Summary
Intracranial vertebral artery dissection (VAD) represents the underlying etiology in a significant percentage of posterior circulation ischemic strokes and subarachnoid hemorrhages. These lesions are particularly challenging in their diagnosis, management, and in the prediction of long-term outcome. Advances in the understanding of underlying processes leading to dissection, as well as the evolution of modern imaging techniques are discussed. The data pertaining to medical management of intracranial VADs, with emphasis on anticoagulants and antiplatelet agents, is reviewed. Surgical intervention is discussed, including, the selection of operative candidates, open and endovascular procedures, and potential complications. The evolution of endovascular technology and techniques is highlighted.
Key words: intracranial, vertebral artery, stroke, hemorrhage, subarachnoid, dissection, dissecting aneurysm, pseudoaneurysm, surgery, endovascular, stent
Introduction
Intracranial vertebral artery dissection (VAD) represents an increasingly realized underlying etiology of ischemic and hemorrhagic intracranial pathology especially in young adults. Complicating the management of intracranial VAD is the development of pseudoaneurysms and the potential for subarachnoid hemorrhage (SAH). Advances in the study of the cellular and biochemical mechanisms of vascular injury have led to an improvement in the understanding of vessel dissection and resultant thromboembolism. Improvements in invasive and non-invasive imaging techniques has led to more rapid diagnoses, enhanced treatment paradigms, and improved long-term management. Multiple controversies related to the medical and surgical management of these traditionally complex lesions currently exist.
A review of the literature reveals a paucity of prospective randomized data pertaining to the management of intracranial VAD. We examine the existing literature with regards to the etiology, pathophysiology, diagnosis, and management of intracranial VAD. Medical management is reviewed with special emphasis on the data regarding anticoagulation and antiplatelet therapy. Furthermore, traditional open surgical management of intracranial VADs is discussed and compared to emerging endovascular interventions.
Epidemiology
Once considered quite rare, vertebral artery dissection (VAD) has now been recognized as a significant source of neurologic morbidity and mortality with an annual incidence of approximately 1-1.5/100,000 1-10. The vertebral artery, which is involved in up to 81.6% of cases, is the most commonly involved artery in spontaneous posterior circulation dissections 11. These dissections are associated with a mortality rate ranging between from 19 to 83% 12. Importantly, VADs represent a significant cause of stroke in the young, accounting for 10-25% of strokes in those aged 25-45 years 13. VADs predominantly affect those patients in the fourth decade of life, an age slightly more advanced than the patient population afflicted with carotid dissections (second and third decades) 3,14,15. Advancing age also appears to be a significant risk factor for the development of VAD-associated ischemia, with those suffering an ischemic stroke averaging an age of 54.6 years 16,17.
Pathophysiology
Multiple etiologies have been implicated in the development of a VAD and these have been associated with inconsistent terminology. VADs begin as a tear in the intimal lining of the vessel, thereby creating a “false lumen” which acts as an alternative conduit for blood flow 2,18-22. Subintimal extravasation of blood or blood between the intima and media result in a true dissection and is the most common form of extracranial dissection 23. Damage to the intimal lining exposes the pro-thrombotic sub-endothelial vessel wall, which may act as a source of thrombus formation and potential embolization 24,25.
If high pressure arterial blood enters the false lumen and dissects through the layers of the tunica media or in between the media and adventitia a dissecting aneurysm is formed 23. An expanding hematoma can accumulates within the vessel wall as pro-thrombotic sub-endothelial vessel wall is exposed to blood. This may result in thrombotic phenomena, parent vessel occlusion due to progressive stenosis or hematoma expansion, or SAH due to extension through the adventitia 1,2,9,12,23,26.
If the dissection continues to involve all three layers of the vessel a pseudoaneurysm may form with subsequent encapsulation of the extravascular hematoma 23. Due to the lack of an external elastic lamina and fewer elastic fibers in the tunica media, intracranial VADs may also present as a hemorrhage, with the pseudoaneurysm wall rupturing into the surrounding subarachnoid space 1,2,9,12,27. Pseudoaneurysms associated with a VAD represent approximately 28% of posterior circulation and 3.3% of all intracranial aneurysms and may rupture acutely or remote from the time of dissection 28-31. Additionally, these lesions represent the underlying causative agent in approximately 10% of all cases of non-traumatic SAH 2,18,26. These pseudoaneurysms have a high propensity for rupture, with series demonstrating up to 73% of pseudoaneurysms presenting with SAH, while only 27% of symptomatic lesions presented with bulbar signs/symptoms or cerebellar ischemia 32.
Anatomical Considerations
The vertebral arteries, which originate as the first branch of the subclavian arteries, most commonly enter the transverse foramina at the level of C6. The arteries then course through the remaining transverse foramina and enter the intracranial compartment at the level of the foramen magnum, piercing the dura prior to their anastomosis at the vertebrobasilar junction (VBJ). Due to their passage through the transverse foramina of the cervical spine, the vertebral arteries, unlike the carotids, are tethered at multiple points along their course, resulting in varying mobility between segments. V1 (origin to C6 transverse foramen) and V3 (C2 transverse foramen to foramen magnum) represent the most mobile segments, while V2 (C6 transverse foramen to C2 transverse foramen) and V4 (foramen magnum to VBJ) are relatively fixed in place. As a result of their mobility, V1 and V3 are most commonly involved in dissections 19. Yamaura et al. 9 further divided the V4 segment into three subdivisions: V4 (1), between C1 and dural penetration, V4 (2), between the dural penetration and the origin of the PICA and V4 (3), between the origin of PICA and the unification with the opposite VA. The authors reported involvement of VA at V4 (3) in 8/19 cases and at V4 (2,3) in 11/19 cases. None of the VADs demonstrated only V4 (1) or V4 (2) involvement in their study.
The left vertebral artery, occurring with an incidence of approximately 80%, is more commonly the dominant of the two vertebral arteries. However, multiple studies have shown the right vertebral artery to be most commonly involved in VADs 33,34. Furthermore, the sidedness of dissection may play an important role in determining the presenting pathology. Bilateral VADs, although significantly less common, have been reported to occur in up to 15% of cases 33-35.
The anatomical and histological classification of intracranial VADs has important clinical implications based on the segment of vessel dissected and the location of the mural hematoma 2. Extracranial VADs tend to result from subintimal extravasation or extravasation between the intima and media. This most commonly results in ischemic phenomena as the pro-thrombotic sub-endothelial vessel wall is exposed to blood.
Intracranial dissection can result in both ischemic phenomena and SAH. Group 1 intracranial VADs which are limited to the V4 segment without involvement of the basilar artery are the most common. These often develop dissecting aneurysms or pseudoaneurysms resulting in SAH. Thrombotic phenomena are also possible as thrombi formed within dissecting aneurysms or pseudoaneurysms may embolize 2,5,6,23. Less commonly VADs can extend to include the basilar artery (Group 2). These commonly result in a subintimal hematoma, thereby leading to signs and symptoms of brainstem ischemia, rather than SAH. With time, these dissections may result in an ischemic stroke or SAH several weeks or months remote from the time of the initial insult 2.
Modifiable and Non-Modifiable Risk Factors for Dissection
Vascular risk factors that contribute to coronary artery disease and stroke have also been found to contribute to the development of VADs. Hypercholesterdemia, smoking, OCPs, a history of migraine, and hypertension have all been linked to VADs 16,35. Hypertension has been reported in as high as 50% of the patients with VADs 33. Smoking may increase the risk of VAD through its pleiotropic effect on vascular homeostasis including platelet activation, inhibition of substance-P-induced tissue plasminogen activator release, impaired vasodilation, and increased inflammation 36-39. These pathologic changes lead to endothelial dysfunction and result in activation of the coagulation cascade, thereby increasing the risk of thrombus formation and embolization.
A thorough review of the literature does not consistently reveal gender predominance for VADs. A male predominance is found in some studies 16,17,27. We found only one study which demonstrated female predominance 40. Due to the protective vascular effects of estrogen, women may potentially possess a lower risk of ischemic disease following VADs when compared to men. This protective effect appears to be lost with aging, as post-menopausal women on hormonal therapy have been shown to be at increased risk of stroke 41. Furthermore, the use of oral contraceptives (OCPs) has been shown to be associated with a higher risk of VADs 16,35.
VADs are associated with connective tissue disorders including Ehler-Danlos Syndrome, Marfan syndrome, polycystic kidney disease, and osteogenesis imperfect type I, fibromuscular dysplasia (FMD), cystic medial necrosis 14,22,35,42,43.
