Summary
In acute ischemic stroke, the ability to estimate the penumbra and infarction core ratio helps to triage those who will potentially benefit from thrombolytic therapies. Flat-panel post-contrast DynaCT imaging can provide both vasculature and parenchymal blood volume within the angio room to monitor hemodynamic changes during the endovascular procedures. We report on an 80-year-old woman who suffered from an acute occlusion of the right distal cervical internal carotid artery. She was transferred to the angio room where in-room post-contrast flat-panel DynaCT imaging (syngo Neuro PBV IR) was performed to access the ischemic tissue, followed by successful mechanical thrombolytic therapy.
Key words: brain, cerebral, anterior communicating, aneurysm
Introduction
In the United States, stroke is the third leading cause of death and the leading cause of disability. Ischemic stroke is the most common type, occurring in about 87% of stroke patients 1. Currently, intravenous recombinant tissue plasminogen activator/altepase (IV r-tPA) thrombolytic therapy within three hours of symptom onset is the only Food and Drug Administration (FDA)-approved pharmacological treatment for acute ischemic strokes 2. Studies have shown only 3-8.5% of patients who present with ischemic stroke are treated with r-tPA 3.
Recent research has revealed that treatment with IV-tPA may be beneficial without increased risk of complications beyond the three-hour window and many protocols are now moving to allow treatment up to 4.5 hours after symptom onset 4-6. Perfusion/diffusion mismatch imaging has been used to identify patients who have a higher chance of reperfusion/recanalization, and thus may potentially benefit from treatment beyond the approved three-hour window 4,7. The rationale is that hemorrhagic transformation is predisposed in those with a large infarction core. In this subgroup of patients, all thrombolytic treatment is contraindicated and by using mismatch imaging, these patients can be identified and excluded from the treatment protocol 8-10. However, validation of the different mismatch selection paradigms is still under investigation. Therefore more extensive clinical trials are still required to prove their clinical benefits. The required dose of tPA for intra-arterial thrombolysis (IAT) is smaller than that for intravenous thrombolysis and has been shown to lead to a higher recanalization rate beyond the three -hour window without an increased rate of complications in carefully selected patients 7,11-13. Previous studies have also shown a lower recanalization rate in patients with large vessel occlusion who were treated by intravenous thrombolysis 9,11-14. These patients may have a greater chance of recovery using IAT.
CT or MR angiography can be used to visualize the vasculature to estimate thrombus burden, determine the extent of infarction and identify brain tissue at risk of infarction. However, the acquisition time of the images and transfer time between modalities can delay door-to-needle time, potentially leading to worse clinical outcomes as more viable brain tissue infarcts 15.
Parenchymal and vascular imaging of the FD-CT dataset can be processed minutes after image acquisition and provide quantitative measurement of perfused cerebral blood volume (CBV) in the angio room where treatment can be immediately initiated, decreasing time from initial assessment to intervention 16.
Case Report
Clinical history: An 80-year-old woman presented to the emergency room with sudden onset of left sided hemi-paralysis and aphasia that began 90 minutes prior with an initial NIHSS score of 13. The patient was on warfarin for previously diagnosed atrial fibrillation and was thus not eligible for intravenous thrombolytic therapy. Initial CT with CT angiography and CT perfusion revealed no flow above the right carotid bifurcation, a thrombus in the right distal cervical ICA, and an infarction in the right caudate nucleus and temporal region with a large penumbra (Figure 1). By the time intra-arterial mechanical thrombolytic therapy was initiated, the onset of symptoms was more than three hours prior. The local guideline and medial reimbursement did not approve the use of intravenous r-tPA between three and 4.5 hours after the onset of symptoms, and thus intra-arterial mechanical thrombolytic therapy was initiated for this patient.
Imaging processing: Biplane angiography (AXIOM-Artis®, Siemens Healthcare) was used for digital subtraction angiography. A 4 French pigtail catheter was threaded into the ascending aorta 2 cm below the carina. Biplane intracranial angiography was initially obtained at six frames/second with administration of 25 ml of contrast medium (340 mgI/ml) for two seconds by a power injector (Liebel-Flarsheim Angiomat®, Illumena) (Figure 2A). The acquired imaging was then analyzed using processing software (syngo iFlow®, Siemens Healthcare) to determine the time at which the superior sagittal sinus achieved highest density of iodined-contrast (Tmax). The C-arm rotated 240° and the images were taken at a rate of 30 frames/second for eight seconds during the mask run. Then 50% diluted contrast was administered via the pigtail catheter at the same position at a rate of 5 ml/s for a duration equal to Tmax with an additional eight seconds for the second rotation of the C-arm. The parenchyma blood volume map was available within one minute after subtraction of the two sets of source images (Leonardo DynaCT, InSpace 3D software; Siemens). An algorithm was applied to further subtract air and bone from the image volume. The steady state arterial input function value was then calculated from an automated histogram analysis of the vessel tree. The axial map also showed decreased blood volume in the right caudate nucleus and temporal region (Figure 2B), and perfusion in less than one-third of the middle cerebral artery (MCA) territory. Those measured values of cerebral blood volume were consistent with those in prior CT perfusion study two hours previously. Both modalities revealed a thrombus in the right distal cervical ICA and inadequate collateral vessels from the previous angiography series, a large amount of brain tissue appeared to be at risk of infarction.
