Abstract
Background
Mexican Americans (MAs) have shown lower post-stroke mortality compared to non-Hispanic whites (NHWs). Limited evidence suggests race/ethnic differences exist in intensive care unit (ICU)admissions following stroke. Our objective was to investigate the association of ethnicity with admission to the ICU following stroke.
Methods
Cases of intracerebral hemorrhage and acute ischemic stroke were prospectively ascertained as part of the Brain Attack Surveillance in Corpus Christi (BASIC) project for the period January, 2000 through December, 2009. Logistic regression models fitted within the generalized additive model framework were used to test associations between ethnicity and ICU admission and potential confounders. An interaction term between age and ethnicity was investigated in the final model.
Results
A total 1,464 cases were included in analysis. MAs were younger, more likely to have diabetes, and less likely to have atrial fibrillation, health insurance, or high school diploma than NHWs. On unadjusted analysis, there was a trend toward MAs being more likely to be admitted to ICU than NHWs (34.6% versus 30.3%; OR=1.22; 95% CI 0.98–1.52; p=0.08). However, on adjusted analysis, no overall association between MA ethnicity and ICU admission (OR=1.13; 95% CI 0.85–1.50) was found. When an interaction term for age and ethnicity was added to this model, there was only borderline evidence for effect modification by age of the ethnicity/ICU relationship (p=0.16).
Conclusions
No overall association between ethnicity and ICU admission was observed in this community. ICU utilization alone does not likely explain ethnic differences in survival following stroke between MAs and NHWs.
Keywords: Intracerebral hemorrhage, Acute ischemic stroke, Mexican Americans, Intensive care unit
Introduction
Multiple studies have demonstrated that minorities are more likely than non-Hispanic whites(NHWs) to utilize life sustaining measures during critical illness [1–4]. However, only a few studies have investigated possible race/ethnic differences in intensive care unit(ICU) admission [1, 5–9]. These studies are conflicting and it is unclear whether differential utilization of the ICU exists between minorities and NHWs during critical illness [1, 5–9]. Even less attention has been given to ICU admission specifically after stroke [7–9]. Existing studies have focused on comparisons between African Americans and NHWs and suggest either that there is no racial difference in ICU admission or that African Americans are less likely than NHWs to be admitted to the ICU [2, 7, 8]. Little is known about possible differences in ICU admission between Mexican Americans (MAs) and NHWs after stroke. MAs represent the largest sub-population of Hispanics, the largest minority group in the United States [10].
Compared to NHWs, MAs have an increased incidence of stroke, greater burden of risk factors such as diabetes, but a paradoxically lower post-stroke mortality [11, 12]. Differences in intensity of medical treatment could be one possible explanation for this paradox. We have previously shown that MAs are less likely to have a do-not-resuscitate order following intracerebral hemorrhage than NHWs; however, ethnic differences in other indices of intensity of medical treatment following stroke have not been well-studied [13].Patients with stroke are often admitted to the ICU for close monitoring and treatment of their brain injury or management of concurrent medical illness. Thus, ICU utilization could be viewed as one index of intensity of medical treatment following stroke.
The objectives of this study were to investigate the association of ethnicity with admission to the ICU after stroke in a bi-ethnic, population-based study and explore factors that may confound this association. We also explored whether the association between ethnicity and ICU admission was modified by age, based on a hypotheses that any ethnic differences would be less prominent at younger ages when most individuals would tend to opt for full intensive care. Better understanding of possible ethnic differences in ICU admission might help explain the survival advantage of MAs compared to NHWs following stroke and provide further data on whether previously observed ethnic differences in do-not-resuscitate orders extend to other indices of treatment intensity [12, 14].
