Abstract
The ability to chronically record from populations of neurons in freely behaving animals has proven an invaluable tool for dissecting the function of neural circuits underlying a variety of natural behaviors, including navigation1 , decision making 2,3, and the generation of complex motor sequences4,5,6. Advances in precision machining has allowed for the fabrication of light-weight devices suitable for chronic recordings in small animals, such as mice and songbirds. The ability to adjust the electrode position with small remotely controlled motors has further increased the recording yield in various behavioral contexts by reducing animal handling.6,7
Here we describe a protocol to build an ultra-light motorized microdrive for long-term chronic recordings in small animals. Our design evolved from an earlier published version7, and has been adapted for ease-of use and cost-effectiveness to be more practical and accessible to a wide array of researchers. This proven design 8,9,10,11 allows for fine, remote positioning of electrodes over a range of ~ 5 mm and weighs less than 750 mg when fully assembled. We present the complete protocol for how to build and assemble these drives, including 3D CAD drawings for all custom microdrive components.
Keywords: Neuroscience, Issue 69, Physiology, Medicine, Anatomy, Mechanical Engineering, microdrive, in-vivo chronic recording, electrophysiology, songbirds
Protocol
1. Overview of Components
A complete microdrive consists of several main components (Figure 1): a chassis that serves as the superstructure for the drive, a motor with a finely threaded output shaft, a threaded shuttle that carries the electrodes and provides a point of electrical connection, and an Omnetics (or equivalent) connector.
The chassis, electrode shuttle, and electrode shuttle tubes are custom components that were designed with 3D CAD software (SolidWorks) and were machined by a local precision machinist. All other components are commercially available.
Chassis and shuttle are machined from lightweight polyetherimide (PEI), while the electrode shuttle tubes are cut to size with a lathe from stainless steel hypodermic tubing (0.0293"OD x 0.00975"ID). Though these components may be cut from other materials, we have found that PEI and stainless steel have the right balance of machinability, strength, and weight for our application.
To assemble the drive, a motor controller, capable of spinning the low DC-voltage stepper motor, is required. We employ a custom solution based on a three-phase motor driver IC (Faulhaber BLD05002 available from the manufacturer) in conjunction with an IC timer that provides the timestep-input. A variety of off-the-shelf solutions are also available (e.g. Faulhaber MCBL05002 or Sutter Instruments MP-285).
2. Drive Chassis Preparation and Assembly
With fine diagonal cutters, trim the contact pins on the bottom of the Omnetics (or equivalent) connector - 1.5 mm for the rear row, 1 mm for the front row (Figure 2A). To improve the strength of the epoxy bond between the drive chassis and the connector, use a scalpel to roughen the rear surfaces of both components.
Using a scalpel, cut four 15 mm long sections of polyimide tubing (0.0113" ID x 0.0133" OD) for use as wire guides. Carefully secure the chassis in a small vice with the open cavity down (Figure 2B). Use cyanoacrylate glue to attach the polyimide tubes to the chassis. They should lay flush with the rear chassis surface and be oriented parallel to each other as shown in Figure 2B.
Once dry, trim the polyimide tubes to approximately 6 mm in length and remove the waste sections. The remaining tubes should be approximately 3 mm from the bottom edge of the chassis.
To ensure proper clearance for mating with the headstage or cable, the connector should be mounted a few millimeters above the polyimide tubes and be parallel with the chassis surface (Figure 2B). Use epoxy (Torr Seal) to construct a small pedestal for the connector, place it on top with the shorter row of pins topmost, and add more epoxy around the connector. Do not proceed until the epoxy has completely cured.
Remove the chassis from the vice, transfer to a jig that allows for connection to the motor controller (i.e., in our case, this jig is simply a rigid rod with the mating Omnetics connector affixed to one end) and orient with the front surface of the drive facing up. Guide the motor wires through the hole in the chassis and slide the motor into the groove in the base. The motor and drive shaft should be parallel to the chassis and fit snuggly against the bottom and side.
Epoxy the motor to the chassis, taking care not to obstruct either the drive shaft or the space where the electrodes will be installed (see Torr Seal placement in Figure 4). Allow the epoxy to cure.
