Abstract
Styrene–acrylonitrile Trimer (SAN Trimer), a by-product in production of acrylonitrile styrene plastics, was identified at a Superfund site in Dover Township, NJ, where childhood cancer incidence rates were elevated for a period of several years. SAN Trimer was therefore tested by the National Toxicology Program in a 2-year perinatal carcinogenicity study in F344/N rats and a bacterial mutagenicity assay; both studies gave negative results. To further characterize its genotoxicity, SAN Trimer was subsequently evaluated in a combined micronucleus (MN)/Comet assay in juvenile male and female F344 rats. SAN Trimer (37.5, 75, 150, or 300 mg/kg/day) was administered by gavage once daily for 4 days. Micronucleated reticulocyte (MN-RET) frequencies in blood were determined by flow cytometry, and DNA damage in blood, liver, and brain cells was assessed using the Comet assay. Highly significant dose-related increases (P < 0.0001) in MN-RET were measured in both male and female rats administered SAN Trimer. The RET population was reduced in high dose male rats, suggesting chemical-related bone marrow toxicity. Results of the Comet assay showed significant, dose-related increases in DNA damage in brain cells of male (P < 0.0074) and female (P < 0.0001) rats; increased levels of DNA damage were also measured in liver cells and leukocytes of treated rats. Chemical-related cytotoxicity was not indicated in any of the tissues examined for DNA damage. The results of this subacute MN/Comet assay indicate induction of significant genetic damage in multiple tissues of weanling F344 male and female rats after oral exposure to SAN Trimer.
Keywords: chromosomal damage, DNA damage, Comet assay, micronuclei, superfund, childhood cancer
INTRODUCTION
Styrene–acrylonitrile Trimer (SAN Trimer; CAS No. SANTRIMER2) is a mixture of stereoisomers produced as a by-product of the production of acrylonitrile styrene plastics and specific manufacturing processes for polymers of acrylonitrile and styrene. The mixture is composed of two structural forms, 4-cyano-1,2,3,4-tetrahydro-a-methyl-1-naphthaleneacetonitrile (THNA, CAS No. 57964-39-3) and 4-cyano-1,2,3,4-tetrahydro-1-naphthalene-propionitrile (THNP, CAS No. 57964-40-6). In many countries, polymers of acrylonitrile and styrene are used as plastics in housewares, toys, electronic and electrical appliances, recreational items, handles, bags, pipes, and inner components of refrigerators (Martinmaa, 1984). Although SAN Trimer was patented in Japan for use as a flow-modifier in the manufacture of the styrene–acrylonitrile polymer [ATSDR, 1997], the chemical is no longer considered commercially useful.
SAN Timer was nominated for carcinogenicity testing following reports by the New Jersey Department of Health and Senior Services (NJDHSS) of a possible childhood cancer cluster in the Toms River section of Dover Township, NJ between 1979 and 1995; the relative risk for acute lymphocytic leukemia (Standardized Incidence Ratio [SIR] = 4.3, 95% Confidence Interval [CI] = 1.2–11) and brain and central nervous system cancer (SIR = 11.6, 95% CI 2.3–34) was high for females under the age of 5 years [NJDHSS, 1997]. The increased cancer incidence in Toms River was linked with the Reich Farm Superfund site’s groundwater plume in which previously unknown semivolatile contaminants were discovered. These were subsequently identified and are now referred to as SAN Trimer. In addition to SAN Trimer, a number of other chemicals were tentatively identified in the Reich Farm ground water plume (http://ntp-apps.niehs.nih.gov/ntp_tox/index.cfm), and it should be noted that the authors of the NJDHSS report concluded that there was considerable uncertainty in the findings linking exposure to this water source with elevated incidence of childhood cancers [NJDHSS, 1997].
In 1997, the SAN Trimer interagency workgroup was established by the US Environmental Protection Agency (EPA) to review existing toxicological data on SAN Trimer, evaluate the need for additional toxicity testing, and develop protocols for any proposed studies. In 1998, the National Toxicology Program (NTP), headquartered at the National Institute of Environmental Health Sciences (NIEHS), was invited to participate in the activities of the workgroup, including designing and conducting perinatal toxicity and carcinogenicity studies with SAN Trimer in laboratory rodents. Results of a 2-year cancer bioassay conducted by the NTP in male and female F344/NTac rats exposed to SAN Trimer via dosed feed (perinatal and postnatal exposure) revealed some rare, but not statistically significant, brain and spinal cord neoplasms (NTP Technical Report 573, 2011). In addition, exposure-related increases in peripheral neuropathy were noted in male and female rats. The results of a bacterial reverse mutation assay conducted by the NTP with the same batch (Batch 3) of SAN Trimer used in the 2-year cancer bioassay were negative, (http://ntp-apps.niehs.nih.gov/ntp_tox/index.cfm), consistent with results that were reported in a summary of unpublished industry-sponsored studies. In addition to the bacterial studies, the industry report [Union Carbide Corporation, 2001] summarized results from other genetic toxicity studies with SAN Trimer including an Hprt mutation assay and a test for induction of chromosomal aberrations in Chinese hamster ovary cells. The Hprt test was reported to be negative, but significant increases in chromosomal aberrations were observed. The industry report further stated that no induction of micronuclei or chromosomal aberrations was observed in bone marrow cells of rats treated with SAN Trimer, but details of the study protocol were not provided.
Vacek et al. [2005] reported results of a human biomonitoring study in which mutation frequencies at the HPRT locus in lymphocytes of children whose siblings were included in the Dover Township pediatric cancer cluster were compared with frequencies in healthy nonexposed children; no difference in mutation frequencies were observed between the two groups of children. These results are consistent with several earlier reports showing no association between environmental chemical exposures and HPRT mutation frequencies in adult study populations [Cole et al., 1996, 1997; Becker et al., 2001; Kyrtopoulos et al., 2001]. Vacek et al. [2005] speculated that the length of time from exposure to measurement of mutation frequencies may have contributed to the negative results.
Because of the observance of rare central nervous system neoplasms above the background incidences in the 2-year carcinogenicity study in rats and the limited published genotoxicity data for SAN Trimer, the NTP designed additional tests in an effort to determine the potential for SAN Trimer to induce genetic damage. Thus, the same batch of SAN Trimer used in the 2-year animal studies was tested in a combined micronucleus (MN) and Comet assay [Witt et al., 2008; Recio et al., 2010] in male and female juvenile F344/N rats to evaluate the ability of SAN Trimer to induce chromosomal damage in bone marrow hematopoietic cells and primary DNA damage in several potential target tissues, including brain. The results of these studies were included in the draft NTP Technical Report 573; they are presented here in greater detail.
