Abstract
Many drugs that target transforming growth factor-β (TGFβ) signalling have disease applications. Preclinical and clinical studies indicate the utility of these agents in fibrosis and oncology, particularly in augmentation of existing cancer therapies, such as radiation and chemotherapy, as well as in tumour vaccines. There are also reports of specialized applications, such as the reduction of vascular symptoms of Marfan syndrome. Here, we consider why the TGFβ signalling pathway is a drug target, the potential clinical applications of TGFβ inhibition, the issues arising with anti-TGFβ therapy and how these might be tackled using personalized approaches to dosing, monitoring of biomarkers as well as brief and/or localized drug-dosing regimens.
The transforming growth factor-β (TGFβ) superfamily of cytokines, which consists of TGFβs, activins, inhibins, Nodal, bone morphogenetic proteins (BMPs), anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting factor) as well as growth and differentiation factors (GDFs), is conserved through evolution and found in all multicellular organisms1. The TGFβs per se are involved in many cellular processes, including growth inhibition, cell migration, invasion, epithelial-mesenchymal transition (EMT), extracellular matrix (ECM) remodelling and immune-suppression2. However, although normally dynamically regulated and involved in maintenance of tissue homeostasis, TGFβs are often chronically over-expressed in disease states, including cancer, fibrosis and inflammation, and this excessive production of TGFβ drives disease progression by modulating cell growth, migration or phenotype. The TGFβ signalling pathway has therefore become a popular target for drug development.
Knowledge about cellular activities gleaned from studying one disease is often applicable to others. For example, inhibition of TGFβ-induced EMT — a process that contributes to cancer progression — is a goal not only of oncologists but also of cardiovascular surgeons to prevent neointimal hyperplasia, and of nephrologists and pneumologists in the treatment of fibrosis3. In addition, the immune-modulatory activities of TGFβ have implications in many diseases, including cancer, cardiovascular disease, asthma, rheumatoid arthritis and multiple sclerosis4.
TGFβ action is highly context-dependent and influenced by cell type, culture conditions, interaction with other signalling pathways, developmental or disease stage in vivo and innate genetic variation among individuals5-9. This makes the pathway a particular challenge for drug development. Nevertheless, over the past decade several drugs targeting the TGFβ signalling pathway have been developed by pharmaceutical companies and biotechnology firms alike. Drug design strategies have been numerous and include the development of small-molecule inhibitors (SMIs) and monoclonal antibodies, as well as the inhibition of gene expression; some drugs have reached Phase III clinical trials for a number of disease applications, particularly fibrosis and oncology. There is an increasing number of preclinical examples of TGFβ inhibitors that are capable of reducing cancer progression and metastasis, and that augment existing cancer therapies (such as radiation therapy in breast cancer) while simultaneously guarding against radiation-induced fibrosis10. Additionally, there are novel reports of targeting TGFβ signalling in less prevalent indications, such as reduction of vascular symptoms of Marfan syndrome (MFS)11,12. Although there have been many reviews on the pleiotropic action of TGFβ during tumorigenesis, which is characterized by tumour-suppressing activity of TGFβ at an early stage of cancer and tumour-promoting activity at later stages13-16, few focus specifically on drug targets, drug classes and possible therapeutic applications beyond the oncology arena. The translation of anti-TGFβ therapies has been pursued most intensively for oncology; however, this Review also discusses the potential of the TGFβ signalling pathway as a target for non-neoplastic disease therapies and addresses the associated challenges in the development and application of these strategies.
The TGFβ family
The vertebrate genome contains more than 30 pleiotropic ligands that belong to the TGFβ superfamily, including TGFβs, BMPs, GDFs, activins, inhibins, Nodal and AMH1. TGFβ has a conserved motif of nine cysteine residues, eight of which form a tight cysteine knot, with the ninth being crucial for homodimerization2. Aberrant expression and activity of many of the ligands of the TGFβ superfamily are associated with developmental defects and human diseases17. Here we focus on TGFβs as there are currently several clinical trials underway involving therapies targeting TGFβ signalling, whereas other members of the TGFβ superfamily are under-represented in current trials.
Three highly homologous isoforms of TGFβ exist in humans: TGFβ1, TGFβ2 and TGFβ3. They share a receptor complex and signal in similar ways but their expression levels vary depending on the tissue18, and their functions are distinct as demonstrated by the phenotypes of knockout mice19-23. Each TGFβ ligand is synthesized as a precursor, which forms a homodimer that interacts with its latency-associated peptide (LAP) and a latent TGFβ-binding protein (LTBP), forming a larger complex called the large latent complex (LLC). The TGFβ activation process involves the release of the LLC from the ECM, followed by further proteolysis of LAP to release active TGFβ to its receptors2. Matrix metalloproteinase 2 (MMP2) and MMP9 are known to cleave latent TGFβ. In addition to MMPs, thrombospondin 1 (THBS1) is known to activate latent TGFβ24. Alternatively, upon mechanical stretch, αVβ6 integrin can activate TGFβ by binding to the RGD motif present in LAP and inducing the release of mature TGFβ from its latent complex25,26.
TGFβ signalling
Proteolytic cleavage, interaction with integrins or pH changes in the local environment are known to activate latent TGFβ and free active TGFβ for binding to its receptors at the cell membrane. TGFβ superfamily members signal via heteromeric complexes of two related transmembrane type I and type II serine/threonine kinase receptors. Five type II receptors and seven type I receptors (also termed activin receptor-like kinases (ALKs)) have been identified. Auxilliary co-receptors (also known as type III receptors) that regulate the access of TGFβ superfamily members to signalling receptors also exist. Each subfamily of the TGFβ superfamily of ligands binds to type I and type II receptors (Box 1). BMPs can bind to type I receptors alone and, in their absence, can weakly bind to type II receptors, but they show highest affinity when both receptors act together. TGFβ and activin display high affinity only for type II receptors and do not normally interact with isolated type I receptors. Binding to the extracellular domains of type I and type II receptors by the dimeric ligand induces close proximity and a productive conformation for the intracellular serine/threonine kinase domains of the receptors, facilitating the phosphorylation and subsequent activation of the type I receptor. The activation of the type I receptor leads to the propagation of signalling by at least two seemingly independent routes: the SMAD-dependent canonical pathway (Box 1; Fig. 1) and the SMAD-independent or non-canonical pathways (Box 2; Fig. 2).
Box 1. Canonical signal transduction pathway of the TGFβ superfamily of growth factors.
The basic framework of the canonical signal transduction pathways of three subfamilies of the transforming growth factor-β (TGFβ3) superfamily — TGFβ3s, activins/inhibins/Nodal and bone morphogenetic proteins (BMPs) — is highly conserved. The ligand binds to a specific set of type I and type II receptors, which are both serine/threonine kinases, followed by signal transduction by SMAD proteins31,205. Although each subfamily transmits the signal through a specific signalling pathway, the interaction among the TGFβ, activins/inhibins/Nodal and BMP subfamilies is well recognized during development and in postnatal homeostasis of various organs (Box 3). Upon ligand binding and resultant heterotetrameric receptor complex formation, the constitutively active type II receptor phosphorylates the type I receptor, which in turn propagates a signal by phosphorylating the receptor-specific SMADs (R-SMADs)31,205. Unlike type I and type II receptors, type III receptors do not possess kinase activity and are not required for signal transduction; however, they bind to specific ligands and modulate the signalling pathway either positively or negatively31,205. Phosphorylation of R-SMADs at two serine residues within the extreme carboxyl terminus by type I receptor kinase activity promotes association with the common mediator SMAD (co-SMAD), SMAD4, resulting in nuclear accumulation and sequence-specific binding to DNA in concert with other DNA-binding transcription factors that bind distinct sequences adjacent to the SMAD-binding element (SBE)27, and together these complexes modulate transcription. The inhibitory SMADs (I-SMADs), SMAD6 and SMAD7, antagonize R-SMAD activation by competing with R-SMADs for type I receptor interaction and/or by recruiting specific ubiquitin ligases or phosphatases to the activated receptor complex, thereby targeting it for proteasomal degradation or dephosphorylation, respectively. SMAD7 inhibits signalling from all branches of the TGFβ superfamily, whereas SMAD6 is a specific inhibitor of the BMP signalling pathway. The table indicates the basic molecules in the signal transduction pathway, including three types of receptors and SMADs, for three subfamilies of the TGFβ superfamily of ligands: TGFβs, activins/inhibins/Nodal and BMPs.
