Abstract
Purpose
To evaluate cortical and retinal activity by pattern visual evoked potentials (PVEP) in patients with type II diabetes mellitus.
Methods
PVEP was recorded in 40 diabetic patients including 20 subjects with non-proliferative diabetic retinopathy (NPDR) and 20 others without any retinopathy on fundus photography, and compared to 40 age- and sex-matched normal non-diabetic controls.
Results
P100 wave latency was significantly longer in diabetic patients as compared to normal controls (P<0.001); both diabetic subjects without retinopathy and those with NPDR had significantly longer P100 latency than controls (P<0.001 for both comparisons). There was significant reduction in N75 (P=0.037) and P100 (P=0.001) amplitudes in diabetic subjects. No correlation was observed between VEP amplitude or wave latency, and the level of glycemia or duration of diabetes mellitus.
Conclusion
Increased PVEP latency may be a sign of retinal ganglion cell damage which takes place before the appearance of the first ophthalmoscopically detectable signs of diabetic retinopathy. PVEP may be considered as a method for detecting prediabetic retinopathy and has the potential to reduce diabetic complications.
Keywords: Pattern VEP, Type II Diabetes Mellitus, Diabetic Retinopathy
INTRODUCTION
The prevalence of diabetes mellitus (DM) in adult populations is 6.6% worldwide and an estimated 438 million people will be affected by DM in the year 2030.1 Considering vasculopathy and neuropathy associated with DM, it is reasonable to expect dysfunction to occur along the visual pathway upstream from the retina.2Visual deficits in DM appear to result from both vascular disease and metabolic abnormalities which can affect the retina, optic nerve and visual pathways.3Metabolic abnormalities in DM can involve ganglionic and preganglionic elements in the entire retina and the macular region. In addition, neural conduction may be delayed along post-retinal central visual pathways.4Optic neuropathy manifesting as optic atrophy due to DM alone is estimated to occur in about 0.6% of cases.5
Diabetic retinopathy is a common complication of DM that affects retinal blood vessels. It is the leading cause of new cases of legal blindness in Americans aged 20 to 74 years despite the fact that visual loss due to DM may be preventable by glycemic control or photocoagulation.6-10Unfortunately, in many cases the patient is asymptomatic until it is too late for effective treatment. Growth of new blood vessels, known as proliferative retinopathy, may lead to blindness through hemorrhage and scarring. Deterioration of retinal vasculopathy causes loss of blood vessel integrity with fluid leakage into the retina leading to maculopathy which causes visual impairment and may progress to blindness.11
Visual dysfunction in DM is multifactorial and depends on predominant pathophysiologic factors in various stages of the disease.4One of the primary goals of management in diabetic patients is to avoid the risk of diabetic retinopathy by maintaining blood glucose levels close to the normal range.12Before the onset of microvascular lesions, the neural retina of diabetic eyes undergoes subtle functional changes that are not detectable by fundus photography.13Analysis of pattern VEP responses may provide early diagnosis of such diabetic changes and determine prognosis during treatment.11Pattern VEP (PVEP) can detect any defect from the optic nerve to the occipital cortex.14There have been reports from Western countries showing alterations in PVEP latencies in diabetic patients.15
The current study aims to investigate the ability of PVEP in detecting preclinical neurodegenerative changes in patients with diabetic retinopathy.
METHODS
The study was conducted on three groups of individuals: two groups of diabetic patients with and without non-proliferative retinopathy (NPDR) consisting of 20 subjects in each subgroup, and one group of healthy age- and sex-matched controls including 40 subjects. All subjects underwent a complete ophthalmological examination including measurement of best corrected visual acuity, slit lamp biomicroscopy, direct and indirect ophthalmoscopy and fundus photography after obtaining a complete history including duration of diabetes and latest fasting blood glucose level in diabetic patients. Exclusion criteria consisted of significant ocular disorders including proliferative retinopathy (PDR), cataract, glaucoma, optic nerve disease, macular disease, best corrected visual acuity less than 20/20 and amblyopia.
Diabetic retinopathy was classified by slit lamp biomicroscopy with a 90D lens and dilated fundus photography which was converted to slides for inspection (based on ETDRS classification).16Examinations and testing of diabetic and control subjects was performed during the same time period. The study protocol was approved by the district ethics committee of Mashhad University of Medical Sciences and written informed consent was obtained from all subjects. Subjective visual acuity was measured monocularly. All participants underwent refraction to ensure an exact optical correction.
