Summary
We describe our experience of the development of contralateral de novo intraosseous AVMs in a ten-year-old girl with AVMs of the mandible who underwent endovascular embolotherapy. She initially presented with intermittent oral bleeding. Computed tomography and digital subtraction angiography demonstrated intraosseous AVMs within the right mandible. The AVMs were treated by transosseous direct-puncture and transarterial embolization with Guglielmi detachable coils and n-butyl cyanoacrylate glue. However, de novo intraosseous AVMs developed within the previously healthy contralateral mandible and resulted in dangerous oral bleeding. Therefore, we suggest regular follow-up and prompt retreatment of any residual mandibular AVMs in patients undergoing endovascular or surgical treatment to prevent subsequent development of “secondary” AVMs and life-threatening oral bleeding.
Key words: mandible, arteriovenous malformation, transarterial embolization, transosseous embolization
Introduction
High-flow arteriovenous malformations (AVMs) are rare in the mandible. Because they may be lethal due to life-threatening intraoral bleeding spontaneously or after tooth extraction 1, prompt treatment is necessary in symptomatic mandibular AVMs. Usually, AVMs of the mandible are managed by endovascular embolization, surgical excision or combined therapy. However, the recurrence of the mandibular AVMs is always a great challenge for surgeons and interventional radiologists. We describe our experience in managing a case with contralateral de novo intraosseous AVMs after initial endovascular embolotherapy of the right mandibular AVMs.
Case Report
A ten-year-old Taiwanese girl was referred to our institution for intermittent gingival bleeding of the right lower jaw after tooth brushing or forceful chewing. Her medical history revealed painless focal skin redness at her right lower jaw since birth. In addition, intermittent right lower jaw pain when chewing and swallowing were noted one year ago. Computed tomography (CT) scan showed an intraosseous lytic expansile lesion with avid enhancement at the body and ramus of the right mandible as well as numerous adjacent engorged adjacent vessels. The bilateral carotid angiogram demonstrated complex intraosseous and extraosseous arteriovenous malformations (AMVs) with multiple dilated racemous venous pouches in body and ramus of the right mandible and medial pterygoid region (Figure 1). The AVMs were supplied by numerous feeding arteries from bilateral external carotid arteries (ECAs) and drained mainly by the right retromandibular vein into right internal jugular vein. Neither AVMs nor engorged venous structure were found in the left portion of the mandible. Enhanced brain CT and complete angiogram survey, including bilateral carotid and vertebral angiogram showed no intracranial AVM or other vasculopathy.
With the patient under general anesthesia, endovascular embolization therapy in the manner of percutaneous transosseous direct puncture was performed. Puncture of the largest venous pouch within the right mandibular body was performed with a 17-gauge coaxial puncture needle. The right retromandibular vein and the intraosseous venous pouches were subsequently completely filled with densely packed Guglielmi detachable coils (GDCs) (Figure 2). However, small residual AVMs at the mandibular symphysis, which was supplied by the bilateral ECAs and drained by the right inferior mandibular vein, were seen on postembolization angiography. One month later, feeders of the residual AVM from bilateral ECAs were successfully occluded transarterially with n-butyl cyanoacrylate glue (NBCA) in another session of embolotherapy. The residual AVM was almost completely obliterated after transarterial superselective catheterization and embolization with glue.
The patient was free of oral discomfort and bleeding after treatment. However, she was admitted again 18 months later for intermittent intraoral bleeding of the left lower jaw. CT scan showed that the marrow space within the left mandible was replaced by engorged vessels accompanied by remodeling and thinning of the bony cortex. Subsequent carotid angiogram demonstrated newly-developed intraosseous AVMs with engorged venous pouches mainly within body and ramus of the left mandible. (Figure 3). Because the patient suffered from long-standing toothache due to dental caries and periodontitis, the dentist requested palliative embolization of the feeders from bilateral ECAs with NBCA before performing dental extraction and dental therapy. Another session of radical transosseous direct-puncture embolization was scheduled two months later. However, sudden massive oral bleeding with hypovolemic shock of the patient occurred following endotracheal intubation and general anesthesia. Transosseous direct-puncture embolization of the left retromandibular vein and intraosseous venous pouches within the left mandibular body were done with GDCs and NBCA for urgent bleeding control. The feeders from bilateral ECAs were also embolized with NBCA. The bleeding was successfully controlled after embolotherapy and concurrent oral packing performed by the maxillofacial surgeon. The patient has no recurrent oral bleeding but dental infection requiring minor surgical management in the left lower jaw during the subsequent two-year follow-up.