| Molecular category |
TGFp pathway* | Activin/inhibin/Nodal pathway* |
BMP pathway* |
|---|---|---|---|
| Ligands | TGFβ1, TGFβ2, TGFβ3 | Activin A, activin B, inhibin A, inhibin B, Nodal |
BMP2, BMP4, BMP5, BMP6, BMP7, BMP8A, BMP8B, BMP9, BMP10 |
| Type I receptors |
TβRI(ALK5), ALK1 (ACVRLlorSKR3) |
ALK4(ACVR1Bor ACTRIB), ALK7 (ACVR1C or ACTRIC) |
ALK1 (ACVRL1, SKR3), ALK2 (ACVR1, ACTRI), ALK3 (BMPR1A), ALK6 (BMPR1B) |
| Type II receptors |
TβRII | ACTRIIA, ACTRIIB | BMPR2, ACTRIIA, ACTRIIB |
| Type III receptors |
TβRIII (betaglycan), endoglin, CRIPT03 (TDGF1P3) |
CRIPT01 (TDGF1), CRIPT03 (TDGF1P3),TβRIII (betaglycan) |
RGMA, RGMB (DRAGON), RGMC (HJV or HFE2), endoglin |
| R-SMADs | SMAD2, SMAD3 | SMAD2, SMAD3 | SMAD1, SMAD5, SMAD8 |
| Co-SMAD | SMAD4 | SMAD4 | SMAD4 |
| I-SMADs | SMAD7 | SMAD7 | SMAD6, SMAD7 |
Alternative protein names are listed in brackets. ACTR, activin receptor; ALK, activin receptor-like kinase; BMP bone morphogenetic protein; BMPR, BMP receptor; RGM, repulsive guidance molecule; Tβ3R, TGFβ receptor; TDGF, teratocarcinoma-derived growth factor.
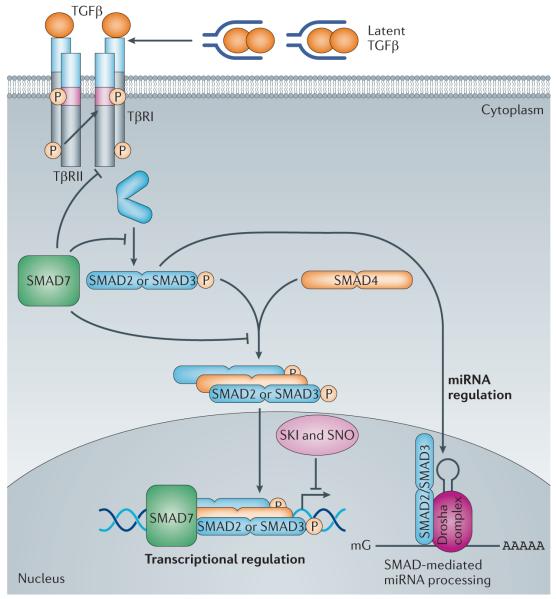
Figure 1. Schematic overview of the canonical, SMAD-dependent TGFβ signalling pathway.
The transforming growth factor-β (TGFβ) ligands are synthesized as a large latent TGFβ complex consisting of mature dimeric TGFβ associated with its latency-associated peptide (LAP) and a latent TGFβ-binding protein (LTBP) (not shown). Upon activation, TGFβ dimers induce heteromeric complex formation between specific type II and type I receptors (such as TGFβ receptortype II (TpRII) andTpRI, respectively). Type II receptors then transphosphorylate the type I receptors, which propagate the signal into the cell by phosphorylating TGFβ receptor-specific SMADs (R-SMADs: SMAD2 and SMAD3). They form heteromeric complexes with the common mediator SMAD (co-SMAD: SMAD4) and translocate to the nucleus. Once in the nucleus, the R-SMAD-co-SMAD complex preferentially associates with the genomic SMAD-binding element (SBE) in a sequence-specific manner. However, high-affinity binding of the R-SMAD-co-SMAD complex with the SBE generally occurs in concert with other DNA-binding transcription factors that bind to distinct sequences adjacent to the SBE27. The nuclear proteins SKI and SNO (also known as SKIL) antagonize the transcriptional regulation by SMADs. An inhibitory SMAD (I-SMAD), SMAD7, inhibits the TGFβ pathway through multiple mechanisms, including by mediating the degradation of the type I receptor, inhibiting phosphorylation of R-SMADs by the type I receptor kinase or inhibiting the formation of the R-SMAD–co-SMAD complex. In addition to regulating transcription, R-SMADs can modulate microRNA (miRNA) biogenesis by facilitating the processing of primary miRNA into precursor miRNA in the nucleus. The co-SMAD is not required for the regulation of miRNA biosynthesis by R-SMADs. ‘mG’ and ‘AAAAA’ represent 5′ capping and 3′ polyadenylation of mRNAs, respectively.
Box 2. Non-canonical TGFβ signalling and crosstalk with other pathways.
In addition to activating SMAD proteins, transforming growth factor-β (TGFβ) signalling can regulate the activity of a number of signalling molecules, such as TNF receptor-associated factor 4 (TRAF4), TRAF6, TGFβ-activated kinase 1 (TAK1), p38 mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), JUN N-terminal kinase (JNK), RHO GTPases, phosphoinositide 3-kinase (PI3K)–AKT and nuclear factor-κB (NF-κB), to transmit a signal6. In addition, these non-canonical signals can crosstalk with the SMAD pathways and mutually modulate each other. Both canonical and non-canonical TGFβ signalling can also be influenced by other signalling pathways, such as the RAS, WNT, Hedgehog, Notch, tumour necrosis factor (TNF) and interferon pathways6. The exact nature of the crosstalk with other pathways and biological outcomes is complex and highly context-dependent6. However, some of the crosstalk has been found to modulate the function and stability of SMAD proteins through post-translational modifications, and to define cell type- and context-specific outcomes by inducing other factors that modulate TGFβ activity.
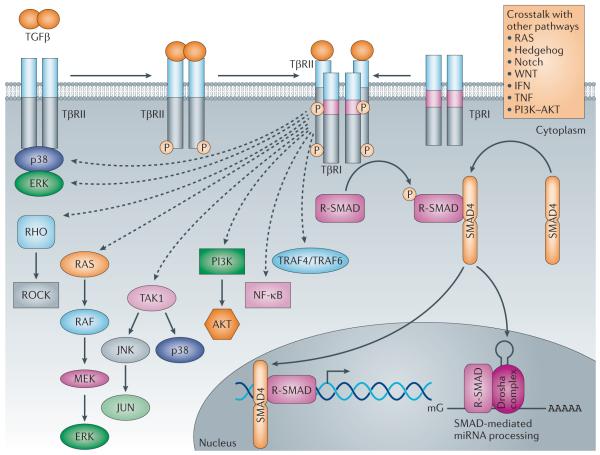
Figure 2. Schematic representation of non-canonical TGFβ signalling and crosstalk with other signalling pathways.
In the non-canonical pathways, the activated transforming growth factor-β (TGFβ) receptor complex transmits a signal through other factors, such as TNF receptor associated factor 4 (TRAF4) or TRAF6, TGFβ-activated kinase 1 (TAK1), p38 mitogen-activated protein kinase (p38 MAPK), RHO, phosphoinositide 3-kinase (PI3K)-AKT, extracellular signal-regulated kinase (ERK), JUN N-terminal kinase (JNK) or nuclear factor-KB (NF-kB). TGFβ signalling can be influenced by pathways other than the canonical and non-canonical TGFβ signalling pathways, such as the WNT, Hedgehog, Notch, interferon (IFN), tumour necrosis factor (TNF) and RAS pathways. The crosstalk between TGFβ and other pathways defines the activities of TGFβ to propagate spatial- and temporal-specific signals. miRNA, microRNA; ROCK, RHO-associated protein kinase; R-SMAD, receptor-specific SMAD; TpR, TGFβ receptor. ‘mG’ and ‘AAAAA’ represent 5′ capping and 3′ polyadenylation of mRNAs, respectively.