Materials and Methods
Case Identification and Data Collection
The study population included individuals with acute ischemic stroke or intracerebral hemorrhage who were identified as part of the Brain Attack Surveillance in Corpus Christi (BASIC) project between January 1, 2000 and December 31, 2009. The BASIC project is an ongoing population-based stroke surveillance study conducted in Corpus Christi, Nueces County, Texas. Ninety percent of the county’s population resides within the city of Corpus Christi. Nueces County is an urban, bi-ethnic, non-immigrant community located over 120 miles from San Antonio and Houston. This distance affords complete case capture for initial stroke contact. The community is devoid of an academic medical center and none of the 7 area hospitals have specialized neuro-intensive care units. The project’s surveillance techniques and study methods have been previously published [12, 15]. Briefly, active and passive surveillance are used to identify all patients evaluated for stroke who present to any emergency department or hospital. Board-certified neurologists blinded to patient age and ethnicity validated all cases using source documentation, published criteria, and definitions. If an individual had multiple hospitalizations during the study period, only the first hospitalization was included in this analysis. Ethnicity was determined from the medical record, as we have previously identified good agreement (kappa = 0.94) between medical record and patient self-reported ethnicity in this community [12, 15]. A small number of patients with race/ethnicity other than MA or NHW were excluded. National Institutes of Health Stroke Scale (NIHSS) score was collected from the medical record or abstracted from the chart based on a previously validated method [16]. Glasgow Coma Scale (GCS) score was similarly obtained from the medical record or retrospectively abstracted from data in the chart if not available. Excessive alcohol use was ascertained from the medical record.
During the time of the study, two methods were used to identify subjects that had an extended abstraction. From January 1, 2000 to June 30, 2007,a random selection of subjects were asked to participate in an interview and a random sample of those interviewed had an extended abstraction. From July 1, 2007 to December 21, 2009, all subjects were approached for an interview and all subjects that participated in the interview had an extended abstraction. The extended abstraction includes information on whether the individual was admitted to the ICU at any point during their hospitalization. Only patients with an extended abstraction were included in our analysis, as ICU admission status would otherwise not have been recorded. Patients with subarachnoid hemorrhage were excluded from analysis due to the expected frequent admission to the ICU as part of routine care.
Statistical Methods
Baseline characteristics were tabulated by ethnicity and by ICU admission status and summarized as frequency and percent for categorical variables and by mean and standard deviation or median and interquartile range (IQR) for continuous variables. Categorical variables were compared by ethnicity and ICU admission status using chi-square statistics. Continuous variables were compared by ethnicity or ICU admission status by Kruskal-Wallis non-parametric tests owing to the skewedness of some variables. The distribution of each variable was examined. Glucose levels demonstrated a particularly skewed distribution with several outliers at higher values; therefore, values greater than the 99th percentile were re-coded to the 99th percentile value (438 mg/dL).
Potential confounders of the relationship between ethnicity and ICU admission were identified in a 2-stage process starting with an a priori list of variables expected to be associated with ICU admission or ethnicity based on known associations or biological plausibility (Table 1). Potential confounders were identified as variables that were associated with both ethnicity and ICU admission in unadjusted analysis using a liberal cut-point for significance (p<~0.2). Next, we constructed separate bivariate logistic regression models predicting ICU admission with ethnicity and each of the potential confounding variables included as covariates. We calculated the percent change in the odds ratio (OR) from the unadjusted association between ethnicity and ICU admission to the adjusted OR for ethnicity in each bivariate model after inclusion of the potential confounder. Confounders were defined as variables that altered the OR for the association between ethnicity and ICU admission by more than 10%. The logistic models were fitted within the generalized additive model (GAM) framework. This approach allows the model to flexibly adjust for a continuous variable without forcing a linear form, since we suspected that certain predictor variables (such as mean arterial pressure) would have a non-linear U-shaped association with admission to the ICU.
Table1.