Clinical Presentation
The clinical presentation of intracranial VAD varies greatly and consists of signs and symptoms referable to hemorrhage, ischemia, and cranial neuropathies. The most common association with VAD is recent or remote trauma 44-52. This may include minor trauma and craniocervical manipulation including massage 42,53-55. These seemingly trivial traumas may cause direct injury to the VA or cause focal stretching at the V3/V4 junction, which is fixed around the dura. VAD that occurs at the craniocervical junction may extend both proximally and distally. As a result, patients may present to various practitioners within multiple specialties and with different levels of acuity. Neurosurgeons most often encounter VAD in the setting of a SAH or acute ischemic stroke, while neurologists and primary care physicians commonly diagnose VAD in the work-up of headache and neck pain 6,9,56,57. As many as 8%, are asymptomatic and represent an incidental finding on imaging of unrelated pathology, such as symptomatic internal carotid dissections and sinus thrombosis 17,58,59. Nevertheless, symptomatic patients may complain of nonspecific symptoms, including headache, vertigo, nausea/vomiting, tinnitus, double vision, hearing loss, dysesthesia, and dysphasia 17,60. Headache, one of the most commonly cited complaints following VAD, often fails to be associated with distinguishing features 61. Due to this variability, it is important to maintain a high level of clinical suspicion and to differentiate between hemorrhagic, ischemic, and compressive signs and symptoms.
VAD in the Pediatric Population
Although occurring less frequently than in adults, traumatic VAD has also been reported in the pediatric population 62,63.
As demonstrated by Nguyen et al. 64, the presence of VAD in a child should raise clinical suspicion of child abuse. Within this population, VAD was found to be more common in males (mean age, 8.24 years) and harbor a higher incidence of hemorrhagic presentations. Although there is a paucity of literature pertaining to the management of pediatric VADs, successful conservative management has been reported in a child as young as three months old 65.
Ischemic Presentation
In a large prospective study, as many as 77% of patients diagnosed with VAD presented with findings secondary to ischemia. On admission 67% of these patients had suffered an ischemic stroke, while the additional 10% presented following a transient ischemic attack (TIA) 16,35. Head and/or neck pain was associated with about 88% of patients presenting with ischemia, while 5% of patients complained of pulsatile tinnitus 35. Isolated occipital head and/or neck pain has been reported in up to 12% of patients suffering an ischemic event in the wake of a VAD 16,17,35.
There is significant variability in the severity of the neurologic deficit encountered in patients suffering an ischemic stroke secondary to a VAD. Studies have reported a National Institutes of Health Stroke Score ranging from 1 to 35, with an average admission score of 3.
In patients first suffering a TIA (13%), the median time interval from TIA to stroke onset was 24 hours (range 1h-17 days) 35. Overall, more than 50% of patients with intracranial VAD present with brain stem ischemia. Wallenberg syndrome is commonly seen in patients afflicted with intracranial VAD, being reported in 26%-43% of cases (Figure 1) 40,57,66.
Old age and basilar artery involvement have been shown to be independent predictors of poor outcome in symptomatic intracranial VAD with ischemic presentation 67.
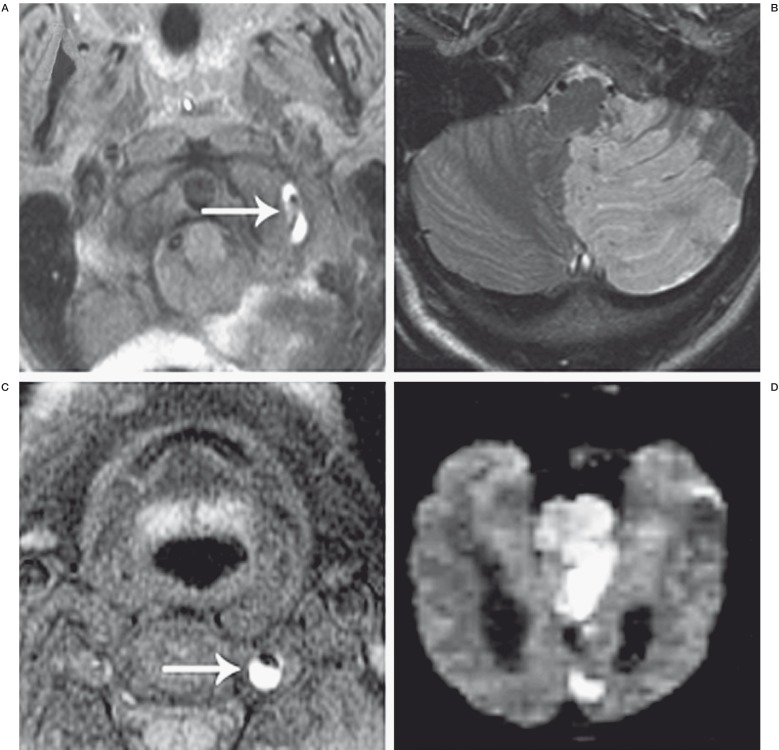
Figure 1.
Ischemic complications of vertebral artery dissection. A 46-year-old woman presented with worsening headaches, nausea, and dizziness one week after the acute onset of left-sided neck pain during chiropractic manipulation. A) Axial T1 fat saturation MRI demonstrates a hyperintense signal surrounding the left vertebral artery (white arrow) indicating dissection. B) Axial FLAIR MRI shows a subacute infarct in the distribution of the left PICA. C) Axial T1 fat saturation MRI in a 32-year-old man who suffered the acute onset of left-sided neck pain without preceding trauma. The left vertebral artery is shown to be dissected, as evidenced by the hyperintense intimal flap and associated thrombus (white arrow). D) Diffusion-weighted MRI demonstrates diffuse acute infarcts in the pons, left occipital lobe, and left mesial temporal lobe. The patient presented intubated and was found to be locked-in on examination.
Subarachnoid Hemorrhage
Whereas extracranial VAD almost exclusively presents with ischemic disease, subarachnoid hemorrhage represents a significant complication of intracranial VAD (Figure 2) 56,68-71. Intracranial pseudoaneurysms associated with VAD frequently rupture and result in massive SAH 2,5,7,12,18,29. Furthermore, these aneurysms have shown a propensity to rebleed, with re-hemorrhage rates ranging from 30 to 58% within 24 hours of the initial bleed 7,12,18,22,34,72. Yamada et al. reported rebleeding in 71.4% of patients within six hours and 93% within 24 hours 34. The vascular healing process is long, starting one week after the rupture which might explain this high recurrent bleeding rate 18. In some cases these aneurysms are incidental findings without clinical evidence of rupture 28,57,73,74. Lee et al. in their review of 28 patients showed that 79% (22/28) of patients presented with SAH and 21% (6/28) patients presented with unruptured aneurysms 33. Lesions were proximal to the PICA in 46.4% (13/28), distal in 39.3% (11/28), and 14.3% (4/28) involved the PICA. Recurrent hemorrhages occurred 18.2% (4/22) of the patients who presented with SAH. Hydrocephalus was identified in 50% (11/22) and 18.2% (4/22) had cerebral and cerebellar infarction.
Imaging Findings
Multiple imaging modalities currently exist to aid in the diagnosis of VAD dissection. Catheter angiography (DSA) remains the “gold standard”, however, the increasing sophistication of non-invasive techniques has improved the reliance on magnetic resonance (MR) imaging, MR angiography (MRA), and computed tomography angiography CTA) 33,35,75.
Invasive Techniques
Using catheter angiography, VAD demonstrates a number of classical findings, including, the pearl and string sign, a double lumen or rosette, or a simple fusiform dilatation of the dissected segment. Angiography may also reveal a delayed clearance of contrast from the false lumen of the dissected vessel 57,73,76-79. In cases of vessel occlusion secondary to dissection, the dissected vessel may exhibit an abrupt or tapered “cut off” beyond which the flow of contrast cannot be visualized 17,32. The pearl and string sign, which is representative of a dilatation adjacent to the narrowed dissected segment, has been demonstrated in as many as 91% of VAD 72. Additional studies have shown the double lumen and pearl and string signs to be the only reliable angiographic findings diagnostic of VAD 9,17,56.