MATERIALS AND METHODS
Animal Husbandry and Experimental Design
A coded sample of SAN Trimer (NTP bioassay sample, Batch 3), (Union Carbide Corporation, South Charleston, WV) was sent to the testing laboratory for use in in vivo MN/Comet assays. Male and female F344/NTac rats (Taconic Farms, Germantown, NY) were ~–4 weeks of age at the beginning of treatment. Although the study protocol called for five rats per treatment group, the number of female rats available at the beginning of the study was less than anticipated, and therefore only four rats were included in the vehicle control and low dose groups. Rats were singly housed in polycarbonate cages with Beta-Chip hardwood bedding (Northeastern Products Corp, Warrensburg, NY) in an AAALAC-accredited Specific Pathogen Free facility with a 12-hr light/12-hr dark cycle. Temperature and humidity were monitored continuously. Certified Rodent Chow No. 5002 (Ralston Purina, St. Louis, MO) and water were provided ad libitum. The ILS Institutional Animal Care and Use Committee approved the design of the study, and all procedures were completed in compliance with the Animal Welfare Act Regulations, 9 CFR 1-4, and the Guide for the Care and Use of Laboratory Animals [ILAR, 1996].
The treatment protocol and tissue sample preparation procedures followed in this study have been described in detail previously [Recio et al., 2010]. In brief, following 1 week of acclimatization, four to five rats per treatment group (randomized using a body weight stratification scheme) were dosed by oral gavage with SAN Trimer once daily for 4 days, at 24-hr intervals, and humanely sacrificed 4 hr after the fourth dose. Dosing solutions of the test article (37.5, 75, 150, or 300 mg/kg/day body weight/day) were prepared fresh daily in corn oil. The lower doses (37.5 and 75 mg/kg/day) approximated those used in the NTP 2-year feeding study; additional higher doses (150 and 300 mg/kg/day) were included to provide adequate exposure in this short-term study, as per OECD guidelines (OECD 474). The top dose of 300 mg/kg/day was selected based on preexisting data indicating that this dose approximates the maximum tolerated dose. Ethyl methanesulfonate (EMS; 150 mg/kg/day), used as a concurrent positive control, was prepared fresh daily in 0.9% saline. Corn oil and EMS were purchased from Sigma–Aldrich (St. Louis, MO). Four hours after the final dose, peripheral blood samples were collected for flow cytometric evaluation of micronucleated reticulocytes (MN-RET), [Witt et al., 2008], and blood and tissue samples from liver and brain (left and right cerebrum and cerebellum) were collected for DNA damage assessment using the Comet assay [Tice et al., 2000; Ghanayem et al., 2005; Burlinson et al., 2007]. Blood samples, 50 μL per animal collected during exsanguinations, were placed into tubes containing 1 mL of mincing solution [Mg+2 and Ca+2 free Hank’s Balanced Salt Solution (Life Technologies, Carlsbad, CA) with 20 mM ethylenediaminetetraacetic acid (EDTA) pH 7.4–7.7 and 10% v/v fresh dimethyl sulfoxide (DMSO)]. A portion of each solid tissue to be analyzed for DNA damage (left lobe of the liver; right and left cerebrum and cerebellum) was placed in a vial containing 1 mL of mincing solution and rapidly minced. Blood and tissue samples were flash frozen in liquid nitrogen and stored at −80°C until slides were prepared.
Comet Assay
After thawing, each tissue sample was empirically diluted with phosphate buffered saline (PBS) as necessary, mixed with 0.5% low melting point agarose (Lonza, Walkersville, MD) at 37°C, layered onto each well of a two-well CometSlide™ (Trevigen, Gaithersburg, MD), and placed in cold lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM tris(hydroxymethyl)aminomethane (Tris), pH 10, with freshly added 10% DMSO and 1% Triton X-100) overnight. After rinsing in 0.4 M Trizma base, pH 7.5, slides were treated with cold alkali (300 mM NaOH, 1 mM Na2EDTA, pH > 13) for 20 min, then electrophoresed at 4–10°C for 20 min at 1.0 V cm−1, 300 mA. Following electrophoresis, slides were neutralized with 0.4 M Trizma base (pH 7.5) for 5 min, incubated for 5 min in ice-cold 100% ethanol (McCormick Distilling, Weston, MO) and allowed to air-dry. Slides were stored at room temperature in a desiccator with a relative humidity of <60% until stained and scored. After staining slides with SYBR® Gold (Molecular Probes, Invitrogen, Carlsbad, CA), 200 cells were scored per brain quadrant and 100 cells were scored for blood and liver at 200 × magnification using Comet Assay IV Imaging Software, Version 4.11 (Perceptive Instruments, Suffolk, UK). Slides were scored without knowledge of their identity. For each cell, the extent of DNA migration was characterized using the % tail DNA endpoint measurement (intensity of all tail pixels divided by the total intensity of all pixels in the Comet, expressed as a percentage). PBS, NaCl, Na2EDTA, Triton X-100 and Trizma base were purchased from Sigma–Aldrich (St. Louis, MO); NaOH and DMSO were purchased from Fisher Scientific (Pittsburgh, PA).
Neutral Diffusion Assay
A neutral diffusion assay was conducted in parallel with the Comet assay to assess chemical-induced cytotoxicity. This assay measures the percentage of cells containing fragments of low molecular weight (LMW) DNA, considered indicative of necrosis or early stages of apoptosis (Burlinson et al., 2007; Tice et al., 2000). To determine the proportion of cells with LMW DNA, one slide for each sample was removed from lysis buffer after 1 hr, neutralized with 0.4 M Trizma base (pH 7.5), fixed in 100% ethanol, air-dried, and then stored in a desiccator at room temperature. After staining slides with SYBR® Gold, 100 cells per slide were scored microscopically as having either condensed DNA (Type I) or diffused LMW DNA (Type II).
Erythrocyte Micronucleus (MN) Assay
Peripheral blood samples were collected at the time of sacrifice and processed for flow cytometric evaluation of MN-RET as described previously [Witt et al., 2008]. Briefly, cells were fixed and labeled using a MicroFlowPLUS Kit (Litron Laboratories, Rochester, NY) according to the manufacturer’s directions. For each blood sample, 20,000 immature CD71+ RET were analyzed using a FACSCalibur™ flow cytometer (Becton-Dickinson Biosciences, San Jose, CA) to determine the frequency of MN-RET, defined as propidium iodide+ RET [Kissling et al., 2007]. Aggregates were excluded based on forward and side scatter, platelets based on staining with an anti-CD61 antibody, and nucleated leukocytes based on intense propidium iodide staining. More than 106 mature erythrocytes were enumerated concurrently during MN-RET analysis, and the percentage of RET (%RET) among total erythrocytes was calculated as a measure of bone marrow toxicity. The top three exposure groups were analyzed. To evaluate aneugenic potential, propidium iodide associated fluorescence (i.e., DNA content) was determined for 100 MN-RET and analyzed as a single-parameter histogram. This value, termed “median channel” intensity, provides a quantitative description of relative MN size distribution based on DNA content [Torous et al., 1998; Witt et al., 2008; Bryce et al., 2011]. DNA content provides an indication of whether a MN contains a whole chromosome or small chromosomal fragment.
