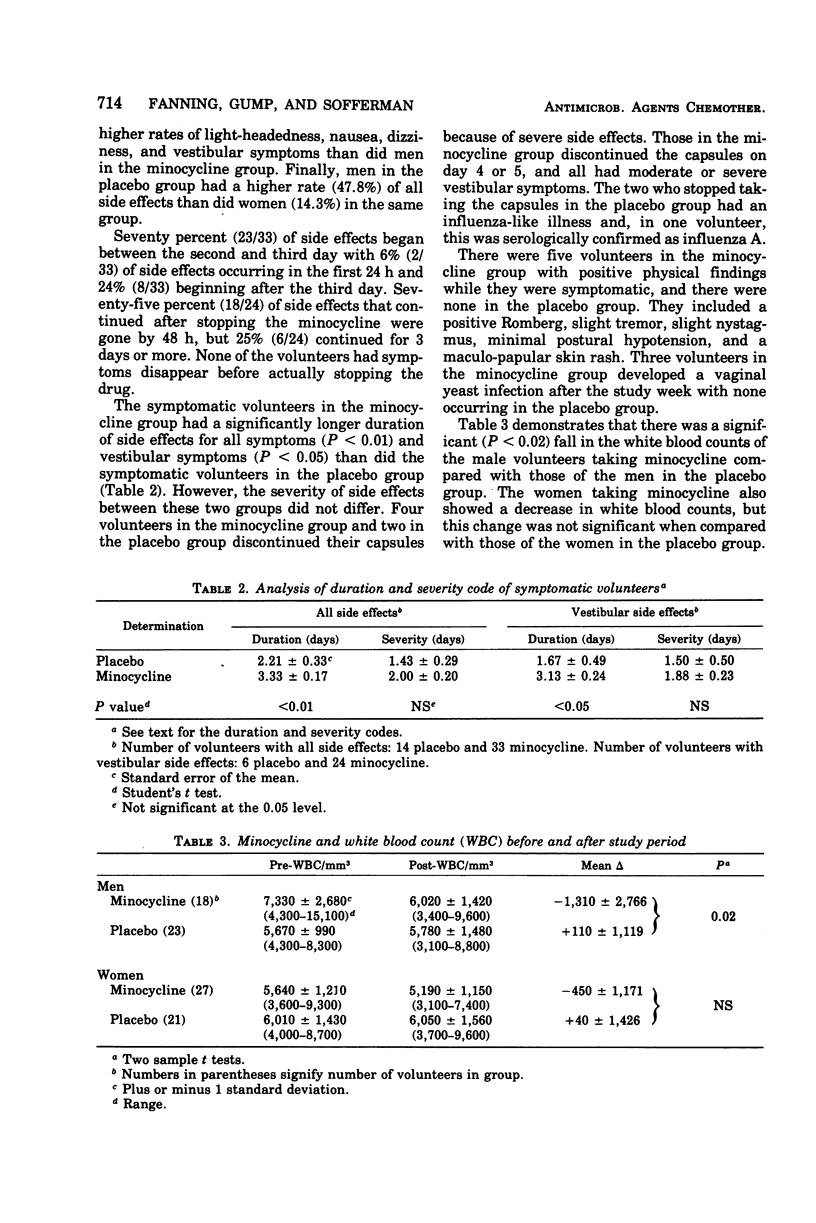
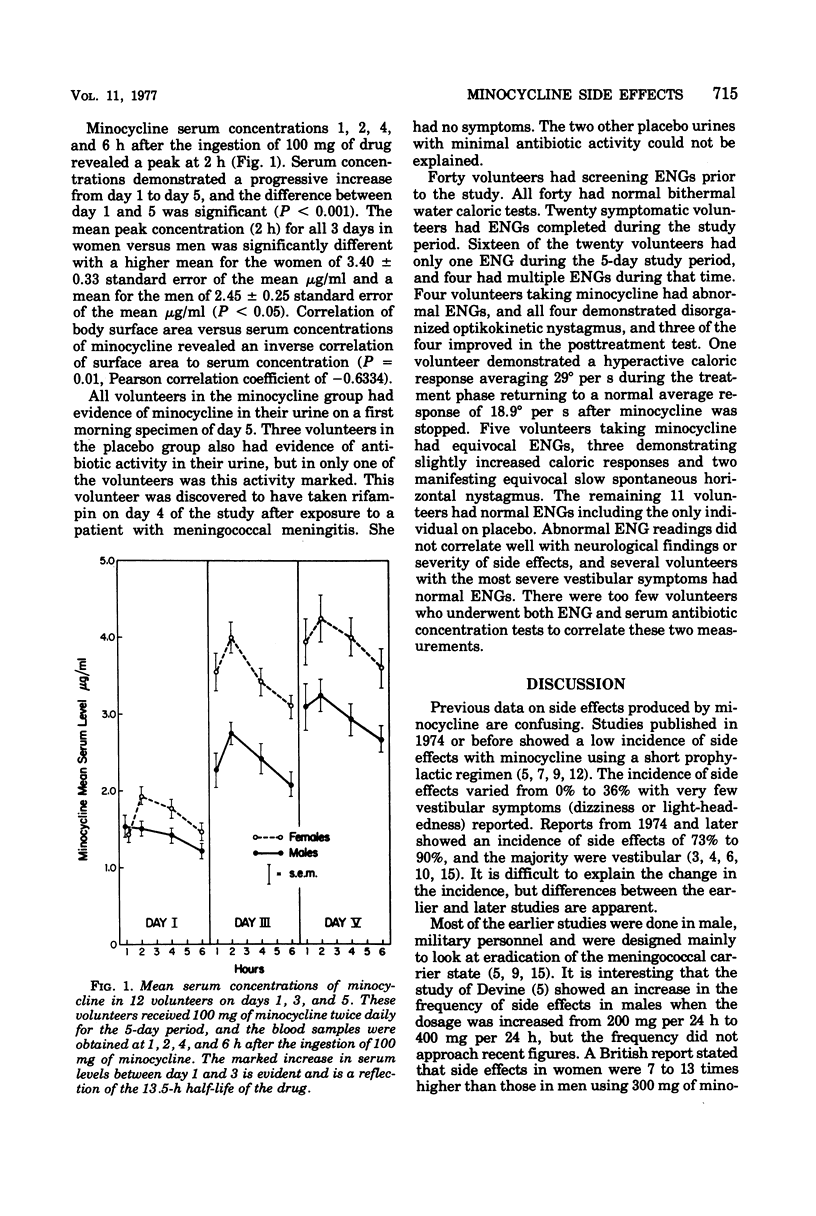
Abstract
We studied the incidence and type of side effects of minocycline in a double-blind study. A total of 45 volunteers (18 men and 27 women) were given minocycline, and 44 volunteers (23 men and 21 women) were given placebo. The men in both the minocycline and placebo groups were significantly (P < 0.0001) larger than the women in the comparable groups. Minocycline dosage was 100 mg every 12 h for 5 days, and placebo was administered in an identical manner. Minocycline serum concentrations were determined in 12 volunteers at 1, 2, 4, and 6 h after the morning doses on days 1, 3, and 5 of the study. Side effects were recorded by volunteers in diaries and also through daily interviews and were evaluated by examination and electronystagmography. Peak minocycline serum concentrations were seen by day 3 and correlated with the peak onset of side effects. These concentrations were significantly higher in women than in men. Vestibular side effects occurred in 70.4% of the women on minocycline and significantly (P < 0.0001) exceeded the rate of the women on placebo (9.5%). Only loss of balance was significantly (P < 0.05) increased in the men taking minocycline as contrasted with men on placebo. Electronystagmography generally revealed no abnormalities. Side effects were usually not severe: four volunteers in the minocycline group and two in the placebo group discontinued their capsules because of side effects. It is concluded that women experience an unacceptably high incidence of side effects from minocycline, and this may be related to their higher serum concentrations, which in turn may relate to their smaller size.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barza M., Brown R. B., Shanks C., Gamble C., Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother. 