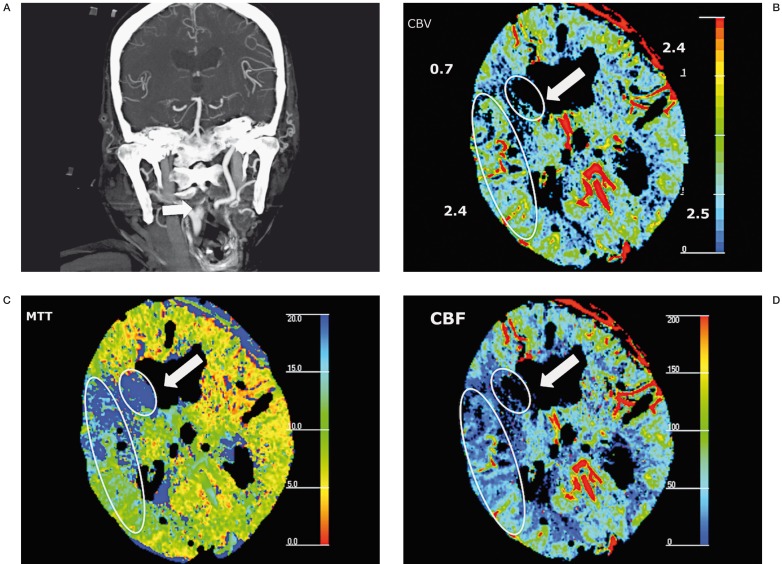
Figure 1.
A) Maximal intensity projection of coronal CTA showed occlusion of right distal cervical ICA 0.5 cm above carotid bifurcation. B) Decreased CBV (0.7 ml/100 g) was noted in caudate nucleus and right frontal lobe (arrow) with unchanged CBV in temporal region. Prolonged MTT (C) and decreased CBF (D) were noted in the area (ellipsis) surrounding the infarction zone (circle), suggestive of penumbra. MTT: mean transit time, CBF: cerebral blood flow, CBV: cerebral blood volume.
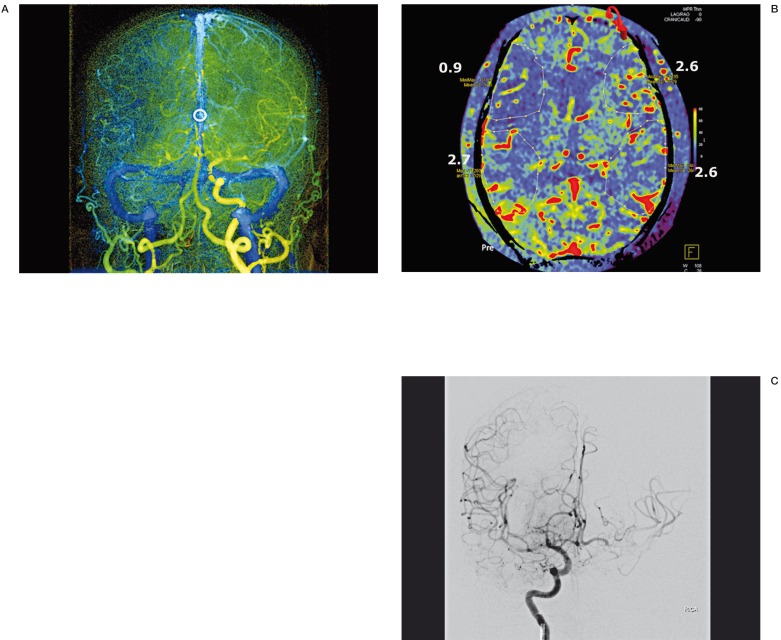
Figure 2.
A) Parametric color-coded syngo iFlow® of aortic angiogram of intracranial vessels with a pigtail catheter in ascending aorta revealing non-visualization of right ICA without adequate collateral flow from the Circle of Willis and ipsilateral external carotid artery. The time of maximal intensity of region of interest in superior sagittal sinus (circle) plus 8 seconds (rotation time of first mask run) was chosen as duration of contrast administration during the second rotation of the C-arm. B) Axial view of parenchymal blood volume at the same level as CT perfusion demonstrating decreased cerebral blood volume (0.9 ml/100 g) in the right caudate nucleus and frontal lobe region. It correlated well with the cerebral blood volume obtained from conventional CT perfusion (Figure 1D). C) Right internal carotid angiography after successful removal of thrombus by Penumbra System® showed recanalization of right ICA and MCA.