Data Analysis
Comet and Neutral Diffusion (LMW DNA) Assays
Using individual animal data, statistical analysis using Analyse-it® software for Microsoft Excel was conducted on the % tail DNA data and % cells with LMW DNA. For brain samples, the mean data from the four regions, left and right cerebrum and cerebellum, were pooled (“total brain”) for analysis. The Shapiro–Wilk test, with a confidence level of 95%, was used to assess normality of the vehicle control group. Data that were normally distributed were analyzed using an independent sample’s t-test to compare each dose level to the concurrent control and linear regression to determine the presence of a dose response. Normally distributed data were also tested for homogeneity of variances using the F test; for data of unequal variances, the Welch’s approximation for unequal variances t-test value was used for determination of a one-tailed significant (P < 0.05) increase in DNA migration. Data that were not normally distributed were analyzed by the Mann–Whitney test [Mann and Whitney, 1947] comparing each dose level to the concurrent control, followed by the Kendall rank correlation test [Kendall, 1938] to determine the presence of a dose response. Using a conservative approach, trend tests were considered statistically significant at P = 0.025 and pairwise comparisons were significant at P = 0.006 (0.025/4) to correct for multiple comparisons. Criteria for a positive test were at least one dose group significantly elevated over the vehicle control and a significant trend test. A test was judged to be equivocal if only a significant trend or a single significant dose group was observed. Negative tests were those in which neither a significant trend nor a significant dose group were seen. A one-tailed independent t-test was used to verify a significant (P = 0.05) induction of DNA damage by the positive control compound, EMS.
MN Assay
Because measurements of MN frequency are obtained from a large number of cells using flow cytometry, it is reasonable to assume that the proportion of micronucleated cells is approximately normally distributed within each sample [Kissling et al., 2007]. The NTP uses Levene’s test at α = 0.05 to test for equal variances among the treatment groups. In the case of equal variances, linear regression was used to test for a dose-related trend, and Williams’ test [Williams, 1971, 1972] was used to test for pairwise differences between each treatment group and the vehicle control group. In the case of unequal variances, Jonckheere’s test [Jonckheere, 1954] was used to test for a linear trend and pairwise differences with the control group were tested using Dunn’s test [Dunn, 1964]. To correct for multiple pairwise comparisons, the P value for each comparison was multiplied by the number of comparisons made. Trend tests and pairwise comparisons with the controls were considered statistically significant at P = 0.025. A one-tailed independent t-test was used to verify a positive response (P = 0.05) to the control compound, EMS.
RESULTS
Clinical Observations
Male and female F344/NTac rats (four to five animals per dose group) at 3–4 weeks of age were administered SAN Trimer once daily for 4 consecutive days by oral gavage. Some adverse clinical symptoms (e.g., three moribund animals; lethargy, uncoordinated movement, piloerection, and/or hunched posture) were noted in male and female rats in the top dose groups (300 mg/kg/day) during the course of the study. A female rat was found to be moribund 1 hr after the second administration of test chemical; following euthanasia, tissues were collected and analyzed for DNA migration only. A second female rat, observed moribund 4 hr after the third treatment, was-sacrificed and tissue was collected for both DNA migration studies and MN-RET frequency determination. A moribund male rat was euthanized 1 hr following the third dose of SAN Trimer; no blood or other tissues were collected for analysis. One female animal in the vehicle control group had preexisting diarrhea that resolved prior to administration of test chemical. One male animal in the vehicle control group had a preexisting ear scab that was observed into the second day of the study. All other male and female animals appeared normal throughout the entire course of the study.
Comet Assay
DNA damage, measured as % tail DNA, was evaluated in blood leukocytes, and cells of the brain (left and right cerebrum and cerebellum) and liver of male and female juvenile rats following administration of 37.5, 75, 150, or 300 mg/kg/day SAN Trimer by oral gavage for 4 days. Images typical of the extent of damage observed in cells from the brain tissue of high dose animals are provided in Figure 1. Results of the Comet assays are summarized in Figure 2. In both male and female rats, significant increases in the % tail DNA (P = 0.0074 and P < 0.0001, respectively) were observed in brain cells over the tested dose range. When the two female animals in the top dose group that were sacrificed before receiving the fourth treatment were excluded from the analysis, the overall results did not change (P < 0.0001; Table I). Although the analyses were performed using pooled data for four regions of the brain, similar results were observed when the cerebrum and cerebellum were evaluated separately (P = 0.0004 and 0.0718 for male cerebrum and cerebellum and P = 0.0015 and 0.0036 for female cerebrum and cerebellum; Table I). Comparison of the results for the two different brain regions suggests that more DNA damage may have been induced in the cerebrum of male rats exposed to SAN Trimer.
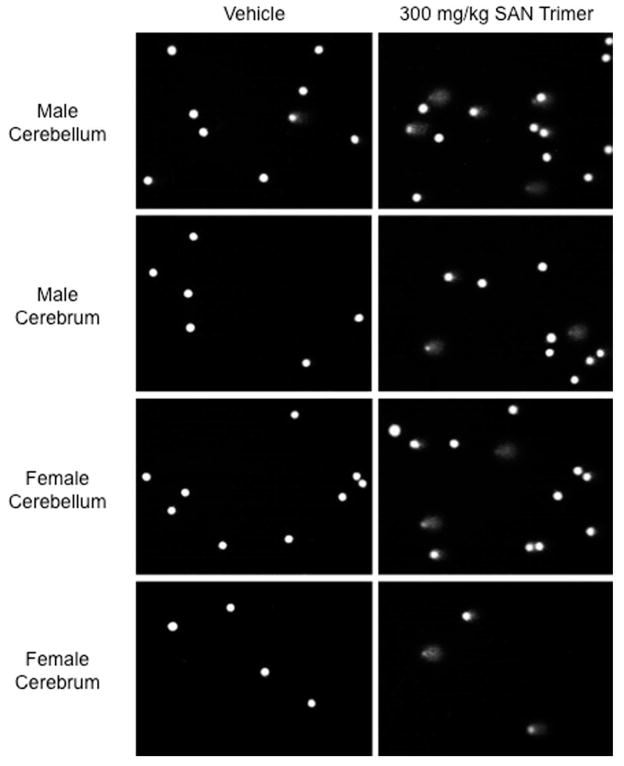
Fig.1.
Images of Comet cells from the brains of male and female rats administered SAN Trimer by oral gavage once daily for 4 consecutive days. Some examples of damaged cells in the cerebrum and cerebellum of animals in the 300 mg/kg/day dose group are shown in comparison to cells from vehicle control animals. A minimum of 10 random fields/coded slide were scored by the imaging software. For each animal, 200 cells were scored from each of four brain sections (left and right cerebrum and cerebellum).
Fig. 2.

DNA damage in male and female juvenile rats following administration of SAN Trimer. DNA damage was measured in blood leukocytes, brain, and liver cells following administration of SAN Trimer by oral gavage daily for 4 consecutive days. Blood and tissues were harvested 4 hr after the final administration of test chemical and stored frozen until assessed for induction of DNA damage using the Comet assay. Percent (%) tail DNA was used as the measure of DNA damage. (a) Male rat brain tissues; (b) female rat brain tissues; (c) male rat blood and liver; (d) female rat blood and liver. *Significant at P < 0.006; **significant for the total brain sample; #significant at P < 0.006 based on exclusion of the outlier animal in the vehicle control group.
TABLE I.
