Abstract
Neointimal hyperplasia is one the primary causes of stenosis in arterialized veins that are of great importance in arterial coronary bypass surgery, in peripheral arterial bypass surgery as well as in arteriovenous fistulas.1-5 The experimental procedure of vein graft interposition in the common carotid artery by using the cuff-technique has been applied in several research projects to examine the aetiology of neointimal hyperplasia and therapeutic options to address it. 6-8 The cuff prevents vessel anastomotic remodeling and induces turbulence within the graft and thereby the development of neointimal hyperplasia.
Using the superior caval vein graft is an established small-animal model for venous arterialization experiment.9-11 This current protocol refers to an established jugular vein graft interposition technique first described by Zou et al., 9 as well as others.12-14 Nevertheless, these cited small animal protocols are complicated.
To simplify the procedure and to minimize the number of experimental animals needed, a detailed operation protocol by video training is presented. This video should help the novice surgeon to learn both the cuff-technique and the vein graft interposition. Hereby, the right external jugular vein was grafted in cuff-technique in the common carotid artery of 21 female Sprague Dawley rats categorized in three equal groups that were sacrificed on day 21, 42 and 84, respectively. Notably, no donor animals were needed, because auto-transplantations were performed. The survival rate was 100 % at the time point of sacrifice. In addition, the graft patency rate was 60 % for the first 10 operated animals and 82 % for the remaining 11 animals. The blood flow at the time of sacrifice was 8±3 ml/min. In conclusion, this surgical protocol considerably simplifies, optimizes and standardizes this complicated procedure. It gives novice surgeons easy, step-by-step instruction, explaining possible pitfalls, thereby helping them to gain expertise fast and avoid useless sacrifice of experimental animals.
Keywords: Medicine, Issue 69, Anatomy, Physiology, Immunology, Surgery, microsurgery, neointimal hyperplasia, venous interposition graft, external jugular vein, common carotid artery, rat
Protocol
1. Experimental Animals
Obtain a Local Ethics Committee approval for all experiments which will be performed, in accordance with the animal protection FELASA guidelines.
Use female Sprague-Dawley rats available to purchase from any commercial breeder (e.g. Charles River Wiga GmbH, Sulzfeld, Germany). We used 21 animals divided in three groups of 7 animals each that were sacrificed at 3, 6 and 12 weeks postoperatively. At the time of purchase, the animals should be 12 weeks of age and weigh 250-300 g.
Keep the animals in climate-controlled rooms having an ambient temperature of 21 °C, 60% relative humidity and a 12-hr light-dark cycle with free access to food and water.
2. Anesthesia and Operative Preparation
Induce general anesthesia by using a Plexiglas box connected to the anesthesia machine, set at an oxygen flow rate of 1 L/min, and an isofluorane vaporizer with 5 % anesthetic gas.
Maintain the anesthesia with the isofluorane vaporizer at 1.5 % as soon as the deep sensory reflexes of the animals are suppressed.
Fix the rats in supine position on a body warming plate.
Prepare the operation region of each animal by removing its fur with a depilation cream, from the jaw angle down to 2 cm below the manubrium sterni.
- Prepare a sterile surgical field by paying attention to the surgical area, the area prepared on the animal and the front of the surgeon:
- Clean the benchtop with a disinfectant.
- Prepare the incision site by using multiple applications of surgical scrub solution (e.g. Chlorhexidine and 70% isopropyl alcohol). Apply the Chlorhexidine first, the alcohol second and, finally, the scrub solution.
- Wear a disposable surgical gown and surgical gloves, or alternatively, exam gloves that have been previously washed with a surgical disinfectant.
Use sterile microsurgery instruments in the operation procedure.
Use an operation microscope (Wild Heerbrugg M650, Gais, Switzerland) in 10-20-fold magnification.
3. Anatomy
Figure 1 depicts the anatomy of the rat's neck:
1a) Situs before transection of the right sternocleidomastoid muscle.
1b) Situs after transection of the right sternocleidomastoid muscle.