Rotate the chassis so that the connector is accessible. To support the motor wires and prevent accidental breakage, add a small drop of fast-set epoxy to the wires where they emerge from the chassis. Trim, strip, and solder the wires to the appropriate contacts on the connector (Figure 2A). Test the motor to ensure smooth shaft rotation in both directions.
3. Electrode Shuttle Assembly
To minimize the potential for motion-induced artifacts in the recordings, it is essential that the electrode shuttle fit snuggly in the chassis. Test the fit of the shuttle by threading it onto the motor shaft and running it up and down the full length of the shaft at low speed. Be careful not to wedge the shuttle in the drive and thus over-torquing the motor- it is very easy to permanently damage the plastic gearhead at this stage. If a previously spinning motor shaft ceases turning, the gearhead may be damaged.
If the shuttle does not run smoothly down the shaft, inspect the chassis surfaces and the drive screw for debris. Remove with fine forceps or compressed air, coat the threading in some light mineral oil and recheck the shuttle fit.
If the shuttle is too tight, use forceps and a fine grain whetstone to remove obstructing material from the side of the shuttle. Take care to remove the material evenly.
If the shuttle is too loose such that it tilts and chatters during travel, glue a small sliver of transparency film to the shuttle to fill the gap between it and the chassis surface.
Once the shuttle has been properly fit, remove the shuttle from the shaft and press one of the stainless steel shuttle tubes into each of the four large holes in the shuttle. Each should be centered on the shuttle with the ends of all four tubes flush (Figure 3). Secure them with a small drop of cyanoacrylate glue applied to the base of the tube. Be very careful not to allow the glue to get on the edges of the shuttle or into the tubes.
Thread the completed electrode shuttle back on to the drive shaft and bring it all the way down to the base of the threading.
4. Guide Tube and Electrode Installation
Cut four 25 mm long sections of polyimide tubing (0.0045" ID x 0.006" OD) for use as electrode guides. In addition, cut four electrodes to 40 mm sections for use as dummy electrodes which will hold the guide tubes in position during assembly. (Note that these dummy electrodes are for assembly only and will not be used for recording, so you may reuse the same ones for each assembly.) Slide each section of tube onto on to a dummy electrode such that the electrode extends ~10 mm beyond the end of the tube.
Arrange each of the polyimide tubes in the chassis as shown in Figure 4A. This will result in the ends of the electrode guides, flush with each other and aligned to the base of the motor shaft, splaying out toward the electrode shuttle and the opposite ends converging at the bottom of the drive. Mix a small amount of Kwik-Cast and apply approximately 2 mm below the top end of the electrode guides. Allow to dry.
Remove the dummy electrodes and reposition the free ends of the electrode guides such that they form a tight bundle at the bottom of the drive. It may be helpful to use a fine wire to hold them in place temporarily (Figure 4B). Fix the new position with more Kwik-Cast. Allow to dry.
With a sharp scalpel, trim the electrode guide tubes where they extend beyond the bottom of the microdrive. When implanted, these tubes will guide the electrodes from the bottom of the drive (resting on the skull) to the surface of the brain. Thus, the length at which the tubes should be trimmed depends on the anatomy of the implantation site (Figure 5). For songbirds, this is approximately 1.5 mm.
To connect an electrode to the connector, cut a 30 mm long section of insulated platinum wire (0.003" dia.) and strip 1 mm of insulation from one end. Solder it to one of the signal pins on the connector. Bend the wire under the connector and push it through one of the wire guide tubes. Pull the wire taunt and wrap it around the guide slot in the top of the chassis.
With scissors or fine wire cutters, cut an electrode to ~25 mm in length. Take care to do this without bending the electrode or damaging the tip; if either occur, the electrode cannot be used. With the electrode shuttle now at the top of the shaft, insert the electrode into a polyimide tube and pull it up through the stainless steel tube in the shuttle. Position it such that the tip of the electrode is flush with the bottom end of the polyimide tube. Trim the electrode 1 mm above the shuttle tube.