Comet Assay Data Summary
| Dose (mg/kg/day) | Male rats
|
Female ratsa
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of animals | % Tail DNA
|
No. of animals | % Tail DNA
|
|||||
| Mean | SEM | P valueb | Mean | SEM | P valueb | |||
| Total brain | ||||||||
| 0 | 5 | 9.0 | 0.68 | — | 4 | 8.7 | 0.43 | — |
| 37.5 | 5 | 9.0 | 0.82 | 0.5962c | 4 | 8.3 | 0.39 | 0.7479 |
| 75 | 5 | 9.4 | 0.72 | 0.2009c | 5 | 10.3 | 0.55 | 0.0182 |
| 150 | 5 | 10.1 | 0.95 | 0.2085c | 5 | 10.7 | 0.43 | 0.0011*** |
| 300 | 4 | 12.7 | 1.16 | 0.0037c*** | 5 (3) | 11.6 (11.7) | 0.52 (0.71) | <0.0001*** (0.0004***) |
| Trend testd | P = 0.0074e** | P < 0.0001** (P < 0.0001**) | ||||||
| EMS | 5 | 36.1 | 1.12 | <0.0001c* | 4 | 31.3 | 0.93 | <0.0001* |
| Cerebrum | ||||||||
| 0 | 5 | 7.0 | 0.42 | — | 4 | 8.1 | 0.55 | — |
| 37.5 | 5 | 7.0 | 0.88 | 0.4722 | 4 | 8.5 | 0.41 | 0.2926 |
| 75 | 5 | 7.3 | 0.53 | 0.3294 | 5 | 9.9 | 0.71 | 0.0373 |
| 150 | 5 | 7.9 | 0.60 | 0.1145 | 5 | 10.6 | 0.69 | 0.0083 |
| 300 | 4 | 10.3 | 0.91 | 0.0015*** | 5 (3) | 10.7 (11.2) | 0.57 (0.83) | 0.0034*** (0.0039***) |
| Trend testd | P = 0.0004** | P = 0.0042** (0.0015**) | ||||||
| EMS | 5 | 32.5 | 1.21 | <0.0001* | 4 | 29.1 | 0.64 | <0.0001* |
| Cerebellum | ||||||||
| 0 | 5 | 10.9 | 0.94 | — | 4 | 9.2 | 0.64 | — |
| 37.5 | 5 | 11.0 | 1.09 | 0.5441c | 4 | 8.1 | 0.69 | 0.8833 |
| 75 | 5 | 11.6 | 0.94 | 0.3421c | 5 | 10.6 | 0.88 | 0.1177 |
| 150 | 5 | 12.3 | 1.53 | 0.1763c | 5 | 10.9 | 0.55 | 0.0343 |
| 300 | 4 | 15.2 | 1.79 | 0.0610c | 5 (3) | 12.6 (12.1) | 0.78 (1.19) | 0.0028*** (0.0202) |
| Trend testd | P = 0.0718e | P = 0.0002** (0.0036**) | ||||||
| EMS | 5 | 39.8 | 0.90 | <0.0001c* | 4 | 33.6 | 1.36 | <0.0001* |
| Liver | ||||||||
| 0 | 5 | 10.6 | 2.95 | — | 4 | 19.4 | 0.98 | — |
| 37.5 | 5 | 13.6 | 0.90 | 0.1894 | 4 | 21.2 | 0.71 | 0.0994 |
| 75 | 5 | 12.1 | 1.33 | 0.3326 | 5 | 23.0 | 3.01 | 0.1729 |
| 150 | 5 | 15.0 | 0.67 | 0.1088 | 5 | 25.7 | 1.79 | 0.0121 |
| 300 | 4 | 17.4 | 1.33 | 0.0474 | 5 (3) | 31.6 (28.8) | 4.58 (2.83) | 0.0277 (0.0080) |
| Trend testd | P = 0.0078** | P = 0.0018** (P = 0.0032**) | ||||||
| EMS | 5 | 30.0 | 0.59 | 0.0011 (<0.0001*) | 4 | 38.5 | 1.75 | <0.0001* |
| Blood | ||||||||
| 0 | 5 | 3.3 | 0.23 | — | 4 | 6.2 | 0.36 | — |
| 37.5 | 5 | 2.6 | 0.19 | 0.9780 | 4 | 5.1 | 0.63 | 0.9208 |
| 75 | 5 | 3.1 | 0.24 | 0.7436 | 5 | 6.2 | 0.65 | 0.5217 |
| 150 | 5 | 3.7 | 0.32 | 0.1978 | 5 | 7.6 | 0.65 | 0.0012*** |
| 300 | 4 | 4.3 | 0.14 | 0.0047*** | 5 (3) | 7.0 (7.4) | 1.08 (1.61) | 0.2766 (0.2234) |
| Trend testd | P = 0.0006** | P = 0.1345 (P = 0.0706) | ||||||
| EMS | 5 | 32.3 | 0.67 | <0.0001* | 4 | 35.3 | 0.97 | <0.0001* |
Table modified from NTP TR 573, page C-6.
Data are presented as mean ± standard error of the mean (SEM).
Significant at P ≤ 0.05.
Significant at P ≤ 0.025.
Significant at P ≤ 0.006.
Values in parentheses were generated after excluding data for two animals that did not receive the final dose of SAN Trimer.
One-tailed independent t-test.
One-tailed Mann–Whitney.
Linear regression.
Kendall rank correlation.
Dose-related increases in DNA damage were also measured in liver cells of male and female SAN Trimer-treated rats (P = 0.0078 and p = 0.0018, respectively), but the absolute increases were smaller than those observed in brain cells. The % tail DNA value (21.9) measured in the liver of one male animal in the vehicle control group was determined to be an outlier by the Dixon–Massey and Grubbs’ statistical tests (personal communication, Dr. Grace Kissling, NTP); no experimental error was identified to account for this outlier and therefore, the data from this animal were retained. On this basis, SAN Trimer was concluded to give an equivocal response in the Comet assay in male rat liver (Table I). However, exclusion of the outlier data from the control group analysis results in a lower mean control value of 7.8 ± 1.09, a highly statistically significant dose-response (P = 0.0001), and significant increases (P < 0.006) for all treatment groups except the 75 mg/kg/day group.
Analysis of liver data for all scored female rats showed a significant dose response (P = 0.0018), but none of the individual dose groups showed a significant (at P < 0.006) increase in DNA damage (Table I). Exclusion of the two female rats in the 300 mg/kg/day dose group that were sacrificed early did not change the overall result, and the Comet assay result for liver cells was considered to be equivocal for female rats.
Indications of DNA damage following exposure to SAN Trimer were also seen in leukocytes of treated rats (Table I). Dose-related increases in male rats were significant (P = 0.0006) and the mean % tail DNA value for the top dose group was significantly elevated over the control value; in females, the only statistically significant increase in % tail DNA was measured in the 150 mg/kg/day group (with or without inclusion of the two animals sacrificed early) and the trend statistic was not significant. Therefore, the Comet assay results for blood leukocytes were judged to be positive in males and equivocal in females.
In the neutral diffusion assay, no dose-related increases in the percentage of cells containing LMW DNA were observed in any of the examined tissues in male or female rats exposed to SAN Trimer (Table II). These data suggest that exposure to SAN Trimer at the doses used in this study did not induce cytotoxicity in brain cells, blood leukocytes, or liver cells of male or female rats. The positive control chemical, EMS, induced cytotoxicity (LMW DNA) only in the blood of male rats (Table II).
