Summary
We report our experience with endovascular treatment and follow-up results of a ruptured blood blister-like aneurysm (BBA) in the supraclinoid internal carotid artery. We performed a retrospective review of ruptured blood blister-like aneurysm patients over a 30-month period. Seven patients (men/women, 2/5; mean age, 45.6 years) with ruptured BBAs were included from two different institutions. The angiographic findings, treatment strategies, and the clinical (modified Rankin Scale) and angiographic outcomes were retrospectively analyzed.
All seven BBAs were located in the supraclinoid internal carotid artery. Four of them were ≥ 3 mm in largest diameter. Primary stent-assisted coiling was performed in six out of seven patients, and double stenting was done in one patient. In four patients, the coiling was augmented by overlapping stent insertion. Two patients experienced early re-hemorrhage, including one major fatal SAH. Complementary treatment was required in two patients, including coil embolization and covered-stent placement, respectively. Six of the seven BBAs showed complete or progressive occlusion at the time of late angiographic follow-up. The clinical midterm outcome was good (mRS scores, 0-1) in five patients.
Stent-assisted coiling of a ruptured BBA is technically challenging but can be done with good midterm results. However, as early re-growth/re-rupture remains a problem, repeated, short-term angiographic follow-up is required so that additional treatment can be performed as needed.
Key words: blood blister-like aneurysm, internal carotid artery, endovascular treatment, stent-assisted coil embolization
Introduction
Blood blister-like aneurysm (BBA) is classically described as small, bleblike, very broad-neck lesions developing at non-branching sites within the supraclinoid internal carotid artery (ICA). These lesions present with acute subarachnoid hemorrhage (SAH) and generally have a malignant course 1-4.
The angiographic diagnosis of BBAs may be difficult because they are often tiny lesions which can be overlooked, mistaken for artifacts or focal atheromatous irregularities, or missed altogether due to an overlap of the vessel curvature. As the literature indicates, routine microsurgical and endovascular treatment approaches carry the risk of a high morbidity and mortality 4, thus leading to attempts to develop numerous, novel treatment techniques 1,5. Endovascular techniques usually use a combination of coil embolization and/or stent placement, with definitive endovascular treatment limited to parent artery occlusion, with or without surgical bypass 3,6-8.
Since Kim et al. 7 reported a case of successful BBA treatment using stent-assisted coil embolization followed by stent-in-stent deployment, an increasing number of cases of reconstructive endovascular treatment using a stent have been reported as effective for durable occlusion of a BBA 9-11. The present study evaluated the clinical and angiographic outcomes in seven consecutive patients with BBAs who underwent reconstructive endovascular treatment.
Patients and Methods
Patients
This retrospective study was approved by our Institutional Review Board and informed consents were waived. Seven, consecutive patients with confirmed ruptured BBAs underwent treatment at two institutions between January 2009 and August 2011 and using reconstructive endovascular methods.
We reviewed all of the relevant imaging studies and records pertaining to the diagnosis and treatment of these aneurysms, including admission and discharge summaries, progress notes, initial diagnostic imaging studies, procedural images and reports, and follow-up imaging studies.
Our patients included five women and two men, aged 37-54 years (mean 45.6 years). The BBAs were diagnosed if they had the following morphological features, i.e. a typical small, hemispheric bleb-like, very broad-neck bulge, which was classically described at non-branching sites of the supraclinoid ICA segments. Reconstructive endovascular treatment using stents with or without coiling was primarily considered due to the poor collateral blood supply, extreme proximity of the BBA to the origin of the anterior choroidal artery, or both.
Procedure for the Reconstructive Endovascular Treatment of BBA
All procedures were performed with the patients under general anesthesia using the transfemoral approach. The activated coagulation time was maintained two to three times above the baseline during the procedure. After systemic heparinization, a 6-French Envoy guiding catheter (Cordis Neurovascular, Miami Lakes, FL, USA) was placed into the distal internal carotid artery. Using diagnostic angiography and 3D angiographic reconstruction, the morphologic characteristics of the aneurysm were carefully evaluated, including its location and size, as well as all major branches originating from the distal ICA adjacent to the aneurysm. Stent placement or stent-assisted coiling (SAC) for treatment of the ruptured BBA was performed using a self-expanding stent (Enterprise; Cordis Neurovascular, Miami Lakes, FL, USA).