In the SMAD-dependent pathway, activation of TGFβ receptor type I (TβRI; also known as TGFBR1 and ALK5) leads to phosphorylation of receptor-specific SMAD (R-SMAD) proteins. SMAD2 and SMAD3 are substrates of TβRI, whereas type I receptors for BMPs utilize SMAD1, SMAD5 and SMAD8 (Fig. 1). Upon phosphorylation by the receptor, R-SMADs together with the common mediator SMAD4 (co-SMAD) translocate to the nucleus, where they interact with other transcription factors (cofactors) to regulate transcriptional responses27 (Fig. 1). In addition to the canonical role of SMADs as transcription factors, a novel role for R-SMADs in the post-transcriptional regulation of microRNA (miRNA) biogenesis has been identified28 (Fig. 1). Therefore, the canonical TGFβ-SMAD pathway modulates gene expression both transcriptionally and post-transcriptionally to propagate the physiological and pathological activities of TGFβ. In the non-canonical pathway, the activated TGFβ receptor complex transmits a signal through other factors, such as tumour necrosis factor (TNF) receptor-associated factor 4 (TRAF4), TRAF6, TGFβ-activated kinase 1 (TAK1; also known as MAP3K7), p38 mitogen-activated protein kinase (p38 MAPK), RHO, phosphoinositide 3-kinase (PI3K), AKT (also known as protein kinase B), extracellular signal-regulated kinase (ERK), JUN N-terminal kinase (JNK) or nuclear factor-κB (NF-κB) (Box 2; Fig. 2). Thus, cellular responses to TGFβ signalling result from the dynamic combination of canonical and non-canonical signalling cascades. In addition to the complexity generated by the canonical and non-canonical TGFβ signalling pathway, TGFβ signalling can be influenced by different signalling pathways, including the PI3K-AKT, WNT, Hedgehog (HH), Notch, interferon (IFN), TNF and RAS pathways (Box 2; Fig. 2). Interactions with several of these pathways can change the output of TGFβ signalling from suppressing growth to inducing cellular plasticity29. Nuclear accumulation and transcriptional activity of R-SMADs can also be negatively regulated through phosphorylation of multiple Ser-Pro and Thr-Pro residues (in the linker region connecting the MH1 and MH2 domains) by ERK, MAPKs, calcium/calmodulin-dependent protein kinase II and cyclin-dependent kinases (CDKs)30. The mode and outcome of the crosstalk between TGFβ and other signalling pathways vary considerably but are essential to define the activities of TGFβ in propagating spatially and temporally specific outputs6,31,32.
Biological actions of TGFβ
TGFβ is involved in a range of biological processes both during embryogenesis and in adult tissue homeostasis. Although the physiological roles of TGFβ have been extensively reviewed elsewhere16,33-36, the major functions of TGFβ that are relevant to the topic of this Review are briefly outlined below.
Inhibition of cell proliferation
TGFβ strongly inhibits the growth of many cell types, including epithelial, endothelial, haematopoietic and immune cells37,38. TGFβ also has pro-apoptotic and differentiation-inducing actions on epithelial cells; together, these actions result in tumour suppression in the context of cancer34. TGFβ in epithelial cells activates transcription of cyclin-dependent kinase inhibitor 1A (CDKN1A) and CDKN2A (which encode p21CIP1 and p15INK4B, respectively) to mediate cell cycle arrest at the G1 phase39. Conversely, TGFβ represses the transcription of MYC, which encodes a potent transcriptional activator of genes that is required for cell proliferation and growth, and inhibitor of DNA binding (ID) family genes, which encode transcription factors that promote cell differentiation and determination40. In oncology, many tumours attenuate TGFβ growth-inhibitory effects but respond to this ligand in a pro-tumorigenic manner. Thus, depending on the tumour type and the stage of tumour progression, TGFβ may provide potent tumour-suppressive or tumour-promoting functions directly on the tumour cell, presumably by mediating differential gene expression programmes (Fig. 3).
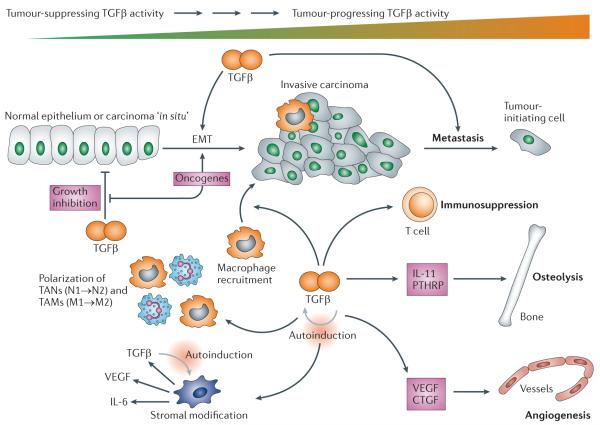
Figure 3. Biphasic activities of the TGFβ signalling pathway during tumorigenesis: from the tumour suppressor to the tumour promoter.
Transforming growth factor-β (TGFβ) has biphasic actions during tumorig enesis, suppressing tumorigenesis at early stages but promoting tumour progression later on, which is the underlying paradigm for the action of TGFβ during disease progression in general and thus complicates the development of therapies targeting TGFβ signalling. The light grey arrows indicate a positive feedforward loop resulting in higher levels of TGFβ, which is a feature of non-neoplastic as well as neoplastic diseases. The current goal in cancer therapy is to downmodulate excessive levels of TGFβ ligands and to target the tumour-progressing versus the tumour-suppressing arm of TGFβ action; the latter goal will almost certainly require more-specific second-generation drugs. CTGF, connective tissue growth factor; EMT, epithelial-mesenchymal transition; IL, interleukin; PTHRP, parathyroid hormone-related protein; TAMs, tumour-associated macrophages; TANs, tumour-associated neutrophils; VEGF, vascular endothelial growth factor.
Unlike the role of TGFβ signalling during tumorigenesis, the contribution of TGFβ to vascular disease is more complex and confusing. Studies on clinical samples from vascular disorders, such as atherosclerosis, hypertension and pulmonary hypertension, often find signatures of both upregulation and downregulation of TGFβ signalling, as well as complex interactions between this pathway and other ligands of the TGFβ family, such as BMPs (Box 3). This has been confirmed by in vitro studies, demonstrating the contradictory effects of TGFβ in the regulation of vascular cells36,41. Furthermore, the TGFβ pathway often exhibits contrasting effects in different vascular cell types, such as endothelial versus vascular smooth muscle cells36. The promiscuous and cell type-specific action of the TGFβ pathway on vascular cells makes the application of targeted TGFβ signalling therapies for cardiovascular disease a particular challenge.
Box 3. BMP-TGFβ-activin crosstalk in endothelial cells.
Within the transforming growth factor-β (TGFβ) superfamily, the crosstalk between three subfamilies — activins/inhibins/Nodal, TGFβ3s and bone morphogenetic proteins (BMPs) — is well established during development and postnatal homeostasis of various organs206-207. In vertebrates, the BMP-SMAD1/SMAD5/SMAD8 and activin–Nodal–TGFβ-SMAD2/SMAD3 signalling pathways execute antagonistic actions in different developmental contexts by inducing the expression of antagonistic factors, such as inhibitory SMADs (I-SMADs: SMAD6 and SMAD7). Some studies have shown that the common mediator SMAD (co-SMAD), SMAD4, is rate-limiting; therefore, when one of the two pathways is activated, it can negatively influence the other pathway by sequestering SMAD4. In endothelial cells, TGFβ can signal not only via canonical TGFβ receptor type I (TβRI)-SMAD2/SMAD3 but also via activin receptor-like kinase 1 (ALK1)-SMAD1/SMAD5/SMAD8 (Ref. 36). In contrast to TGFβ-TβRI signalling-mediated activation of SMAD2 or SMAD3, which leads to endothelium quiescence, TGFβ-ALK1 signalling induces SMAD1/SMAD5/SMAD8 activation and has been shown to stimulate endothelial cell migration, proliferation and tube formation, thus promoting angiogenesis208. BMP9 was shown to induce SMAD2/SMAD3 and SMAD1/SMAD5/SMAD8 phosphorylation via signalling mediated by BMP receptor 2 (BMPR2), activin receptor type II (ACTRII) and ALK1 or ALK2. Cross-activation of TGFβ-specific and BMP-specific receptor-specific SMADs (R-SMADs) by a single ligand is believed to provide a mechanism for the ligand to fine-tune endothelial cell behaviour and function36. In summary, the crosstalk among signalling pathways mediated by different TGFβ family ligands exists in every tissue. However, the mechanism and the biological outcome of this crosstalk are highly species-, tissue- and context-dependent.
Induction of epithelial-mesenchymal transition and the myofibroblast phenotype
TGFβ can induce an EMT of both epithelial and endothelial cells. This has consequences for disease progression in both cancer and fibrosis3. EMT enhances cellular migration and invasive properties, as cell migration requires loss of cell-cell contacts and acquisition of fibroblastic characteristics. E-cadherin is commonly downregulated in many cancers, and its overexpression can suppress invasion by tumour cells. The TGFβ-SMAD pathway mediates the expression of high mobility group AT-hook 2 (HMGA2), which is important for the induction of SNAIL (also known as SNAI1) and SLUG (also known as SNAI2): two zinc-finger transcription factors that are known to repress the E-cadherin gene33. In breast and skin cancer, tumour cell EMT contributes to cancer progression as cells consequently become more migratory and invasive, and they can ultimately transition to a myofibroblastic phenotype3. The myofibroblast further modulates the basic biology of the tumour by increasing ECM elaboration and eliciting a tissue contraction process, which results in increased interstitial fluid pressure (IFP). This has consequences for the efficiency of drug delivery to the tumour42, as drugs cannot penetrate tissue under positive IFP. EMT can also polarize carcinoma cells towards ‘stem cell-like’ properties, such as increased tumour-initiating capacity and tumour cell drug resistance43. Blocking the TGFβ pathway can thus have a threefold benefit: the reduction of tumour invasion and metastasis; the suppression of cancer stem cell-like properties; and the restoration of negative IFP to enhance chemotherapeutic drug delivery44.