TABLE II.
Summary of Neutral Diffusion Assay Results
| Dose (mg/kg/day) | Male rats
|
Female rats
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of animals | % of cells with LMW DNA
|
No. of animals | % of cells with LMW DNA
|
|||||
| Mean | SEM | P valuea | Mean | SEM | P valuea | |||
| Total brain | ||||||||
| 0 | 5 | 1.1 | 0.22 | — | 4 | 0.6 | 0.20 | — |
| 37.5 | 5 | 1.2 | 0.28 | 0.4887b | 4 | 0.7 | 0.25 | 0.3594b |
| 75 | 5 | 1.0 | 0.30 | 0.7309b | 5 | 0.8 | 0.22 | 0.2952b |
| 150 | 5 | 0.8 | 0.30 | 0.9042b | 5 | 0.2 | 0.09 | 0.9161b |
| 300 | 4 | 0.8 | 0.28 | 0.8195b | 3 | 0.7 | 0.26 | 0.3476b |
| Trend testc | P = 0.1372d | P = 0.5117 | ||||||
| EMS | 5 | 1.2 | 0.34 | 0.6447b | 4 | 0.5 | 0.20 | 0.5781b |
| Liver | ||||||||
| 0 | 5 | 0.8 | 0.37 | — | 4 | 0.5 | 0.29 | — |
| 37.5 | 5 | 0.6 | 0.40 | 0.6378 | 4 | 0.0 | 0.00 | 0.9000b |
| 75 | 5 | 1.8 | 0.66 | 0.1128 | 5 | 0.8 | 0.49 | 0.4524b |
| 150 | 5 | 1.0 | 0.32 | 0.3469 | 5 | 0.4 | 0.24 | 0.6349b |
| 300 | 4 | 1.0 | 0.71 | 0.3990 | 3 | 1.0 | 0.58 | 0.3143b |
| Trend testc | P = 0.8279 | P = 0.3880d | ||||||
| EMS | 5 | 1.4 | 0.93 | 0.2826 | 4 | 0.8 | 0.25 | 0.3429b |
| Blood | ||||||||
| 0 | 5 | 0.2 | 0.20 | — | 4 | 1.0 | 0.41 | — |
| 37.5 | 5 | 0.0 | 0.00 | 0.7262b | 4 | 1.0 | 0.41 | 0.5000 |
| 75 | 5 | 0.6 | 0.24 | 0.2103b | 5 | 0.6 | 0.24 | 0.7965 |
| 150 | 5 | 0.2 | 0.20 | 0.5794b | 5 | 1.2 | 0.37 | 0.3647 |
| 300 | 4 | 0.8 | 0.75 | 0.4524b | 3 | 0.3 | 0.33 | 0.8572 |
| Trend testc | P = 0.4991d | P = 0.7701 | ||||||
| EMS | 5 | 4.0 | 0.45 | 0.0040b* | 4 | 1.0 | 0.41 | 0.5000 |
Data are presented as mean ± standard error of the mean (SEM).
LMW = low molecular weight.
Significant at P ≤ 0.05.
One-tailed independent t-test.
One-tailed Mann–Whitney.
Linear regression.
Kendall rank correlation.
MN Test
The results of the MN assays are summarized in Figure 3. Under the conditions used in these assays, significant (P < 0.0001) dose-related increases in the frequencies of MN-RET were observed in the peripheral blood of both male (2.7-fold) and female (3.1-fold) juvenile rats administered SAN Trimer (Fig. 3a). To evaluate aneugenic potential, the median channel propidium iodide fluorescence intensity was used as a measure of relative MN size distribution based on DNA content [Torous et al., 1998; Witt et al., 2008]. No upward shift in the MN-RET median fluorescence was observed, suggesting no evidence of aneuploidy (Table III); however, for a more definitive assessment of a nonaneugenic mechanism, use of antikinetochore [Miller and Adler, 1990] or centromeric [Pinkel et al., 1986] probe methods should be employed. Decreases in the percentage of RET among erythrocytes, an indication of bone marrow toxicity, were seen in the highest dose groups of male and female rats (60.7% and 35.5%, respectively; Fig. 3b); only the decrease in male rats was significant. The extent of bone marrow cytotoxicity was within the acceptable range outlined in OECD guidelines for a valid in vivo MN test [OECD, 1997]. The positive control chemical, EMS, induced a significant (P = 0.0001) 12.6-and 13.4-fold increase in MN-RET in both males and females, respectively (Table III).
Fig. 3.
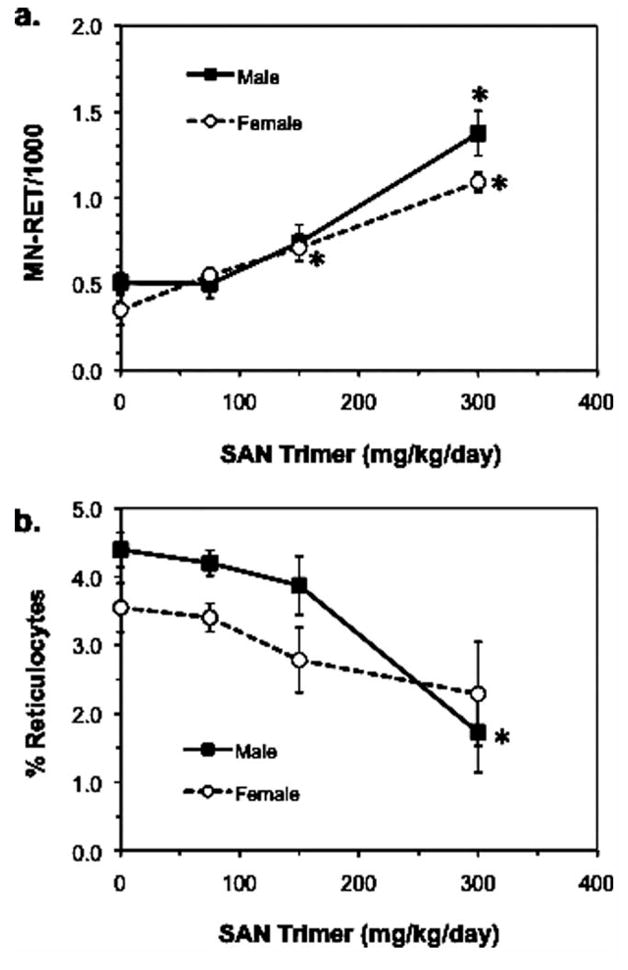
Frequency of MN-RET and % RET in juvenile rats administered SAN Trimer once daily for 4 days. Data obtained by flow cytometric analysis of peripheral blood. *Significant at P < 0.025.
TABLE III.