For stent placement, a delivering microcatheter (Prowler Select Plus; Cordis Neurovascular, Miami Lakes, FL, USA) was navigated to the distal branch of the middle cerebral artery using a guidewire. The Enterprise stent was then loaded into the microcatheter. The tip of another second microcatheter was placed near the aneurysm neck facing the dome of the aneurysm sac. After introducing the coil (not detached) through the second microcatheter, the stent was deployed to push the pre-deployed coil loops into the sac, and the coil finally detached 12.
Subsequent coils were then introduced into the aneurysmal sac, after which, depending on the operator's preference and each individual patient's anatomical factors, e.g. (tortuosity and atherosclerotic changes in the ICA), a second stent was inserted using the stent-within-a-stent (SWS) technique 7. For the patients with a ruptured BBA, a loading dose of antiplatelet medication, i.e. (aspirin plus clopidogrel) was given following completion of the treatment.
Clinical and Angiographic Follow-up
Patients were clinically assessed at the time of their hospital admission, again when the patient's neurological status deteriorated, and finally at discharge by neurosurgeons. For the patients with subarachnoid hemorrhage, their clinical status upon admission was evaluated according to the Hunt and Hess grading system. Each patient's clinical outcome was evaluated and graded according to the modified Rankin Scale (mRS) score 13, and their clinical outcome at the latest clinical follow-up was defined as the final outcome. Angiographic follow-up was performed following treatment at one to four weeks, two to four months, and six to 12 months postoperatively.
Results
The demographics are summarized in Table 1. A pre-morbid condition included arterial hypertension found in five patients (patients 1, 2, 4, 5, and 7). All seven patients presented with subarachnoid hemorrhage, diagnosed by unenhanced head CT. Four of the seven aneurysms were ≥ 3 mm in their largest diameter. The Hunt and Hess grade was II in four patients, III in two, and IV in one. The average Hunt and Hess grade at the time of the patient's admission was 2.7 (range 2 - 4), and the average Fisher grade was 2.9 (range 2 - 4). All patients subsequently underwent diagnostic CT angiography (CTA) prior to their digital subtraction angiography (DSA) evaluation. Of the seven patients diagnosed with BBA, six (85%) were prospectively identified on CTA, while the other patient (15%) not prospectively identified by CTA (false negative). All were identified on DSA. On retrospective review of the initial CTA images, all aneurysms could be identified including the one aneurysm which was initially missed on CTA and misinterpreted as a vascular irregularity caused by artherosclerosis of the carotid artery. An increase in aneurysm size was observed in one out of seven patients who underwent angiographic follow-up within four days after the initial study (Figure 1).
Table 1.
Patient demographics.
| Age | Sex | Aneurysm location | Aneurysm size (mm) |
HH grade |
Fisher grade |
Diagnosis | Diagnosis- to-treatment (days) |
Follow-up (months) |
|
|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | Left ICA: medial wall | 3.5 × 4.0 | 3 | 3 | DSA | 4 | 24 |
| 2 | 37 | M | Left ICA: anterior wall | 1.2 × 1.5 | 2 | 2 | CTA | 1 | 16 |
| 3 | 52 | M | Left ICA: lateral wall | 2 × 1 | 4 | 4 | CTA | 1 | NA |
| 4 | 41 | F | Right ICA: anterior wall | 2 × 2.5 | 2 | 2 | CTA | 2 | 10 |
| 5 | 53 | F | Left ICA: medial wall | 2.7 × 4.5 | 2 | 2 | CTA | 1 | 18 |