4. Cuff Preparation
Cut the cuffs in a length of 3 mm by hand from a plastic tube (Portex, inner diameter 0.75 mm, outer diameter 0.94 mm, Smiths Medical, UK). These cuffs consist of a tubular cuff body including the complete volume of the plastic tube, and a cuff handle consisting of half of the cuff volume. Both the cuff body and the cuff handle should be equally long. The cuff handle is essential for manipulating the cuff during the surgical procedure. After the cuff is slipped over the vessel, a mini Bulldog clamp is placed simultaneously on the cuff handle and the vessel, in order to prevent from bleeding, keep the cuff in position and ensure stability.
5. Cuff Technique
Slip the cuff over the vessel.
Immediately afterwards, evert the vessel, pull it over the outside of the cuff body and fix it with a 7-0 silk ligature. In this manner, the lumen of the cuff is covered with endothelium. Thereafter, slip the venous graft over the cuff body and fix it with a new 7-0 silk ligature. To prevent thrombus formation, it is essential to fasten the vein graft tie very close to the cuff margin in order to avoid the blood from contacting the tie holding the artery in place. This is crucial for the distal anastomosis due to the blood flow direction. However, according to the authors, it is not considered essential for the proximal anastomosis for the same reason.
6. Surgical Procedure
6.1 Preparation of the right external jugular vein
Make an approximate 2.5 cm-long median cervical incision direct above the manubrium sterni.
Completely separate a 1 cm-long segment of the right external jugular vein from the surrounding tissues
Ligate the venous tributaries with 7-0 silk sutures.
6.2 Preparation of the right common carotid artery
Prepare the sternocleidomastoid muscle and transect it in the middle.
Prepare the right common carotid artery carefully, making sure not to compress the artery or the Vagus nerve. Mobilize it from the carotid bifurcation down to the brachiocephalic trunk.
Subsequently, ligate it twice in the middle with 7-0 silk sutures before transection.
Slip a nylon cuff consisting of a body and a handle (Figure 2a) over the distal end of the common carotid artery, whereby it is important to ensure that the cuff handle is behind the vessel (Figure 2b).
Clamp the cuffed end of the distal common carotid artery with a mini Bulldog clamp exactly on the cuff handle to ensure stability (Figure 2c).
Subsequently, cut the artery directly behind the ligature, whereby the resulting arterial stump should be as long as the cuff body.
Rinse the arterial lumen with heparin-saline solution (20 IU heparin/ ml 0.9% NaCl). Set a ligation loop aside in the proper position for later use.
Pull the arterial stump up and slip it like a sleeve over the cuff body.
Subsequently, pull the ligation loop and knot it tightly.
To prevent the artery from slipping out of its sleeve, you may implement a trick: grasp both the arterial wall and the suture with the right hand simultaneously and with the left-hand forceps (Figure 2d) make the knot (Figure 2e).
Repeat the same procedure for the proximal arterial stump (Figure 2f).
6.3 Venous graft positioning
Excise a 1 cm-long segment of the clamped external jugular vein
Since the two cuffed arteries are too far apart from each other for the venous interposition, approximate the correct distance for this procedure using a 3-0 Vicryl-suture which you should loop around each mini Bulldog clamp.
Criss-cross both sutures.
Subsequently, pull the sutures in opposite directions, enabling the proper positioning of the cuffed arterial stumps (Figure 2g)
Rinse the vein segment with the heparin-saline solution.
Slip one end of the previously reversed vein segment over the cuffed distal carotid artery and secure it in the correct position with a silk 7-0 ligature.
It is important to note here that the circumference of the vein must be completely and evenly grasped.
Rinse the vein segment with heparin-saline-solution, and slip the other end of the vein segment over the proximal carotid stump.
Complete the ligation (Figure 2h);
Remove the mini Bulldog clamps one after the other, first on the distal carotid artery stump and then on the proximal one, thereby allowing unobstructed blood flow and the arterialization of the venous graft (Figure 2i). The surgery is considered successful if blood flow and pulsation are observed within the graft and the common carotid artery (assessed visually).