Patient demographics and clinical characteristics by ethnicity and ICU admission status (January 1, 2000 to December 31, 2009)
| Ethnicity | ICU Admission Status | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Overall N=1464 |
NHW n=657 |
MA n=807 |
P-value | Not Admitted n=986 |
Admitted n=478 |
P-value |
| Admitted to ICU | 478 (32.7%) | 199 (30.3%) | 279 (34.6%) | 0.08 | - | - | - |
| Mexican American | 807 (55.1%) | - | - | - | 528 (53.6%) | 279 (58.4%) | 0.08 |
| Mean age(SD) | 71.0 (12.8) | 74.0 (12.3) | 68.7 (12.7) | <0.001 | 72.1 (12.6) | 68.9 (13.0) | <0.001 |
| Female | 757 (51.7%) | 343 (52.2%) | 414 (51.3%) | 0.73 | 509 (51.6%) | 248 (51.9%) | 0.93 |
| Intracerebral hemorrhage | 231 (15.8%) | 102 (15.5%) | 129 (16.0%) | 0.81 | 59 (6.0%) | 172 (36.0%) | <0.001 |
| Hypertension* | 1114 (76.2 %) | 489 (74.5%) | 625 (77.5%) | 0.18 | 742 (75.3%) | 372 (78.2%) | 0.22 |
| Coronary artery disease* | 460 (31.5%) | 216 (32.9%) | 244 (30.3%) | 0.28 | 307 (31.1%) | 153 (32.1%) | 0.7 |
| Atrial fibrillation* | 184 (12.6%) | 104 (15.9%) | 80 (9.9%) | <0.001 | 117 (11.9%) | 67 (14.1%) | 0.23 |
| Diabetes* | 586 (40.1%) | 179 (27.3%) | 407 (50.5%) | <0.001 | 409 (41.5%) | 177 (37.2%) | 0.12 |
| Hyperlipidemia* | 456 (31.2%) | 201 (30.6%) | 255 (31.6%) | 0.68 | 318 (32.3%) | 138 (29.0%) | 0.21 |
| Prior stroke or TIA* | 419 (28.7%) | 194 (29.6%) | 225 (27.9%) | 0.49 | 290 (29.4%) | 129 (27.1%) | 0.36 |
| Current smoker* | 264 (18.4%) | 121 (18.9%) | 143 (18.0%) | 0.67 | 179 (18.6%) | 85 (18.2%) | 0.86 |
| Excessive alcohol use* | 89 (6.1%) | 36 (5.5%) | 53 (6.6%) | 0.40 | 52 (5.3%) | 37 (7.8%) | 0.06 |
| Treated with tPA | 46 (3.1%) | 25 (3.8%) | 21 (2.6%) | 0.19 | 3 (0.3%) | 43 (9.0%) | <0.001 |
| Insurance coverage | 1316 (89.9%) | 617 (93.9%) | 699 (86.6%) | <0.001 | 902 (91.5%) | 414 (86.6%) | 0.004 |
| High school education* | 802 (55.4%) | 520 (80.1%) | 282 (35.3%) | <0.001 | 550 (56.4%) | 252 (53.3%) | 0.27 |
| Mean arterial pressure, mm HG(SD) | 112.6 (21.4) | 112.2 (20.9) | 112.9 (21.8) | 0.63 | 110.3 (19.2) | 117.4 (24.8) | <0.001 |
| Median glucose(IQR)* | 129 | 123 | 137 | <0.001 | 124 | 136 | <0.001 |
| (107, 172) | (103, 155) | (110, 194) | (104, 168) | (112, 181) | |||
| Median NIHSS score* | 4 (2, 9) | 4 (2, 10) | 4 (2, 8) | <0.001 | 3 (2, 7) | 8 (3, 15) | 0.04 |
| Median Glasgow Coma Scale score* | 15 (14, 15) | 15 (14, 15) | 15 (14,15) | 0.16 | 15 (14, 15) | 14 (10, 15) | <0.001 |
ICU, intensive care unit; IQR, interquartile range; MA, Mexican American; NHW, non-Hispanic White; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TIA, transient ischemic attack; tPA, tissue plasminogen activator.
Hypertension, coronary artery disease, atrial fibrillation, diabetes, hyperlipidemia, prior stroke or TIA, median NIHSS score and Median Glasgow Coma Scale Score all had 2 missing values; current smoker, 31 missing values; excessive alcohol use, 5 missing values; high school education, 15 missing values; median glucose, 55 missing values.
A final multivariable model was constructed using confounders identified from the prior 2-stage process as well as variables associated with ICU admission on unadjusted analysis (at p<0.2). A GAM approach was again used for continuous variables. GCS score and glucose did not have significant deviation from linear associations with ICU admission, so these variables were entered into the final model as linear terms. Based on an a priori hypothesis that age may modify the association between ethnicity and ICU admission, an interaction term between age (centered at the mean value) and ethnicity was also investigated in the final model. A plot demonstrating the interaction of age and ethnicity was generated. Statistical analysis was performed using SAS software, version 9.2 (SAS Institute, Cary, NC) and R 2.13.1 for Windows. This project was approved by the Institutional Review Board of the University of Michigan and the individual Corpus Christi hospitals.