| 6 | 54 | F | Left ICA: anterior wall | 1.3 × 3.3 | 3 | 4 | CTA | 0 | 13 |
| 7 | 43 | F | Right ICA: anterior wall | 2.5 × 5. 3 | 2 | 3 | CTA | 0 | 12 |
| ICA, internal cerebral artery; HH, Hunt and Hess; CTA, computed tomographic angiography; DSA, digital subtraction angiography; N/A, not applicable | |||||||||
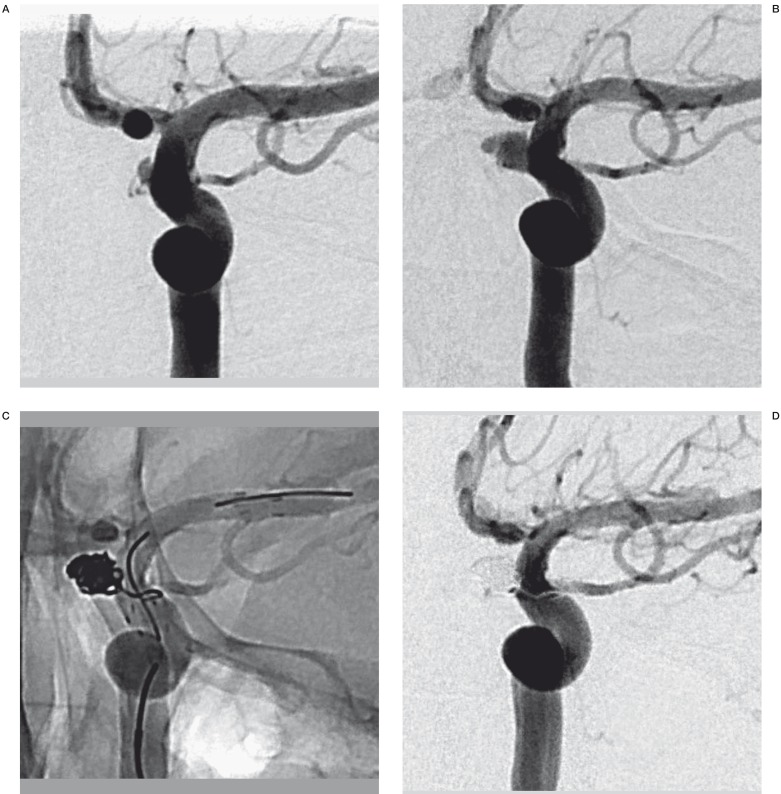
Figure 1.
A 39-year-old woman (patient No. 1) presenting with HH grade III SAH. A) Preprocedural DSA shows a blister aneurysm along the medial wall of the supraclinoid segment of the left ICA. B) Subsequent DSA shows enlargement of the blister aneurysm 4 days later. C) The patient was treated with stent-assisted coiling and followed by the stent-within-a-stent technique. D) Final DSA shows complete occlusion of BBA.
Table 2 summarizes the treatment options, as well as angiographic and clinical follow-up results. Among the seven study patients, five were treated within one day following admission. The average interval between the diagnostic and initial treatment procedures was 1.3 days (range, 0 – 4 days). SAC or SWS was initially attempted for all patients. In the six patients who underwent SAC embolization, a second stent insertion using the SWS procedure was attempted during the same session and was successful in four patients. Therefore, two patients received initial treatment consisting of only SAC embolization. On the other hand, one patient received stent monotherapy without coil embolization. There were no treatment-related complications or neurologic deterioration during or after treatment.
Table 2.
Results.
| Initial treatment | Clinical course and re-treatment | Status at last FU angiogram (months) |
Clinical outcome (mRS score) |
|
|---|---|---|---|---|
| 1 2 3 4 5 6 7 |
SAC + SWS SWS SAC + SWS SAC + SWS SAC SAC SAC + SWS |
No recurrence No recurrence Regrowth & covered stent (12 days) No recurrence No recurrence No recurrence Regrowth & SAC (15 days) |
CR (12 mos) CR (12 mos) NA CR (3 mos) CR (13 mos) CR (11 mos) CR (3 mos) |
0 0 6 1 1 1 4 |
| SAC, stent-assisted coil embolization; SWS, stent-within-a-stent; CR, complete resolution of BBA with ICA reconstruction; NA, not applicable; mRS, modified Rankin Scale | ||||
Of the four patients treated using SWS technique, excellent outcomes were achieved in two (mRS score of 1). These two patients also showed complete resolution of their BBA with reconstruction of the affected ICA segment. However, two patients showed BBA re-growth with bleeding 12 and 15 days, respectively, following their initial treatment. One of the two recurrent BBAs was re-treated by coil embolization (Figure 2), and the other by covered stent insertion (Figure 3). The first of these two patients was moderately disabled (mRS score of 4), while the latter patient died from early re-hemorrhage. The two patients who received SAC embolization alone showed excellent outcomes (mRS score of 1). These two patients also showed complete resolution of the BBA with smooth reconstruction of the affected ICA segment. One patient who received SWS monotherapy without coiling also showed an excellent outcome. This patient showed residual aneurysmal contrast stasis after completion of the procedure, but no delayed BBA recanalization or re-growth was found on follow-up angiography.
All of the six surviving patients were clinically followed for a mean of 15.5 months (range, 10 - 24 months). Five of the seven patients who were treated using the endovascular method had a favorable outcome (mRS score of 0-1).