6.4. Wound closure
For the subcutaneous running suture, use 4-0 Vicryl. For a cutaneous running suture, use 3-0 Vicryl.
7. Recovery and Postoperative Management
Let the animals recover from anesthesia on a flat paper bedding under an infrared warming lamp. Do not return the operated animals to the animal holding area until all animals appear normal.
Postoperatively administer Buprenorphine (Temgesic) in the concentration of 0.03-0.05 mg/kg b.w. sc every 8-12 hr for 2 days. Buprenorphine can be administered just before isofluorane induction.
Monitor animals postoperatively for their general condition and signs of illness or infections.
8. Blood Flow Measurement
The postoperative patency of the graft was proved by blood flow measurement, by using a research Transonic blood flow measurement system (Model: TS420, Transonic Systems Europe B.V., Maastricht, The Netherlands) with a perivascular flowprobe (3 mm, MA3PSB). The system is based on the Doppler principle.
9. Representative Results
By faithfully keeping the instructions aforementioned, the mean duration of the surgical operations was about 65 min. The animals presented an excellent physical condition and gained weight as expected throughout the whole experiment: at the starting point of operation the animals weighed 312±20.39 g. At the sacrifice time point of 3 weeks post-operatively the animals weighed 325.2±28.7 g, at 6 weeks 333±39.9 g and at 12 weeks 362.22±29 g.
No infections were observed at the operation site and no sores developed. The operation wounds healed completely at the expected time. The neurological control showed no signs of brain infarction or motoric deficiency. The later was an important point since the animals were operated in the carotid artery and one of the two external jugular veins was removed and used as auto-transplant. No allergic reactions to the postoperative pain alleviation therapy were observed.
All animals were vigilant and social throughout the whole experiment. Their habits concerning receiving food and water remained unchanged throughout the whole time.
The survival rate of all experiments was 100 % at the time point of sacrifice. The graft patency rate was 60 % for the first 10 operated animals and accordingly 82 % for the remaining 11 animals. The total graft patency was 71 %. The blood flow in the arterialized venous grafts was measured at the time point of sacrifice and was 8±3 ml/min without any statistically significant differences between the three sacrifice time points.
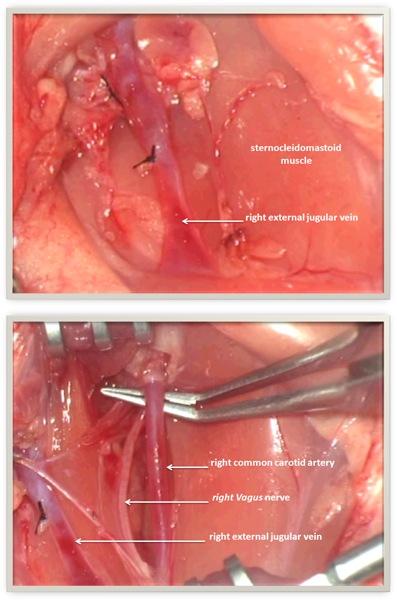 Figure 1. Anatomy of the rat's neck. 1a) Situs before transection of the right sternocleidomastoid muscle. 1b) Situs after transection of the right sternocleidomastoid muscle.
Figure 1. Anatomy of the rat's neck. 1a) Situs before transection of the right sternocleidomastoid muscle. 1b) Situs after transection of the right sternocleidomastoid muscle.