Results
During the study period, BASIC identified a total of 4,077 cases of acute ischemic stroke and intracerebral hemorrhage. Of these, 1,464 were randomly chosen for extended chart abstractions and were included in the analysis. There was no difference between the study population and those who did not undergo extended abstraction in age (p = 0.93), ethnicity (p = 0.69) gender (p = 0.31), NIHSS (p=0.30), or the proportion with GCS less than or equal to 8 (p=0.50). Demographic and baseline characteristics by ethnicity and by ICU admission status are shown in Table 1. MAs were younger, more likely to have diabetes, and less likely to have atrial fibrillation, health insurance, and less likely to have completed a high school education than NHWs. There was a trend toward MAs being more likely to be admitted to the ICU than NHWs in unadjusted analysis (34.6% versus 30.3%; OR = 1.22; 95% CI 0.98–1.52; p = 0.08; Table 1).
The analysis of potential confounders of the ethnicity/ICU relationship is shown in Table 2. Each row displays the OR for the association between ethnicity and ICU admission, when adjusted for each of the potential confounding variables individually in a bivariate model. Of the potential confounding variables identified on unadjusted analysis (Table 1), only age, NIHSS score, and glucose approached our pre-specified definition of a confounder (a 10% change in the OR). The relationship between ethnicity and ICU admission was attenuated when including either age or glucose in the model. In contrast, adjusting for NIHSS score strengthened the association between ethnicity and ICU admission and the relationship became significant (OR =1.33; 95% CI 1.05–1.69).
Table 2.
Bivariate models of potential confounders in the ethnicity/ICU relationship
| Potential Confounding Variables |
Odds Ratio for MA |
95% Confidence Interval |
P-value for MA |
% Change in MA Odds Ratio with Adjustment |
|---|---|---|---|---|
| None* | 1.22 | 0.98–1.52 | 0.08 | NA |
| Age† | 1.10 | 0.88–1.38 | 0.41 | −9.54% |
| NIHSS score | 1.33 | 1.05–1.69 | 0.02 | 9.37% |
| Glucose | 1.11 | 0.88–1.39 | 0.39 | −9.13% |
| Hypertension | 1.21 | 0.97–1.51 | 0.09 | −0.25% |
| Atrial fibrillation | 1.24 | 0.99–1.54 | 0.06 | 1.56% |
| Diabetes | 1.29 | 1.03–1.62 | 0.03 | 5.92% |
| Treated with tPA | 1.28 | 1.02–1.60 | 0.04 | 5.02% |
| Insurance | 1.17 | 0.94–1.47 | 0.16 | −3.54% |
| High school education | 1.16 | 0.91–1.50 | 0.23 | −4.36% |
| Glasgow Coma Scale score | 1.29 | 1.02–1.62 | 0.03 | 5.76% |
CI, confidence interval; ICU, intensive care unit; MA, Mexican American; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator
Unadjusted model with MA as the only predictor of ICU admission
Note that this association should be interpreted with caution due to possible effect modification of age on the ethnicity/ICU relationship. See Table 3 and text for additional details.