The PVEP recording equipment consisted of a Roland Reti (Roland Company, ISXEV 60, Berlin, Germany) signal averager connected to a 2 to 8 channel amplifier for storing and summating the waves. The stimulus for this study was a checkerboard with equal black and white checks, 15 minutes of arc in size at a viewing distance of one meter. Mean screen luminance was 100cd/m2 with 99% contrast and a full field display. The temporal frequency was 1.5 Hz (3 reversals per second). Mean luminance of the test room was 80cd/m2 and recording conditions were kept in accordance with International Society of Clinical Electrophysiology of Vision (ISCEV) standards. The amplifier band-pass filters were set at 1-50Hz. In recording the PVEP, the active electrode was positioned one inch above the inion (oz), referencing to the center of the forehead with a ground electrode on the vertex of the head (cz). Inter-electrode impedance was maintained below 5 K Ohm in all recordings. PVEP was recorded monocularly for each subject. In each recording 200 sweeps were averaged. All electrophysiological tests were performed at the Electrophysiology Laboratory of the Optometry Clinic at Mashhad University of Medical Sciences. All tests were performed with the subjects wearing best refractive correction.
Data analysis was performed using SPSS software version 11.5 (SPSS Inc., Chicago, USA) utilizing Spearman's correlation coefficient, t-test, Mann-Whitney test, one-way analysis of variances (ANOVA), Kruskal-Wallis test and Tukey test. Significance level was set at P<0.05.
RESULTS
Overall 80 patients including 20 diabetic subjects with NPDR, 20 individuals without diabetic retinopathy, and 40 matched normal controls were evaluated. Mean age was 54.8±8.2 years in diabetic subjects with NPDR, 51.6±10.7 years in diabetics without retinopathy and 51.3±8.5 years in controls (P=0.113). Fifty percent of diabetics with NPDR, 65% of diabetics without retinopathy and 60% of controls were female (P=0.375).
As detailed in Table 1, there was a significant difference between diabetic subjects and controls in terms of P100 latency (P<0.001), N75 amplitude (P=0.037) and P100 amplitude (P=0.001). However, there was no significant difference between the two groups regarding N75 latency (P=0.77).
Table 1.
Mean and standard deviation for N75 and P100 latency and amplitude in the study groups
| Diabetics | Controls | P-value | Statistical Test | |
|---|---|---|---|---|
| N75 latency (ms) | 80.73±10.00 | 81.55±7.85 | 0.77 | Mann-Whitney |
| t-Test | ||||
| N75 amplitude (µv) | 8.58 ± 4.72 | 10.24±5.20 | 0.037 | t-Test |
| P100 latency (ms) | 120.52±7.71 | 111.65±6.22 | <0.001 | t-Test |
| P100 amplitude (µv) | 7.08±5.92 | 9.60±6.02 | 0.001 | Mann-Whitney |
ms, millisecond; μv, microvolt
One-way ANOVA was used to test significant differences between the three groups. Significant differences between each paired groups were then evaluated by Post Hoc Tukey test (in case of data with normal distribution). Kruskal-Wallis test was performed to test differences among all the three groups. Significant differences between each paired group were then evaluated by Mann-Whitney test (for data without normal distribution). The results are shown in Table 2. Differences between diabetics with and without retinopathy was statistically significant in terms of P100 latency and amplitude and N75 amplitude (P<0.001). Differences between diabetics without retinopathy and controls was also significant regarding P100 latency (P<0.001). The differences between diabetics with retinopathy and controls were significant in terms of P100 latency and amplitude and N75 amplitude (P<0.001).
Table 2.
