications for the use of FD stents and the problems raised by several possible complications, namely haemorrhage 5,7,16,27 and recent reports of early 14,15,28 or late thrombo-embolism of the vertebrobasilar artery.
This study reviews our first 30 consecutive patients treated over the three-year period from December 2007 to December 2010 analysing the technical difficulties and the early (less than ten days after treatment) and late complications encountered.
Materials and Methods
Our cohort has already been described in detail 6,18. Briefly, between December 2007 and December 2010 we treated 29 unruptured aneurysms and one blister-like aneurysm 30 days after SAH deploying a Silk stent to cover the aneurysm neck. Patients comprised 27 women of whom 26 had carotid siphon aneurysms and one had an aneurysm at the origin of the posteroinferior cerebellar artery (PICA), and three men all with vertebrobasilar aneurysms. Patients were aged between 34 and 81 years. Dual antiplatelet therapy was administered four days prior to the endovascular procedure following a daily protocol of ticlopidine × 2, aspirin 300 mg × 1 and a gastric protector × 2 prolonged with stepwise tapering for six to four months. All procedures were carried out under general anaesthesia with anticoagulant therapy (heparin bolus (3500-5000 IU) combined with maintenance therapy every 30 min (1500-2000 IU) and antiplatelet medication (1 g aspirin bolus). Treatment was performed in most patients using a 90 cm long introducer with a 4.2F lumen (Leonardi – Chapot; Balt), whereas we opted for a 6F (Fargo; Balt) guiding catheter to enhance the stability of the system in six patients with marked vessel tortuosity.
The Silk stent comes with its own microcatheter varying in size in relation to the size of the stent (calibres ranging from 0.0021'' for stents with diameters up to 4.5 mm or 0.0025'' for stents with diameters equal to or larger than 5 mm). We always used a Synchro Boston-Scientific 0.0014'' microguide wire. Only one stent was released in all cases with associated coil deployment in the aneurysmal sac in one patient 23. All patients were followed with MR and/or CT angiograms 26.
Results
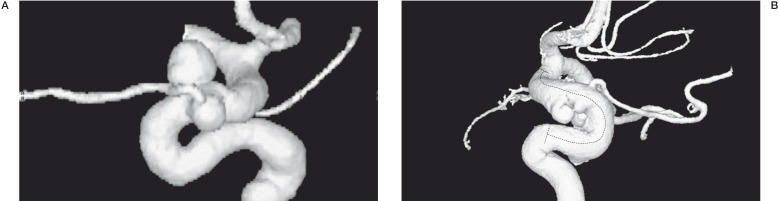
We treated 30 patients with 30 aneurysms comprising 13 giant lesions, 12 large, five with maximum diameters <10 mm and one blister-like aneurysm. The large aneurysms and lesions with a transverse diameter <10 mm all had wide necks and sac or vessel morphology deemed unsuitable for traditional endovascular treatment with coils alone (Figure 1A,B). Twenty-six aneurysms were located in the anterior circulation divided into nine intracavernous and 17 supraclinoid lesions including one blister-like aneurysm. Only four lesions were located in the posterior circulation and comprised two basilar artery aneurysms with involvement of the vertebrobasilar junction, one aneurysm at the origin of the PICA and one on the intradural segment of the vertebral artery.
Over a follow-up period of between 15 and 31 months, we observed 23 positive results (16 aneurysms were completely closed and seven had a very small residual neck), whereas four aneurysms still have a residual flow in the aneurysmal sac (Table 1).
Table 1.
| Patient | Aneurysm site | Aneurysm size |
Silk | Stent opening | Complications | Results |
|---|---|---|---|---|---|---|
| 1 | Supraclinoid | >10 | 4.5×20 | Correct | No | Complete occlusion |
| 2 | Supraclinoid | >10 | 4.5×15 | Correct | No | Complete occlusion |
| 3 | Supraclinoid | <10 | 4.5×35 | Correct | No | Residual neck |
| 4 | Supraclinoid | >10 | 4.5×15 | Correct | No | Complete occlusion |
| 5 | Supraclinoid | <10 | 4.5×25 | Wrong | No | Residual sac |
| 6 | Supraclinoid | <10 | 4.5×30 | Wrong | No | Residual sac |
| 7 | Intracavernous | >25 | 4×20 | Correct | No | Residual neck |
| 8 | Supraclinoid | <10 | 4.5×20 | Correct | No | Complete occlusion |
| 9 | Supraclinoid | <10 | 4×20 | Correct | No | Complete occlusion |
| 10 | Supraclinoid | >25 | 4×25 | Correct | Yes | Died |
| 11 | Supraclinoid | >10 | 4.5×20 | Correct | No | Residual neck |
| 12 | Intracavernous | >25 | 4×30 | Correct | No | Complete occlusion |
| 13 | Supraclinoid | >10 | 4×35 | Wrong | No | Residual sac |
| 14 | Supraclinoid | >10 | 4×30 | Correct | No | Complete occlusion |
| 15 | Supraclinoid | >10 | 4×25 | Correct | Yes | Residual sac |
| 16 | Intracavernous | >25 | 4×30 | Correct | No | Complete occlusion |
| 17 | Intracavernous | >25 | 4×35 | Correct | No | Complete occlusion |
| 18 | Intracavernous | >25 | 4×30 | Correct | No | Complete occlusion |
| 19 | Supraclinoid | <10 | 4.5×40 | Correct | Yes | Died |
| 20 | Intracavernous | >25 | 4×35 | Correct | No | Complete occlusion |
| 21 | Supraclinoid | >10 | 3.5×25 | Correct | Yes | Complete occlusion |
| 22 | Intracavernous | >25 | 4×30 | Correct | No | Residual neck |
| 23 | Basilar artery | >25 | 5.5×60 | Correct | Yes | Died |
| 24 | PICA | >10 | 3×25 | Correct | Yes | Complete occlusion |
| 25 | Intracavernous | >25 | 4×30 | Correct | No | Residual neck |
| 26 | Intracavernous | >25 | 5.5×60 | Correct | No | Residual neck |
| 27 | Basilar artery | >25 | 4.5×40 | Correct | Yes | Residual neck |
| 28 | Blister like | <10 | 4.5×40 | Correct | No | Complete occlusion |
| 29 | Supraclinoid | >25 | 3.5×25 | Correct | Yes | Complete occlusion |
| 30 | Vertebral artery (V4) | >10 | 4.5×50 | Correct | No | Complete occlusion |
Figure 1.