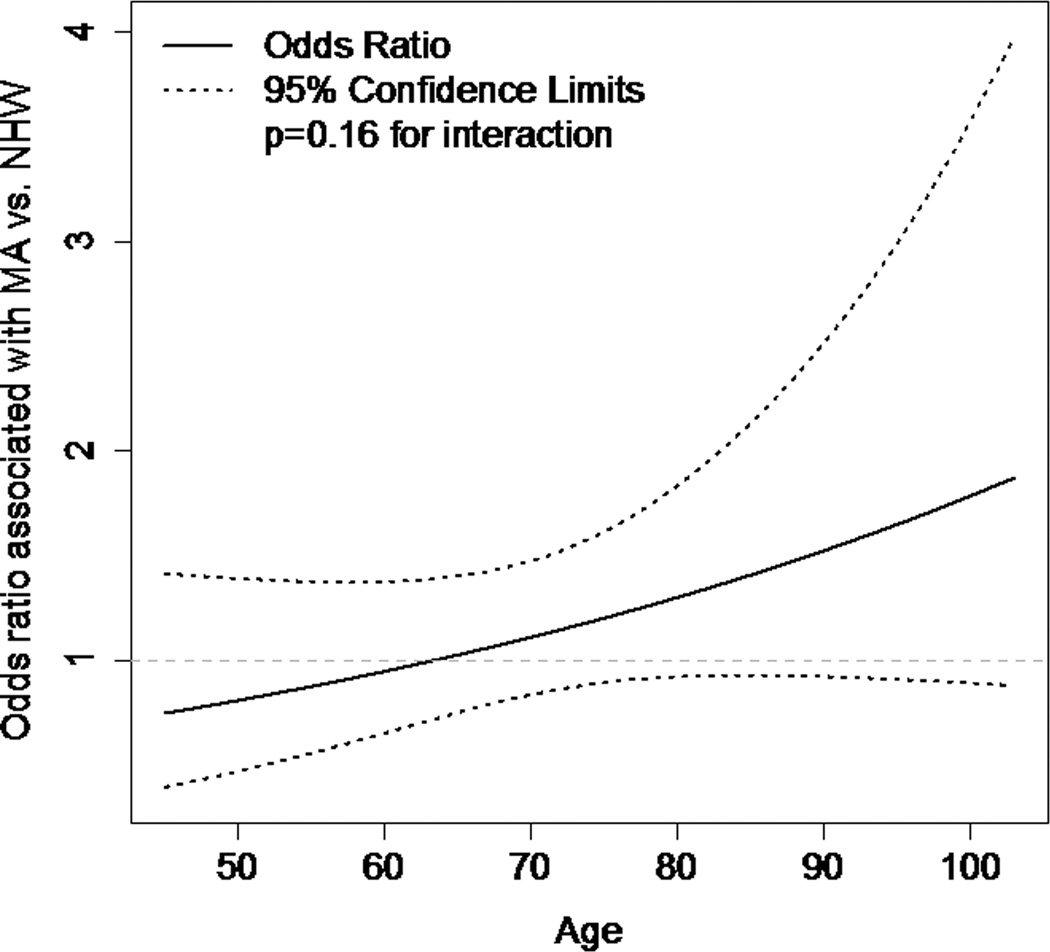
An adjusted model containing the identified confounders of the ethnicity/ICU relationship and factors independently associated with ICU admission found no overall association between MA ethnicity and ICU admission (OR = 1.13; 95% CI 0.85–1.50). When an interaction term for age and ethnicity was added to this model, there was only borderline evidence for effect modification by age of the ethnicity/ICU relationship (p = 0.16). There did not appear to be an association between ethnicity and ICU admission at younger ages (age 56, MA OR = 0.89; 95% CI 0.58–1.37), though there was a trend toward MAs being more likely to be admitted to the ICU at older ages (age 86, MA OR = 1.43; 95% CI 0.93–2.20). The results of this final model with the interaction term are shown in Table 3, and the interaction is shown graphically in Figure 1.
Table 3.