Non-Invasive Techniques
Magnetic Resonance Imaging Techniques
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) offer sensitive non-invasive means by which to detect cervical and intracranial dissections 80. One of the advantages of MRA is the reproducibility of the technique, the uniformity between vendors and the minimal interoperator variation. A recently published study has shown higher accuracy for predicting intracranial VAD with T2-weighted MRI and basi-parallel anatomical scanning (BPAS-MRI) compared to T2-weighted MRI and conventional angiography 75. The diagnosis of VAD generally depends on the demonstration of intramural hematoma and alteration in the caliber of the patent lumen. The shape of intramural hematoma varies, depending on the relationship between the axis of the affected vessel and the imaging plane. The following shapes have been demonstrated: curvilinear, crescentic (circumferential), bamboo-cut, band-line and spotty 81. The signal intensity on T1-weighted, fat suppressed (or “black blood” imaging) images of intramural hematoma changes from isointense/slightly hyperintense in the acute setting to hyperintense in the subacute and back to isointense in chronic settings. Fat suppression is a beneficial component of the imaging sequence in order to aid in discrimination between periaterial atheroma from intramural hematoma 82. Other complementary MRI findings include an identifiable intimal flap on proton density-or T2-weighted images, an overall increase in the diameter of the affected vessel compared with the normal side, a double lumen, and enhancement of the wall and the septum on contrast-enhanced images that are obtained with a 3D spoiled gradient-recalled acquisition in the steady state (SPGR) 17.
On MRA vascular dissection may appear as a tapered or narrowed vessel lumen. Medium to large sized pseudoaneurysms or sacculations are reliably identified with non-contrast MRA 80,83,83. When dissection results in thrombosis of the vessel, it can be difficult to distinguish from occlusion by other etiologies (e.g. atheroma, emboli). In some cases VAD may be a diagnosis of exclusion. Sensitivity and specificity of MRA in VAD is 20% and 100%, respectively and 60% and 98% for MRI, making it a valuable tool for screening, diagnosis, and follow-up 85,86.
Dynamic contrast enhanced MRA (DCE-MRA) is often used to improve visualization of the complex anatomy of the cervical vasculature. A properly timed bolus of MR contrast agent (Gadolinium-DTPA) is imaged using a special rapid technique as it passes through the arterial tree. This provides greatly improved visualization of the entire cerebrovascular circulation; from the aortic arch through the circle of Willis in less than one-minute. Moreover, the areas of anatomy challenging to interrogate with non-contrast MRA are visualized reliably using this technique.
The disadvantage of MRI/MRA is the significantly lower spatial resolution compared to CT angiography or DSA. This is notably apparent with the larger slice partitions used in MRA over CTA. Therefore, subtle or short segment dissections may be difficult to discern reliably. Moreover, the complex anatomy of the V3 and V4 segments may cause artifactual loss of flow in the segments that orient horizontally to the slice plane. This may render key portions of the VA invisible on MRA. In addition, the fat-suppression techniques relied upon for conventional MR imaging to identify subintimal thrombus can fail at the cervico-thoracic and cranio-cervical junctions. This can lower the sensitivity of the method.
Computed Tomographic Imaging
The technical evolution to multi-detector computed tomography (MDCT) has facilitated the development of CT angiography (CTA). These efficient scanners are able to image large segments of the body during the few seconds that intravascular contrast agent floods the arterial tree. CTA evaluation cranio-cervical circulation can be obtained in less than thirty seconds. The reconstructed images are of angiographic quality. Moreover, unlike catheter angiography, an unlimited number of projections can be obtained from one data acquisition. This allows the physician the luxury of interrogating the vasculature off-line without the need for additional contrast, radiation or catheterizations. CTA is particularly beneficial as an emergent screening method for instances of suspected VAD. The combination of diagnosing hemorrhage or early evidence of ischemia by non-contrast CT and the underlying vascular pathology on CTA results in significant reductions in time to treatment, morbidity and cost.
Modern CTA reliably demonstrates many of the findings seen on traditional DSA, including changes in vessel caliber, false lumens, fusiform dilatations, and pseudoaneurysms 84,87. The most reliable criterion for the diagnosis of acute arterial dissection is likely a narrowed, eccentric lumen in association with enlargement of the overall diameter of the dissected artery from mural thickening (compared to contralateral side) 88. Zenteno et al. summarized CTA and MRA findings in their series as 1) a focal dilatation 2) an intimal flap 3) increase in the outer diameter of the vessel and 4) narrowing or an occlusion of the vessel lumen 72. Vertinsky et al. 89 compared the sensitivity of CTA and MRA in 25 VAD and found that CTA excelled in identification of intimal flaps, pseudoaneurysms and high grade stenoses. Lum et al. showed that alteration in wall thickness on CTA is a more sensitive criterion than change in luminal diameter in VAD 90. A meta-analysis of 91 articles that compared the performance of MRI, MRA and CTA with DSA for detection of carotid and vertebral artery injury found that the reported sensitivity and specificity for CTA and MRA are fairly similar 91.
Limitations of Imaging
It must be realized that arterial dissection represents a dynamic condition that may demonstrate spontaneous improvement, gradual progression, or immediate deterioration, as is seen in the growth of pseudoaneurysms and aneurysmal rupture 57,92. As a result, no single imaging study is definitive, and arterial dissections must be followed radiographically until resolved or stable for a prolonged period of time 73,87. Although catheter angiography is still considered to be the gold standard in neurovascular imaging, it has the intrinsic limitations of being operator dependent, invasive, carrying an inherent risk of stroke or vascular injury, and a substantially larger contrast load than CT angiography is required. Clinical guidelines generally recommend prudent follow-up and evaluation of VAD using non-invasive means.
Spontaneous resolution of VAD is well-documented within the literature and the incidence of spontaneous thrombosis of the pseudo-lumen may not be uncommon. In such instances, imaging may fail to detect an intramural hematoma due to the observation of the thrombosed wall within the subacute phase 17. The variable most critical to the detection of dissection on MRI/A is the amount of time elapsed between symptom onset and imaging 17. The sensitivity of both angiography and MRI for the detection of a dissection or intramural hematoma is limited in patients presenting less than a week or over one month out from the onset of symptoms 17,83,84.
In summary, significant advances have been made in non-invasive imaging techniques for the diagnosis of intracranial vertebral artery dissections. Definitive diagnosis is often made with a combination of non-invasive imaging and/or DSA. Non-invasive imaging techniques play an essential role in follow-up imaging of VAD.
Management of Intracranial VAD
Medical Management
A large number of patients presenting with ischemic symptoms referable to a VAD may be managed medically. The Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group published the most comprehensive review of the existing literature in regards to the medical management of dissections 24,93. As a result, the practice guidelines developed from this data largely represent the current medical management paradigms for VAD. A thorough review of the literature supports thromboembolism rather than hypo-perfusion, as the primary etiology of ischemia in the wake of a VAD 94. Multiple non-invasive imaging modalities, including, CT and MRI, exhibit infarct patterns consistent with thromboembolism 24. Additionally, angiographic data demonstrates branch occlusion of the intracranial vasculature distal to the dissected vessel suggestive of thromboembolic phenomena 24. Some authors recommend refraining from anticoagulant treatment in all intracranial dissections or to perform lumbar puncture to rule out SAH prior to initiation of anticoagulant 95-98. This approach has not been, evaluated in randomized trials. In a study by Arnold et al. 35 the majority of patients of intracranial VADs were treated with Aspirin 300 mg per day for three to six months and stopped when evidence of recanalization was found on imaging. This stands in contrast to the majority of patients with spontaneous extracranial VAD who were usually treated with intravenous heparin followed by oral warfarin with a target international normalization ratio (INR) of 2-3 for three to six months. Favorable outcomes were achieved in 82% of patients treated with Aspirin, 77% for Aspirin/Warfarin, and 8% for Heparin/Warfarin. Although controversial, Metso et al. reported favorable outcomes (79% modified Rankin Scale, 0-2) in patients treated with a heparin to warfarin bridge for non-aneurysmal intracranial VADs 99. After three months of warfarin therapy, the authors found only one de novo pseudo-aneurysm on follow-up imaging, while no patient suffered an intracranial hemorrhage during the period of anticoagulation.
There have been limited studies to assess outcomes of patients with intracranial VAD who were monitored without intervention 100,101. In the largest study by Mizutani, 155 of 190 patients with intracranial dissections involved the vertebral artery. In the 54 unruptured cases presenting with infarction 23 were treated with antiplatelet or anticoagulant, and the unruptured IADs without infarction were followed with blood pressure control. In the follow-up with 93 patients with unruptured intracranial artery dissection (IAD), one patient died to rupture of a dilated IAD and one patient died of brainstem infarction due to a basilar artery dissection. Three patients received surgical treatment due to IAD that enlarged within the first three months. Interestingly, dissections recurred in 18 of 190 patients in different arteries from the first dissection (9.5%) 100.