With a fine set of forceps, strip the insulation off the last 2 mm of the electrode and the last 2 mm of the platinum wire. Reposition the electrode in the shuttle tube, insert the platinum wire into the tube, and pin the two together by sliding a short 1 mm section of tungsten wire (0.008" dia) into the tube. This should be a tight fit that will provide both a mechanical and an electrical connection between the electrode and the wire (Figure 3).
Run the shuttle up and down the length of the drive shaft to ensure continued movement.
Repeat 4.5-4.8 until all electrodes have been installed.
Cut a 30 mm long section of silver wire (0.005" dia) for use as a ground wire. Strip 1 mm of insulation off one end and solder to the ground pin.
5. Final Assembly and Pre-Implantation Preparation
To reduce the friction of the electrodes against the guide tubes, fill the guide tubes with light mineral oil. Capillary action is sufficient to fill the tubes from a drop of oil placed at one end.
To create a protective cover for the motor and electrodes, cut a 12 mm x 25 mm rectangle of transparency and fold it into a wide U-shape. Use cyanoacrylate to glue the cover to the outer surfaces of the chassis (Figure 6). Cut a 15 mm x 6 mm rectangle of transparency and use cyanoacrylate to glue it around the connector. To prevent interference with the connector, the long edge of the transparency should be flush with the top edge of the connector.
Prior to implantation, sterilize the electrodes and guide tubes by lowering the extended electrodes into a 10:1 DI water-bleach solution for 10 min. Then, flush thoroughly with sterile water.
A jig similar to that used in step 2.5 can be used to hold the microdrive in a stereotaxic manipulator during implantation.
6. Representative Results
This protocol requires approximately 5 hr of hands-on assembly time with an additional 6-8 hr interspersed for the epoxy and glue to dry. However, once the microdrive has been assembled the first time, it can be prepared for reuse (i.e., the electrodes, electrode guide tubes, and electrode wires can be replaced) in less than 2 hr. The quality and character of the recordings obtained by following this protocol will, of course, be in part dependent on the recording target, the choice of electrodes, and any processing performed at the headstage or further upstream of the microdrive. This aside, it is possible to obtain stable recordings from a behaving animal over a time-span of more than ten weeks. Examples of single unit recordings from the robust nucleus of the archistriatum (RA) in a behaving songbird is shown in Figure 7A; multi-unit activity from the same region is shown in Figure 7B.
 Figure 1. 3D model of the assembled motorized microdrive. The microdrive consists of several major components: (i) the chassis, (ii) motor with threaded output shaft, (iii) electrode shuttle, (iv) shuttle tubes, (v) electrodes, (vi) electrode guide tubes, and (vii) connector.
Figure 1. 3D model of the assembled motorized microdrive. The microdrive consists of several major components: (i) the chassis, (ii) motor with threaded output shaft, (iii) electrode shuttle, (iv) shuttle tubes, (v) electrodes, (vi) electrode guide tubes, and (vii) connector.
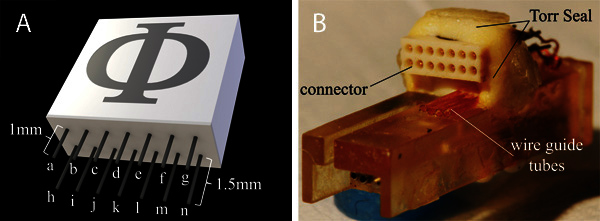 Figure 2. A) Model and connection diagram for the connector. Motor wires: b, i, l. Electrode wires: c,d,e,f. Ground wire: k. All other pins available for auxiliary instrumentation. B) Photo of the connector attached to the chassis with Torr Seal epoxy. Note the position of both the connector and the underlying wire guide tubes. The cyanoacrylate glue holding the guide tubes in position dries clear and, thus, is not visible in the image.
Figure 2. A) Model and connection diagram for the connector. Motor wires: b, i, l. Electrode wires: c,d,e,f. Ground wire: k. All other pins available for auxiliary instrumentation. B) Photo of the connector attached to the chassis with Torr Seal epoxy. Note the position of both the connector and the underlying wire guide tubes. The cyanoacrylate glue holding the guide tubes in position dries clear and, thus, is not visible in the image.