Final model including confounders, independent predictors of ICU admission, and interaction between age and Mexican- American ethnicity
| Odds Ratio | 95% Confidence Interval |
P-value | |
|---|---|---|---|
| Age/MA ethnicity interaction term | 0.16 | ||
| MA at age = 56(mean minus 15) | 0.89 | 0.58– 1.37 | |
| MA at mean age= 71 | 1.13 | 0.85– 1.50 | |
| MA at age = 86(mean plus 15) | 1.43 | 0.93– 2.20 | |
| Age effect for MA, 10- year increase | 0.82 | 0.70– 0.96 | |
| Age effect for NHW, 10- year increase | 0.70 | 0.59– 0.83 | |
| Glucose* | 1.00 | 1.00– 1.00 | 0.82 |
| Intracerebral hemorrhage(ref. ischemic stroke) | 7.44 | 5.08– 10.88 | <0.001 |
| tPA | 36.38 | 10.94– 120.91 | <0.001 |
| Insurance | 0.70 | 0.45– 1.11 | 0.13 |
| Glasgow Coma Scale score | 0.90 | 0.85– 0.97 | 0.003 |
| Diabetes | 0.90 | 0.65– 1.22 | 0.49 |
| Excessive alcohol use | 0.98 | 0.57– 1.69 | 0.94 |
| NIHSS score (spline, df=2.8) | <0.001 | ||
| 10 vs. 0 | 2.82 | 1.17– 6.83 | |
| 20 vs. 10 | 1.76 | 0.72– 4.42 | |
| 30 vs. 20 | 0.64 | 0.24– 1.76 | |
| MAP (spline, df=2.5) | 0.01 | ||
| 100 vs. 70 mm Hg | 0.74 | 0.29– 1.89 | |
| 130 vs. 100 mm Hg | 1.33 | 0.64– 2.73 |
df, degrees of freedom; ICU, intensive care unit; MA, Mexican American; MAP, mean arterial pressure; NHW, non- Hispanic White; NIHSS, National Institutes of Health Stroke Scale; ref., reference; tPA, tissue plasminogen activator
Values over 99th percentile re-coded to 99th percentile value due to skewed distribution
Figure 1.
Effect modification of age on the ethnicity/ICU relationship.
Discussion
We did not find an association between ethnicity and ICU admission following stroke between MAs and NHWs in the Corpus Christi community. The borderline association between ethnicity and ICU admission on unadjusted analysis was eliminated when adjusting for confounders and independent predictors of ICU admission. Previous studies focusing on race/ethnic differences in utilization of the ICU following stroke are limited and have focused on differences between African Americans and NHWs with conflicting results[7–9]. Based on our results, it seems unlikely that differences in ICU utilization are a major contributor to the overall survival advantage of MAs compared with NHWs after stroke [14].
We examined potential confounding variables individually to determine whether the potential confounder strengthened or attenuated the relationship between ethnicity and ICU admission. Results of this analysis found that adjustment for age or glucose attenuated this association. Confounding of the ethnicity/ICU relationship by age could be expected, as MAs were younger than NHWs. Younger stroke patients may be more likely to be admitted to the ICU due to a tendency to accept a higher intensity of medical treatment among younger individuals. Similarly, confounding of the ethnicity/ICU relationship by glucose could be explained if the higher glucose is representative of a higher non-stroke severity of illness [17–20]. In contrast to age and glucose, adjustment for NIHSS score strengthened the association between ethnicity and ICU. Although the median NIHSS score was similar across ethnic groups, the relationship between ethnicity and the NIHSS score distribution was found to be complex. MAs tended to be more likely to have low (0–10) NIHSS scores, while NHWs were more likely to have moderate (11–20)NIHSS scores, with no ethnic difference seen at higher (>20) NIHSS scores.
We also investigated whether any ethnic difference in admission to the ICU after stroke may vary with age by including an interaction term for age and ethnicity in our final model. This analysis was based on a hypothesis that any ethnic differences would be less prominent at younger ages, when individuals of both ethnic groups may tend to opt for full intensive care. We found that there was only borderline evidence for effect modification of the ethnicity/ICU relationship by age (p = 0.16 for interaction), with MAs possibly more likely than NHWs to be admitted to the ICU at older ages. This finding should be interpreted with caution due to the exploratory nature of this analysis and the need for a large sample size to formally assess for effect modification. Future studies with larger sample sizes may wish to investigate this possibility further to determine whether ethnic differences in ICU admission are more prominent at older ages.
Our study has several limitations. Data was obtained from one county in Texas and regional differences in healthcare have been shown to potentially confound the relationship between race/ethnicity and healthcare, which may compromise external validity [5]. However, the fact that this study was in a community without an academic center may make it more representative of real-world treatment. Additionally, we did not have data on other indices of intensity of medical treatment or socioeconomic data such as family income, which would be important to obtain in future studies evaluating ethnic differences in intensity of medical care. Similarly, we did not have data on other potentially important confounding factors such as religiosity, pre-existing do-not-resuscitate orders, or other advance directives. Finally, we assessed ICU admission at any time during the hospitalization, while severity measures (mean arterial pressure, glucose, NIHSS score) were only assessed on hospital admission.