Two different images of small multiple aneurysms of the ophthalmic tract of the internal carotid siphon in two different patients. The siphon tortuosity and the anatomy of aneurysms suggests much difficulty in the catheterization and release of coils in the aneurysm sacs. In particular in one case (A) we found that the ophthalmic artery borns by aneurysm sac.
Complications
We distinguished technical difficulties linked to stent placement and release and intraprocedural complications from complications arising after the procedure.
We failed to obtain optimal stent apposition in three patients. Three intraprocedural complications occurred (acute stent thrombosis, stent migration with subsequent stent thrombosis, perforation of the artery of Percheron) of which two had no clinical consequences.
Three serious early complications occurred within a month after the procedure (spontaneous aneurysm haemorrhage, acute hydrocephalus, cerebral haemorrhage) and two late symptomatic embolic complications three to six months after treatment. There was no late stent occlusion or occlusion of large vessels covered by the stent (ophthalmic artery, PICA).
Analysis of Complications
Assessing the characteristics of the flow-diverting stents deployed (Silk), we sought to explain possible technical difficulties and intra and post-procedural complications to be entertained when opting for these devices in the treatment of cerebral aneurysms.
Two major variables must be addressed:
1) Broad differences among centres in the use of antiplatelet and anticoagulant therapy both in terms of timing and individual patient dose. In addition, patients may be resistant or hypersensitive to the therapy administered after placement of any type of stent;
2) Some of the complications that occurred can be deemed unpredictable, as ours was one of the first centres to use this new device.
Intraprocedural problems
Perforation of a distal arteriole
The Silk stent is a retractable device attached to a high friction delivery system with a 200 cm steel plunger with a 15 mm long radio-opaque floppy portion that extends beyond the stent with a 45° tip. As the stent is released the plunger advances into the vessel for a length equal to that of the stent 18. So before stent deployment it is important to calculate whether the vessel below will allow the plunger to advance without meeting an obstacle.
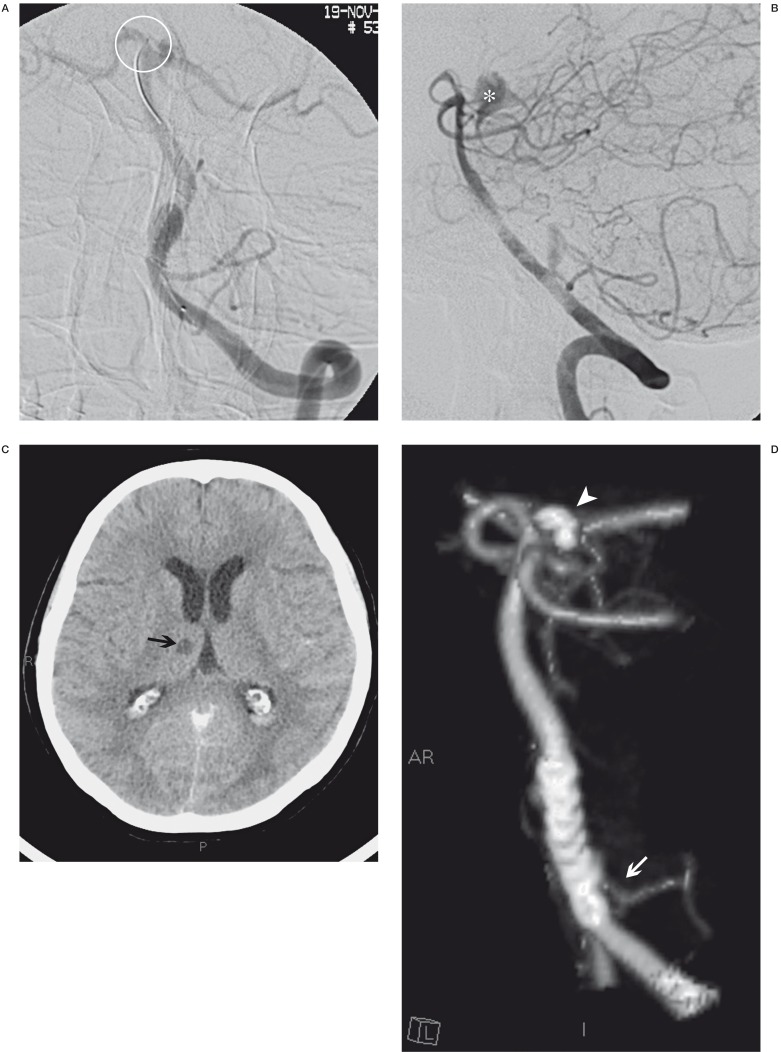
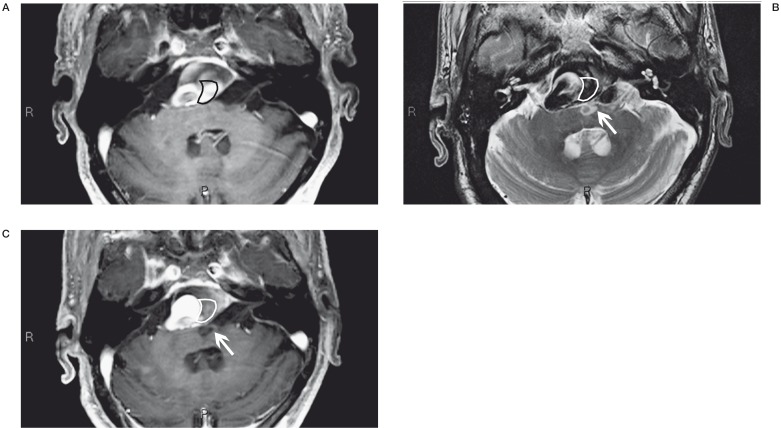