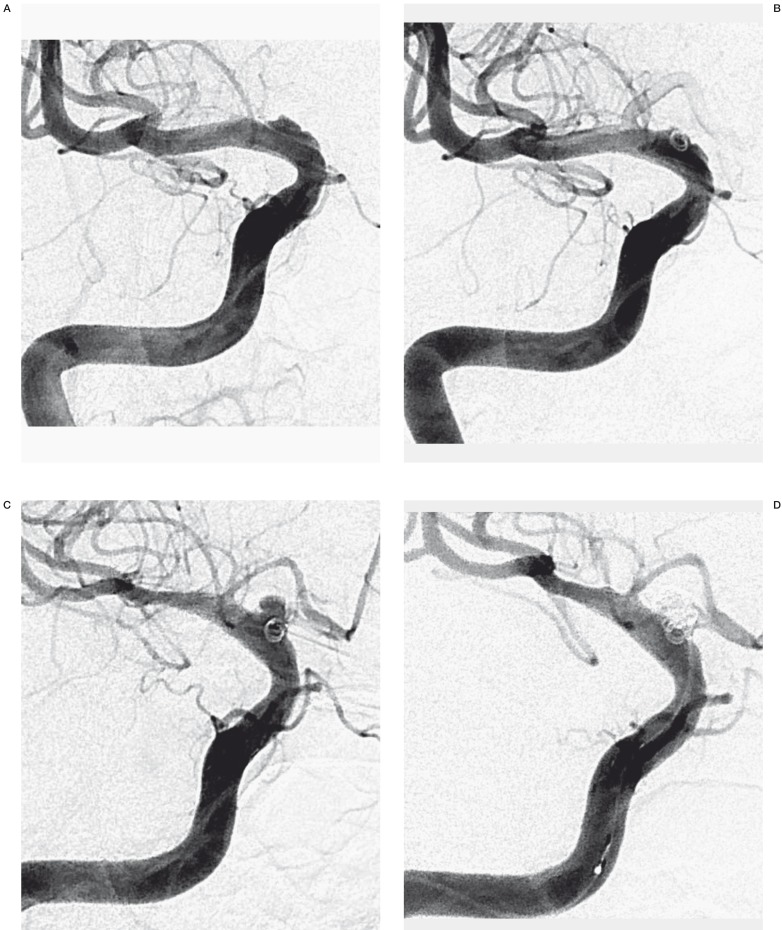
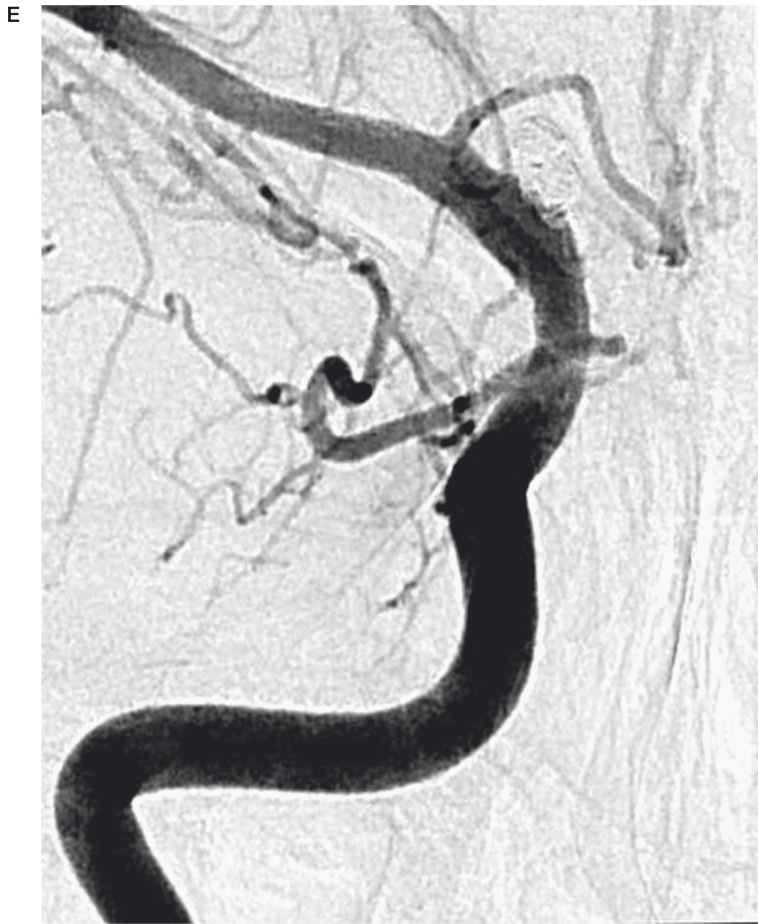
Figure 2.