In summary, the existing data derived from multiple clinical trials has been inconclusive, in part due to a lack of prospective randomized analysis, variable medical and surgical treatment algorithms, and differences in rates of treatment-associated hemorrhage 80,102. Furthermore, specific clinical scenarios, such as recurrent thromboembolic events on antiplatelet agents and complete vascular occlusion, may require further consideration of anticoagulation or endovascular or surgical intervention. In patients with non-SAH VAD, we recommend angiography; in patients without pseudoaneurysms, dissecting aneurysms, or significant stenosis, patients are treated with antiplatelet therapy. In patients with continued thromboembolic symptoms despite antiplatelet therapy, we recommend endovascular or surgical intervention and anticoagulation when patients cannot be treated. In patients with dissecting aneurysms, pseudoaneurysms, significant stenosis, or dissection associated with SAH, we recommend endovascular or surgical intervention. As the best medical therapy remains unclear, a randomized controlled trial is needed and ethically justified, but a large sample size would be necessary making this a tremendous venture.
Surgical Intervention and the Management of VAD
Open Surgical Intervention
The vast majority of these cases whereby operative management is indicated present as true neurosurgical emergencies and, include hemodynamic insufficiency secondary to severe stenosis or occlusion of the dissected vertebral artery, subarachnoid hemorrhage, or new onset cranial neuropathy due to an expanding pseudoaneurysm. SAH caused by dissection, dissecting aneurysms, or pseudoaneurysms may carry a rerupture as high 70% within the first six hours and 93% in first 24 hours from presentation, making prompt vascular treatment a surgical emergency 18,34. Traditionally, these instances could only be addressed with open surgical intervention, consisting of proximal ligation of the dissected vessel, clipping or wrapping of associated pseudoaneurysms, and extracranial to intracranial bypass. These procedures, although effective in re-establishing blood flow, require extended operative time for exposure of the diseased vessel, microsurgical techniques, and careful manipulation of the dissected artery during anastomosis. In the setting of acute hypoperfusion and evolving infarct or SAH, such extensive and time-consuming procedures were of limited utility. Furthermore, these open interventions have been shown to carry relatively high rates of morbidity and mortality 22,103.
Surgical procedures performed for the management of intracranial VAD include proximal clipping, clipping of the aneurysm and trapping. Takemoto et al. 104 reported surgical treatment of 14 patients. Patients presented with hemorrhage (5/14), ischemia (8/14) and headache (1/14). Internal trapping was performed in hemorrhagic patients with severe Glasgow Outcome Scale (GOS). Proximal trapping, internal trapping and bypass were performed in the ischemic group with good outcome. Katsuno et al. 105 presented a case of rupture of VAD when trapping was performed on the contralateral VA aneurysm. VA trapping has shown better results in prevention of recurrent bleeding compared to proximal ligation of VA or wrapping of the aneurysm 1,6,7,18.
Treatment of VA dissection involving the PICA is still controversial and challenging. If trapping is performed for these lesions, revascularization of the PICA such OA-PICA anastomosis and side-to-side PICA anastomosis may be required. If these options cannot be performed, then a VA-PICA anastomosis using a radial artery or saphenous vein graft is possible 106,107.
Endovascular Approach
The evolution of endovascular intervention has provided effective alternative means by which to treat these complex lesions, with reports of favorable outcomes as high as 89% 33,108,109. Endovascular therapy offers the clear advantages of dramatically reduced operative time, minimally invasive technique, and elimination of donor vessel preparation for bypass procedures 80. The utilization of angiography can establish a diagnosis, assess collateral blood flow, and allows for immediate intervention 1,7,29,73. In patients with ischemic symptoms, endovascular procedures may employ the use of antiplatelet agents and systemic anticoagulation to minimize thromboembolic complications that are precluded during microsurgical procedures 7. Finally, when intracranial VAD occurs in the setting of multisystem trauma, endovascular therapy provides access to the diseased vessel at a site remote from additional severe injuries to the chest, neck, and head. VAD presenting with SAH can be successfully treated with endovascular approach, but anticoagulation and antiplatelet therapy must be used more cautiously 110.
Traditional therapies largely consisted of vessel sacrifice with permanent occlusion of the diseased vessel segment (Figure 3). More recent treatments include the use of detachable coils, detachable balloons, liquid embolic agents such as Onyx to completely eliminate blood flow across the diseased segment or obliterate dissecting aneurysms or pseudoaneurysms 1,4,18,74,78,111-116. Prior to complete exclusion of the vessel from the circulation, a diagnostic angiogram is performed to evaluate for the presence of collateral circulation, determine the length of the dissected segment, and identify vertebral artery dominance. A balloon occlusion test is also recommended when possible.
Long-term follow-up of the patients treated with parent vessel sacrifice has often revealed acceptable results, yet these interventions are not performed without significant complications 4,78,117,118. Often these complications stem from failure to completely occlude the vessel at the time of initial treatment, coil compaction and subsequent recanalization, or the use of antiplatelet therapy for the prevention of postoperative thromboembolism.
With the development of increasingly sophisticated stent and balloon technology, the goal of endovascular surgery has often shifted from vessel sacrifice to one of vessel/endoluminal reconstruction, particularly in the setting of ischemic disease, fusiform dilatations, and wide-necked pseudoaneurysms 31,33,12,18,78,115,119-123. Stent deployment offers the advantages of maintained blood flow throughout the course of the procedure and the ability to tack down the intimal flap against the vessel wall, thereby eliminating a source of thromboembolism 124. Multiple studies have demonstrated successful obliteration of pseudoaneurysms (up to 91%) and restoration of the normal vessel caliber solely using stent reconstruction 72. Unfortunately, the time interval from stenting to complete occlusion is unpredictable, thereby exposing patients to continued risk of hemorrhage or thromboembolism until complete obliteration occurs.
Further endovascular techniques have developed to try to preserve parent vessels through vessel reconstruction and flow diversion. Stent-within-stent techniques provide more metal coverage across the dissection thereby causing significant stagnation of flow within the aneurysm and eventual thrombosis and exclusion from the circulation 30,108,125. Shin et al. 124 reported the successful treatment of seven VAD with single, multiple, and double stent (stent-within-stent) techniques (Figure 3). Park et al. found that authors found complete or partial obliteration was more frequent in the multiple stent (Figure 2B) 126.
Most recently through the use of the Pipeline stent (ev3 Neurovascular, Irvine, CA, USA) has been used for endoluminal reconstruction using flow diversion 127-130. Similar to the stent-within-stent technique, the Pipeline stent has more metal coverage allowing for eventual thrombosis and exclusion from the circulation. This stent is designed to occlude aneurysms through increased metal coverage, but uphold flow through perforating vessels. Preliminary studies with dissections, dissecting aneurysms, and pseudoaneurysms have been favorable 127-130, but perforator vessel flow may not always be preserved 131, and further long-term studies are necessary.
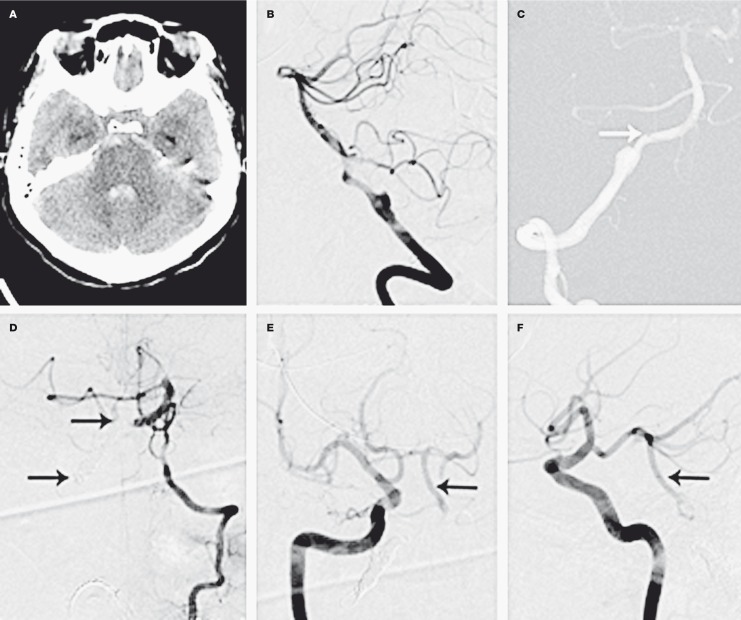
Figure 2.