Mean and standard deviation for N75 and P100 latency and amplitude in the study groups
| WithoutNPDR | WithNPDR | Controls | P-value | Statistical Test | |
|---|---|---|---|---|---|
| N75latency (ms) | 83.80±6.88 | 77.67±11.67 | 81.55±7.85 | 0.080 | Kruskal-Wallis |
| N75amplitude (µv) | 10.77±5.34 | 6.39±2.60 | 10.24±5.20 | <0.001 | ANOVA |
| P100latency (ms) | 117.92±7.69 | 123.12±6.90 | 111.65±6.22 | <0.001 | ANOVA |
| P100amplitude (µv) | 9.60±6.63 | 4.56±3.75 | 9.60±6.02 | <0.001 | Kruskal-Wallis |
NPDR, non-proliferative diabetic retinopathy; ms, millisecond; μv, microvolt
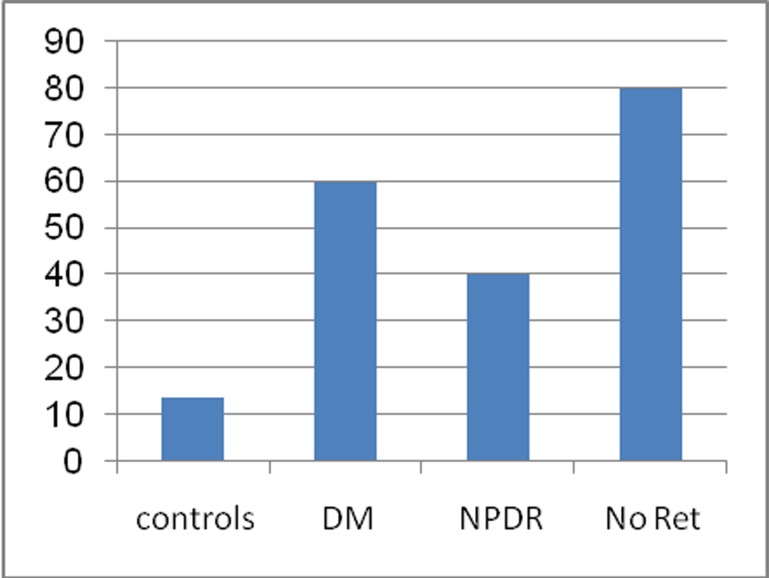
Spearman's test revealed no correlation between VEP wave amplitude or latency, and level of glycemia or duration of diabetes mellitus (Table 3). P100 latency above the normal range was present in 60% of all diabetic patients, 40% of diabetics without retinopathy, 80% of those with NPDR, and 13.75% of controls (Fig. 1).
Table 3.
Spearman’s test for determining correlation between PVEP parameters and duration of diabetes and fasting blood sugar level in diabetic patients
| Duration of diabetes |
Fastingblood sugar level |
|||
|---|---|---|---|---|
| Correlation Coefficient | P-value | Correlation Coefficient | P-value | |
| N75latency (ms) | -0.009 | 0.933 | -0.045 | 0.692 |
| N75amplitude (µv) | -0.181 | 0.108 | -0.014 | 0.900 |
| P100latency (ms) | 0.085 | 0.455 | -0.050 | 0.662 |
| P100amplitude (µv) | -0.163 | 0.150 | 0.076 | 0.503 |
PVEP, pattern visual evoked potentials; ms, millisecond; μv, microvolt
Figure 1.
Percentage of P100 latency abnormalities in the study groups (DM, diabetes mellitus; NPDR, nonproliferative diabetic retinopathy; Ret, retinopathy).
DISCUSSION
PVEP is a simple, sensitive and objective technique for evaluating impulse conduction along the visual pathways. PVEP abnormalities have been described in DM, but the proportion of patients with increased P100 latency is quite variable, ranging from 9% to 77%.17-22This high variability could be explained by several factors, such as criteria for inclusion or diagnosis, the presence of retinopathy or peripheral polyneuropathy and differences in stimulus recording conditions. Algan et al17 reported prolonged P100 latency in 50 DM patients, six of whom had diabetic retinopathy. In 19 subjects with type II DM, they showed an increase in P100 latency. Mariani et al19 reported prolongation of P100 latency in 35 diabetic patients who did not have retinopathy. Ponte et al20 reported prolongation of PVEP latencies in 50 asymptomatic insulin dependent diabetic patients without retinopathy. Puvanendran et al18, Cirillo et al21 and Anastazi et al22 also reported PVEP abnormalities in diabetic patients. Although Collier et al23 found PVEP abnormalities in diabetic patients with retinopathy, they found no abnormalities in patients without retinopathy. However their sample size was small. Yaltkaya et al24 found prolongation of N140 latency and N90-N140 interpeak latencies as well as increased P100 latency. They explained these findings by the presence of retrochiasmal involvement. Millinger et al25 reported similar findings. They stated that abnormal PVEPs could reflect papillomacular bundle or optic nerve involvement. Bortec et al26 found PVEP abnormalities in 77% of diabetic patients and reported that abnormalities did not correlate with level of retinopathy. Lanting et al27 investigated pupillary light reflex latency and P100 latency in 42 diabetic patients and found that pupillary light reflex latency was prolonged in 55%, and P100 latency was increased in 19% of subjects. There was no correlation between diabetic retinopathy and pupillary light reflex latency or P100 latency. Moreo et al28 reported that P100 wave latency increases in non-insulin dependent diabetic patients.