A 71-year-old woman with an aneurysm at the origin of the left PICA was treated by Silk stent deployment across the aneurysm neck. Angiographic control at the end of the procedure disclosed leakage of contrast medium into the interpeduncular cistern due to perforation of the right artery of Percheron, subsequently occluded by releasing two GDC coils. Reviewing the images we realized that during stent deployment the plunger had advanced into the right PICA and accidentally blocked in the ipsilateral artery of Percheron. CT follow-up scans displayed regular resorption of the contrast medium and subarachnoid bleed and the natural evolution of the right thalamic ischaemic lesion. After four days in intensive care the patient presented severe neurological deficits in breathing, swallowing and motor function also due to the large aneurysmal mass in the posterior fossa. Fourteen months later the patient had made a marked neurological improvement but is not self-sufficient (Figure 2A-C).
Figure 2.
A) The position where the guide tip was accidentally blocked during stent release (ring). When we performed a final angiographic control (B) we observed a leakage of contrast into the interpeduncular cistern (*). Two days later, CT control (C) disclosed a right thalamic ischaemic lesion (black arrow) and subtotal reabsorption of leakage of contrast. Two months later, CT angiography follow-up control showed the aneurysm occlusion, regular view of the PICA (white arrow), good position of the Silk stent and presence of the coils (arrowhead) that we used to block the haemorrhage due to the artery of Percheron perforation.
Incorrect stent release
The Silk device is made of 48 nitinol and two platinum braided strands but has weak rigidity thereby allowing good flexibility and navigability as shown in a recent in vitro study. We always managed to reach the position for stent deployment without encountering differences in the stability of the system or vessel wall injury between the VASCO 21 microcatheter (calibre 0.0021”) and the VASCO 25 (calibre 0.0025”) 6,18.
However, weak rigidity also signifies low radial force with respect to devices used for stent-assisted coiling so that the Silk stent may have difficulty opening fully within the vessel lumen based on shape memory alone. To ensure perfect adhesion of the stent to the vessel wall, stent release must be assisted by a continuous push and pull movement requiring time and monitoring in different projections. This is relatively straightforward as the stent can easily be resheathed and repositioned if necessary.
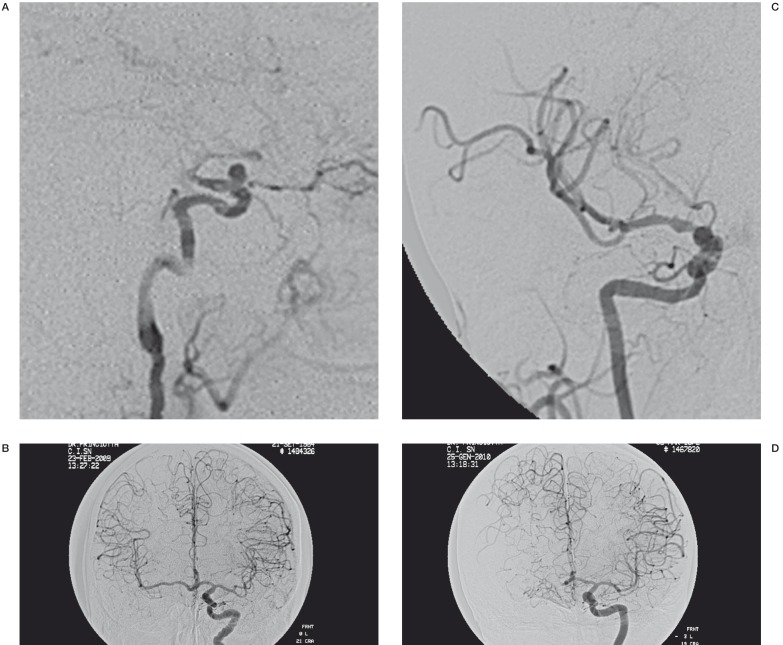
Non-optimal stent aperture responsible for inefficient flow diversion was displayed by MR angiography and CT angiogram in three of our patients. In particular. Suboptimal stent opening in two of these cases led to a significant, but fortunately asymptomatic, reduction in vessel calibre with development of haemodynamic compensation through the circle of Willis in one patient and hypertrophic collateral pial vessels in the other (Figure 3A-D.