A 43-year-old woman (patient No. 7) presenting with HH grade II SAH. A) Initial angiogram shows a broad-based, shallow outpouching arising from a non-branching site along the anterior wall of supraclinoid ICA. B) Stent-assisted coil embolization with a single coil and followed by the stent-within-a-stent technique was performed. The angiogram revealed residual aneurysmal filling. C) Follow-up right ICA angiogram obtained 15 days postoperatively revealed considerable BBA growth. D) After re-treatment using stent-assisted coiling with multiple coils, the angiogram reveals minimal residual aneurysm filling of the aneurysm sac. E) At three months' angiographic follow-up examination, complete occlusion with reconstruction of the ICA was noted.
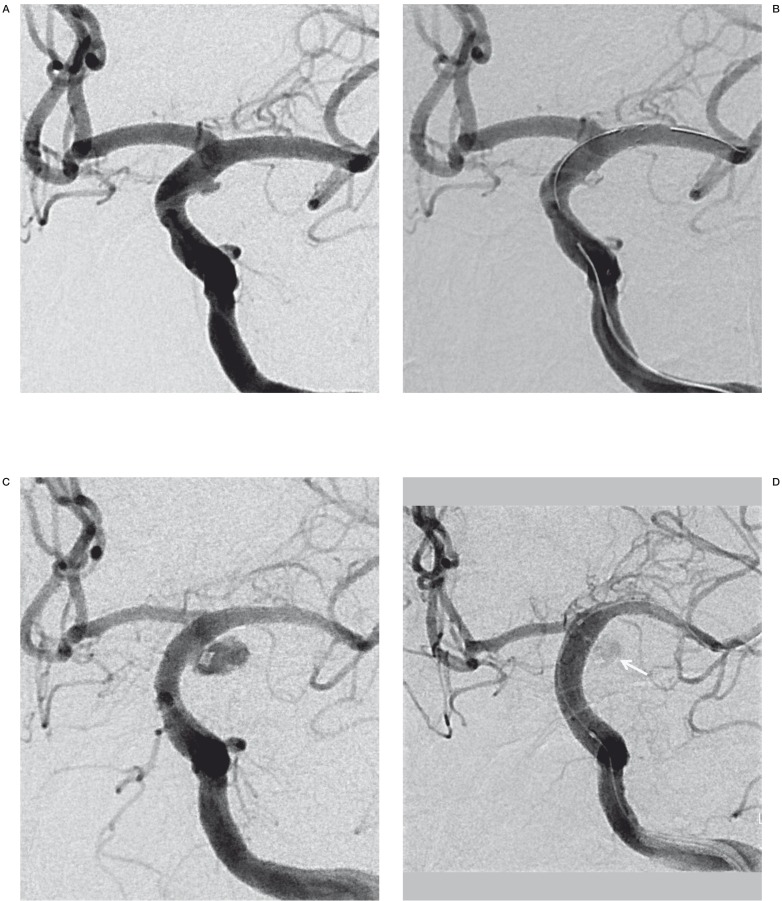
Figure 3.
A 52-year-old man (patient No. 3) presenting with HH grade IV SAH. A) Preprocedural DSA shows a blister aneurysm along the lateral wall of the supraclinoid segment of left ICA. B) A control angiogram obtained after stent-assisted coil embolization, revealed minimal residual aneurismal filling. C) Follow-up left ICA angiogram obtained 12 days postoperatively revealed considerable BBA growth. D) Immediately after re-treatment with a covered stent, the control angiogram revealed subtle contrast media leakage into the recurred sac (arrow).
Discussion
The term “blood blister-like aneurysm” has been used to describe aneurysms arising from non-branching sites of the wall of the supraclinoid internal carotid artery (ICA). These lesions, with an estimated incidence of 0.9% - 6.5% among all ruptured intracranial aneurysms 2, represent a rare but potentially catastrophic cause of SAH. Due to their very unfavorable morphology, small size, ill-defined necks, and friable walls, they pose a particularly challenging problem for surgical as well as endovascular treatment 4.