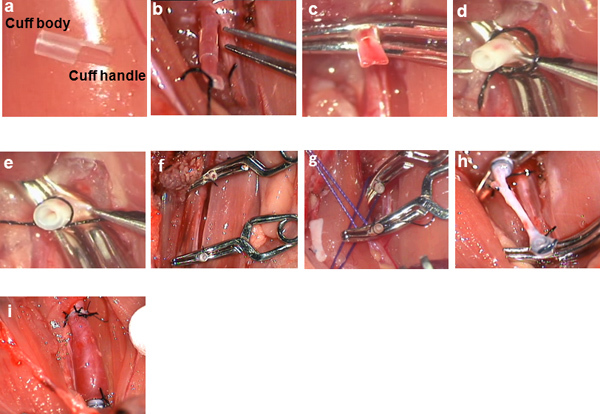 Figure 2. Intraoperative microscopic pictures: a) cuff consisting of cuff-body and cuff-handle; b) cuff slipped over the distal end of the common carotid artery; c) clamping of the cuffed distal common carotid artery stump; d) eversion of the common carotid artery stump over the cuff; e) securing the everted common carotid artery stump with a ligature; f) situs after eversion of both cuffed distal and proximal common carotid artery stumps; g) criss-cross technique for approximation of the two cuffed common carotid artery stumps; h) situs after venous interposition; i) situs after reperfusion of the venous interposition graft.
Figure 2. Intraoperative microscopic pictures: a) cuff consisting of cuff-body and cuff-handle; b) cuff slipped over the distal end of the common carotid artery; c) clamping of the cuffed distal common carotid artery stump; d) eversion of the common carotid artery stump over the cuff; e) securing the everted common carotid artery stump with a ligature; f) situs after eversion of both cuffed distal and proximal common carotid artery stumps; g) criss-cross technique for approximation of the two cuffed common carotid artery stumps; h) situs after venous interposition; i) situs after reperfusion of the venous interposition graft.
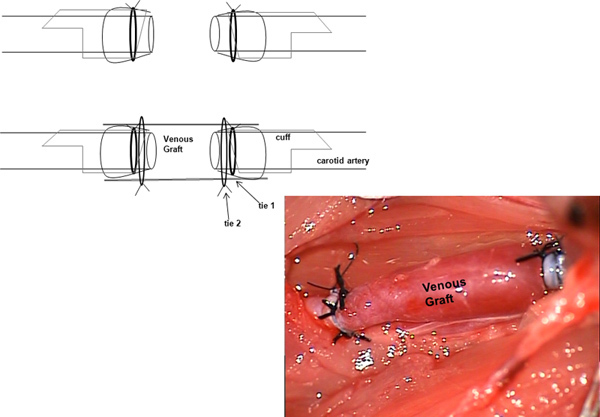 Figure 3. Schematic depiction of the situs after reperfusion of the venous interposition graft. Click here to view larger figure.
Figure 3. Schematic depiction of the situs after reperfusion of the venous interposition graft. Click here to view larger figure.
Discussion
Interposing an external jugular vein graft in the common carotid artery is a sophisticated microsurgery technique. This video helps the novice surgeon master this technique in minimum time with a lower waste of experimental animals.
Inhalative anesthesia is the best option for this operation, since it avoids an excessively deep anesthesia stage, can be well controlled and allows the surgeon enough time to operate. Moreover the rat is an experimental animal that offers a suitable size to practice such complex surgical techniques much easier, in comparison to the mouse.
The advantage of using the cuff-technique is that vessels with different luminal diameters can be anastomosed. It is important to ligate the complete circumference of the cuffed and everted vessels to avoid bleeding after reperfusion. This model is very suitable to simulate hemodynamics of high blood velocity and low shear stress and to study mechanisms which affect the development of neointimal hyperplasia. The cuff prevents vessel anastomotic remodeling and induces turbulence within the graft and thereby the development of neointimal hyperplasia.
To prevent formation of thrombi, it is essential to place the vein graft tie 2 in a suitable position to prevent association of blood with the tie 1 holding the artery in place (Figure 3).9 Tying the vein graft in the recommended way limits thrombotic events to 12-13% and occlusion of the vessel to even lower proportions.15 As it can seen in the operation video, it is not easy to place the tie exactly in that proposed position. The authors consider it to be crucial for the distal anastomosis, in particular, because of the blood flow direction. By contrast, this is not considered absolutely important for the proximal anastomosis for the same reason.