MN Assay Data Summary
| Dose (mg/kg/day) | No. of animals | % RET
|
MN-RET/1000 RET
|
MN-NCE/1000 RET
|
Median channel fluorescence
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | P valuea | Mean | SEM | P valueb | Mean | SEM | P value | Mean | SEM | P valuec | ||
| Male rats | |||||||||||||
| 0 | 5 | 4.40 | 0.251 | — | 0.51 | 0.051 | — | 0.07 | 0.013 | — | 36.71 | 2.375 | — |
| 75 | 5 | 4.20 | 0.186 | 1.0000 | 0.50 | 0.082 | 0.5161 | 0.06 | 0.009 | 1.0000a | — | — | — |
| 150 | 5 | 3.87 | 0.428 | 1.0000 | 0.74 | 0.104 | 0.0533 | 0.09 | 0.016 | 1.0000a | — | — | — |
| 300 | 4 | 1.73 | 0.588 | 0.0162* | 1.38 | 0.130 | <0.0001* | 0.13 | 0.040 | 0.9931a | 42.59 | 1.954 | 0.1042 |
| Trend test | P = 0.002d* | P < 0.0001e* | P = 0.269d | — | |||||||||
| EMS | 5 | 0.33 | 0.029 | 0.009* | 6.40 | 0.836 | 0.0001* | 0.34 | 0.069 | 0.0045a* | — | — | — |
| Female rats | |||||||||||||
| 0 | 4 | 3.55 | 0.355 | — | 0.35 | 0.089 | — | 0.03 | 0.007 | — | 39.72 | 2.691 | — |
| 75 | 5 | 3.40 | 0.206 | 1.0000 | 0.55 | 0.042 | 0.0294 | 0.06 | 0.024 | 0.1442b | — | — | — |
| 150 | 5 | 2.79 | 0.472 | 0.9443 | 0.71 | 0.080 | 0.0012* | 0.04 | 0.005 | 0.1714b | — | — | — |
| 300 | 4 | 2.29 | 0.759 | 0.3831 | 1.09 | 0.057 | <0.0001* | 0.06 | 0.012 | 0.1007b | 41.49 | 0.820 | 0.5034 |
| Trend test | P = 0.077d | P < 0.0001e | P = 0.158e | — | |||||||||
| EMS | 4 | 0.40 | 0.017 | 0.0209* | 4.69 | 0.155 | <0.0001* | 0.22 | 0.007 | <0.0001b* | — | — | — |
Table modified from NTP TR 573, page C-7.
Data are presented as mean ± standard error of the mean (SEM).
Abbreviations: MN, micronucleated; RET, reticulocytes; NCE, normochromatic (i.e., mature) erythrocytes.
Significant at P ≤ 0.025.
Dunn’s test.
William’s test.
One-way ANOVA and Dunnett’s test (tested using Analyze-it for Excel).
Jonckheere’s test.
Linear regression.
DISCUSSION
Although there are no published mutagenicity test data for SAN Trimer, an industry report [Union Carbide Corporation, 2001] concluded that the compound had little if any mutagenic potential; this conclusion was based on negative results in a bacterial reverse mutation assay, a lack of induction of mutations at the Hprt locus in Chinese hamster ovary (CHO) cells, and no increases in the frequencies of micronuclei or chromosomal aberrations in bone marrow cells of rats administered a single gavage dose of 500 (males) or 650 (females) mg/kg SAN Trimer. Furthermore, increased frequencies of structural chromosomal aberrations observed in CHO cells treated with SAN Trimer were dismissed as being secondary to cytotoxicity.
Because of the lack of published genotoxicity data for SAN Trimer and its possible association with a pediatric cancer cluster, the NTP conducted bacterial mutagenicity and rodent carcinogenicity studies. These studies were conducted with Batch 3 of SAN Trimer; identity and purity analyses indicated that Batch 3 contained ~95.5% mixed SAN Trimer isomers and no 2-amino-3-methyl-1-naphthalenecarbonitrile (AMNC), a mutagenic contaminant that can be generated as a by-product of the heat distillation process used to purify SAN Trimer [Union Carbide Corporation, 2001]. No mutagenicity was detected in standard bacterial assays, with or without exogenous metabolic activation, using this lot of SAN Trimer (http://ntp-apps.niehs.nih.gov/ntp_tox/index.cfm?fuseaction=salmonella.overallresults&cas_no=SANTRIMER2&endpointlist=SA; [NTP Technical Report 573, 2011]). We found that high doses (up to 300 mg/kg/day, approaching the maximum tolerated dose) of Batch 3 SAN Trimer administered by gavage to male and female juvenile F344 rats were associated with small but statistically significant increases in DNA damage in brain cells, measured by the Comet assay, and chromosomal damage in proerythrocytes, measured by the peripheral blood MN assay. Additional evidence of DNA damage, measured by the Comet assay, was seen in liver cells and peripheral blood leukocytes of these male and female rats exposed to SAN Trimer. Although no laboratory historical control data are available for juvenile F344 rat Comet samples processed under identical experimental conditions, confidence in the results is reinforced by the fact that positive tests were obtained for multiple tissues in both male and female rats using very conservative statistical methods that included corrections for multiple comparisons.
In an earlier NTP 2-year bioassay, in which dosing began on gestational Day 7, a small number of brain and spinal cord tumors, along with increased severity of peripheral neuropathy, was noted in male and female rats exposed to SAN Trimer. Although rare, the number of CNS tumors observed in rats did not reach the level of statistical significance, and therefore, the results of the cancer bioassay were judged to be negative [NTP Technical Report 573, 2011]. The occurrence of brain neoplasms in an NTP rat bioassay is extremely rare (brain neoplasms have been seen with 10 chemicals out of a total of 550 carcinogenicity studies conducted over a 30-year period); therefore, even though the incidence of CNS tumors in the rat bioassay with SAN Trimer was low, these tumors may not be biologically irrelevant, considering the increased levels of DNA damage detected in the rat brain samples in the acute study. Although clearly elevated, the DNA damage response in the brain was not strong; one possible explanation for this result could be a strong response only in a subpopulation of cells (e.g., glial cells). Metabolic processes and bioavailability may also be important considerations for the outcomes of the acute genotoxicity and the 2-year carcinogenicity studies, which utilized oral gavage and dosed feed, respectively, as routes of chemical administration. Reported results of a toxicokinetic study suggest that SAN Trimer is extensively metabolized and rapidly eliminated in the urine and feces of dosed rats, indicating that the compound probably does not accumulate in blood and tissues, although clearance mechanisms may become saturated at high doses (≥200 mg/kg) [Gargas et al., 2008]. Compared to delivery of a bolus dose by oral gavage, ingestion of smaller fractionated doses over a prolonged period of time via dosed feed may lead to different patterns of bioavailability. The relatively weak responses seen in the in vivo genotoxicity tests conducted with SAN Trimer in juvenile rats and the low incidence of historically rare brain neoplasms in rats in the NTP bioassay might be considered consistent biological events.