In this study, we found significantly longer P100 wave latencies in diabetic patients as compared to controls. Abnormal latencies were found in 60% of diabetic patients which is consistent with other studies reporting this finding in 15%29, 20%30 and 62.5%18 of diabetic subjects.
Our results indicate that optic nerve involvement may develop in patients with type II DM prior to the onset of symptoms. Prolongation of P100 latencies observed in diabetics is an expression of structural damage at the level of myelinated optic nerve fibers. This may be due to different pathogenic mechanisms underlying peripheral nerve versus optic pathway involvement. Our results also imply that there is a definite neurological deficit in type 2 DM which can involve the central nervous system at a much earlier stage. The pathophysiology of central nervous system dysfunction is not clear but is multifactorial, involving metabolic and vascular factors, similar to the pathogenesis of diabetic peripheral neuropathy in which ischemia and reduced protein synthesis may result in nerve fiber loss in peripheral nerves. It is also possible that optic nerve fibers suffer from similar diabetes induced changes. Accumulation of these mediators probably delays conduction in the visual pathway, which may cause the observed delay in latencies found in diabetic subjects as compared to healthy controls.
It has been reported that PVEP abnormalities correlate with hyperglycemia31 but we found no significant correlation between blood glucose levels and P100 wave latencies in diabetic patients. There are some conflicting reports regarding the correlation between duration of diabetes and P100 wave latencies32 but we found no significant correlation between the duration of diabetes and P100 wave latencies.
In conclusion, PVEP latency in diabetic patients with or without NPDR is significantly delayed as compared to non-diabetic controls. Importantly, even in patients without retinopathy, PVEP can detect preclinical microvascular and/or neurodegenerative changes within or upstream the retina.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Silink M. IDF Diabetes Atlas. 4th. Brussels: International Diabetes Federation; 2009. [Google Scholar]
- 2.Biessels GJ, Koffeman A, Scheltens P. Diabetes and cognitive impairment. Clinical diagnosis and brain imaging in patients attending a memory clinic. J Neurol. 2006;253:477–482. doi: 10.1007/s00415-005-0036-4. [DOI] [PubMed] [Google Scholar]
- 3.Algan M, Ziegler O, Gehin P, Got I, Raspiller A, Weber M, et al. Visual evoked potentials in diabetic patients. Diabetes Care. 1989;12:227–229. doi: 10.2337/diacare.12.3.227. [DOI] [PubMed] [Google Scholar]
- 4.Gregori B, Galie E, Pro S, Clementi A, Accornero N. Luminance and chromatic visual evoked potentials in type I and II diabetes: relationships with peripheral neuropathy. Neurol Sci. 2006;27:323–327. doi: 10.1007/s10072-006-0704-x. [DOI] [PubMed] [Google Scholar]
- 5.Locke S. The nervous system and diabetes. In: Marble A, et al., editors. Joslin's Diabetes Mellitus. 11th ed. Philadelphia: Lea and Febiger; 1971. pp. 562–564. [Google Scholar]
- 6.Fankhauser F, Enoch J, Cibis P. Receptor orientation in retinal pathology. A first study. Am J Ophthalmol. 1961;52:767–783. doi: 10.1016/0002-9394(61)90901-1. [DOI] [PubMed] [Google Scholar]
- 7.Flynn HT, Smiddy WE. Diabetes and ocular disease: past, present, and future therapies. Ophthalmology monographs 14. San Francisco: American Academy of Ophthalmology; 2000. [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98:766–785. [PubMed] [Google Scholar]
- 10.The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 11.Sivakumar R, Ravindran G, Muthayya M, Lakshminarayanan S, Velmurughendran CU. Diabetic retinopathy analysis. J Biomed Biotechnol. 2005;2005:20–27. doi: 10.1155/S1110724304310016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scanlon PH, Foy C, Chen FK. Visual acuity measurement and ocular co-morbidity in diabetic retinopathy screening. Br J Ophthalmol. 2008;92:775–778. doi: 10.1136/bjo.2007.128561. [DOI] [PubMed] [Google Scholar]