In fibrotic conditions, excessive TGFβ production induced in the diseased state contributes to EMT elaboration, which can further exacerbate fibrosis, as seen in pulmonary45,46, cardiac47 and renal48,49 fibrosis, and in arterial restenosis following surgical trauma50. TGFβ can also promote a proliferative and/or migratory phenotype on smooth muscle cells that can aggravate some vascular diseases, including neointimal formation following vascular surgery51-53.
Extracellular matrix regulation
The ECM is a complex structure that surrounds mammalian cells. It is the major component of connective tissue and is composed of multiple proteins, such as collagen, elastin, fibrillin, fibronectin, lamin and proteoglycans. Fibrosis is characterized by the accumulation of fibroblasts, which secrete excessive amounts of ECM. As TGFβ is widely documented to increase collagen synthesis and deposition by fibroblasts, TGFβ has become a central therapeutic target for different types of fibrosis. TGFβ activity and the synthesis of ECM proteins are mutually regulated. Several genes encoding ECM proteins that are known to be important in driving fibrosis are directly regulated by TGFβ-SMAD signalling pathways. There is a reciprocal regulation of TGFβ by the ECM: latent TGFβ bound to ECM components, such as fibronectin and fibrillin, is inactive until physiological or pathological processes initiate its release. This is seen in MFS, in which the mutation of a fibrillin-encoding gene results in reduced fibrillin levels and a consequent increase in levels of unbound TGFβ; this, in turn, leads to the activation of TGFβ signalling, which is possibly responsible for the aetiology of many Marfanoid features11,12.
Immune-suppression and inflammation
The lethal postnatal inflammatory phenotype of Tgfb1-knockout mice19,20,54 demonstrates the important immune-suppressor function of this ligand. The widespread expression profile of TGFβ receptors on all immune cell types suggests that they have broad activities, including responses in cytotoxic CD8+ effector T cells, CD4+ effector T helper 1 (TH1) and TH2 cells, suppressive regulatory T (TReg) cells, natural killer (NK) cells, monocytes, macrophages, neutrophils and eosinophils (Fig. 4). Cell type-specific mouse gene knockout studies with Tgfbr2 demonstrate both direct and indirect actions of TGFβ on effector T cells4.
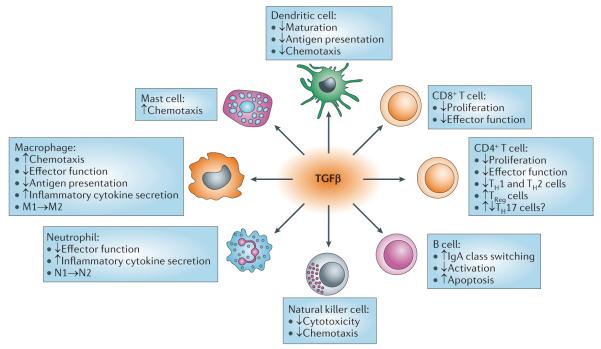
Figure 4. TGFβ effects on immune cells.
Transforming growth factor-p (TGFβ) has effects on most immune cell types. The figure depicts the activity of TGFβ on immune cell subsets that is relevant to human diseases. M1→M2 and N1→N2 indicate polarization of macrophages and neutrophils, respectively, from type I to type II. IgA, immunoglobulin A; TH, T helper; TReg, regulatory T.
TGFβ has potent growth-suppressing activity on most precursor cells of the immune system, particularly T and B cells of the adaptive arm. TGFβ is a potent suppressor of T cell proliferation55 and an inducer of B cell apoptosis56. Additionally, the ligand can alter the course of immune cell differentiation. Suppressive TReg cells that are driven by the expression of the transcription factor forkhead box protein P3 (FOXP3) are crucial for maintenance of peripheral immune tolerance as well as regulation of tumour immunity and infection. In CD4+ T cells, Foxp3 expression is positively but indirectly regulated by TGFβ1 through enhanced binding of the SMAD2-induced transcription factor E2A to the Foxp3 gene promoter, and by relief from GATA3-mediated transcriptional inhibition of the Foxp3 promoter by competition with TGFβ-induced Id3 (Ref. 57). TGFβ suppresses inflammatory TH1 and TH2 cell differentiation while stimulating suppressor TReg cells. Overall, TGFβ-mediated suppression of effector CD8+ cytolytic cells and TH cells, together with TGFβ dependence for suppressive TReg cell differentiation, results in the hyper-inflammatory phenotype seen in Tgfb1-/- mice.
During tumour progression, excess TGFβ suppresses immune surveillance by attenuating the antitumour functions of CD8+ T cells, CD4+ T cells and dendritic cells. CD4+ T cell-specific ablation of TGFβ signalling in transgenic mice expressing dominant negative TβRII (DNRII; also known as CD4-ΔTβRII and CD4-ΔTGFBR2) led to the development of autoimmunity58 and enhanced the differentiation of CD8+ cytotoxic T lymphocytes (CTLs). When challenged with tumour cells, these transgenic mice raised a greater tumour-specific CTL response than wild-type littermates58. Tumour-derived TGFβ also blocks the differentiation of antigen-presenting dendritic cells59 and modifies chemokine receptor expression to blunt dendritic cell chemotaxis60, further suppressing immune surveillance.
In addition to having a predominant immune-suppressive function, TGFβ counterintuitively may have a pro-inflammatory role through its effects on TH17 cells and cells of the innate immune system. TGFβ, together with interleukin-6 (IL-6), was reported to be an essential player in driving pro-inflammatory TH17 lineage differentiation61-63. However, there is considerable controversy surrounding this topic. First, different laboratories cannot agree on the specific functions of various TH1, TH2 and TH17 cell types in disease progression. TH17 cells were implicated as major agonists in inflammatory diseases, including inflammatory cancer, asthma and autoimmune disorders55. However, recent studies suggest that the active player in disease progression is in fact a TH17-derived TH1 cell, or a ‘TH1-TH17’ cell64. Second, the role of TGFβ in regulating the balance between TH1 and TH17 differentiation is in dispute. Despite the widespread acceptance of a role for TGFβ in TH17 differentiation61-63, more recent studies have suggested that TGFβ is totally dispensable for the generation of these cells65,66.
With respect to cells of the innate immune system, TGFβ directly suppresses NK cell-mediated production of IFNγ (which is required for the tumour killing activity of NK cells) through transcriptional effects of SMAD3 on the IFNγ promoter67. It also ‘polarizes’ macrophages68 and neutrophils69 from a type I, productive phenotype (that evolved to attack and devour foreign agents such as cancer cells) towards a type II phenotype (that has reduced effector function but produces large quantities of inflammatory molecules, such as IL-6, IL-11 and TGFβ). These molecules can exacerbate the local diseased state, resulting in solid tumour progression or inflammation associated with fibrosis or atherosclerosis4.
In summary, the regulation of the immune system by TGFβ is highly complex and context-dependent. It delicately regulates the tolerogenic versus immunogenic arms of the immune system to balance adequate host defence while limiting collateral inflammatory tissue damage. The molecular details of this regulation have been recently reviewed in depth4,55,64.
Targeting TGFβ signalling
Virtually every component of the TGFβ pathway has been targeted for drug development (Fig. 5) through numerous design strategies (Fig. 6). Several have been developed through preclinical to clinical trials (Table 1) and many more have been tested only in preclinical systems (Table 2). The drugs that have progressed furthest in clinical development include anti-ligand antisense oligonucleotides (ASOs) from Antisense Pharma70-74, ligand-competitive peptides from Digna Biotech75-78, antibodies that target ligands, receptors or associated proteins spearheaded by Genzyme79-81, and SMIs against TGFβ receptor kinases developed by many companies, with Eli Lilly having an active clinical programme in Phase II development82. The various approaches currently being investigated are discussed in more detail below.