Figure 3.
Two year follow-up post-treatment angiographic control showed a slow and poor blood flow distal to the stent (A,C). However the injection of contrast in the contralateral internal carotid artery demonstrated a good haemodynamic compensation of the flow through the circle of Willis and hypertrophic collateral pial vessels (B,D).
Choice of stent
Choice of stent size is crucial to obtain good adhesion of the device to the vessel wall and thereby divert flow in the parent artery 1,2.
A 4×25 mm stent was released in one patient into the carotid siphon with a maximum diameter of 3.6 mm. Angiographic control just after the procedure showed that the stent had migrated distally in M1 resulting in incorrect cover of the aneurysm neck. Subsequent slowing of flow within the aneurysm was depicted with progressive complete intrastent thrombosis. This was treated by i.v. administration of ReoPro and intra-arterial urokinase and ReoPro leading to a complete resolution of the angiographic picture. On awakening, the patient had no neurological deficit or clinical changes. It was impossible to determine whether the stent had shifted due to an error in the choice of stent diameter or was caused by an initial intrastent thrombization at the distal tip of the device leading to distal shift under the force of blood flow (Figure 4A-C).
Figure 4.
3D reconstruction angiogram of the internal left carotid artery injection showed multiple aneurysms (A). The largest paraophthalmic aneurysm (arrow) was treated with Silk stent apposition. Intraoperative images in lateral projection demonstrated a good position release of the stent (B). Subsequent serial angiographic control showed stent migration in left M1 segment with clip and coils implanted in previous treatments.
Post-procedural complications
As mentioned above, since FD stents are relatively new devices some of the complications we experienced were unpredictable. When we started using FD stents we knew that an aneurysm could be excluded from the circulation by positioning a stent alone in the vessel of origin 24 and that FD devices has been designed for this purpose and supported by in vivo and in vitro experimental evidence. Hence we refer to a thrombogenic endoluminal device diverting flow in the parent artery thereby leading to thrombosis 25.