In vivo assays for genotoxicity are generally less sensitive but more specific than in vitro assays, and positive results in the in vivo rodent MN assay have been shown to be highly predictive of rodent carcinogenicity [Witt et al., 2000]. The Comet assay detects a variety of DNA damage including single and double strand breaks, as well as alkali labile DNA damage, in individual cells [Collins et al., 2008]. A comprehensive study that assessed the correlation between positive Comet assay data in a variety of target tissues of rats and mice concluded that a positive Comet assay response in at least one organ of one species is highly correlated with rodent carcinogenicity [Sasaki et al., 2000]. Use of a combined MN/Comet assay is a recent development in genetic toxicity testing. In 2009, at the fifth meeting of the international workgroup on genotoxicity testing (IWGT), the working group concluded that the combination of the two assays in a short-term protocol was both feasible and scientifically acceptable [Rothfuss et al., 2011]. For the past few years, the NTP has been routinely using this combined test protocol to aid in the characterization of the genotoxicity of chemicals [Witt et al., 2008; Recio et al., 2010]. Although no OECD guideline currently exists for conducting the in vivo Comet assay, an international validation trial is currently underway, led by the Japanese Center for the Validation of Alternative Methods, to evaluate aspects of the protocol and the methods of data analysis, and this effort is expected to result in an OECD guideline in the future. In addition to IWGT, organizations such as the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use and the International Life Sciences Institute/HESI recommend the use of the Comet assay when a second in vivo genotoxicity assay is desired [Eastmond et al., 2009; Wang et al., 2010].
Genotoxicity assay results may be influenced by factors not related to genotoxicity. For example, significant changes in body temperature have been shown to increase the frequency of MN-RET in mice in the absence of direct chemical interaction with chromosomes or the mitotic machinery. In an analysis of MN induction in mice resulting from chemical-induced hypothermia, spindle fiber disruption during anaphase appears to be the primary mechanism of MN induction [Tweats et al., 2007]. MN arising from this mechanism contain whole chromosomes and are typically larger in size than those containing chromosome fragments. Two points relative to the current study are worth noting in this context. First, rats are more refractory to the hypothermic effects of the chemicals that were assessed for this phenomenon in mice. Second, the size distribution of the MN observed in our study was similar between the vehicle control and high dose groups; no shift toward larger MN in the high dose group rats was observed (Table III).
Cytotoxicity may potentially confound interpretation of DNA damage levels measured in the Comet assay because apoptosis and necrosis result in DNA fragmentation. In this rat study, we did not perform histopathological assessment of the tissues examined in the Comet assay to assess cytotoxicity, but we did conduct tests assessing the frequency of cells with LMW DNA (fragmented DNA characteristic of apoptosis and necrosis). No indication of an increase in cells with LMW DNA was seen in any of the tissues analyzed in the Comet assay in either male or female rats. Interestingly, the IWGT that met in 2009 did not express concern for cytotoxicity as a confounding factor in the in vivo Comet assay. The working group found no clear examples where cytotoxicity alone convincingly affected the extent of DNA migration in the in vivo Comet assay.
Typically, 100 cells are scored per animal per tissue in a standard Comet assay; this number of cells is the minimum recommended to provide an accurate assessment of DNA migration in a tissue [Hartmann et al., 2003]. In this study, 100 liver cells and blood leukocytes were counted per animal, but for the brain, 200 cells per quadrant (left and right cerebrum and cerebellum) were evaluated for each animal. We scored this greater number of cells in the brain samples to increase the precision of the measurement for each animal (to minimize sampling error) and to provide higher confidence in the event of a “no effect” conclusion. Because there was no obvious biological reason to focus on one particular section of the brain, the data from each of the four regions per animal were combined into an assessment of “total brain.”
In addition to the role that DNA damage plays in carcinogenesis, it has been implicated in the pathogenesis of many progressive, degenerative neurological disorders [Martin, 2008]. Furthermore, it has been suggested that potentially lower rates of DNA repair in CNS tissue might result in an accumulation of DNA damage, eventually leading to symptoms of neurotoxicity [Fishel et al., 2007]. Thus, the positive results seen in the rat MN and Comet assays resulting from exposure to high levels of SAN Trimer may be evidence of an exposure hazard and suggest that brain cells may be especially sensitive to SAN Trimer-induced DNA damage. It should be pointed out, however, that the increase in DNA migration detected using the Comet assay might represent the direct effect of DNA-damaging agents and/or might be intermediate steps in normal DNA repair processes [Frenzilli et al., 2006; Liao et al., 2009]. Additional studies are needed to elucidate the mechanism and potential risks of SAN Trimer-induced genotoxicity.
Acknowledgments
Contract grant sponsor: National Institute of Environmental Health Sciences (NIEHS)/National Toxicology Program (NTP); Contract grant number: N01-ES-35514.
The authors are indebted to Kim Shepard, Amanda Green, Cathy Baldetti, John Winters, and Patricia Allen for expert technical assistance with scoring Comet slides and evaluating blood samples for micronucleus frequency, and/or critical review of the data. The authors are also very grateful for the advice of Dr. Grace Kissling regarding statistical analyses. The authors also acknowledge the contributions of members of the Genetic Toxicology, Investigative Toxicology and Necropsy programs at ILS. Special thanks are due to Drs. Raymond Tice, Andrew Kligerman, and David Malarkey who provided critical review of the manuscript.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
This article is the work product of a group of employees of the NIEHS, National Institutes of Health (NIH); however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH, or the United States government.
References
- ATSDR. Toxicological Issues Related to Chemicals by the New Jersey Department of Health and Senior Services, September 12, 1997. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1997. Chemical Specific Health Consultation. [Google Scholar]
- Becker R, Nikolova T, Wolff I, Lovell D, Huttner E, Foth H. Frequency of HPRT mutants in humans exposed to vinyl chloride via an environmental accident. Mutat Res. 2001;494:87–96. doi: 10.1016/s1383-5718(01)00182-6. [DOI] [PubMed] [Google Scholar]
- Bryce SM, Avlasevich SL, Bemis JC, Dertinger SD. Miniaturized flow cytometry-based CHO-K1 micronucleus assay discriminates aneugenic and clastogenic modes of action. Environ Mol Mutagen. 2011;52:280–286. doi: 10.1002/em.20618. [DOI] [PubMed] [Google Scholar]
- Burlinson B, Tice RR, Speit G, Agurell E, Brendler-Schwaab SY, Collins AR, Escobar P, Honma M, Kumaravel TS, Nakajima M, Sasaki YF, Thybaud V, Uno Y, Vasquez M, Hartmann A. Fourth International Workgroup on Genotoxicity testing: Results of the in vivo Comet assay workgroup. Mutat Res. 2007;627:31–35. doi: 10.1016/j.mrgentox.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Cole J, Green MH, Bridges BA, Waugh AP, Beare DM, Henshaw D, Last R, Liu Y, Cortopassi G. Lack of evidence for an association between the frequency of mutants or translocations in circulating lymphocytes and exposure to radon gas in the home. Radiat Res. 1996;145:61–69. [PubMed] [Google Scholar]