Pathology studies suggest that BBAs may not be saccular aneurysms but a specific type of pseudoaneurysm. BBAs have been shown to represent focal arterial wall lacerations with a gap in the internal elastic lamina and media at the border between the eccentrically sclerotic and normal carotid wall. The focal wall defects may be the result from laceration of the ICA wall caused by ulceration and penetration into the internal elastic lamina, resulting from arteriosclerosis 1,14. This defect is covered with thin fibrous tissue and adventitia, lacking the usual collagen layer as in classic berry aneurysm. Therefore, these aneurysms have a high risk of intraoperative rupture and postoperative hemorrhage, thus resulting in significant disability and mortality caused by the surgical repair 1,3,14.
All lesions in our study arose from non-branching sites of the supraclinoid ICA, and were primarily located in the anterior and medial walls of the ICA, where the ICA curves in lateral and superior directions and where hemodynamic stress is thus thought to be high. In our study, five patients' aneurysms demonstrated a characteristic, shallow, triangular appearance with a broad-based attachment to the parent vessel, as seen on the initial CTA image. These aneurysms were diagnosed on CTA and closely resembled their DSA counterparts. On the other hand, one patient showed a tiny hemispheric bulge at the medial wall of the parent artery and the other patient's lesion was missed on CTA. Subsequent DSA images identified all aneurysms in all cases. Because CTA lacks the spatial resolution of DSA imaging, optimizing the CTA techniques used to identify BBAs is important 15. In addition, timing of the image acquisition may be another important factor for identifying the lesion, as BBAs tend to grow rapidly during a relatively short period of time. Furthermore, repeated DSA, including 3D rotational angiographic studies, is necessary to disclose the presence of BBAs, as they can be obscured by luminal irregularities related to atherosclerosis in adjacent arteries 11.
Various surgical and endovascular techniques have already been identified for the treatment of ruptured BBAs. Primary surgical clipping or wrapping is considered too dangerous due to the highly fragile BBA wall, as these surgical techniques are often associated with high surgical mortality and morbidity 1,2,3,14. Likewise, primary coiling may be very hazardous given the lesion's shallow, wide-necked morphology and very weak wall lacking collagenous tissue 4,6. Deconstructive endovascular trapping after a balloon occlusion test has been suggested as a definitive and safe approach for the treatment of BBAs in the ICA 8. However, this option may not be suitable for lesions in which important branching arteries arise close to the BBA or if there is an insufficient collateral blood supply. Furthermore, parent artery occlusion may also interfere with the endovascular access required for later vasospasm treatment.
Recently, there have been some reports on the stent-assisted coil embolization of BBA 9-11,17. Table 3 shows a summary of BBAs treated by stent-assisted embolization described in the literature. Gaughen et al. 10 reported that double-stent placement can prevent re-bleeding and can heal ruptured BBAs. However, Meckel et al. 11 did not advocate double-stent placement without coiling as a primary treatment option as the likelihood of a residual or recurrent aneurysm requiring further treatment was reported to be high at 50% in the report of Gaughen et al. 10. Similarly, our treatment strategy in the acute setting focused on stent-assisted coil embolization to diminish the hemodynamic stress placed on an aneurysm rather than use of double-stent monotherapy alone.
Table 3.
Summary of previous studies involving SAC or SWS in blood blister-like aneurysms, previously described in the literature and our series.
| Authors & year |
No. of patients |
Procedure | FU duration | Clinical outcome |
|---|---|---|---|---|
| Fiorella et al., 2006 |
2 | single stent / multiple SWS | 9.8 mos (1-24 mos) | good recovery |
| Lee et al., 2009 |
6 | 1 SAC + covered stent 5 SAC + SWS |
11 mos (4-26 mos) | good recovery (GOS 5) |
| Gaughen et al., 2010 |
6 | 3 SWS + SAC / 3 SWS | 20 days-12 mos | 5 (mRS1), 1 (mRS 3) |
| Meckel et al., 2011 |
12 | 11 SAC / 1 SWS | 12 mos (5-27 mos) | 12 (mRS 0-2) |
| Our cases | 7 | 4 SAC + SWS 2 SAC / 1 SWS |
7.7 mos (3 -13 mos) | 5 (mRS 0-1), 1 (mRS 4), 1 (mRS 6), |
| SAC, stent-assisted coil embolization; SWS, stent-within-a-stent; GOS, Glasgow Outcome Scale; mRS, modified Rankin Scale | ||||
Stenting has been seen to promote neo-intima formation along the stent struts in experimental models 16. As this may also prevent re-bleeding in the acute stage by redirecting the flow and lowering the wall stress of BBAs, it may be another option for treating ruptured BBAs, especially when stent-assisted coiling is not feasible 10,17. Though our data are limited to a single case, double-stent monotherapy has shown complete occlusion of a BBA.