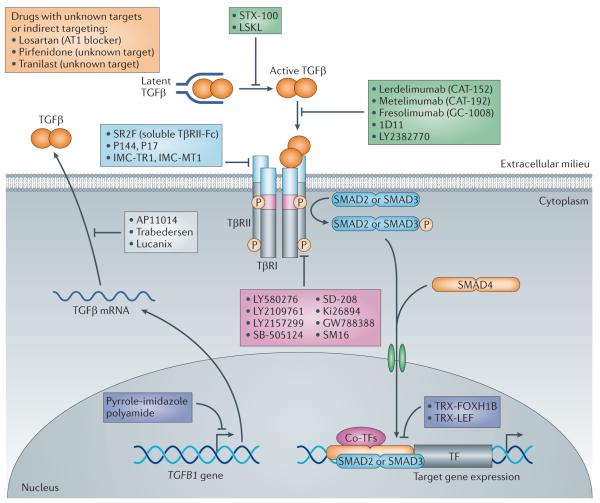
Figure 5. Schematic representation of therapeutic approaches for blocking TGFβ signalling.
Transforming growth factor-p (TGFβ) signalling can be inhibited by: sequestering ligands using soluble receptor ectodomain constructs (ligand traps) derived from TGFβ receptortype II (TpRII) or TpRIII; using TGFβ-neutralizing antibodies; or with TpRII or TpRI kinase inhibitors. Furthermore, translation of TGFβ mRNA can be blocked by targeting TGFβ mRNA with antisense oligonucleotides, thus preventing the production of the ligand. Different small-molecule kinase inhibitors against TpRI have been developed to block its kinase activity. Peptide inhibitors against specific TGFβ ligands are also used. Other approaches block the transformation of TGFβ from the latent to the active form. Three molecules are shown that either affect TGFβ signalling indirectly (losartan) or that have an as-yet-unidentified target (tranilast and pirfenidone). All of these approaches decrease the initiation of intracellular receptor signalling pathways, such as phosphorylation of downstream receptor-specific SMADs (R-SMADs), and thereby blunt the transcriptional regulation of target genes. ATI, angiotensin II type 1 receptor; co-TFs, co-transcription factors; FOXH1B, forkhead box protein H1B; LEF, lymphoid enhancer-binding factor; LSKL, Leu-Ser-Lys-Leu peptide; TRX, thioredoxin.
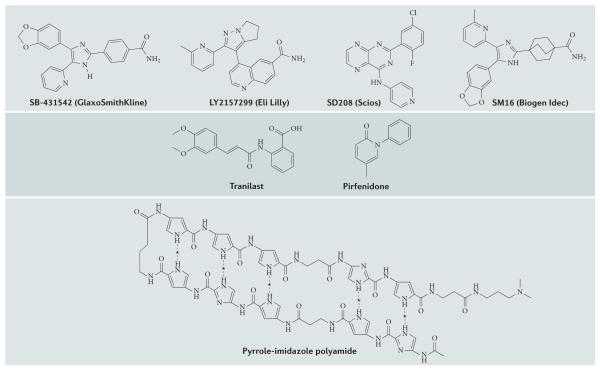
Figure 6. Structures of representative small-molecule inhibitors of TGFβ signalling.
Depicted are the molecular structures of a selection of small-molecule inhibitors identified to target the transforming growth factor-p (TGFβ) signalling pathway. SB-431542, LY2157299, SD208 and SM16 are all ATP mimetics that inhibit TGFβ receptortype I (TpRI; also known asTGFBRl) kinase activity. Pyrrole-imidazole polyamide blocks transcription of the TGFB1 gene. Pirfenidone and tranilast have unknown molecular mechanisms of action. Dashed lines denote putative hydrogen bonding with bases in DNA; asterisks indicate positions where hydrogen bonds form with nucleotide residues of DNA within the TGFB1 gene promoter.
Table 1. Summary of clinical trials for TGFβ inhibitory drugs.
| Drug; company |
Type | Targets | Disease applications |
Stage | Clinical trial identifiers |
Summary of results | Refs |
|---|---|---|---|---|---|---|---|
| Trabedersen (AP12009); Antisense Pharma |
Antisense oligo | TGFβ2 ligand |
Glioblastoma | Phase I/IIb | NCT00431561 | Safe | 70,73,74 |
| Pancreatic cancer, MetM, colon cancer |
Phase I | NCT00844064 | Pancreatic cancer trials continue |
84 | |||
| Glioblastoma | Phase III | NCT00761280 | Glioblastoma trials stopped in March 2012 owing to advances in standard of care and neurosurgery (Box 4) |
||||
| Belagen- pumatucel-L (Lucanix); NovaRx |
Antisense gene-modified allogeneic tumour cell vaccine |
TGFβ2 | NSCLC | Phase III | NCT00676507 | Well tolerated in 75 patients; survival advantage justifies further Phase III evaluation |
85-87 |
| Disitertide (P144); Digna Biotech |
Peptide | Peptide based on TβRIIIthat blocks ligand binding to receptors |
Skin fibrosis in systemic sclerosis |
Phase II |
NCT00574613, NCT00781053 |
Preclinical efficacy in peritoneal fibrosis associated with peritoneal dialysis, renal and cardiac fibrosis, corneal haze and retinal AMD; safety and efficacy in Phase IIa clinical trial for scleroderma/ skin fibrosis |
75-78 |
| Lerdelimumab (CAI-152); Cambridge Antibody Technology |
Humanized antibody |
TGFβ2 ligand |
Reduction of scarring after glaucoma surgery |
Phase III (complete) | Safe; ineffective in reducing scarring in Phase III trial |
88,89 | |
| Metelimumab (CAI-192); Cambridge Antibody Technology |
Humanized Antibody |
TGFβ1 ligand |
Diffuse systemic sclerosis |
Phase I/II | NCT00043706 | Ineffective when systemically administered in doses up to 10 mg per kg |
90 |
| Fresolimumab (GC-1008); Cambridge Antibody Technology/ Genzyme/ Sanofi |
Humanized antibody |
TGFβ1, TGFβ2 and TGFβ3 ligands |
Focal segmental glomerulosclerosis |
Phasel | NCT00464321 | Completed and safe; plans to progress |
92 |
| Systemic sclerosis | Phasel | NCT01284322 | Still recruiting | - | |||
| Study ongoing | - | ||||||
| Completed, no results | - | ||||||
| Myelofibrosis | Phasel | NCT01291784 | See Box 4 | 93 | |||
| TPF | Phasel | NCT00125385 | See Box 4 | 93 | |||
| Renal cell carcinoma |
Phasel | NCT00356460 | See Box 4 | 93 | |||
| Malignant melanoma |
Phasel | NCT00356460 | See Box 4 | 81 | |||
| Metastatic breast cancer (with radiotherapy) |
Phasel | NCT01401062 | Active and recruiting patients | - | |||
| Relapsed malignant pleural, mesothelioma |
Phase II | NCT01112293 | Ongoing but not recruiting participants |
- | |||
| LY2382770; Eli Lilly |
Humanized Antibody |
TGFβ1 | Diabetic kidney disease (fibrosis) |
Phase II | NCT01113801 | Safety and efficacy in protecting kidney function in patients with diabetic kidney disease; still recruiting |
- |
| SIX-100; Stromedix |
Antibody | aVβ6 integrin | Fibrosis | Phase II | NCT01371305 | Significant antifibrotic activity in preclinical models of lung, kidney and liver disease |
168 |
| LY2157299; Eli Lilly |
Small molecule | TβRI kinase |
Advanced-stage melanoma |
Phase II | NCI10038320 | See Box 4 | 82 |
| Recurrent glioblastoma |
Phase II | NCT01582269 | Recruiting; LY2157299 alone or with lomustine therapy versus lomustine alone in recurrent glioblastoma |
213 | |||
| Glioblastoma | Phase II | NCT01220271 | Recruiting; LY2157299 with temozolomide-based radiochemotherapy in newly diagnosed malignant glioma |
212 | |||
| Hepato cellular carcinoma |
Phase II | NCT01246986 | Recruiting | - | |||
| Advanced pancreatic carcinoma |
Phase II | NCT01373164 | Recruiting; comparison of gemcitabine with gemcitabine plus LY2157299 |
- | |||
| Dominant negative TGFBR2-modified CILs |
Recombinant T cells |
TβRII | Adoptive transfer of T cells expressing HER2 and ΔNTGFBR2 for lung cancer (HERCREEM) |
Phasel | NCT00889954 | No update on clinical trials | - |
| Dominant negative TGFBR2-modified CILs |
Recombinant T cells (a clinical grade retrovirus vector encoding dominant negative TβRII) |
TβRII | TGFβ-resistant LMP2A-specific CILs for EBV-positive lymphoma |
Phasel | NCT00368082 | Preclinical efficacy in tumour killing of TGFβ-secreting EBV-positive lymphoma; no update on clinical trials |
107 |
| Avotermin (Juvista); Renova |
Recombinant protein |
TGFβ3 | Scarring | Phase II | NCT004322111, NCT00656227 |
Thejuvista Phase II trial had not reached its primary or secondary efficacy end points as of February 2011 |
214 |
| Pirfenidone; lnterMune |
Small molecule, not TGFβ-specific |
IPF, glomerulosclerosis and diabetic kidney disease, pathological skin scarring |
Phase III | Multiple trials | First drug approved for IPF in Europe |
162 | |
| Losartan; Merck and Co. |
Small molecule, not TGFβ-specific |
AT1 | Marfan syndrome (MFS) |
Phase I/II |
NCT00723801, NCT00763893, NCT00782327 |
Reduction of aortic aneurysm in mouse model of MFS; clinical trials in progress to reduce aortic root dilation and cardiac muscle stiffness in patients with MFS |
12,182 |
| Tranilast; Kissei Pharmaceuticals |
Small molecule, not TGFβ-specific |
Unknown | Corneal primary pterygium |
Phase III | NCT01003613 | Tranilast reduces myofibroblast proliferation of corneal myofibroblasts in vitro and may be a novel adjuvant therapy for corneal keloid |
185, 215,216 |
| IMC-TR1; lmClone Systems/ Eli Lilly |
Humanized antibody |
TβRII | Mammary and colon cancer |
Phasel | NCT01646203 | Preclinical efficacy against primary tumour growth and metastasis through multiple effects on tumour, stroma and immune cells |
94 |
AMD, age-related macular degeneration; AT1, angiotensin II type 1 receptor; CTL, cytotoxic T lymphocyte; EBV, Epstein–Barrvirus; IPF, idiopathic pulmonary fibrosis; LMP2A, an EBV-specific antigen; MetM, metastatic melanoma; NSCLC, non-small-cell lung cancer; oligo, oligonucleotide; Tβ3R, TGFβ3 receptor; TGFβ, transforming growth factor-β; TGFBR2, gene encoding TβRII.