 Figure 3. Electrode shuttle assembly.
Figure 3. Electrode shuttle assembly.
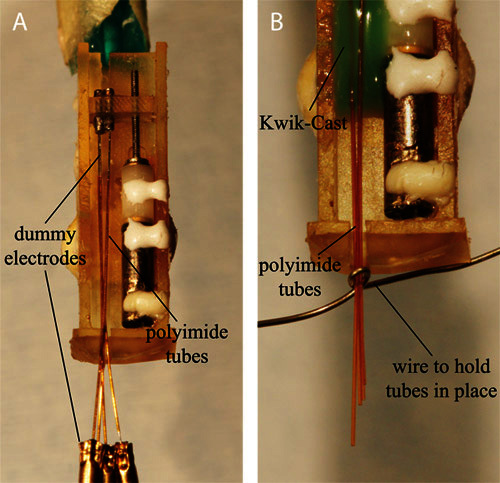 Figure 4. A) The electrode guide tubes positioned in the chassis prior to applying Kwik-Cast. B) To ensure that the electrodes exit the bottom of the drive in parallel, bring the bottom end of the guide tubes into a tight bundle before adding the last drop of the Kwik-Cast.
Figure 4. A) The electrode guide tubes positioned in the chassis prior to applying Kwik-Cast. B) To ensure that the electrodes exit the bottom of the drive in parallel, bring the bottom end of the guide tubes into a tight bundle before adding the last drop of the Kwik-Cast.
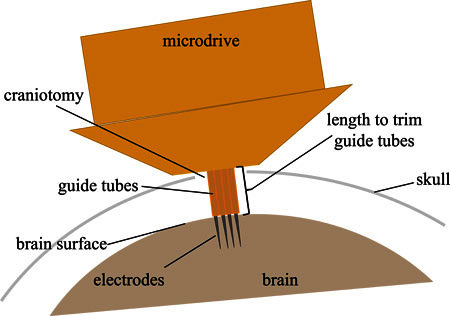 Figure 5. The amount that the electrode guide tubes extend beyond the bottom of the drive is specified by the anatomy of the implantation site. The diagram illustrates the dimensions that are relevant for determining the length of the guide tubes. For implanting in the songbird, 1.5 mm is sufficient.
Figure 5. The amount that the electrode guide tubes extend beyond the bottom of the drive is specified by the anatomy of the implantation site. The diagram illustrates the dimensions that are relevant for determining the length of the guide tubes. For implanting in the songbird, 1.5 mm is sufficient.
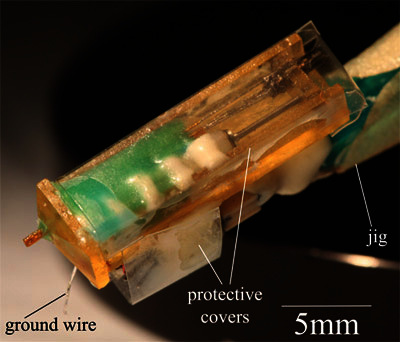 Figure 6. The finished motorized microdrive with protective covers attached.
Figure 6. The finished motorized microdrive with protective covers attached.
 Figure 7. Representative recordings from the robust nucleus of the archistriatum (RA) in the behaving zebra finch. A) Single-unit activity recorded with 10MΩ platinum-iridium electrodes. Top: example recording made one week after implantation. Bottom: example recording from the same electrode as above, nine weeks later. B) Multi-unit activity recorded with 1MΩ platinum electrodes. Click here to view larger figure.
Figure 7. Representative recordings from the robust nucleus of the archistriatum (RA) in the behaving zebra finch. A) Single-unit activity recorded with 10MΩ platinum-iridium electrodes. Top: example recording made one week after implantation. Bottom: example recording from the same electrode as above, nine weeks later. B) Multi-unit activity recorded with 1MΩ platinum electrodes. Click here to view larger figure.
Additional Files: The chassis, shuttle, and electrode tubes were designed with SolidWorks 2010 CAD software. These part files are provided in both the proprietary (*.sldprt) and vendor-neutral (*.iges) formats and dimensioned production drawings are provided in PDF format.