First and foremost, flow adjustments slow down blood flow into the aneurysm resulting in changes to shear stress and possibly also increased pressure within the aneurysm.
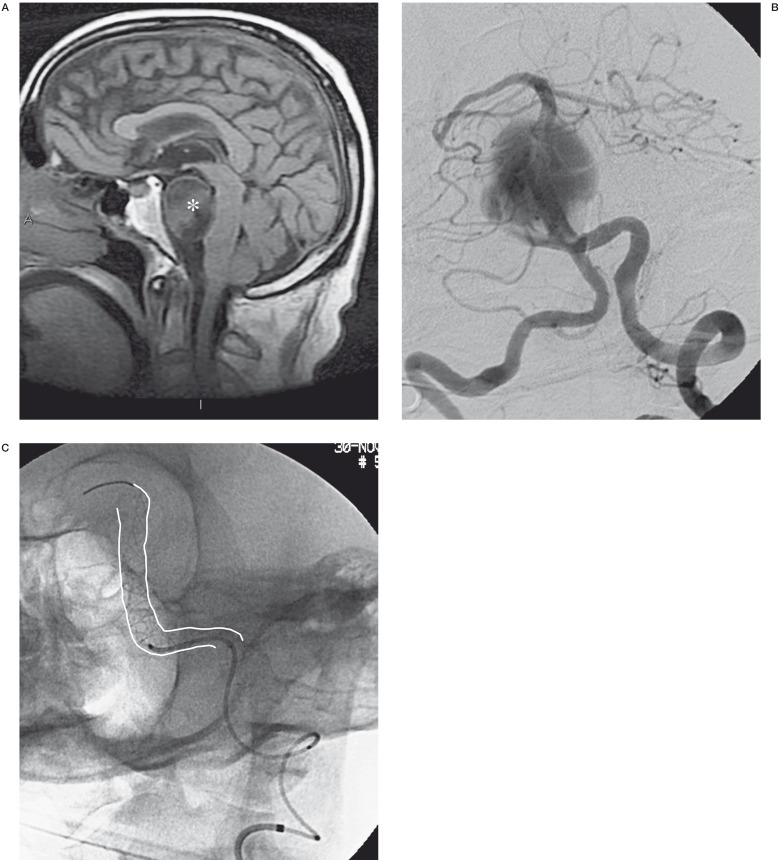
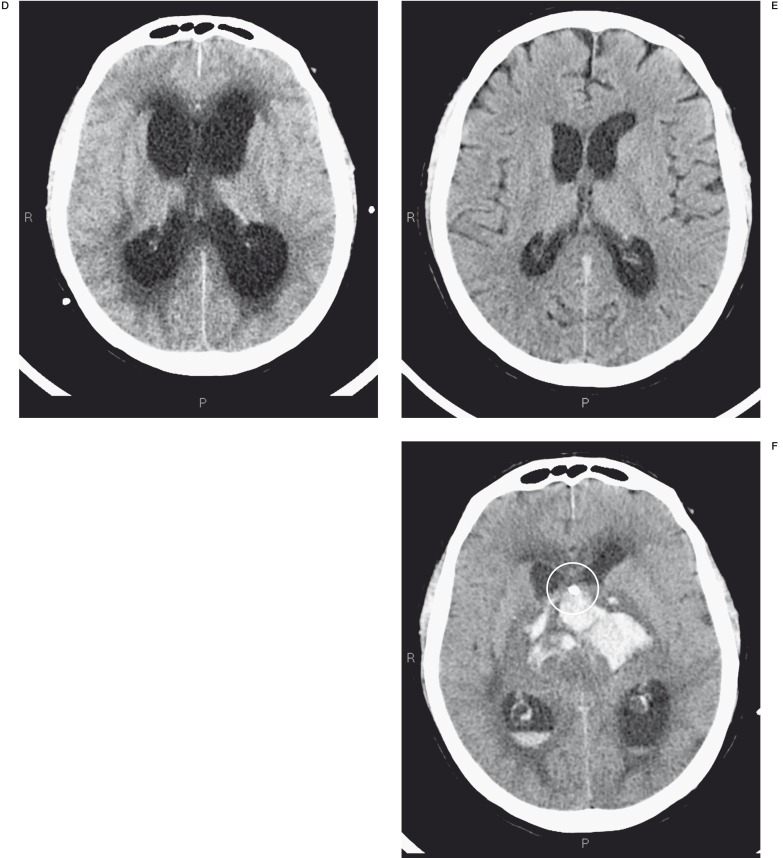
A male patient with a giant aneurysm at the vertebrobasilar junction developed acute hydrocephalus one week after endovascular treatment. This complication was probably due to the increased compression on the Sylvian acqueduct. Emergency external CSF drainage was positioned but resulted in massive intra-axial and intraventricular haemorrhage leading to the patient’s death (Figure 5A-C).
Figure 5.
A-C) The MR image shows a giant basilar aneurysm and its massive compression on the bulb (*). Angiographic examination confirms the giant aneurysm that was treated with one Silk stent (white lines). D-F) CT controls post endovascular treatment showed acute hydrocephalus that was treated by external CSF drainage (ring), but it resulted in massive intra-axial and intraventricular haemorrhage.
Slowing of flow within the aneurysmal sac allows the formation of the thrombus which attracts water by means of osmolarity. This leads to a further unpredictable increase in pressure within the aneurysmal sac. There are literature reports of fatal rupture of the aneurysmal sac after FD placement maybe due to haemodynamic conditions 5,10,13 and/or thrombotic events 16. The need for antiplatelet and anticoagulant therapy before during and after the endovascular procedure significantly worsens any spontaneous and/or traumatic bleeding.
A female patient died from sudden spontaneous cerebral haemorrhage seven days after discharge from hospital. Autopsy disclosed rupture of the FD-treated carotid artery aneurysm.
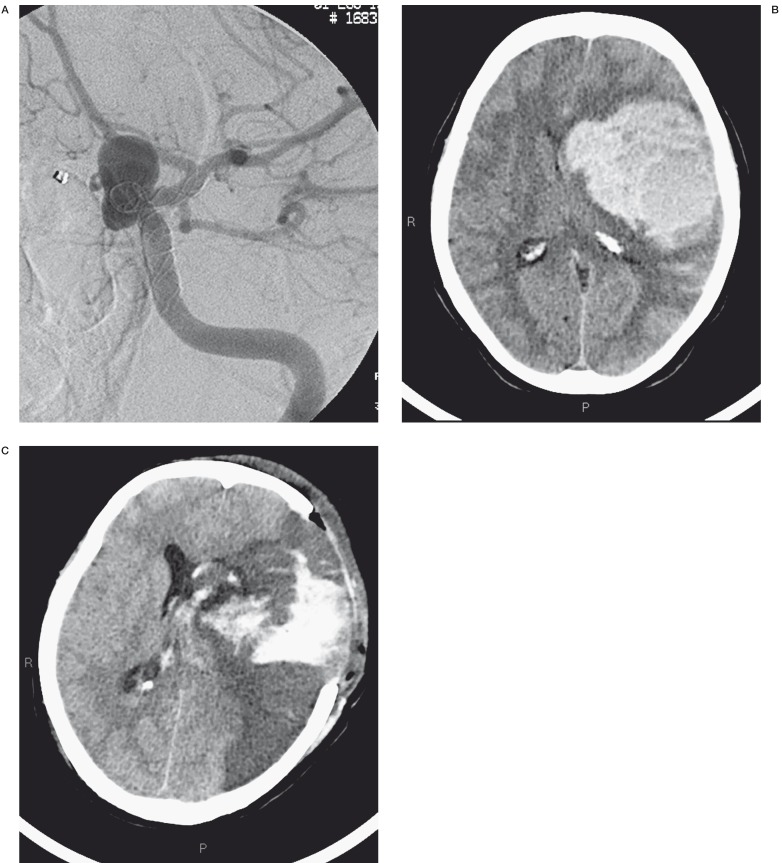
A 55-year-old woman with a carotid siphon aneurysm showed severe clinical worsening 20 minutes after treatment. CT scan disclosed an intra-axial cerebral haematoma with no clear cause-effect relation with the procedure showing minimum subarachnoid bleeding. The patient underwent emergency neurosurgical evacuation of the haematoma and the surgeon deemed it essential to complete the internal carotid artery occlusion but this led to a massive cerebral hemispheric ischaemic lesion. The patient died four days after surgery (Figure 6A-C.