Conclusions
In summary, we did not find an ethnic difference between MAs and NHWs in ICU admission following stroke in the Corpus Christi community. This suggests ICU utilization alone does not likely explain the ethnic difference in survival following stroke; however, other measures of life-sustaining therapy in these populations should be explored.
Acknowledgments
This study was funded by the National Institute on Neurological Disorders and Stroke, Grant No. R01 NS38916. Dr. Zahuranec is supported by grant K23AG038731from the National Institute on Aging
References
- 1.Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Crit Care Med. 2003;31:S373–S378. doi: 10.1097/01.CCM.0000065121.62144.0D. [DOI] [PubMed] [Google Scholar]
- 2.Hopp FP, Duffy SA. Racial variations in end-of-life care. J Am Geriatr Soc. 2000;48:658–663. doi: 10.1111/j.1532-5415.2000.tb04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139:1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero LJ, Lindeman RD, Koehler KM, Allen A. Influence of ethnicity on advance directives and end-of-life decisions. JAMA. 1997;277:298–299. [PubMed] [Google Scholar]
- 5.Barnato AE, Berhane Z, Weissfeld LA, Chang CC, Linde-Zwirble WT, Angus DC. Racial variation in end-of-life intensive care use: a race or hospital effect? Health Serv Res. 2006;41:2219–2237. doi: 10.1111/j.1475-6773.2006.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caralis PV, Davis B, Wright K, Marcial E. The influence of ethnicity and race on attitudes toward advance directives, life-prolonging treatments, and euthanasia. J Clin Ethics. 1993;4:155–165. [PubMed] [Google Scholar]
- 7.Feng W, Nietert PJ, Adams RJ. Influence of age on racial disparities in stroke admission rates, hospital charges, and outcomes in South Carolina. Stroke. 2009;40:3096–3101. doi: 10.1161/STROKEAHA.109.554535. [DOI] [PubMed] [Google Scholar]
- 8.Golestanian E, Liou JI, Smith MA. Long-term survival in older critically ill patients with acute ischemic stroke. Crit Care Med. 2009;37:3107–3113. doi: 10.1097/CCM.0b013e3181b079b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xian Y, Holloway RG, Noyes K, Shah MN, Friedman B. Racial differences in mortality among patients with acute ischemic stroke: an observational study. Ann Intern Med. 2011;154:152–159. doi: 10.1059/0003-4819-154-3-201102010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Accessed February 2012];United States Census. 2010 at http://www.census.gov/.)
- 11.Eden SV, Meurer WJ, Sanchez BN, et al. Gender and ethnic differences in subarachnoid hemorrhage. Neurology. 2008;71:731–735. doi: 10.1212/01.wnl.0000319690.82357.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgenstern LB, Smith MA, Lisabeth LD, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahuranec DB, Brown DL, Lisabeth LD, et al. Ethnic differences in do-not-resuscitate orders after intracerebral hemorrhage. Crit Care Med. 2009;37:2807–2811. doi: 10.1097/CCM.0b013e3181a56755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisabeth LD, Smith MA, Brown DL, Uchino K, Morgenstern LB. Family history and stroke outcome in a bi-ethnic, population-based stroke surveillance study. BMC Neurol. 2005;5:20. doi: 10.1186/1471-2377-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MA, Risser JM, Moye LA, et al. Designing multi-ethnic stroke studies: the Brain Attack Surveillance in Corpus Christi (BASIC) project. Ethn Dis. 2004;14:520–526. [PubMed] [Google Scholar]
- 16.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 17.Bejot Y, Aboa-Eboule C, Hervieu M, et al. The deleterious effect of admission hyperglycemia on survival and functional outcome in patients with intracerebral hemorrhage. Stroke. 2012;43:243–245. doi: 10.1161/STROKEAHA.111.632950. [DOI] [PubMed] [Google Scholar]
- 18.Christensen H, Boysen G. Blood glucose increases early after stroke onset: a study on serial measurements of blood glucose in acute stroke. Eur J Neurol. 2002;9:297–301. doi: 10.1046/j.1468-1331.2002.00409.x. [DOI] [PubMed] [Google Scholar]
- 19.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33:2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]