Hemorrhagic complications of vertebral artery dissection. A 65-year-old woman presented following the acute onset of the worst headache of her life. A) Noncontrast axial head CT demonstrating subarachnoid hemorrhage and intraventricular hemorrhage within the fourth ventricle. B) Right vertebral artery injection revealing a dissection and associated ruptured pseudoaneurysm. C) The tip of the microcatheter (white arrow) parked within the right vertebral artery. Coil deposition is begun distal to the aneurysm and proximal to the vertebrobasilar junction. D) Control angiogram from the nondominant left vertebral artery demonstrates some filling of the basilar artery. The black arrows denote the coil mass used to trap the dissecting aneurysm and occlude the right vertebral artery. E,F) Control angiograms from the right ICA demonstrate a large right posterior communicating artery filling the distal basilar artery and basilar trunk (black arrows).
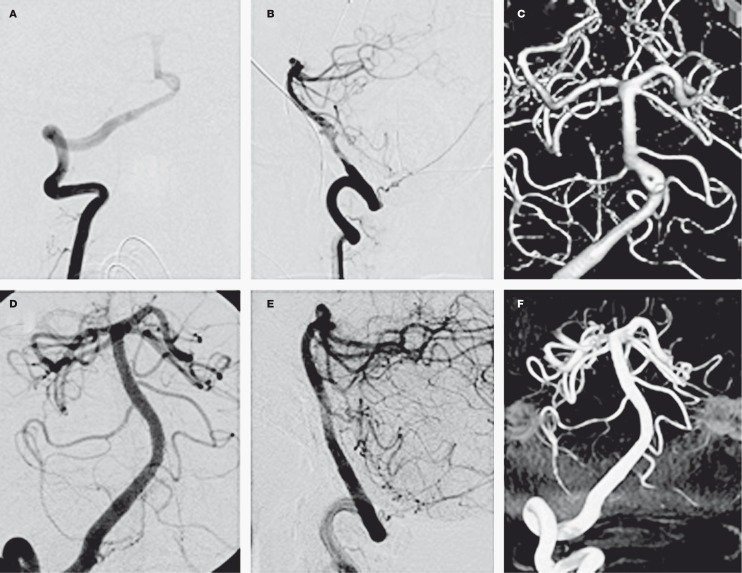
Figure 3.
Treatment of a vertebral artery dissection with a self-expanding stent. A,B) AP and lateral projections revealing a dissection and associated pseudoaneurysm of the dominant right vertebral artery. C) Three-dimensional reconstruction. D,E) AP and lateral projections showing the right vertebral artery status post stenting. The intimal flap has been trapped by the stent, thereby restoring the normal caliber of the vessel lumen. The pseudoaneurysm is no longer visible secondary to diversion of flow. F) Three-dimensional reconstruction post-stenting.
Conclusions
Intracranial VAD is an important cause of morbidity and mortality. These lesions present with variable signs and symptoms which need to be readily identified to prevent complications such as recurrent hemorrhage or ischemia. Improvement in the noninvasive diagnostic techniques has allowed early detection and treatment of this condition. Management is based on clinical presentation, the location of the dissection, and the presence of associated pseudoaneurysms. In the setting of acute hemorrhage, early intervention is critical in order to prevent significant morbidity and mortality associated with rehemorrhage. With an ischemic presentation, early medical management may be started, and close clinical and radiological follow-up is mandatory. Endovascular intervention is reserved for patients with refractory ischemia, severe stenosis, diminished collateral supply to the vascular territory at risk, and associated dissecting aneurysms and pseudoaneurysms. Surgical intervention is often necessary in the treatment of complex dissections and those with associated dissecting aneurysms and pseudoaneurysms. The complexity of these lesions warrants transfer of patient to centers where care can be carried out by a multimodality team trained in neurocritical care, endovascular therapy, and microsurgery.
Disclosure
Industry affiliations: Pascal Jabbour: Consultant for ev3, Codman, Mizuho; Stavropoula Tjoumakaris: Consultant for Stryker; L. Fernando Gonzalez: Consultant for ev3; Aaron S. Dumont: Consultant for ev3; Robert Rosenwasser: Consultant for Boston Scientific. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Halbach VV, Higashida RT, Dowd CF, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993;79:183–191. doi: 10.3171/jns.1993.79.2.0183. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki O, Ogawa H, Koike T, et al. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75:874–882. doi: 10.3171/jns.1991.75.6.0874. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 4.Yamaura I, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg. 1999;90:853–856. doi: 10.3171/jns.1999.90.5.0853. [DOI] [PubMed] [Google Scholar]
- 5.Yasui T, Komiyama M, Nishikawa M, et al. Fusiform vertebral artery aneurysms as a cause of dissecting aneurysms. Report of two autopsy cases and a review of the literature. J Neurosurg. 1999;91:139–144. doi: 10.3171/jns.1999.91.1.0139. [DOI] [PubMed] [Google Scholar]
- 6.Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg. 1984;60:325–334. doi: 10.3171/jns.1984.60.2.0325. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani T, Aruga T, Kirino T, et al. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905–911. doi: 10.1227/00006123-199505000-00003. discussion 912-903. [DOI] [PubMed] [Google Scholar]
- 8.Tsukahara T, Wada H, Satake K, et al. Proximal balloon occlusion for dissecting vertebral aneurysms accompanied by subarachnoid hemorrhage. Neurosurgery. 1995;36:914–919. doi: 10.1227/00006123-199505000-00004. discussion 919-920. [DOI] [PubMed] [Google Scholar]
- 9.Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183–188. doi: 10.3171/jns.1990.72.2.0183. [DOI] [PubMed] [Google Scholar]
- 10.Sano H, Kato Y, Okuma I, et al. Classification and treatment of vertebral dissecting aneurysm. Surg Neurol. 1997;48:598–605. doi: 10.1016/s0090-3019(97)00022-0. [DOI] [PubMed] [Google Scholar]
- 11.Huang YC, Chen YF, Wang YH, et al. Cervicocranial arterial dissection: experience of 73 patients in a single center. Surg Neurol. 2009;72(Suppl 2):S20–27. doi: 10.1016/j.surneu.2008.10.002. discussion S27. [DOI] [PubMed] [Google Scholar]
- 12.Ramgren B, Cronqvist M, Romner B, et al. Vertebrobasilar dissection with subarachnoid hemorrhage: a retrospective study of 29 patients. Neuroradiology. 2005;47:97–104. doi: 10.1007/s00234-005-1346-z. [DOI] [PubMed] [Google Scholar]
- 13.Ducrocq X, Lacour JC, Debouverie M, et al. Cerebral ischemic accidents in young subjects. A prospective study of 296 patients aged 16 to 45 years. Rev Neurol (Paris) 1999;155:575–582. [PubMed] [Google Scholar]
- 14.Schievink WI, Wijdicks EF, Michels VV, et al. Heritable connective tissue disorders in cervical artery dissections: a prospective study. Neurology. 1998;50:1166–1169. doi: 10.1212/wnl.50.4.1166. [DOI] [PubMed] [Google Scholar]
- 15.Thanvi B, Munshi SK, Dawson SL, et al. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383–388. doi: 10.1136/pgmj.2003.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold M, Kurmann R, Galimanis A, et al. Differences in demographic characteristics and risk factors in patients with spontaneous vertebral artery dissections with and without ischemic events. Stroke. 2010;41:802–804. doi: 10.1161/STROKEAHA.109.570655. [DOI] [PubMed] [Google Scholar]
- 17.Hosoya T, Adachi M, Yamaguchi K, et al. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke. 1999;30:1083–1090. doi: 10.1161/01.str.30.5.1083. [DOI] [PubMed] [Google Scholar]
- 18.Albuquerque FC, Fiorella DJ, Han PP, et al. Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus. 2005;18:E3. [PubMed] [Google Scholar]
- 19.Fratkin JD ZA. A pathological spectrum: Basilar dolichoectasia and vertebral dissection, each with fatal subarachnoid hemorrhage. Semin Cerebrovasc Dis Stroke. 2044;4:69–75. [Google Scholar]
- 20.Mizutani T, Kojima H, Asamoto S, et al. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001;94:712–717. doi: 10.3171/jns.2001.94.5.0712. [DOI] [PubMed] [Google Scholar]
- 21.Yasui T, Komiyama M, Nishikawa M, et al. Subarachnoid hemorrhage from vertebral artery dissecting aneurysms involving the origin of the posteroinferior cerebellar artery: report of two cases and review of the literature. Neurosurgery. 2000;46:196–200. discussion 200-191. [PubMed] [Google Scholar]
- 22.Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke. 1994;25:889–903. doi: 10.1161/01.str.25.4.889. [DOI] [PubMed] [Google Scholar]