Interventional procedure and outcome: Thrombolytic therapy was immediately initiated by placing a Neuron Guiding catheter (Penumbra Inc., Alemada, CA, USA) in the right common carotid artery via the right femoral approach, and a 041 reperfusion catheter-separator pair was delivered to the right ICA to remove the thrombus just proximal to the carotid bifurcation. After we advanced to the right MCA, we changed to a 033-reperfusion catheter-separator to accommodate the smaller caliber of the vessel to retrieve the other thrombus in the right middle cerebral artery as well. Recanalization of the right distal cervical ICA and right middle cerebral artery was achieved six hours after the onset of symptoms and one hour and ten minutes after the patient arrived in the angio room (Figure 2B). CT perfusion study 48 hours after treatment showed no further extension of the ischemic core or hemorrhage transformation. The patient's NIHSS score decreased to 9 and her modified Rankin score was 1 at the three-month follow-up.
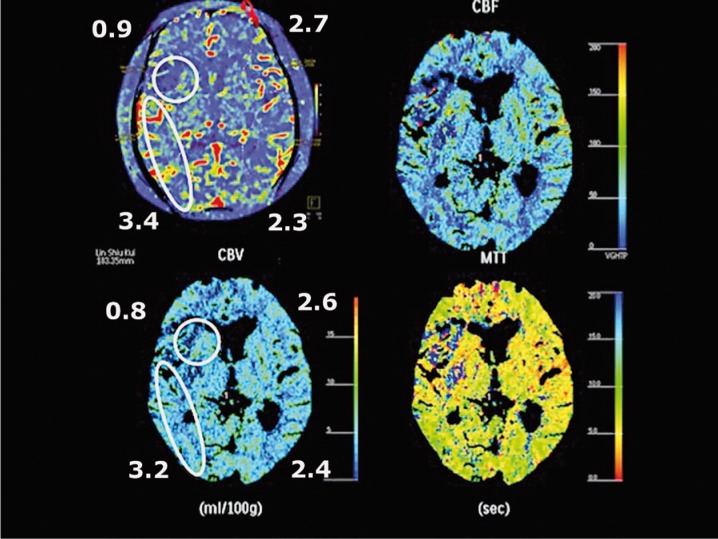
Figure 3.
The cerebral blood volume after recanalization showed consistent findings as compared with CT perfusion study in 48 h. The ischemic core was confined to the original area in the right caudate nucleus and frontal region with no further extension. The original right temporal region which was at risk tissue is adequately perfused with even more collateral circulation, so called re-perfused 'luxury flow'.
Discussion
Perfusion study is critical in the management of acute ischemic stroke, especially to identify patients who have the potential to benefit from intra-arterial thrombolytic therapy beyond the normal three-hour window. Before the application of syngo Neuro PBV IR was available, there was no practical method to monitor the status of brain viability during revascularization procedures. The ability to measure cerebral blood volume (CBV) in the angio room can facilitate the following: first, whenever there is a significant delay between the diagnosis and the beginning of the endovascular procedure; second, whenever the procedure has been too long or unsuccessful, the operator can assess the evolution of the infarct core; third, immediately after finishing the procedure, to access the prognosis factors and to rule out hemorrhage. In this way, follow-up imaging can be delayed to 48 hours instead of having to do an early follow-up. From the hemodynamic points of view, the differences of CT perfusion parameters generated from intra-arterial and intravenous administrations were not significant because both created a bolus from the left ventricles. During the deconvolution process, arrival time does not affect the concentration in relation to time sequence, and thus the values of CBV are comparable. The advantages of intra-arterial contrast administration is to save the contrast use and radiation dose because no fluoroscopy was needed to monitor the optimal starting time point of scan. The disadvantage of intra-arterial contrast administration is the concern of patients' comfort. During the last two years, in vitro and animal studies have proven the feasibility of using flat-panel detectors to acquire volumetric soft-tissue imaging which demonstrates consistent accuracy as compared with conventional CT perfusion study 17. The initial performance applied on the acute revasculization procedures also showed promising results 18,19. As a result, in flat panel CT perfusion imaging, the infarct core in defined as any region of CBV with less than 2 ml/100 g according to the conventional CT perfusion criteria. Although C-arm CT lacks the temporal resolution to assess cerebral blood flow (CBF) or mean transit time (MTT), the penumbra was considered large because CTA and DSA both confirmed the occlusion level at ipislateral proximal ICA without adequate collateral flows. It is so called “angiography–infarction core mismatch 15. The average contrast dose used was 12 to 14 ml for each PBV acquisition. It is acceptable compared with the average use of 100-150 ml contrast in endovascular treament. The radiation dose is 0.6 mSV according to the preliminary results from the manufacturer. The rationale for performing the PBV is to re-assess the progression of ischemic core because the two-hour interval between initial CT perfusion and puncture time. More than 20 Roentgen videodensitometric techniques can measure blood flow and velocity from attenuations changes in digital subtraction angiography in vitro. Some of these techniques can access intraluminal flow quantitatively and display cerebral blood flow in two-dimension ischemic brain models 20. However these techniques typically require computers and are thus time-consuming and not ideal for immediate hemodynamic analysis within the angio room. Further, additional investigation is needed to validate their use with human subjects.