Theoretically, overlapping double stents would create better flow diversion than a single stent. The greater the strut density and stent thickness, the more easily neo-intima formation is promoted. In our series, it was not always feasible to insert a second stent for the SWS procedure immediately following SAC embolization, and we failed in two of the six patients in whom we attempted to insert a second stent immediately following embolization. However, these two patients showed excellent outcomes without recurrence. Contrary to the report of Lee et al. 9, occlusion of a BBA by SAC embolization and subsequent use of an overlapping stent were not sufficient to prevent a BBA from reforming, as seen in our series and in the report of Gaughen et al. 10. According to our visual inspection on angiogram, overlapping stents markedly decrease the flow into aneurysms, although theoretically this method cannot be guaranteed to completely block the blood flow by sealing off the affected wall of the ICA around a BBA treated in the acute stage.
Because BBA walls are very fragile and thin, coil embolization without stents carries the potential associated risks of aneurysm neck disruption and distal coil migration. In addition, it is not generally advocated to perform double-stent placement without coiling as a primary treatment option because the likelihood of a residual or a recurrent aneurysm requiring further treatments was reported to be as high as 50% 10. The principal indication for using stents with coiling in our series was a combination of a shallow aneurysmal sac and a wide neck. When the BBA anatomy is even more unfavorable (i.e. with a neck / dome ratio ranging from 1 to 2), a single stent may only help to hold the coils in place but not provide sufficient additional flow-diversion to exclude an extremely wide aneurysm neck. If BBAs are presumed to be a specific type of dissection or pseudoaneurysm, treatment should not be focused only on the BBA sac, but also on the affected wall of the ICA around the BBA. Therefore, we would generally advocate stent-within-a-stent placement after stent-assisted coil embolization.
In our series, two of seven aneurysms showed BBA re-growth with bleeding as seen on short-term follow-up. In one patient, subsequent coil embolization was achieved during one coiling session by the use of multiple coils. In another patient, covered stent (Jostent Graftmaster, Abbott Vascular devices) placement was achieved. Lee et al. 9 reported a small, mixed series of endovascular reconstructive treatment including covered stent placement. They showed durable occlusion of BBAs using a covered stent. However, ICA rupture occurred once during the procedure and a patient died. There are several limitations inherent in the use of covered stents according to the current technology, and their application in the treatment of BBAs may put patients at additional risk for vessel injury to the fragile nature of BBAs and ICA wall 7. In addition, as BBAs are often located in close proximity to the origin of the anterior choroidal artery, and to the posterior communicating arteries, using a covered stent is less appropriate. Alternatively, particularly in areas with important branching arteries, multiple overlapping stents or the recently introduced flow-diverting stents may be used. The benefits and potential drawbacks of using flow-diverting stents (e,g., Pipeline, Chestnut Medical Technologies, Menlo Park, CA, USA; or Silk devices, Balt Extrusion, Montmorency, France) in ruptured BBAs will need to be assessed in future studies 18-20.
As for the timing of the treatment for BBA, acute treatment is still controversial. Given the fragile nature of these aneurysms, it might be better to wait until the subacute or chronic phase when the wall of the lesion becomes more stable and when the aneurysm may also progress to a more saccular appearance which allows a more successful coil embolization, while not overlooking the risk of aneurysm rerupture. Thus, we strongly recommend vigorous and repeated short-term angiographic follow-up of all incompletely occluded BBAs.
Parent artery occlusion of the ICA as a definitive treatment for a ruptured blister aneurysm, should be considered a salvage procedure when initial treatments using endovascular stent placement with or without subsequent coiling, has failed. However, we would not recommend PAO as the primary therapy option for ruptured BBAs, unless the target BBA involves a very long segment with associated dysplastic wall changes. PAO remains an option, although it should be avoided until there is no longer a risk of secondary SAH-induced vasospasm subsides 11.
The limitations of our study should be noted, including its retrospective evaluation, small study size, and lack of a control group for comparison. Further investigation with larger patient populations and prospective evaluation is clearly warranted in order to validate our results.
Conclusions
Our study results suggest that ruptured BBAs have a high risk for re-growth and re-bleeding, thus resulting in a potentially severe and unfavorable outcome. Other than fatal early re-hemorrhage, the mid-term angiographic and clinical outcomes were favorable in our series. Stent placement with or without coiling thus become a viable treatment option and is technically feasible. However, we strongly recommend early angiographic follow-up of all incompletely occluded BBAs, both initially and at very short time intervals, so that additional treatment can be performed as required.