- 23.Yamaura A. Nontraumatic Intracranial arterial dissection: natural history, diagnosis, and treatment. Contemp. Neurosurg. 1994;16:1. [Google Scholar]
- 24.Engelter ST BT, Debette S, et al. Antiplatelets verus anticoagulation in cervical artery dissection. Stroke. 2007;38:2605–2611. doi: 10.1161/STROKEAHA.107.489666. [DOI] [PubMed] [Google Scholar]
- 25.Anson J, Crowell RM. Cervicocranial arterial dissection. Neurosurgery. 1991;29:89–96. doi: 10.1097/00006123-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Ro A, Kageyama N, Abe N, et al. Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage: clinical and histopathological investigations from a medicolegal perspective. J Neurosurg. 2009;110:948–954. doi: 10.3171/2008.11.JNS08951. [DOI] [PubMed] [Google Scholar]
- 27.Ro A, Kageyama N, Takatsu A, et al. Differential diagnosis between traumatic and nontraumatic rupture of the intracranial vertebral artery in medicolegal autopsy. Leg Med (Tokyo) 2009;11(Suppl 1):S66–S70. doi: 10.1016/j.legalmed.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 28.de Bray JM, Penisson-Besnier I, Dubas F, et al. Extracranial and intracranial vertebrobasilar dissections: diagnosis and prognosis. J Neurol Neurosurg Psychiatry. 1997;63:46–51. doi: 10.1136/jnnp.63.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lylyk P, Cohen JE, Ceratto R, et al. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001;94:427–432. doi: 10.3171/jns.2001.94.3.0427. [DOI] [PubMed] [Google Scholar]
- 30.Mehta B, Burke T, Kole M, et al. Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. Am J Neuroradiol. 2003;24:1814–1818. [PMC free article] [PubMed] [Google Scholar]
- 31.Wakhloo AK, Lanzino G, Lieber BB, et al. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery. 1998;43:377–379. doi: 10.1097/00006123-199808000-00126. [DOI] [PubMed] [Google Scholar]
- 32.Santos-Franco JA, Zenteno M, Lee A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg Rev. 2008;31:131–140. doi: 10.1007/s10143-008-0124-x. discussion 140. [DOI] [PubMed] [Google Scholar]
- 33.Lee JW, Jung JY, Kim YB, et al. Spontaneous dissecting aneurysm of the intracranial vertebral artery: management strategies. Yonsei Med J. 2007;48:425–432. doi: 10.3349/ymj.2007.48.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada M, Kitahara T, Kurata A, et al. Intracranial vertebral artery dissection with subarachnoid hemorrhage: clinical characteristics and outcomes in conservatively treated patients. J Neurosurg. 2004;101:25–30. doi: 10.3171/jns.2004.101.1.0025. [DOI] [PubMed] [Google Scholar]
- 35.Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37:2499–2503. doi: 10.1161/01.STR.0000240493.88473.39. [DOI] [PubMed] [Google Scholar]
- 36.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 37.Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 38.Newby DE, Wright RA, Labinjoh C, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 39.Rubenstein D, Jesty J, Bluestein D Differences between mainstream and sidestream cigarette smoke extracts and nicotine in the activation of platelets under static and flow conditions. 2004. 2004;109:78–83. doi: 10.1161/01.CIR.0000108395.12766.25. [DOI] [PubMed] [Google Scholar]
- 40.Mokri B, Houser OW, Sandok BA, et al. Spontaneous dissections of the vertebral arteries. Neurology. 1988;38:880–885. doi: 10.1212/wnl.38.6.880. [DOI] [PubMed] [Google Scholar]
- 41.Bath PM, Gray LJ. Association between hormonal replacement therapy and subsequent stroke: a meta-analysis. Br Med J. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris JW, Beletsky V, Nadareishvili G. Sudden neck movement and cervical artery dissection. The Canadian Stroke Consortium. CMAJ. 2000;163:38–40. [PMC free article] [PubMed] [Google Scholar]
- 43.Schievink WI, Bjornsson J, Piepgras DG. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan’s syndrome and bilateral carotid artery dissections. Stroke. 1994;25:2492–2496. doi: 10.1161/01.str.25.12.2492. [DOI] [PubMed] [Google Scholar]
- 44.Adaletli I, Sirikci A, Ulus S, et al. Traumatic bilateral vertebral artery dissection at the dural entry point site in a 10-year-old Boy. Pediatr Surg Int. 2006;22:468–470. doi: 10.1007/s00383-006-1640-9. [DOI] [PubMed] [Google Scholar]
- 45.Akay KM, Izci Y, Ugurel S, et al. [Traumatic dissection of bilateral vertebral arteries]. Ulus Travma Acil Cerrahi Derg. 2003;9:72–75. [PubMed] [Google Scholar]
- 46.Bartels E, Knauth M, Liebetanz D, et al. Traumatic dissection of the vertebral artery-value of sonographic diagnostics. Cerebrovasc Dis. 2006;22:209–213. doi: 10.1159/000093811. [DOI] [PubMed] [Google Scholar]
- 47.Bowen J, Patz J, Bailey J, et al. Dissection of vertebral artery after cervical trauma. Lancet. 1992;339:435–436. [PubMed] [Google Scholar]
- 48.Cals N, Devuyst G, Jung DK, et al. Uncommon ultrasound findings in traumatic extracranial vertebral artery dissection. Eur J Ultrasound. 2001;12:227–231. doi: 10.1016/s0929-8266(00)00114-2. [DOI] [PubMed] [Google Scholar]
- 49.DeBehnke DJ, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12:27–31. doi: 10.1016/0736-4679(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 50.Galtés I, Borondo JC, Cos M, et al. Traumatic bilateral vertebral artery dissection. Forensic Sci Int. 2012;214:e12–15. doi: 10.1016/j.forsciint.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Lleva P, Ahluwalia BS, Marks S, et al. Traumatic and spontaneous carotid and vertebral artery dissection in a level 1 trauma center. J Clin Neurosci. 2012;19:1112–1114. doi: 10.1016/j.jocn.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159–160. doi: 10.1016/S0140-6736(05)75289-2. [DOI] [PubMed] [Google Scholar]
- 53.Chakrapani AL, Zink W, Zimmerman R, et al. Bilateral carotid and bilateral vertebral artery dissection following facial massage. Angiology. 2008;59:761–764. doi: 10.1177/0003319707309653. [DOI] [PubMed] [Google Scholar]
- 54.Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1:155–182. [PubMed] [Google Scholar]
- 55.Fisher CM, Ojemann RG, Roberson GH. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci. 1978;5:9–19. [PubMed] [Google Scholar]
- 56.Yonas H, Agamanolis D, Takaoka Y, et al. Dissecting intracranial aneurysms. Surg Neurol. 1977;8:407–415. [PubMed] [Google Scholar]
- 57.Shimoji T, Bando K, Nakajima K, et al. Dissecting aneurysm of the vertebral artery. Report of seven cases and angiographic findings. J Neurosurg. 1984;61:1038–1046. doi: 10.3171/jns.1984.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 58.Kim CH, Son YJ, Paek SH, et al. Clinical analysis of vertebrobasilar dissection. Acta Neurochir (Wien) 2006;148:395–404. doi: 10.1007/s00701-006-0742-4. [DOI] [PubMed] [Google Scholar]
- 59.Hagiwara N, Kamouchi M, Inoue T, et al. Dissection of bilateral intracranial vertebral artery with basilar artery involvement: a case report of a patient free from neurological deficits. Intern Med. 2007;46:1467–1470. doi: 10.2169/internalmedicine.46.0210. [DOI] [PubMed] [Google Scholar]
- 60.Shibata K, Matsui K, Ito H, et al. Bilateral intracranial vertebral artery dissection presenting as sudden bilateral hearing loss. Clin Neurol Neurosurg. 2012;114:1266–1269. doi: 10.1016/j.clineuro.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 61.Manabe H, Yonezawa K, Kato T, et al. Incidence of intracranial arterial dissection in non-emergency outpatients complaining of headache: preliminary investigation with MRI/MRA examinations. Acta Neurochir Suppl. 2010;107:41–44. doi: 10.1007/978-3-211-99373-6_6. [DOI] [PubMed] [Google Scholar]