1975 Dec;8(6):713–720. doi: 10.1128/aac.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard B., Yin E. J., Simon H. J. Clinical pharmacologic studies with minocycline. J Clin Pharmacol New Drugs. 1971 Sep-Oct;11(5):332–348. [PubMed] [Google Scholar]
- Devine L. F., Johnson D. P., Hagerman C. R., Pierce W. E., Rhode S. L., 3rd, Peckinpaugh R. O. The effect of minocycline on meningococcal nasopharyngeal carrier state in naval personnel. Am J Epidemiol. 1971 May;93(5):337–345. doi: 10.1093/oxfordjournals.aje.a121266. [DOI] [PubMed] [Google Scholar]
- Fanning W. L., Gump D. W. Distressing side-effects of minocycline hydrochloride. Arch Intern Med. 1976 Jul;136(7):761–762. [PubMed] [Google Scholar]
- Guttler R. B., Beaty H. N. Minocycline in the chemoprophylaxis of meningococcal disease. Antimicrob Agents Chemother. 1972 May;1(5):397–402. doi: 10.1128/aac.1.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttler R. B., Counts G. W., Avent C. K., Beaty H. N. Effect of rifampin and minocycline on meningococcal carrier rates. J Infect Dis. 1971 Aug;124(2):199–205. doi: 10.1093/infdis/124.2.199. [DOI] [PubMed] [Google Scholar]
- Jacobson J. A., Daniel B. Vestibular reactions associated with minocycline. Antimicrob Agents Chemother. 1975 Oct;8(4):453–456. doi: 10.1128/aac.8.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Sussuarana de Vasconcelos Z. J., Phillips C. J., Gelli D. S., Gorman G. W., Risi J. B., Feldman R. A. Eradication of carriage of Neisseria meningitidis in families: a study in Brazil. J Infect Dis. 1974 Jun;129(6):644–649. doi: 10.1093/infdis/129.6.644. [DOI] [PubMed] [Google Scholar]
- Williams D. N., Laughlin L. W., Lee Y. H. Minocycline: Possible vestibular side-effects. Lancet. 1974 Sep 28;2(7883):744–746. doi: 10.1016/s0140-6736(74)90941-6. [DOI] [PubMed] [Google Scholar]