Figure 6.
Post-treatment angiographic control showed a good position release of the Silk stent (A). CT control performed because the patient had a severe clinical worsening revealed a massive cerebral hemorrage (B). The patient underwent emergency neurosurgical evacuation of the haematoma and the surgeon deemed it essential to complete the internal carotid artery occlusion but this led to a massive cerebral hemispheric ischaemic lesion (C).
Medical management is essential after Silk stent deployment to avoid possible platelet aggregation on the stent walls or occlusion of the smallest collateral vessels covered by the stent.
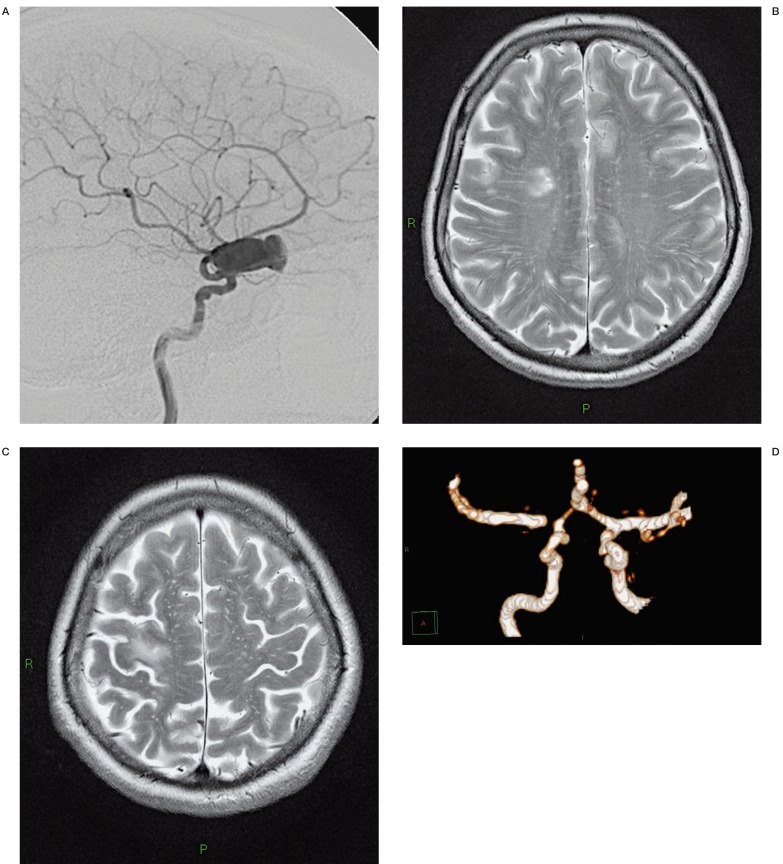
A 62-year-old woman was referred to us for sudden right vision loss. CT and MR angiograms displayed bilateral multiple large partially thrombosed aneurysms with calcified walls, the largest located in the right carotid siphon. The patient was treated by FD deployment into the right carotid siphon resulting in immediate significant flow reduction. Three months later she suddenly presented left hemiparesis due to multiple embolic ischaemic lesions with stenosis of the distal portion of the stent displayed by emergency CT scan and CT angiogram (Figure 7A-D). Three months later the patient has recovered almost completely and was clinically self-sufficient with minor deficits.
Figure 7.
LL cerebral angiography releaved a giant paraophthalmic aneurysm (A). Three months after endovascular treatment the patient presented left hemiparesis due to some ischaemia and a M1 stenosis displayed respectively by MR scan (B-C) and MR angiography (D).
We treated a giant fusiform aneurysm of the basilar artery by FD deployment in the dominant vertebral artery. Two months after treatment the patient presented acute symptoms caused by bulbar ischaemia that subsequently resolved. Interestingly, MR scans and MR angiogram showed a congruence between the thrombosed portion of the aneurysm and the site of the ischaemic lesion (Figure 8 A-C).
There are literature reports of complete stent thrombosis describing the difficulty in adjusting medical therapy 14. No late stent thrombosis occurred in our series. This may be due to the fact that we never deployed more than one Silk stent in the same vessel. Of the two patients described above we wondered whether the first had truly complied with the prescribed therapy whereas the second is plainly a case of thrombosis of a perforating artery. However, the specific causes remain unsettled.