- 62.Tekin S, Aykut-Bingol C, Aktan S. Case of intracranial vertebral artery dissection in young age. Pediatr Neurol. 1997;16:67–70. doi: 10.1016/s0887-8994(96)00252-4. [DOI] [PubMed] [Google Scholar]
- 63.Songsaeng D, Srivatanakul K, Krings T, et al. Symptomatic spontaneous vertebrobasilar dissections in children: review of 29 consecutive cases. J Neurosurg Pediatr. 2010;6:233–243. doi: 10.3171/2010.6.PEDS09290. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen PH, Burrowes DM, Ali S, et al. Intracranial vertebral artery dissection with subarachnoid hemorrhage following child abuse. Pediatr Radiol. 2007;37:600–602. doi: 10.1007/s00247-007-0479-0. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Orbach DB. Traumatic dissecting aneurysm at the vertebrobasilar junction in a 3-month-old infant evaluation and treatment strategies. Case report. J Neurosurg Pediatr. 2008;1:415–419. doi: 10.3171/PED/2008/1/5/415. [DOI] [PubMed] [Google Scholar]
- 66.Sturzenegger M. [Vertebral artery dissection. Clinical aspects, non-invasive diagnosis, therapy: observations in 14 patients] Nervenarzt. 1994;65:402–410. [PubMed] [Google Scholar]
- 67.Kim BM, Kim SH, Kim DI, et al. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology. 2011;76:1735–1741. doi: 10.1212/WNL.0b013e31821a7d94. [DOI] [PubMed] [Google Scholar]
- 68.Chiche L, Praquin B, Koskas F, et al. Spontaneous dissection of the extracranial vertebral artery: indications and long-term outcome of surgical treatment. Ann Vasc Surg. 2005;19:5–10. doi: 10.1007/s10016-004-0149-8. [DOI] [PubMed] [Google Scholar]
- 69.Grosman H, Fornasier VL, Bonder D, et al. Dissecting aneurysm of the cerebral arteries. Case report. J Neurosurg. 1980;53:693–697. doi: 10.3171/jns.1980.53.5.0693. [DOI] [PubMed] [Google Scholar]
- 70.Manz HJ, Luessenhop AJ. Dissecting aneurysm of intracranial vertebral artery: case report and review of literature. J Neurol. 1983;230:25–35. doi: 10.1007/BF00313594. [DOI] [PubMed] [Google Scholar]
- 71.Price RF, Sellar R, Leung C, et al. Traumatic vertebral arterial dissection and vertebrobasilar arterial thrombosis successfully treated with endovascular thrombolysis and stenting. Am J Neuroradiol. 1998;19:1677–1680. [PMC free article] [PubMed] [Google Scholar]
- 72.Zenteno MA, Santos-Franco JA, Freitas-Modenesi JM, et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg. 2008;108:1104–1118. doi: 10.3171/JNS/2008/108/6/1104. [DOI] [PubMed] [Google Scholar]
- 73.Joo JY, Ahn JY, Chung YS, et al. Treatment of intra- and extracranial arterial dissections using stents and embolization. Cardiovasc Intervent Radiol. 2005;28:595–602. doi: 10.1007/s00270-004-0199-x. [DOI] [PubMed] [Google Scholar]
- 74.Amagasaki K, Yagishita T, Yagi S, et al. Serial angiography and endovascular treatment of dissecting aneurysms of the anterior cerebral and vertebral arteries. Case report. J Neurosurg. 1999;91:682–686. doi: 10.3171/jns.1999.91.4.0682. [DOI] [PubMed] [Google Scholar]
- 75.Katsuno M, Kobayashi S. Diagnosis of vertebral artery dissection with basiparallel anatomical scanning magnetic resonance imaging. J Nihon Med Sch. 2011;78:367–373. doi: 10.1272/jnms.78.367. [DOI] [PubMed] [Google Scholar]
- 76.Ahn JY, Han IB, Kim TG, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. Am J Neuroradiol. 2006;27:1514–1520. [PMC free article] [PubMed] [Google Scholar]
- 77.Drapkin AJ. The double lumen: a pathognomonic angiographic sign of arterial dissection? Neuroradiology. 2000;42:203–205. doi: 10.1007/s002340050046. [DOI] [PubMed] [Google Scholar]
- 78.Hamada J, Kai Y, Morioka M, et al. Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg. 2003;99:960–966. doi: 10.3171/jns.2003.99.6.0960. [DOI] [PubMed] [Google Scholar]
- 79.Shin JH, Suh DC, Choi CG, et al. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics. 2000;20:1687–1696. doi: 10.1148/radiographics.20.6.g00nv081687. [DOI] [PubMed] [Google Scholar]
- 80.Amenta P, Jabbour P, Rosenwasser R. Approaches to extracranial and intracranial dissections. In: Bendok BR, Batjer HH, Naidech R, Walker MT, editors. Hemorrhagic and ischemic stroke: surgical, interventional, imaging, and medical approaches. New York: Thieme; 2011. [Google Scholar]
- 81.Kitanaka C, Tanaka J, Kuwahara M, et al. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke. 1994;25:571–575. doi: 10.1161/01.str.25.3.571. [DOI] [PubMed] [Google Scholar]
- 82.Bousson V, Levy C, Brunereau L, et al. Dissections of the internal carotid artery: three-dimensional time-of-flight MR angiography and MR imaging features. Am J Roentgenol. 1999;173:139–143. doi: 10.2214/ajr.173.1.10397114. [DOI] [PubMed] [Google Scholar]
- 83.Boet R WH, Yu SCH, et al. Vertebrobasilar artery dissection: current practice. HKMJ. 2002;8:33–38. [PubMed] [Google Scholar]
- 84.Provenzale JM, Barboriak DP, Taveras JM. JM. Exercise-related dissection of craniocervical arteries: CT, MR, and angiographic findings. J Comput Assist Tomogr. 1995;19:268–276. doi: 10.1097/00004728-199503000-00019. [DOI] [PubMed] [Google Scholar]
- 85.Mascalchi M, Bianchi MC, Mangiafico S, et al. MRI and MR angiography of vertebral artery dissection. Neuroradiology. 1997;39:329–340. doi: 10.1007/s002340050418. [DOI] [PubMed] [Google Scholar]
- 86.Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology. 1994;190:97–103. doi: 10.1148/radiology.190.1.8259436. [DOI] [PubMed] [Google Scholar]
- 87.Kraus RR, Bergstein JM, DeBord JR. Diagnosis, treatment, and outcome of blunt carotid arterial injuries. Am J Surg. 1999;178:190–193. doi: 10.1016/s0002-9610(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 88.Zuber M, Meary E, Meder JF, et al. Magnetic resonance imaging and dynamic CT scan in cervical artery dissections. Stroke. 1994;25:576–581. doi: 10.1161/01.str.25.3.576. [DOI] [PubMed] [Google Scholar]
- 89.Vertinsky AT, Schwartz NE, Fischbein NJ, et al. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. Am J Neuroradiol. 2008;29:1753–1760. doi: 10.3174/ajnr.A1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lum C, Chakraborty S, Schlossmacher M, et al. Vertebral artery dissection with a normal appearing lumen at multisection CT angiography: the importance of identifying wall hematoma. Am J Neuroradiol. 2009;30:787–792. doi: 10.3174/ajnr.A1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. Am J Roentgenol. 2009;193:1167–1174. doi: 10.2214/AJR.08.1688. [DOI] [PubMed] [Google Scholar]
- 92.Kasner SE, Hankins LL, Bratina P, et al. Magnetic resonance angiography demonstrates vascular healing of carotid and vertebral artery dissections. Stroke. 1997;28:1993–1997. doi: 10.1161/01.str.28.10.1993. [DOI] [PubMed] [Google Scholar]
- 93.Georgiadis D, Arnold M, von Buedingen HC, et al. Aspirin vs anticoagulation in carotid artery dissection: a study of 298 patients. Neurology. 2009;72:1810–1815. doi: 10.1212/WNL.0b013e3181a2a50a. [DOI] [PubMed] [Google Scholar]
- 94.Srinivasan J, Newell DW, Sturzenegger M, et al. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke. 1996;27:1226–1230. doi: 10.1161/01.str.27.7.1226. [DOI] [PubMed] [Google Scholar]
- 95.Chen M, Caplan L. Intracranial dissections. Front Neurol Neurosci. 2005;20:160–173. doi: 10.1159/000088166. [DOI] [PubMed] [Google Scholar]
- 96.Guillon B, Levy C, Bousser MG. Internal carotid artery dissection. an update. J Neurol Sci. 1998;153:146–158. doi: 10.1016/s0022-510x(97)00287-6. [DOI] [PubMed] [Google Scholar]