Conclusions
This case illustrates that parenchyma blood volume assessment with a flat-panel detector can accurately estimate infarction core. When combined with angiographic findings and clinical presentation, it can serve as an alternative tool to evaluate the penumbra to facilitate intra-arterial thrombolytic therapy in the angio room without the need to delay treatment while waiting for other diagnostic imaging to be completed.
The shortened door-to-needle time decreases the chance of infarct extension and hemorrhagic transformation, which may lead to better clinical outcomes.
Acknowledgments
This research was co-sponsored by Taipei Veterans General Hospital and Siemens Healthcare (grant number: T1100200).
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Tissue plasminogen activator for acute ischemic stroke The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Reeves MJ, Arora S, Broderick JP, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Albers G, Al-Rawi J, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 5.Asplund K, Glader E-L, Norrving B, et al. Effects of extending the time window of thrombolysis to 4.5 hours: observations in the Swedish stroke register (riks-stroke) Stroke. 2011;42:2492–2497. doi: 10.1161/STROKEAHA.111.618587. [DOI] [PubMed] [Google Scholar]
- 6.Lansberg MG, Thijs VN, Bammer R, et al. The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008;39:2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra N, Albers G, Davis SM, et al. Mismatch-based delayed thrombolysis. A meta-analysis. Stroke. 2010;41:e25–33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- 8.Molina CA, Montaner J, Abillera S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 9.Singe OC, Kurre W, Humpich MC, et al. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40:2743–2748. doi: 10.1161/STROKEAHA.109.550111. [DOI] [PubMed] [Google Scholar]
- 10.Lisboa RC, Jovanovic BD, Alberts MJ. Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke. 2002;33:2866–2871. doi: 10.1161/01.str.0000038987.62325.14. [DOI] [PubMed] [Google Scholar]
- 11.Jakubowska MM, Michels P, Müller-Jensen A, et al. Endovascular treatment in proximal and intracranial carotid occlusion 9 hours after symptom onset. Neuroradiology. 2008;50:599–604. doi: 10.1007/s00234-008-0385-7. [DOI] [PubMed] [Google Scholar]
- 12.Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–3840. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke. final results of the Multi MERCI trial-Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 14.Strother CM, Bender F, Deuerling-Zheng Y, et al. Parametric color coding of digital subtraction angiography. Am J Neuroradiol. 2010;31:919–924. doi: 10.3174/ajnr.A2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61:321–330. doi: 10.1159/000210544. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed AS, Zellerhoff M, Satrother CM, et al. C-arm CT measurement of cerebral blood volume an experimental study in canines. Am J Neuroradiol. 2009;30:917–922. doi: 10.3174/ajnr.A1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Cerebral blood volume imaging by flat detector computed tomography in comparison to conventional multislice perfusion CT. Eur Radiol. 2011;21:882–889. doi: 10.1007/s00330-010-1957-6. [DOI] [PubMed] [Google Scholar]
- 18.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat detector CT in the evaluation of brain parenchyma, intracranial vasculature, and cerebral blood volume: a pilot study in patients with acute symptoms of cerebral ischemia. Am J Neuroradiol. 2010;31:1462–1469. doi: 10.3174/ajnr.A2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struffert T, Deuerling-Zheng Y, Engelhorn T, et al. Feasibility of cerebral blood volume mapping by flat panel detector CT in the angiography suite: first experience in patients with acute middle cerebral artery occlusions. Am J Neuroradiol. 2012;33:618–625. doi: 10.3174/ajnr.A2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shpilfoygel SD, Close RA, Valentino DJ, et al. X-ray videodensitometric methods for blood flow and velocity measurement a critical review of literature. Med Phys. 2000;27:2008–2023. doi: 10.1118/1.1288669. [DOI] [PubMed] [Google Scholar]