Unlike the procedure using the caval vein as a graft, this autotransplantation using the external jugular vein as the graft reduces the necessary number of experimental animals by half. Ultimately, interposing an external jugular vein graft in the common carotid artery is a sophisticated microsurgery technique. This video helps the novice surgeon master this technique in minimum time with a lower waste of experimental animals. This model is an excellent means for novice surgeons to practice the cuff-technique that is currently used in solid organ transplantation in small animals.
Disclosures
No conflicts of interest declared.
Acknowledgments
This study was funded by the research promotion program "START" of the medical faculty of RWTH Aachen University, Germany (Title: "Die Arterialisation venöser Gefäße" START 690753). We would like to thank Mrs. Mary-Joan Blümich for her excellent editing assistance.
References
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: Pathogenesis, Predisposition, and Prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- Bonatti J, Oberhuber A, Schachner T, Zou Y, Hammerer-Lercher A, Mittermair R, Laufer G. Neointimal hyperplasia in coronary vein grafts: Pathophysiology and prevention of a significant clinical problem. The Heart Surg. 2004. pp. E72–E87. [DOI] [PubMed]
- Roy-Chaudhury P, Kelly BS, Zhang J, Narayana A, Desai P, Melhem M, Duncan H, Heffelfinger SC. Hemodialysis Vascular Access Dysfunction: From Pathophysiology to Novel Therapies. Blood Purif. 2003;21:99–110. doi: 10.1159/000067863. [DOI] [PubMed] [Google Scholar]
- Dixon BS. Why don't fistulas mature. Kidney Int. 2006;70:1413–1422. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J. Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Schachner T, Heiss S, Zipponi D, Tzankov A. Perivascular treatment with azathioprine reduces neointimal hyperplasia in experimental vein grafts. Heart Surg. 2006;9:E515–E517. doi: 10.1532/HSF98.20051168. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, Peppel K. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler. Thromb. Vasc. Biol. 2008;28:284–289. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- Tzankov A, Mairinger T, Laufer G, Bonatti JO. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann. Thorac. Surg. 2004;77:1580–1585. doi: 10.1016/j.athoracsur.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse Model of Venous bypass graft arteriosclerosis. A.J.P. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner T, Oberhuber A, Zou Y, Tzankov A, Ott H, Laufer G, Bonatti J. Rapamycin treatment is associated with an increased apoptosis rate in experimental vein grafts. Eur. J. Cardiothorac. Surg. 2005;27:302–306. doi: 10.1016/j.ejcts.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Ali Z, Bursill C, Hu Y, Choudhury RP, Xu Q, Greaves DR, Channon KM. Gene transfer of a broad spectrum CC-chemokine inhibitor reduces vein graft atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2005;30:1235–1241. doi: 10.1161/CIRCULATIONAHA.104.526129. [DOI] [PubMed] [Google Scholar]
- Zou J, Zhang X, Yang H, Zhu Y, Ma H, Wang S. Rapamycin-loaded nanoparticles for inhibition of neointimal hyperplasia in experimental vein grafts. Ann. Vasc. Surg. 2011;25:538–546. doi: 10.1016/j.avsg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu K, Shen L, Wu H, Jing H. Small interfering RNA to c-myc inhibits vein graft restenosis in a rat vein graft model. J. Surg. Res. 2011;169:e85–e91. doi: 10.1016/j.jss.2011.03.060. [DOI] [PubMed] [Google Scholar]
- Schachner T, Zou Y, Oberhuber A, Mairinger T, Tzankov A, Laufer G, Ott H, Bonatti J. Perivascular application of C-type natriuretic peptide attenuates neointimal hyperplasia in experimental vein grafts. Eur. J. Cardiothorac. Surg. 2004;25:585–590. doi: 10.1016/j.ejcts.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Thomas AC, Newby AC. Effect of matrix metalloproteinase-9 knockout on vein graft remodelling in mice. J. Vasc. Res. 2010;47:299–308. doi: 10.1159/000265564. [DOI] [PMC free article] [PubMed] [Google Scholar]