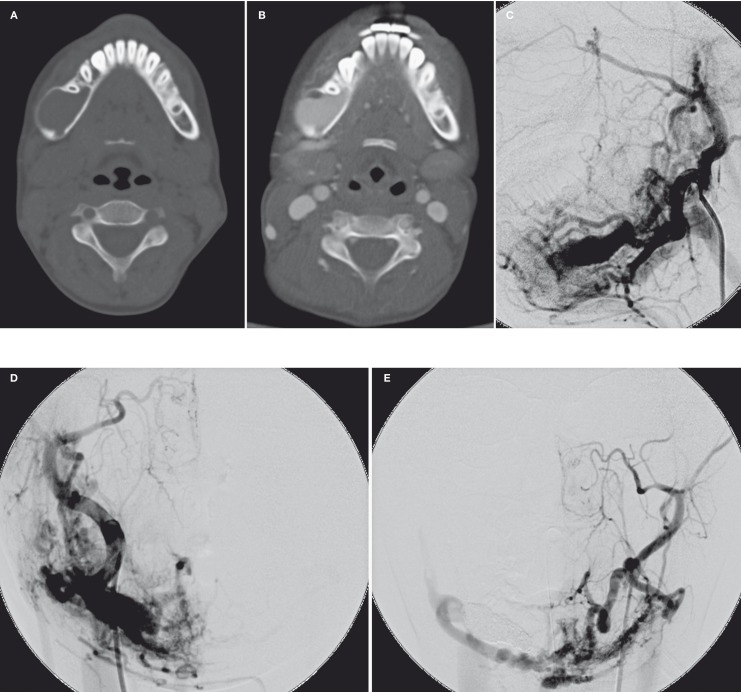
Figure 1.
Axial CT images of head and neck without (A) and with (B) i.v. contrast medium enhancement showed an intraosseous lytic expansile lesion with avid enhancement at body and ramus of the right mandible. The right carotid angiogram in lateral (C) and AP (D) projection and left carotid angiogram in AP projection (E) demonstrated complex intraosseous and extraosseous AMV in right mandible and pterygoid region. The AVMs was supplied from bilateral ECAs and drained mainly by the right retromandibular vein.
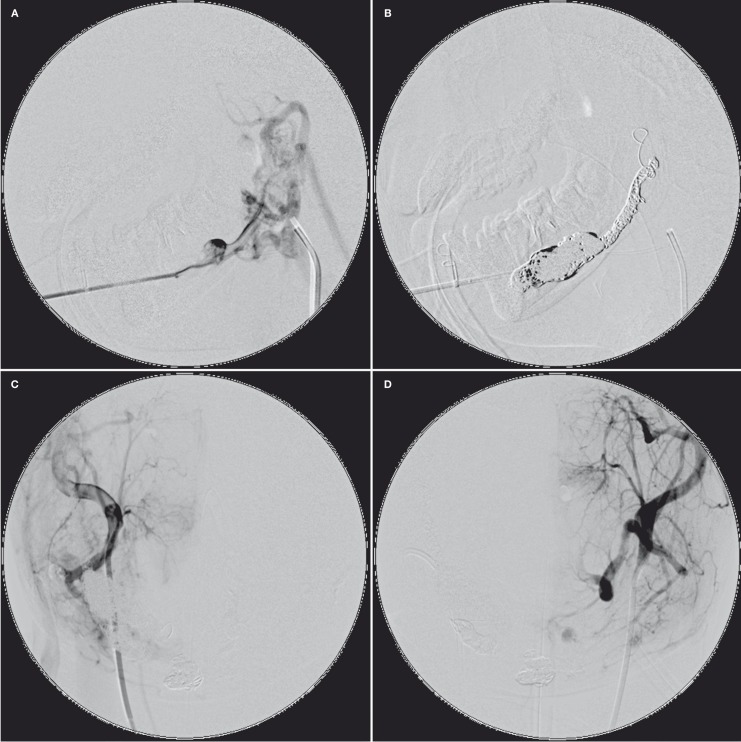
Figure 2.
After direct transosseous puncture with a 17-gauge coaxial puncture needle (A), the right retromandibular vein and the intraosseous venous pouches were completely occluded with densely packed GDCs (B). However, small residual AVM at the mandibular symphysis, supplied by the bilateral ECAs, was seen on the postembolization angiography (C, D).