Table 2. Summary of TGFp inhibitory drugs in preclinical development.
| Drugs; company |
Type | Targets | Disease applications | Stage | Summary of results | Refs |
|---|---|---|---|---|---|---|
| AP11014; Antisense Pharma |
Antisense oligo |
TGFβ1 ligand | Prostate cancer, NSCLC, colorectal cancer |
Preclinical | AP11014 significantly reduced TGFβ1 secretion by 43-100% in different NSCLC, colon and prostate cancer cell lines |
217 |
| P17; Digna Biotech |
Peptide | Peptide derived from Phage Display Technology that targets TGFβ1 binding to receptor |
Liver and pulmonary fibrosis, metastatic lung cancer, angiogenesis, melanoma, immuno suppression, wet AMD |
Preclinical | Preclinical efficacy in peritoneal fibrosis associated with peritoneal dialysis, lung fibrosis, corneal haze and retinal AMD |
76 |
| LSKL (academic only) |
Peptide | Ihrombospondin | - | Preclinical | Preclinical efficacy in reducing renal injury and proteinuria in a murine model of diabetic nephropathy |
97 |
| 1D11; R&D Systems |
Mouse antibody |
Mouse TGFβ1, TGFβ2 and TGFβ3 ligands |
Breast cancer | Preclinical | Safe and efficacious in tumour metastasis in mice |
79,80, 218 |
| SR2F (academic only) |
Ligand trap | TGFβ1, TGFβ3 | Breast cancer | Preclinical | Very safe after lifetime exposure in mice; not progressing to clinical trial |
125 |
| Soluble TβR2-Fc; Genzyme |
Ligand trap | TGFβ1, TGFβ3 | Breast cancer | Preclinical | Safe and efficacious in suppressing metastasis in preclinical model of breast carcinoma; not progressing to clinical trial |
96 |
|
LY580276, LY550410, LY364947, LY2109761*; Eli Lilly |
Small molecule |
TβRI kinase | Cancer | Preclinical | LY2109761 is safe in long-term dosing of tumour-bearing mice, and efficacious in reducing metastasis and TICs in mouse cancer models |
80,156, 219-222 |
| SB-505124, SB-431542; GlaxoSmithKline |
Small molecule |
IPRI kinase | - | Preclinical | Extensively used in vitro; pharmacokinetically unstable in vivo |
223-225 |
| SD208, SD093; Scios |
Small molecule | TβRI kinase | Cancer | Preclinical | Efficacious in suppressing tumour metastasis in rodent models; programme discontinued after merger with Johnson & Johnson |
146, 226,227 |
| Ki26894; Kirin Pharma ceuticals |
Small molecule |
TβRI kinase | Breast cancer | Preclinical | Not progressing to clinical trial | 148,150 |
| SM16; Biogen Idec |
Small molecule |
TβRI kinase | Mesothelioma | Preclinical | Not progressing to clinical trial | 10,155, 228,229 |
|
GW788388; GlaxoSmithKline |
Small molecule |
TβRI kinase | Fibrosis | Preclinical | Not progressing to clinical trial | 230-232 |
| GB1201, GB1203 (academic) |
Pyrrole- imidazole polyamide |
TGFB1 gene promoter |
Cutaneous and corneal scarring, arterial restenosis, kidney fibrosis |
Preclinical | Preclinical efficacy in inhibition of TGFB1 gene expression, which reduced corneal scarring and carotid artery restenosis |
50, 102,103 |
Antisense oligonucleotides and antisense RNA
Antisense Pharma uses the strategy of targeting mRNA translation using ASOs to downregulate ligand synthesis70,83. Its focus has been on targeting TGFβ2, which is produced in excessive quantities by glioblastoma and pancreatic carcinoma cells. Trabedersen (AP12009), a synthetic 18-mer phosphorothioate ASO, binds specifically to human TGFB2 mRNA, and this drug has progressed to a Phase III clinical trial for oncology applications (Box 4). One of the challenges of this drug is delivering it directly to the tumour to avoid the off-target toxicity associated with systemic delivery of first-generation ASOs. In the case of glioblastoma, this was achieved using intrathecal catheter delivery directly into the tumour74. More recently, the company has started developing intravenous delivery approaches for pancreatic cancer, which appear to be effective in mouse models73 and were recently shown to be safe in humans84.
Box 4. Oncology trials to date.
A two-part clinical trial of GC-1008 for the treatment of advanced metastatic melanoma (MetM) and renal cell carcinoma (22 patients) found the drug to be safe and well tolerated with no dose-limiting toxicities (DTLs). Five patients achieved at least stable disease as assessed by RECIST (response evaluation criteria in solid tumours) criteria, and therefore received extended treatment. One patient achieved a partial response with a greater than 75% reduction in the target lesion. The only adverse effect was keratoacanthoma-like lesions in sun-damaged skin of two of the patients with MetM. However, these resolved on cessation of drug treatment and were not malignant93. Despite these promising results, the pursuit of GC-1008 for oncology was terminated after Genzyme was acquired by Sanofi in late 2011.
Antisense Pharma has had success with trabedersen (AP12009) in glioblastoma, pancreatic cancer and colon cancer. Preclinical and clinical studies70,71,73 indicate that neutralization of transforming growth factor-β2 (TGFβ2)-mediated immunosuppression, leading to activation of tumour-infiltrating natural killer cells, is the major mode of action. Intra-tumoural administration of trabedersen to glioblastoma led to shrinkage of the targeted tumour as well as tumours elsewhere in the brain. Three Phase I/II studies of trabedersen for recurrent or refractory high-grade glioma (glioblastoma) and anaplastic astrocytoma showed survival benefit compared with conventional chemotherapy209. A randomized, controlled Phase IIb study evaluating the efficacy and safety of two doses (10 and 80 mM) of trabedersen in comparison with standard therapy concluded that patients with glioblastoma on trabedersen had a threefold enhancement in cognitive function 2 and 3 years after therapy compared to standard chemotherapy74. However, questions have been raised about this most recent study210,211. Wick and Weller211 conceded that although trabedersen was clinically safe and that TGFβ inhibitors, in general, show promise for cancer therapy, the conclusions drawn by Bogdahn et al.74 were premature. Because of other advances in both neurosurgical procedures and first-line standard of care for patients with glioblastoma212, the SAPPHIRE Phase III trial of trabedersen was recently halted owing to patient recruitment issues (ClinicalTrials.gov identifier: NCT00761280). Nevertheless, the drug has undergone a Phase I/II trial for patients with advanced pancreatic cancer, MetM or metastatic colorectal carcinoma, and showed excellent safety and encouraging survival results (ClinicalTrials.gov identifier: NCT00844064)84.