- 13.Lieth E, Gardner TW, Barber AJ, Antonetti DA. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000;28:3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 14.Heravian J, Daneshvar R, Dashti F, Azimi A, Ostadi-Moghaddam H, Yekta AA, et al. Simultaneous pattern visual evoked potential and pattern electroretinogram in strabismic and anisometropic amblyopia. IRCMJ. 2011;13:21–26. [PMC free article] [PubMed] [Google Scholar]
- 15.Moreo G, Mariani E, Pizzamiglio G, Colucci GB. Visual evoked potential in NIDDM: a longitudinal study. Diabetologia. 1995;38:573–576. doi: 10.1007/BF00400726. [DOI] [PubMed] [Google Scholar]
- 16.Retina and vitreous, Section 9.Basic and clinical science course. American Academy of Ophthalmology. American Academy of Ophthalmology; San Francisco: 2004. pp. 99–119. [Google Scholar]
- 17.Algan M, Ziegler O, Gehin P, Got I, Raspiller A, Weber M, et al. Visual evoked potentials in diabetic patients. Diabetes Care. 1989;12:227–229. doi: 10.2337/diacare.12.3.227. [DOI] [PubMed] [Google Scholar]
- 18.Puvanendran K, Devathasan G, Wong PK. Visual evoked responses in diabetes. J Neurol Neurosurg Psychiatry. 1983;46:643–647. doi: 10.1136/jnnp.46.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariani E, Moreo G, Colucci GB. Study of visual evoked potentials in diabetics without retinopathy: correlations with clinical findings and polyneuropathy. Acta Neurol Scand. 1990;81:337–340. doi: 10.1111/j.1600-0404.1990.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 20.Ponte F, Giuffrè G, Anastasi M, Lauricella M. Involvement of the visual evoked potentials in type I insulin-dependent diabetes. Metab Pediatr Syst Ophthalmol. 1986;9:77–80. [PubMed] [Google Scholar]
- 21.Cirillo D, Gonfiantini E, De Grandis D, Bongiovanni L, Robert JJ, Pinelli L. Visual evoked potentials in diabetic children and adelescents. Diabetes Care. 1984;7:273–275. doi: 10.2337/diacare.7.3.273. [DOI] [PubMed] [Google Scholar]
- 22.Anastasi M, Lodats G, Cillino S. VECPs and optic disc damage in diabetes. Doc Ophthalmol. 1987;66:331–336. doi: 10.1007/BF00213661. [DOI] [PubMed] [Google Scholar]
- 23.Collier A, Reid W, McInnes A, Cull RE, Ewing DJ, Clarke BF. Somatosensory and visual evoked potentials in insulin-dependent diabetics with mild peripheral neuropathy. Diabetes Res Clin Pract. 1988;5:171–175. doi: 10.1016/s0168-8227(88)80084-6. [DOI] [PubMed] [Google Scholar]
- 24.Yaltkaya K, Balkan S, Baysal AI. Visual evoked potentials in diabetes mellitus. Acta Neurol Scand. 1988;77:239–241. doi: 10.1111/j.1600-0404.1988.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 25.Millingen KS, Yeo PT, Kamaldeen S. Visual evoked responses in diabetes. Clin Exp Neurol. 1987;24:153–158. [PubMed] [Google Scholar]
- 26.Bártek L, Gat'ková A, Rybka J, Kalita Z, Smecka Z. Visual evoked potentials in diabetics. Cesk Ophthalmol. 1989;45:192–196. [PubMed] [Google Scholar]
- 27.Lanting P, Strijers RL, Bos JE, Faes TJ, Heimans JJ. The cause of increased pupillary light reflex latencies in diabetic patients: the relationship between pupillary light reflex and visual evoked potential latencies. Electroencephalogr Clin Neurophysiol. 1991;78:111–115. doi: 10.1016/0013-4694(91)90110-p. [DOI] [PubMed] [Google Scholar]
- 28.Moreo G, Mariani E, Pizzamiglio G, Culucci GB. Visual evoked potentials in NIDDM: a longitudinal study. Diabetologia. 1995;38:573–576. doi: 10.1007/BF00400726. [DOI] [PubMed] [Google Scholar]
- 29.Khardori R, Soler NG, Good DC, DevlescHoward AB, Broughton D, Walbert J. Brainstem auditory and visual evoked potentials in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1986;29:362–365. doi: 10.1007/BF00903345. [DOI] [PubMed] [Google Scholar]
- 30.Ponte F, Anastasi M, Lauricella M, Bompiani GD. Optic pathway conduction in insulin-dependent diabetics. Doc Ophthalmol. 1986;63:313–319. doi: 10.1007/BF00220221. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Yang Y. Pattern reversal visual evoked potentials analysis in patients with noninsulin-dependent diabetes mellitus. Hunan Yi Ke Da Xue Xue Bao. 2001;26:283–284. [PubMed] [Google Scholar]
- 32.Chopra D, Gupta M, Manchanda KC, Sharma RS, Sidhu RS. A study of visual evoked potentials in patients of type 2 diabetes mellitus. JCDR. 2011;5:519–522. [Google Scholar]