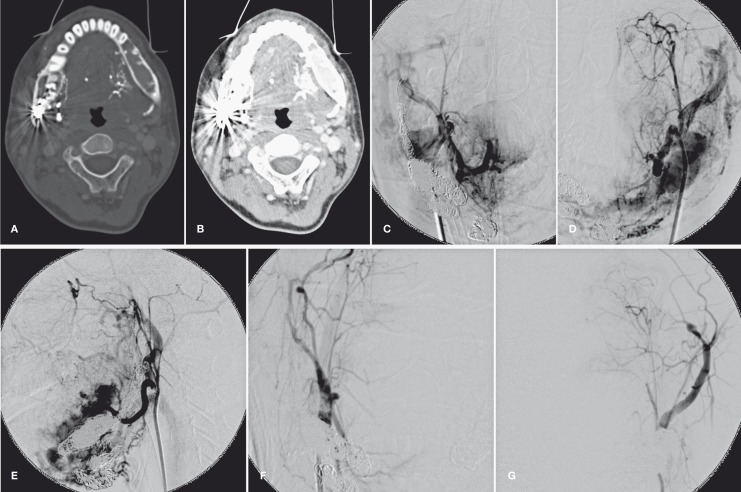
Figure 3.
Post-enhancement axial CT images of head and neck in bone window (A) and soft tissue window (B) showed that the marrow space within the left mandible was replaced by engorged vessels, as well as remodeling and thinning of the bony cortex. Some dense serpiginous structures at the inner aspect of the left mandible (the mouth floor) were glue deposited in the left ECA branches due to prior transarterial embolization. Right (C) carotid angiogram and left carotid angiography in A-P (D) and lateral (E) projection demonstrated newly-developed intraosseous AVM with engorged venous pouches mainly within body and ramus of the left mandible. Right (F) and left (G) external carotid angiogram after the last embolotheraphy showed complete obliteration of the intraosseous mandibular AVM.
Discussion
Optimal planning and decision-making for the treatment of mandibular AVMs depends on age of the patient, the size, flow characteristics and the site of the lesions. Endovascular embolization, surgical excision, or combined therapy are usually performed for patients with mandibular AVMs. Successful treatment of the mandibular AVMs is possible after obliteration of the venous pouches and the drainage veins. Different surgical procedures are used in cases of mandibular AVMs, including ligation of external carotid arteries, simple curettage and partial or complete resection of the mandible followed by reconstructive surgery. Their benefit and disadvantage are discussed in the literature 2. Endovascular embolization procedures are effective in the management of mandibular AVMs 3,4. The overall successful cure rate of mandibular AVMs by endovascular embolization is as high as seventy percent 5. Usually, there are numerous small tortuous feeders arising from ECA for an intraosseous mandibular AVM. Therefore, successful selective catheterization and transarterial embolization through these arterial feeders would be very time-consuming and technique-dependent. In contrast, the transvenous route is an alternative endovascular access directly into the venous pouches of mandibular AVM. After total embolization of the venous pouches and the drainage veins, spontaneous regression of the arterial network may subsequently occur 6,7. Transosseous direct-puncture approach is a modified transvenous vascular access 8. The length of the endovascular route is much shorter in the transosseous approach than in the transvenous approach. In addition, the time-consuming procedure of transvenous catheterization is much simplified in the transosseous approach 9,10. Variable materials, such as NBCA, coils and ethanol have been used in embolization procedures 11,12. Although endovascular embolization could be very difficult to perform, it avoids mutilating surgery and its sequela in pediatric patients.
In our reported case, transosseous direct-puncture embolization of the mandibular AVM was performed with GDCs. GDCs are mainly used in the embolotherapy of intracranial aneurysms, carotid-cavernous fistula, or intracranial AVM 13,14. With the advantage of GDCs, the operator can easily retrieve and redeploy the suboptimally placed coils 15. Therefore, complete obliteration of small intraosseous AVMs can be done easily in the manner of transosseous direct-puncture embolization with GDCs. Although the right mandibular AVM of this patient is large with complex intraosseous and extraosseous components, the intraosseous venous sacs were completely occluded with GDCs alone.
Residual intraosseous or extraosseous AVM is not infrequently seen after embolotherapy or surgical management of the mandibular AVM. The recurrent lesions are usually due to recannulation of feeding arteries or recuritment of collateral blood supply. Locations of recurrence usually correspond to the original AVM. However, in this patient with repeated CT scan and carotid angiography for recurrent contralateral lower jaw bleeding 18 months after initial embolotherapy, the left portion of mandible was initially healthy with intact marrow space and bony cortex, and the intraosseous AVM in the body and ramus of right mandible was almost completely obliterated by embolotherapy. But the progressive enlarged recurrent AVM developed at the previously healthy left portion of mandible. Although the etiology of the mandibular AVMs is not well-understood, they are generally believed to be congenital vascular lesions. However, we believe that de novo intraosseous AVM of mandible could develop within the previously intact bone in response to certain factors. Some authors proposed that an environmental trigger (“second hit”) in addition to genetic predisposition may have a role in the development of de novo brain AVMs 16. According to the imaging evidence in this patient, it is possible that a similar mechanism exists for intraosseous AVMs. In addition to the genetic predisposition, the residual AVMs, the hemodynamic changes after embolotherapy, or the embolotherapy itself may serve as the environmental trigger that induced the development of another AVM within previously healthy bony structure. Therefore, regular imaging follow-up and prompt management of residual AVM after embolotherapy or surgery despite its small size are suggested to prevent recurrent life-threatening bleeding as seen in this case.