- Cole J, Beare DM, Waugh AP, Capulas E, Aldridge KE, Arlett CF, Green MH, Crum JE, Cox D, Garner RC, Dingley KH, Martin EA, Podmore K, Heydon R, Farmer PB. Biomonitoring of possible human exposure to environmental genotoxic chemicals: Lessons from a study following the wreck of the oil tanker Braer. Environ Mol Mutagen. 1997;30:97–111. [PubMed] [Google Scholar]
- Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R. The Comet assay: Topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. [Google Scholar]
- Eastmond DA, Hartwig A, Anderson D, Anwar WA, Cimino MC, Dobrev I, Douglas GR, Nohmi T, Phillips DH, Vickers C. Mutagenicity testing for chemical risk assessment: Update of the WHO/IPCS harmonized scheme. Mutagenesis. 2009;24:341–349. doi: 10.1093/mutage/gep014. [DOI] [PubMed] [Google Scholar]
- Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: So if they don’t divide what’s to repair? Mutat Res. 2007;614:24–36. doi: 10.1016/j.mrfmmm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Frenzilli G, Scarcelli V, Fornai F, Paparelli A, Nigro M. The Comet assay as a method of assessment of neurotoxicity: Usefulness for drugs of abuse. Ann N Y Acad Sci. 2006;1074:478–481. doi: 10.1196/annals.1369.048. [DOI] [PubMed] [Google Scholar]
- Gargas ML, Collins B, Fennell TR, Gaudette NF, Jr, Sweeney LM. Disposition of styrene-acrylonitrile (SAN) trimer in female rats: Single dose intravenous and gavage studies. Toxicol Lett. 2008;178:1–8. doi: 10.1016/j.toxlet.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Witt KL, Kissling GE, Tice RR, Recio L. Absence of acrylamide-induced genotoxicity in CYP2E1-null mice: Evidence consistent with a glycidamide-mediated effect. Mutat Res. 2005;578:284–297. doi: 10.1016/j.mrfmmm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR. Recommendations for conducting the in vivo alkaline Comet assay. 4th International Comet Assay Workshop. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- ILAR. Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- Kendall M. A new measure of rank correlation. Biometrika. 1938;30:81–89. [Google Scholar]
- Kissling GE, Dertinger SD, Hayashi M, MacGregor JT. Sensitivity of the erythrocyte micronucleus assay: Dependence on number of cells scored and inter-animal variability. Mutat Res. 2007;634:235–240. doi: 10.1016/j.mrgentox.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrtopoulos SA, Georgiadis P, Autrup H, Demopoulos NA, Farmer P, Haugen A, Katsouyanni K, Lambert B, Ovrebo S, Sram R, Stephanou G, Topinka J. Biomarkers of genotoxicity of urban air pollution. Overview and descriptive data from a molecular epidemiology study on populations exposed to moderate-to-low levels of polycyclic aromatic hydrocarbons: the AULIS project. Mutat Res. 2001;496:207–228. doi: 10.1016/s1383-5718(01)00222-4. [DOI] [PubMed] [Google Scholar]
- Liao W, McNutt MA, Zhu WG. The Comet assay: A sensitive method for detecting DNA damage in individual cells. Methods. 2009;48:46–53. doi: 10.1016/j.ymeth.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Mann H, Whitney D. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- Martin LJ. DNA damage and repair: Relevance to mechanisms of neurodegeneration. J Neuropathol Exp Neurol. 2008;67:377–387. doi: 10.1097/NEN.0b013e31816ff780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinmaa JM. Synthetic polymers: Main classes of plastics and their current uses. Prog Clin Biol Res. 1984;141:3–10. [PubMed] [Google Scholar]
- Miller BM, Adler ID. Application of antikinetochore antibody staining (CREST staining) to micronuclei in erythrocytes induced in vivo. Mutagenesis. 1990;5:411–415. doi: 10.1093/mutage/5.4.411. [DOI] [PubMed] [Google Scholar]
- NJDHSS. Childhood Cancer Incidence Health Consultation: A Review and Analysis of Cancer Registry Data, 1979–1995 for Dover Township (Ocean County), New Jersey. Trenton, NJ: New Jersey Department of Health and Senior Services, Division of Environmental and Occupational Health Services Consumer and Environmental Health Services; 1997. [Google Scholar]
- NTP Technical Report 573. NTP Technical Report on the Toxicology and Carcinogenesis Study of Styrene–Acrylonitrile Trimer in F344/N Rats (Perinatal and Postnatal Feed Studies) National Institutes of Health Publication No 11-5915. 2011 http://ntp.niehs.nih.gov/go/36150. [PubMed]
- OECD. Test No 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing; 1997. [DOI] [Google Scholar]
- Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA. 1986;83:2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio L, Hobbs C, Caspary W, Witt KL. Dose-response assessment of four genotoxic chemicals in a combined mouse and rat micronucleus (MN) and Comet assay protocol. J Toxicol Sci. 2010;35:149–162. doi: 10.2131/jts.35.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfuss A, Honma M, Czich A, Aardema MJ, Burlinson B, Galloway S, Hamada S, Kirkland D, Heflich RH, Howe J, Nakajima M, O’Donovan M, Plappert-Helbig U, Priestly C, Recio L, Schuler M, Uno Y, Martus HJ. Improvement of in vivo genotoxicity assessment: Combination of acute tests and integration into standard toxicity testing. Mutat Res. 2011;723:108–120. doi: 10.1016/j.mrgentox.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Sekihashi K, Izumiyama F, Nishidate E, Saga A, Ishida K, Tsuda S. The Comet assay with multiple mouse organs: Comparison of Comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and US NTP carcinogenicity database. Crit Rev Toxicol. 2000;30:629–799. doi: 10.1080/10408440008951123. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/Comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Torous DK, Dertinger SD, Hall NE, Tometsko CR. An automated method for discriminating aneugen- vs. clastogen-induced micronuclei. Environ Mol Mutagen. 1998;31:340–344. [PubMed] [Google Scholar]
- Tweats DJ, Blakey D, Heflich RH, Jacobs A, Jacobsen SD, Morita T, Nohmi T, O’Donovan MR, Sasaki YF, Sofuni T, Tice R. Report of the IWGT working group on strategies and interpretation of regulatory in vivo tests I. Increases in micronucleated bone marrow cells in rodents that do not indicate genotoxic hazards. Mutat Res. 2007;627:78–91. doi: 10.1016/j.mrgentox.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Union Carbide Corporation. Analytical Characterization of SAN Trimer, Batch 3, March 30, 2001. South Charleston: WV Union Carbide Corporation; 2001. [Google Scholar]
- Wang T, Jacobson-Kram D, Pilaro AM, Lapadula D, Jacobs A, Brown P, Lipscomb J, McGuinn WD. ICH guidelines: Inception, revision, and implications for drug development. Toxicol Sci. 2010;118:356–367. doi: 10.1093/toxsci/kfq286. [DOI] [PubMed] [Google Scholar]
- Williams DA. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971;27:103–117. [PubMed] [Google Scholar]
- Williams DA. The comparison of several dose levels with a zero dose control. Biometrics. 1972;28:519–531. [PubMed] [Google Scholar]
- Witt KL, Knapton A, Wehr CM, Hook GJ, Mirsalis J, Shelby MD, MacGregor JT. Micronucleated erythrocyte frequency in peripheral blood of B6C3F(1) mice from short-term, prechronic, and chronic studies of the NTP carcinogenesis bioassay program. Environ Mol Mutagen. 2000;36:163–194. [PubMed] [Google Scholar]
- Witt KL, Livanos E, Kissling GE, Torous DK, Caspary W, Tice RR, Recio L. Comparison of flow cytometry- and microscopy-based methods for measuring micronucleated reticulocyte frequencies in rodents treated with nongenotoxic and genotoxic chemicals. Mutat Res. 2008;649:101–113. doi: 10.1016/j.mrgentox.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]