Eli Lilly’s clinical small-molecule inhibitor LY2157299 was found to be safe and well tolerated in a Phase I glioblastoma trial82. Of 28 patients treated in a dose escalation study (14 days on/14 days off treatment), at least three patients showed antitumour effects with durable responses beyond 1 year. As a result, the Eli Lilly anti-TGFβ signalling programme for oncology continues to be pursued with an ongoing Phase II trial of LY2157299, with or without gemcitabine, for hepatocellular carcinoma, glioblastoma and advanced pancreatic cancer, and with lomustine in patients with treatment-refractory malignant glioma213, plus a new Phase I trial of IMC-TM1, an anti-TGFβ receptor type II (TβRII) antibody.
NovaRx’s belagenpumatucel-L (Lucanix) has completed an open-label clinical trial of 75 patients with non-small-cell lung cancer (NSCLC) with a median follow-up of 14.5 months (44 months for patients with stable disease). One-year, two-year and five-year survivals were 55%, 35% and 20%, respectively. Individuals who demonstrated an increase in both cellular and humoral immune reactivity had a significant survival advantage over individuals who showed an increase in only one measure of immunity (32.5 months versus 11.6 months; p = 0.015). On the basis of these findings, an international, randomized Phase III trial to evaluate the efficacy of belagenpumatucel-L in a maintenance setting has been initiated for patients with stage III/IV NSCLC who have stable disease following frontline chemotherapy87.
An anti-TGFB2 antisense strategy has also been used to generate augmented tumour vaccines. Belagenpumatucel-L (Lucanix; NovaRx) is such a drug, in which an ~900-nucleotide TGFB2 antisense construct is transfected into allogeneic non-small-cell lung cancer (NSCLC) cells, which are then used as a tumour vaccine. Here, drug delivery is not an issue as the ‘drug’ is in fact genetically engineered NSCLC tumour cell lines. This tumour vaccine has superior activity compared to conventional tumour vaccination approaches85,86. A significant dose-related survival difference was seen in patients who received 2.5 × 107 cells per injection, allowing progression to a Phase III clinical trial87.
Monoclonal antibodies
The advantages of monoclonal antibodies are their specificity and extracellular mechanism of action — an advantage when trying to mop up excess extracellular ligand. This is tempered by the less con venient intravenous mode of delivery. However, prolonged pharmacokinetic stability permits infrequent drug administration. Cambridge Antibody Technologies and Genzyme developed humanized (or murinized for preclinical studies) monoclonal antibodies specific to individual ligands, such as lerdelimumab (CAT-152)88,89 and metelimumab (CAT-192)90, or with pan-ligand specificity, such as fresolimumab (GC-1008)91-93. These antibodies have proceeded through various stages of preclinical and clinical development. Of these three humanized antibodies, fresolimumab has progressed furthest in the clinic for both neoplastic and non-neoplastic applications. This drug was found to be well tolerated and safe at 15 mg per ml in Phase I trials for metastatic melanoma (MetM) plus renal cell carcinoma93 and at 1 mg per ml for the fibrotic disorder focal segmental glomerulosclerosis92. Lerdelimumab88,89 and metelimumab90, despite passing safety tests, failed to show efficacy in fibrotic models of corneal scarring and systemic sclerosis, respectively, and were therefore discontinued90. Despite a promising Phase I oncology trial of fresolimumab, after Genzyme was acquired by Sanofi the company made the decision to focus on fibrotic applications of this drug.
Eli Lilly entered the monoclonal antibody arena with a TGFβ1 ligand-selective blocking antibody, LY2382770, which has progressed to Phase II trials for kidney fibrosis (Table 1). Since merging with ImClone, Eli Lilly has also developed a TβRII-blocking antibody, IMC-TR1 (Ref. 94), which has just entered clinical trials for breast and colon cancer (ClinicalTrials.gov identifier: NCT01646203). In addition, Biogen Idec and Stromedix have developed an anti-integrin β6 antibody that prevents the activation of TGFβ and has been used efficaciously in preclinical studies of fibrosis and cancer95; it is in a Phase II trial for fibrosis (ClinicalTrials.gov identifier: NCT01371305).
Ligand traps and peptides
Genzyme developed a ligand trap by fusing Fcγ to the extracellular domain of TβRII, but this construct never reached clinical trials96. However, an alternative ligand trap approach, pursued by Digna Biotech, using peptide mimetics of TβRIII (also known as betaglycan and TGFBR3), completed a Phase IIa clinical trial for scleroderma and skin fibrosis, showing safety and efficacy when topically applied to skin (Table 1; Box 4). This company has plans to extend to Phase IIb/III trials in 2013 (J. Dotor, personal communication). A peptide antagonist of TGFβ activation, LSKL (Leu-Ser-Lys-Leu), binds to a conserved sequence in the LAP region of the latent complex and has demonstrated efficacy in reducing TGFβ signalling in vitro97. This antagonist is based on thrombospondin and specifically blocks TGFβ activation. The issue of peptide drug delivery is not a problem for topical application; however, to progress to systemic delivery, Digna Biotech has partnered with Flamel Technologies to investigate proprietary peptide delivery systems.
Small-molecule inhibitors
There are a plethora of SMIs that specifically target the type I receptor of TGFβ to inhibit the phosphorylation of SMAD2 and SMAD3 while keeping at least some non-canonical responses, such as TAK1 activation, intact. These drugs are generally ATP mimetics that bind competitively within the hydrophobic ATP binding pocket of the receptor kinase. The chemistry of these compounds has been extensively reviewed98,99 and some molecular structures are shown in Fig. 6. The obvious advantages of these molecules over most others are their economical production, stability and ease of oral administration, set against a possible disadvantage of cross-inhibition of other kinases. The short half-life of these drugs provides the possibility of rapid drug withdrawal should adverse events arise. Many successful preclinical studies for metastatic cancer have been undertaken with these SMIs, as reviewed previously100,101. However, the only company to continue pursuit of a TβRI-targeted SMI into clinical trials for oncology is Eli Lilly with LY2157299 (Ref. 82) (ClinicalTrials.gov identifier: NCT01373164).
Other approaches
A novel approach to the suppression of ligand production has been the preclinical development of pyrrole-imidazole polyamides that bind with sequence specificity to the TGFB1 gene promoter to attenuate gene expression50,102,103. These large ~17 kDa polymeric molecules (Figs 5,6) bind within the minor groove of DNA to prevent transcription factor binding. Challenges associated with these drugs include the specificity of promoter binding, along with drug delivery issues owing to their large molecular size and the high local concentration required for activity. However, preclinical studies suggest that these molecules might be used in drug-eluting stents for the purpose of reducing restenosis after coronary or carotid artery surgery50.
An alternative approach to suppress TGFβ signalling is gene transfer of antagonizing signalling molecules, such as the inhibitory SMAD7. This approach has been applied in model systems to treat or prevent various pathological conditions, including colonic and hepatic fibrosis, vascular remodelling and diabetic kidney disease104,105. Such an application has the potential to be applied to many other systemic diseases to attenuate the activity of the TGFβ pathway, with the caveat that gene therapy is still far from being widely accepted as a therapeutic approach106.
As an approach to stimulate immune destruction of cancer cells by tumour-infiltrating T cells, human tumour antigen-specific CTLs have been engineered to express DNRII using a clinical grade retrovirus vector. TGFβ-resistant CTLs were found to have a functional advantage over unmodified CTLs in clearing TGFβ-secreting Epstein-Barr virus (EBV)-positive lymphoma in vitro and in vivo107, and this approach to therapy has progressed to a Phase I clinical trial for EBV-positive lymphoma. A further modification of the CTLs, by engineering in an HER2 (also known as ERBB2) chimeric receptor as well as a DNRII, allows the CTLs to target HER2-positive tumour cells108-111. This approach is in a Phase I clinical trial for advanced HER2-positive lung malignancy, labelled the HERCREEM trial (ClinicalTrials. gov identifier: NCT00889954).
Finally, Renova has developed a recombinant TGFβ3 ligand as an anti-scarring agent on the basis of the hypothesis that this ligand has activity that is independent of and antagonistic to TGFβ1 (Ref. 112). The drug, administered by injection around a surgical wound site, progressed to a Phase III clinical trial, but unfortunately it did not reach its primary or secondary efficacy end points.
Pre-existing drugs that inhibit TGFβ
Pre-existing drugs that have been extensively used for other applications may act, in part, by inhibiting TGFβ. Examples are losartan and candesartan, which are angiotensin type II receptor inhibitors that were originally developed for the treatment of hypertension. Both appear to reduce TGFβ signalling, although the precise molecular mechanisms of this action are still unclear12,113-115. Pirfenidone acts in part by reducing the fibrotic effects of TGFβ116 via unknown targets. It is the first approved drug in Europe for idiopathic pulmonary fibrosis (IPF), and is in a Phase III trial in the United States117,118. On the other side of the coin, some common drugs, including aspirin, elevate circulating TGFβ levels, which — in certain cases such as arteriosclerosis — correlates with disease suppression119.