References
- 1.Abe M, Tabuchi K, Yokoyama H, et al. Blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 1998;89:419–424. doi: 10.3171/jns.1998.89.3.0419. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 2000;47:578–586. doi: 10.1097/00006123-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Sim SY, Shin YS, Cho KG, et al. Blood blister-like aneurysms at nonbranching sites of the internal carotid artery. J Neurosurg. 2006;105:400–405. doi: 10.3171/jns.2006.105.3.400. [DOI] [PubMed] [Google Scholar]
- 4.Meling TR, Sorteberg A, Bakke SJ, et al. Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg. 2008;108:662–671. doi: 10.3171/JNS/2008/108/4/0662. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa T, Mizoi K, Sugawara T, et al. Direct repair of a blisterlike aneurysm on the internal carotid artery with vascular closure stable clips: technical note. J Neurosurg. 2004;100:146–149. doi: 10.3171/jns.2004.100.1.0146. [DOI] [PubMed] [Google Scholar]
- 6.Tanoue S, Kiyosue H, Matsumoto S, et al. Ruptured “isterlike” aneurysm with a pseudoaneurysm formation requiring delayed intervention with endovascular embolization: case report. J Neurosurg. 2004;101:159–162. doi: 10.3171/jns.2004.101.1.0159. [DOI] [PubMed] [Google Scholar]
- 7.Kim BM, Chung EC, Park SI, et al. Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent: within-a-stent technique. J Neurosurg. 2007;107:1211–1213. doi: 10.3171/JNS-07/12/1211. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Park IS, Han DH, et al. Endovascular treatment of blood like aneurysms of the internal carotid artery. J Neurosurg. 2007;106:812–819. doi: 10.3171/jns.2007.106.5.812. [DOI] [PubMed] [Google Scholar]
- 9.Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009;110:431–436. doi: 10.3171/2008.7.JNS08257. [DOI] [PubMed] [Google Scholar]
- 10.Gaughen JR, Hasan D, Dumont AS, et al. The efficacy of endovascular stenting in the treatment of supraclinoid internal carotid artery blister aneurysms using a stent-In-stent technique. Am J Neuroradiol. 2010;31:1132–1138. doi: 10.3174/ajnr.A2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meckel S, Singh TP, Undren P, et al. Endovascular treatment using predominantly stent-assisted coil embolization and antiplatelet and anticoagulation management of ruptured blood blister-like aneurysms. Am J Neuroradiol. 2011;32:764–771. doi: 10.3174/ajnr.A2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Paula Lucas C, Piotin M, Spelle L, et al. Stent-jack technique in stent-assisted coiling of wide-neck aneurysms. Neurosurgery. 2008;62(5) Suppl 2:ONS 414–416. doi: 10.1227/01.neu.0000326028.47090.5f. [DOI] [PubMed] [Google Scholar]
- 13.Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patient. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa T, Nakamura N, Houkin K, et al. Pathological consideration of a “blister-like” aneurysm at the superior wall of the internal carotid artery: case report. Neurosurgery. 1997;40:403–406. doi: 10.1097/0006123-199702000-00038. [DOI] [PubMed] [Google Scholar]
- 15.Gaughen JR, Raghavan P, Jensen ME, et al. Utility of CT angiography in the identification and characterization of supraclinoid internal carotid artery blister aneurysm. Am J Neuroradiol. 2010;31:640–644. doi: 10.3174/ajnr.A1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geremia G, Brack T, Brennecke L, et al. Occlusion of experimentally created fusiform aneurysms with porous metallic stents. Am J Neuroradiol. 2000;21:739–745. [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysm. Neurosurgery. 2006;59:291–300. doi: 10.1227/01.NEU.0000223650.11954.6C. [DOI] [PubMed] [Google Scholar]
- 18.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the Pipeline embolization device. Neurosurgery. 2008;62:1115–1121. doi: 10.1227/01.neu.0000325873.44881.6e. [DOI] [PubMed] [Google Scholar]
- 19.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632–643. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 20.Rasskazoff S, Silvaggio J, Brouwer PA, et al. Endovascular treatment of a ruptured blood blister-like aneurysm with a flow-diverting stent. Interv Neuroradiol. 2010;16:255–258. doi: 10.1177/159101991001600304. [DOI] [PMC free article] [PubMed] [Google Scholar]