Discussion
The protocol presented here will result in a device capable of high-quality recordings with minimal motion artifacts only if proper care is taken with construction. The fit of the shuttle in the chassis if of critical importance: too tight and the risk of overloading the motor is high; too loose and the risk of significant motion artifact is high. An ideal fit will allow the shuttle to travel the entire length of the threaded shaft without tilting out of position or chattering.
Selection of recording electrodes is similarly important; the choice of material, impedance, insulation, and tip profile may affect tissue reactivity, long-term stability and signal to noise ratio. For our application, we have found that high impedance (5-10MΩ) platinum-iridium microelectrodes produce stable single-unit recordings with a high signal-to-noise ratio (Figure 7); different applications may be better served by other electrodes. Within a relatively large range of electrode choices, a simple change in electrode guide tube sizing is likely the only required modification to adapt this microdrive.
Though recording neural signals can be informative in its own right, much nuanced insight can be gained by merging this with other behavioral or bio-signal data. One advantage of using such an ultra-light design is that it opens up the possibility of adding additional head-mounted sensors or effectors to the animal without fear of overloading. For example, this microdrive may be implanted in conjunction with a microphone, an audio receiver, stimulation electrodes, or microdialysis probes to provide a richer dataset of neural activity in various contexts. The spare contacts on the Omnetics connector (Figure 2A) provide a convenient interface for this additional instrumentation.
Like all electrophysiology devices, the quality of the recordings made with this microdrive are limited by the upstream signal conditioning and data acquisition equipment. Though the specifications for this equipment will be dictated by the requirements of the experiment, it is essential that a headstage preamplifier is employed immediately upstream of the microdrive to amplify the very small currents induced at the tip of the electrode to voltages measurable by standard acquisition instrumentation. There are a variety of commercial products that may be suitable for particular applications, though guidance on custom solutions is available in previously published work.5,12,13
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the Ester and Joseph Klingenstein Fund, the McKnight Endowment Fund, and NINDS 1R01NS066408-01A1.
References
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Berke JD, Graybiel AM, Ito R, Lansink CS, van der Meer M, Redish AD, Smith KS, Voorn P. Corticostriatal Interactions during Learning, Memory Processing, and Decision. 2009;29:12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Zariwala H, Mainen ZF. Neural correlates, computation, and behavioural impact of decision confidence. Nature. 2008;455:227–2231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble Coding of Vocal Control in Birdsong. J. Neurosci. 2005;25(3):652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Wilson MA. Large-scale chronically implantable precision motorized microdrive array for freely behaving animals. J. Neurophysiol. 2008;100(4):2430–2440. doi: 10.1152/jn.90687.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Leonardo A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J. Neurosci. Methods. 2001;112:83–94. doi: 10.1016/s0165-0270(01)00426-5. [DOI] [PubMed] [Google Scholar]
- Ölveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J. Neurophys. 2011;106:386–397. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Ölveczky BP. Effects of Sensory Experience on the Development and Maintenance of a Motor Program Underlying a Complex Motor Sequence. Soc. for Neurosci. Abstr. 2011.
- Goldberg JH, Adler A, Bergman H, Fee MS. Singing-related neural activity distinguishes two putative pallidal cell types in the songbird basal ganglia: comparison to the primate internal and external pallidal segments. J. Neurosci. 2010;30(20):7088–7098. doi: 10.1523/JNEUROSCI.0168-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. Singing-related neural activity distinguishes four classes of putative striatal neurons in the songbird basal ganglia. J. Neurophys. 2010;103(4):2002–2014. doi: 10.1152/jn.01038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam S, Fee MS, Kleinfeld D. Miniature headstage with 6-channel drive and vacuum-assisted microwire implantation for chronic recording from neocortex. J. Neurosci. Methods. 1999;90:37–46. doi: 10.1016/s0165-0270(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Shirvalkar PR, Shapiro ML. Design and Construction of a Cost Effective Headstage for Simultaneous Neural Stimulation and Recording in the Water Maze. J. Vis. Exp. 2010. p. e2155. [DOI] [PMC free article] [PubMed]