Figure 8.
Partially thrombosed giant aneurysm of basilar artery compressing the bulb (A). Two months after treatment the follow-up MRA showed a increase and change in the thrombosis portion (red rings) and a congruent bulb ischaemic lesion (white arrows) (B,C).
Discussion
Endovascular therapy is now accepted as a valid alternative therapeutic modality. The array of new endovascular techniques has extended beyond the Guglielmi Detachable Coil to include new stents and flow-diverting devices. The FD stents are designed to reduce inflow in the aneurysmal sac there by leading to spontaneous thrombosis without occluding the vessels covered by the stent. Whereas the FD devices have a higher thrombogenicity than older stents, we have always used a single stent, in order for reduce the risk of thrombosis of the vessel. The Silk stent is characterized by structural and technical differences (i.e. low radial force, sliding plunger).
Over a three-year period we treated 30 aneurysms by releasing a Silk stent, and observed some technical difficulties and complications (Table 2).
Table 2.
| Intracavernous aneurysm |
Supraclinoid aneurysm | Vertebrobasilar circulation aneurysm |
|
|---|---|---|---|
|
Intraprocedural Complications |
None | 3 suboptimal stent deployment 1 stent migration 1 acute intrastent thrombosis |
1 perforation of artery of Percheron |
|
Post-procedural Complications |
None | 1 SAH 1 intra-axial cerebral haematoma 1 multiple embolic ischaemic events |
1 acute hydrocephalus 1 ischaemic event |
The recent availability of the new FD devices and the normal learning curve for their deployment may have a significant impact on the technical difficulties rate. No significant correlation with the location or type of aneurysm was observed. We have already described our cases in detail 6,18.
Specifically, we observed suboptimal stent apposition in three carotid siphon aneurysm during follow-up with MR or CT angiograms because we could not perform Dyna CT. We obtained a marked flow reduction in all intracavernous aneurysms treated; five were completely closed and four still show a very small neck residual flow.
The blister-like aneurysm was first treated by stent-assisted coiling in acute stage and 30 days after SAH we placed a Silk stent. We thought that treatment of blister-like aneurysms by FD stent was less dangerous in the chronic stage than in the acute phase.
In the remaining 16 supraclinoid aneurysms treated, we observed one cerebral haemorrhage seven days after Silk stent deployment caused by unpredictable spontaneous aneurysm rupture. Early and/or late tromboembolic events occurred in two supraclinoid aneurysms.
Haemorrhagic or thromboembolic events are potentially serious complications that should be clearly explained to patients. It is particularly important to clarify the major risks of bleeding in the case of extracavernous carotid siphon aneurysms.
Finally, we treated two symptomatic vertebrobasilar aneurysms for which few treatment alternatives were available. One of these patients (Figure 5) died from an unpredictable flow-diverting stent effect: an acute increase in aneurysm size with obstructive hydrocephalus. Instead, Silk stent release caused a flow reduction inside the aneurysm in the second patient (Figure 8). We are still unable to define exactly which part of the aneurysm sac will thrombose after positioning an FD stent. Without a doubt, vertebrobasilar fusiform and giant aneurysms must be considered a complex category to treat with FD devices 14.
Conclusions
The development of FD stents is currently revolutionizing the treatment of intracranial aneurysms, namely wide-neck, giant and fusiform or tiny lesions lateral to a large artery, carotid siphon or vertebrobasilar artery.
Many aneurysms treated with FD devices are particularly complex lesions difficult to treat by conventional endovascular techniques or surgery 7. Nevertheless larger patient cohorts have demonstrated that the complication rate of FD deployment is similar to that of other endovascular procedures such as coiling, remodelling and stent-assisted coiling 3,12,22.
In our experience the indications for the use of FD stents could be summarised as follows:
Primary indication: symptomatic intracavernous giant aneurysms for which bleeding should be considered a less serious risk.
Secondary indication: extracavernous carotid siphon aneurysms have a major risk of bleeding. FD stents are indicated clearly explaining the risks to the patient in case of severe mass effect. The use of FD stents can result in a marked reduction in mass size.
Complex category: FD stent placement is probably appropriate for vertebrobasilar fusiform or giant aneurysms given the concrete possibility of vascular remodelling. More caution is warranted in the case of major aneurysms that could enlarge resulting in hydrocephalus as in one of our patients.
However, a more critical analysis of complications and more experience with this new device are needed to establish the true indications for FD stents in the treatment of intracranial aneurysms.