In conclusion, we described a case of de novo mandibular AVM after initially endovascular embolotherapy. Another AVM developed within the previously healthy bony structure of contralateral mandible and caused life-threatening oral bleeding. Our experience in this patient suggests the importance of regular imaging follow-up and prompt management of residual AVMs after embolotherapy or surgery despite their size or location in a patient treated by embolization for mandibular AVMs.
References
- 1.Lamberg MA, Tasanen A, Jaaskelainen J. Fatality from central hemangioma of the mandible. J Oral Surg. 1979;37:578–584. [PubMed] [Google Scholar]
- 2.Behnia H, Ghodoosi I, Motamedi MH, et al. Treatment of arteriovenous malformations: assessment of 2 techniques--transmandibular curettage versus resection and immediate replantation. J Oral Maxillofac Surg. 2008;66:2557–2565. doi: 10.1016/j.joms.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 3.Frame JW, Putnam G, Wake MJ, et al. Therapeutic arterial embolisation of vascular lesions in the maxillofacial region. Br J Oral Maxillofac Surg. 1987;25:181–194. doi: 10.1016/s0266-4356(87)80019-0. [DOI] [PubMed] [Google Scholar]
- 4.van den Akker HP, Kuiper L, Peeters FL. Embolization of an arteriovenous malformation of the mandible. J Oral Maxillofac Surg. 1987;45:255–260. doi: 10.1016/0278-2391(87)90124-8. [DOI] [PubMed] [Google Scholar]
- 5.Persky MS, Yoo HJ, Berenstein A. Management of vascular malformations of the mandible and maxilla. Laryngoscope. 2003;113:1885–1892. doi: 10.1097/00005537-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Benndorf G, Campi A, Hell B, et al. Endovascular management of a bleeding mandibular arteriovenous malformation by transfemoral venous embolization with NBCA. Am J Neuroradiol. 2001;22:359–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Kawano K, Mizuki H, Mori H, et al. Mandibular arteriovenous malformation treated by transvenous coil embolization: a long-term follow-up with special reference to bone regeneration. J Oral Maxillofac Surg. 2001;59:326–330. doi: 10.1053/joms.2001.21005. [DOI] [PubMed] [Google Scholar]
- 8.Fan X, Zhang Z, Zhang C, et al. Direct-puncture embolization of intraosseous arteriovenous malformation of jaws. J Oral Maxillofac Surg. 2002;60:890–896. doi: 10.1053/joms.2002.33858. discussion 896-897. [DOI] [PubMed] [Google Scholar]
- 9.Resnick SA, Russell EJ, Hanson DH, et al. Embolization of a life-threatening mandibular vascular malformation by direct percutaneous transmandibular puncture. Head Neck. 1992;14:372–379. doi: 10.1002/hed.2880140506. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum GP, Halbach VV, Fraser KW, et al. Direct-puncture coil embolization of maxillofacial high-flow vascular malformations. Laryngoscope. 1994;104:1397–1400. doi: 10.1288/00005537-199411000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, Ma XC. Clinical study of embolization of arteriovenous malformation in the oral and maxillofacial region. Chin J Dent Res. 2000;3:63–70. [PubMed] [Google Scholar]
- 12.Fan XD, Su LX, Zheng JW, et al. Ethanol embolization of arteriovenous malformations of the mandible. Am J Neuroradiol. 2009;30:1178–1183. doi: 10.3174/ajnr.A1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guglielmi G, Viñuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmi G, Viñuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 15.Tong FC, Cloft HJ, Dion JE. Endovascular treatment of intracranial aneurysms with Guglielmi Detachable Coils-emphasis on new techniques. J Clin Neurosci. 2000;7:244–253. doi: 10.1054/jocn.1999.0211. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez H, Perry V, Solle M, et al. De novo cerebral arteriovenous malformation in a child with previous cavernous malformation and developmental venous anomaly. J Neurosurg Pediatrics. 2012;9:327–330. doi: 10.3171/2011.12.PEDS11312. [DOI] [PubMed] [Google Scholar]