- 97.Anwer U. Therapy for unusual causes of stroke. In: Fisher M, editor. Stroke therapy. Waltham, MA: Butterworth-Heinemann; 2001. pp. 347–363. [Google Scholar]
- 98.Mokri B. Cervicocephalic arterial dissections. In: Bogousslavsky J, Caplan L, editors. Uncommon causes of stroke. Cambridge: Cambridge University Press; 2001. pp. 211–229. [Google Scholar]
- 99.Metso TM, Metso AJ, Helenius J, et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke. 2007;38:1837–1842. doi: 10.1161/STROKEAHA.106.479501. [DOI] [PubMed] [Google Scholar]
- 100.Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011;114:1037–1044. doi: 10.3171/2010.9.JNS10668. [DOI] [PubMed] [Google Scholar]
- 101.Kitanaka C, Tanaka J, Kuwahara M, et al. Nonsurgical treatment of unruptured intracranial vertebral artery dissection with serial follow-up angiography. J Neurosurg. 1994;80:667–674. doi: 10.3171/jns.1994.80.4.0667. [DOI] [PubMed] [Google Scholar]
- 102.Arnold M, Nedeltchev K, Mattle HP. [Anticoagulation and antiaggregation in neurological patients]. Ther Umsch. 2003;60:33–35. doi: 10.1024/0040-5930.60.1.33. [DOI] [PubMed] [Google Scholar]
- 103.Müller BT, Luther B, Hort W, et al. Surgical treatment of 50 carotid dissections: indications and results. J Vasc Surg. 2000;31:980–988. doi: 10.1067/mva.2000.104586. [DOI] [PubMed] [Google Scholar]
- 104.Takemoto K, Abe H, Uda K, et al. Surgical treatment of intracranial VA dissecting aneurysm. Acta Neurochir Suppl. 2010;107:51–56. doi: 10.1007/978-3-211-99373-6_8. [DOI] [PubMed] [Google Scholar]
- 105.Katsuno M, Mizunari T, Kobayashi S, et al. Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: case report. Neurol Med Chir (Tokyo) 2009;49:468–470. doi: 10.2176/nmc.49.468. [DOI] [PubMed] [Google Scholar]
- 106.Czabanka M, Ali M, Schmiedek P, et al. Vertebral artery: posterior inferior cerebellar artery bypass using a radial artery graft for hemorrhagic dissecting vertebral artery aneurysms: surgical technique and report of 2 cases. J Neurosurg. 2011;114:1074–1079. doi: 10.3171/2010.5.JNS091435. [DOI] [PubMed] [Google Scholar]
- 107.Chwajol M, Munson TA, Alaraj A, et al. Extracranial carotid-vertebral bypass for endovascular access to complex posterior circulation aneurysms: a novel management approach. Neurosurgery. 2012;70:1296–1303. doi: 10.1227/NEU.0b013e318241374b. discussion 1302-1303. [DOI] [PubMed] [Google Scholar]
- 108.Xu J, Li W, Chen XY, et al. [Early management of intracranial ruptured vertebral dissecting aneurysms] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41:93–98. doi: 10.3785/j.issn.1008-9292.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 109.Deng D, Jin D, Zhou J, et al. Characteristics and endovascular treatment of intracranial vertebral artery aneurysms. Neurol India. 2011;59:833–838. doi: 10.4103/0028-3886.91360. [DOI] [PubMed] [Google Scholar]
- 110.Shin YS, Kim BM, Kim SH, et al. Endovascular treatment of bilateral intracranial vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2012;70:75–81. doi: 10.1227/NEU.0b013e31822ed1f0. discussion 81. [DOI] [PubMed] [Google Scholar]
- 111.Cohen JE, Gomori JM, Umansky F. Endovascular management of symptomatic vertebral artery dissection achieved using stent angioplasty and emboli protection device. Neurol Res. 2003;25:418–422. doi: 10.1179/016164103101201599. [DOI] [PubMed] [Google Scholar]
- 112.Coley SC, Clifton A. Dissecting vertebral artery aneurysm: diagnosis and coil embolization. Br J Radiol. 1999;72:408–411. doi: 10.1259/bjr.72.856.10474507. [DOI] [PubMed] [Google Scholar]
- 113.Iihara K, Sakai N, Murao K, et al. Dissecting aneurysms of the vertebral artery: a management strategy. J Neurosurg. 2002;97:259–267. doi: 10.3171/jns.2002.97.2.0259. [DOI] [PubMed] [Google Scholar]
- 114.Kurata A, Ohmomo T, Miyasaka Y, et al. Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. Am J Neuroradiol. 2001;22:11–18. [PMC free article] [PubMed] [Google Scholar]
- 115.Peluso JP, van Rooij WJ, Sluzewski M, et al. Endovascular treatment of symptomatic intradural vertebral dissecting aneurysms. Am J Neuroradiol. 2008;29:102–106. doi: 10.3174/ajnr.A0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oh DC, Hirsch JA, Yoo AJ. Novel use of Onyx for treatment of intracranial vertebral artery dissection. J Neurointerv Surg. 2012;4:31–33. doi: 10.1136/jnis.2011.004952. [DOI] [PubMed] [Google Scholar]
- 117.Anxionnat R, de Melo Neto JF, Bracard S, et al. Treatment of hemorrhagic intracranial dissections. Neurosurgery. 2008;62:1525–1531. doi: 10.1227/01.neu.0000333818.25520.90. [DOI] [PubMed] [Google Scholar]
- 118.Sugiu K, Tokunaga K, Watanabe K, et al. Emergent endovascular treatment of ruptured vertebral artery dissecting aneurysms. Neuroradiology. 2005;47:158–164. doi: 10.1007/s00234-005-1341-4. [DOI] [PubMed] [Google Scholar]
- 119.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291–300. doi: 10.1227/01.NEU.0000223650.11954.6C. discussion 291-300. [DOI] [PubMed] [Google Scholar]
- 120.Hanel RA, Boulos AS, Sauvageau EG, et al. Stent placement for the treatment of nonsaccular aneurysms of the vertebrobasilar system. Neurosurg Focus. 2005;18:E8. doi: 10.3171/foc.2005.18.2.9. [DOI] [PubMed] [Google Scholar]
- 121.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 122.Lylyk P, Ceratto R, Hurvitz D, et al. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery. 1998;43:385–388. doi: 10.1097/00006123-199808000-00132. [DOI] [PubMed] [Google Scholar]
- 123.Wells-Roth D, Biondi A Janardhan, V Endovascular procedures for treating wide-necked aneurysms. Neurosurg Focus. 2005;18:E7. doi: 10.3171/foc.2005.18.2.8. [DOI] [PubMed] [Google Scholar]
- 124.Shin YS, Kim HS, Kim SY. Stenting for vertebrobasilar dissection: a possible treatment option for nonhemorrhagic vertebrobasilar dissection. Neuroradiology. 2007;49:149–156. doi: 10.1007/s00234-006-0169-x. [DOI] [PubMed] [Google Scholar]
- 125.Benndorf G HU, et al. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. Am J Neuroradiol. 2001;22:1844–1848. [PMC free article] [PubMed] [Google Scholar]
- 126.Park SI, Kim BM, Kim DI, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. Am J Neuroradiol. 2009;30:1351–1356. doi: 10.3174/ajnr.A1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yeung TW, Lai V, Lau HY, et al. Long-term outcome of endovascular reconstruction with the Pipeline embolization device in the management of unruptured dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 2012;116:882–887. doi: 10.3171/2011.12.JNS111514. [DOI] [PubMed] [Google Scholar]
- 128.Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012;54:369–382. doi: 10.1007/s00234-011-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ducruet AF, Crowley RW, Albuquerque FC, et al. Reconstructive endovascular treatment of a ruptured vertebral artery dissecting aneurysm using the Pipeline embolization device. J Neurointerv Surg. 2012 Jun 20; doi: 10.1136/neurintsurg-2012-010358. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 130.de Barros Faria M, Castro RN, Lundquist J, et al. The role of the pipeline embolization device for the treatment of dissecting intracranial aneurysms. Am J Neuroradiol. 2011;32:2192–2195. doi: 10.3174/ajnr.A2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roszelle BN, Babiker MH, Hafner W, et al. In vitro and in silico study of intracranial stent treatments for cerebral aneurysms: effects on perforating vessel flows. J Neurointerv Surg. 2012 doi: 10.1136/neurintsurg-2012-010322. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]