References
- 1.Augsburger L, Farhat M, Reymond P, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Klin Neuroradiol. 2009;19:204–214. doi: 10.1007/s00062-009-9005-0. [DOI] [PubMed] [Google Scholar]
- 2.Aurboonyawat T, Blanc R, Schmidt P, et al. An in vitro study of silk stent morphology. Neuroradiology. 2010;53:659–667. doi: 10.1007/s00234-010-0784-4. [DOI] [PubMed] [Google Scholar]
- 3.Bodily KD, Cloft HJ, Lanzino G, et al. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. Am J Neuroradiol. 2011;32:1232–1236. doi: 10.3174/ajnr.A2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. 2010;2:5–(9). doi: 10.1371/journal.pone.0012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. Am J Neuroradiol. 2011;32:27–33. doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirillo L, Dall’Olio M, Princiotta C, et al. The use of flow-diverting stents in the treatment of giant cerebral aneurysms: preliminary results. Neuroradiol J. 2010;23:220–224. doi: 10.1177/197140091002300212. [DOI] [PubMed] [Google Scholar]
- 7.Cloft HJ. Flow diversion for cerebral aneurysms: a cautionary tale. Am J Neuroradiol. 2011;32:26. doi: 10.3174/ajnr.A2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorella D, Kelly ME, Albuquerque FC, et al. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64:212–217. doi: 10.1227/01.NEU.0000337576.98984.E4. [DOI] [PubMed] [Google Scholar]
- 9.Fiorella D, Lylyk P, Szikora I, et al. Curative cerebrovascular reconstruction with the Pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Sur. 2009;1:56–65. doi: 10.1136/jnis.2009.000083. [DOI] [PubMed] [Google Scholar]
- 10.Fiorella D, Sadasivan C, Woo HH, et al. Regarding “aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment”. Am J Neuroradiol. 2011;32:E95–E97. doi: 10.3174/ajnr.A2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115–1120. doi: 10.1227/01.neu.0000325873.44881.6e. [DOI] [PubMed] [Google Scholar]
- 12.Wong GK, Kwan MC, Ng RY, et al. Flow diverters for treatment of intracranial aneurysms: Current status and ongoing clinical trials. J Clin Neurosci. 2011;18:737–740. doi: 10.1016/j.jocn.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Hassan T, Ahmed YM, Hassan AA. The adverse effects of flow-diverter stent-like devices on the flow pattern of saccular intracranial aneurysm models: computational fluid dynamics study. Acta Neurochir. 2011;153:1633–1640. doi: 10.1007/s00701-011-1055-9. [DOI] [PubMed] [Google Scholar]
- 14.Klisch J, Turk A, Turner R, et al. Very late thrombosis of flow-diverting constructs after the treatment of large fusiform posterior circulation aneurysms. Am J Neuroradiol. 2011;32:627–632. doi: 10.3174/ajnr.A2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulcsár Z, Ernemann U, Wetzel SG, et al. High-profile flow diverter (silk) implantation in the basilar artery: efficacy in the treatment of aneurysms and the role of the perforators. Stroke. 2010;41:1690–1696. doi: 10.1161/STROKEAHA.110.580308. [DOI] [PubMed] [Google Scholar]
- 16.Kulcsár Z, Houdart E, Bonafé A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol. 2011;32:20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulcsár Z, Wetzel SG, Augsburger L, et al. Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery. 2010;67:789–793. doi: 10.1227/01.NEU.0000372920.39101.55. [DOI] [PubMed] [Google Scholar]
- 18.Leonardi M, Cirillo L, Toni F, et al. Treatment of intracranial aneurysms using flow-diverting silk stents (BALT): a single centre experience. Interv Neuroradiol. 2011;17:306–315. doi: 10.1177/159101991101700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke. 2010;41:2247–2253. doi: 10.1161/STROKEAHA.110.589911. [DOI] [PubMed] [Google Scholar]
- 20.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632–642. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 21.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol. 2011;32:34–40. doi: 10.3174/ajnr.A2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierot L, Cognard C, Spelle L, et al. Safety and efficacy of balloon remodeling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. Am J Neuroradiol. 2011;33:12–15. doi: 10.3174/ajnr.A2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Princiotta C, Dall’Olio M, Cirillo L, et al. Staged treatment of a blister-like aneurysm with stent assisted coiling followed by flow diverter in-stent insertion. A case report. Interv Neuroradiol. 2011;17:365–370. doi: 10.1177/159101991101700314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pumar JM, Castineira JA, Vazquez F, et al. Exclusion of a cavernous aneurysm by Leo stent. Intervent Neuroradio. 2006;12:57–60. doi: 10.1177/159101990601200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadasivan C, Cesar L, Seong J, et al. An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase. Stroke. 2009;40:952–958. doi: 10.1161/STROKEAHA.108.533760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toni F, Marliani AF, Cirillo L, et al. 3T MRI in the evaluation of brain aneurysms treated with flow-diverting stents: preliminary experience. Neuroradiol J. 2009;22:588–599. doi: 10.1177/197140090902200512. [DOI] [PubMed] [Google Scholar]
- 27.Turowski B, Macht S, Kulcsár Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (Silk-Stent): Do we need to rethink our concepts? Neuroradiology. 2011;53:37–41. doi: 10.1007/s00234-010-0676-7. [DOI] [PubMed] [Google Scholar]
- 28.van Rooij WJ, Sluzewski M. Perforator Infarction after Placement of a Pipeline Flow-Diverting Stent for an Unruptured A1 aneurysm. Am J Neuroradiol. 2010;31:E43–E44. doi: 10.3174/ajnr.A2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walcott BP, Pisapia JM, Nahed BV, et al. Early experience with flow diverting endoluminal stents for the treatment of intracranial aneurysms. J Clin Neurosci. 2011;18:891–894. doi: 10.1016/j.jocn.2011.01.002. [DOI] [PubMed] [Google Scholar]