Therapeutic uses of TGFβ signalling inhibition
Cancer
TGFβ has a biphasic action during tumorigenesis, suppressing tumorigenesis at early stages but promoting tumour progression later on (Fig. 3). This is a paradigm for the action of TGFβ during disease progression in general, including that of fibrosis, inflammation and cardiovascular disease, and it is rooted in the fact that the normal function of this ligand is in the regulation of homeostasis. During disease progression, TGFβ signalling can go into override and, once unharnessed, results in more damage than good. The main goal in cancer therapy is therefore to downmodulate excessive levels of TGFβ ligands.
A major challenge in developing TGFβ inhibitors for cancer therapy has been the fact that these compounds are not cytotoxic or cytostatic to most tumour cells in vitro. They were developed to target properties of the tumour that are required for cancer progression, including migration, invasion and metastasis, as well as effects on the tumour microenvironment (Figs 3,4). Standard cytotoxic screens used by the pharmaceutical industry to identify anticancer drugs were therefore not relevant, and therapeutic utility could only be determined by in vivo efficacy in animal models and ultimately in the clinic.
Two major concerns in TGFβ drug development have been the inadvertent inhibition of the tumour-suppressing arm of TGFβ signalling in cancer120-122 and the development of adverse side effects unrelated to cancer, such as widespread inflammation, autoimmunity or cardiovascular defects that have been revealed by mouse gene knockout studies19-21,123. Preclinical studies suggested that attenuation of TGFβ-mediated growth inhibition would not be a major issue96,124,125. However, clinical trials to date82 have not revealed the cardiac valvulopathy126 or hyperostosis and chondrocyte hypertrophy and hyperplasia127 observed in rat preclinical toxicology studies. Moreover, there has been no widespread evidence of inflammatory complications in clinical trials reported to date54,82. These reassuring safety findings are supported by evidence from patients with the rare disease multiple self-healing squamous epithelioma (MSSE), who have germline-null mutations in the gene encoding TβRI but develop only self-limiting and mostly non-malignant skin lesions128. Intriguingly, in a Phase I clinical trial of GC-1008 for the treatment of MetM, patients developed skin lesions, keratoacanthoma or squamous cell carcinoma (SCC) that were similar to the skin abnormalities reported in MSSE, with the appearance of keratoacanthoma and SCC seemingly influenced by the extent of exposure to GC-1008. These lesions, which appeared on sun-damaged skin, were manifested in approximately 25% of patients who received higher dose levels of GC-1008 and/or longer exposure to the drug, and the lesions resolved on drug withdrawal91,93. To put this toxicity into context, non-melanoma skin cancers, such as SCC and keratoacanthoma, develop in approximately 15-30% of patients with MetM who are treated with BRAF inhibitors such as vemurafenib and dabrafenib129, and therapy with sorafenib and TNF antagonists produced similar findings130,131. Recent data from studies with vemurafenib for MetM therapy suggest that these lesions arise from pre-existent mutant RAS-containing cells within sun-damaged skin132. Intriguingly, one study of keratoacanthoma that appeared in sorafenib-treated patients showed somatic TGFBR1 missense mutations133, one of which was also identified as a causative germline mutation for MSSE128.
Cancer ‘stem cells’, or tumour-initiating cells (TICs), are defined by their capacity to self-renew and to initiate and persistently propagate the entire tumour. Targeting the cancer stem cell for destruction or irreversible quiescence is therefore the Holy Grail of oncology, especially as these cells are exceedingly resistant to both chemotherapy and radiotherapy, and are responsible for tumour metastasis and recurrence after therapy134. Several groups have now reported the phenomenon that TGFβ-induced EMT can drive tumour cells towards a more ‘stem cell-like’ phenotype characterized by increased expression of stem cell markers and enhanced tumour-initiating activity in vitro and in vivo43,135. In breast cancer135, the TGFβ and WNT signalling pathways were shown to be the most commonly activated signalling pathways in cancer stem cells that had been fractionated from the bulk tumour on the basis of expression of stem cell markers such as CD44hi and CD24low. In preclinical studies, TGFβ inhibitors have been shown to deplete the stem cell compartment in various cancers — including breast cancer135, glioblastoma136-138 and chronic myeloid leukaemia139 — which leads to increased lifespan in several mouse models of metastatic cancer. Anido et al.137 showed that glioblastoma-initiating cells (GICs, which express the stem cell markers CD44, ID1, ID3, SOX2 and SOX4) responded to LY2109761 by downregulating the expression of ‘stem cell’ genes. Moreover, patient-derived glioblastoma neurospheres transplanted orthotopically into non-obese diabetic/severe combined immunodeficient mice (NOD/SCID mice, which do not have T cells or B cells) responded to LY2109761 by decreasing in size and reducing their expression of stem cell markers137. The same research team is currently undertaking a Phase I/II clinical trial for glioblastoma using the closely related drug LY2157299 (Ref. 137). Importantly, they showed a reduction of CD44 and ID1 RNA levels after 2 months of LY2157299 treatment in tumour biopsy material from one patient with glioblastoma for whom a salvage surgical resection was performed both before and after 2 months on the trial137. The ability to reduce the number of stem cells in an aggressive tumour such as glioblastoma is a major coup.
It has been argued that TGFβ inhibitors might, however, release isolated and disseminated tumour (stem) cells from dormancy by initiating proliferation and/or disrupting the stem cell niche. A couple of recent studies may give credence to this notion, as systemic TGFβ inhibition resulted in increased numbers of circulating tumours as well as micro- and macro-metastases in mouse models of head and neck SCC and mammary cancer in vivo140,141. It might therefore be wise to use TGFβ inhibitors in combination with cytotoxic drugs to coax tumour cells out of their quiescent niche while simultaneously targeting those that respond proliferatively to TGFβ inhibition using chemotherapy. This strategy may be highly beneficial for ‘flushing out’ dormant disseminated tumour cells, as alluded to by Carlos Arteaga many years ago142. A further cautionary note is warranted, however, on the basis of two reports indicating that TGFβ may decrease the cancer-initiating cell population of diffuse type gastric carcinoma143 and breast carcinoma144 despite having little or no effect on cellular proliferation. Finally, TGFβ inhibitors might act on the stem cell niche by recruiting bone marrow mesenchymal stem cell-derived myofibroblasts that home in on the primary tumour, contribute to the tumour microenvironment as cancer-associated fibroblasts and consequently promote tumour progression145. Clearly there are tissue- and cell type-specific effects of TGFβ inhibition that can influence the action of TGFβ on the cancer stem cell and its niche146. Understanding the differential molecular mechanisms that elicit these variable responses will be critical to a judicious choice of treatment with TGFβ inhibitors or their derivatives. As TGFβ inhibitors are not directly cytotoxic, the use of these inhibitors in combination with cytotoxic chemotherapeutics may be particularly efficacious. The activation of TGFβ signalling in response to chemotherapeutics may drive the generation of cancer stem cells (via EMT), resulting in their chemoresistance134. This event may be targeted with TGFβ inhibitors, as demonstrated by the synergistic activity of doxorubicin and TGFβ inhibitor combination therapy on breast cancer growth and metastasis147. Studies in multiple myeloma also suggest that TGFβ inhibitors could potentiate the cytotoxic effects of melphalan and dexamethasone148. In vitro, the exposure of multiple myeloma cells to differentiated versus immature MC3T3-E1 pro-osteoblastic cells potentiated chemotherapy-induced multiple myeloma cell death. As TGFβ inhibition acts within the bone microenvironment to elicit osteoblastic differentiation148,149, this combinatorial approach holds great promise for the treatment of multiple myeloma and other bone metastatic cancers. Likewise, in a mouse model of serous gastric cell carcinoma, Ki26894 had an additive effect with a fluorouracil analogue in reducing tumour growth150. Finally, another mechanism whereby TGFβ inhibition can augment conventional therapies is in enhancing drug delivery to the tumour. There are reports that TGFβ inhibition can reduce interstitial tumour pressure44, which enhances the delivery of SMIs, and regulates vascular leakiness, which enhances the delivery of nanoparticle-encapsulated drugs, particularly in highly fibrotic and drug-refractile tumour types such as pancreatic cancer151.
Adoptive T cell therapy involves the harvesting and ex vivo expansion of autologous tumour-specific CTLs followed by their reintroduction into the patient to stimulate tumour killing152. Used most extensively in the treatment of MetM and lung cancer, this therapy often fails owing to the apoptosis of re-grafted CTLs. Preclinical studies suggest that failure may be due to the direct effects of TGFβ on CTLs153, and strategies to prevent such failure include the use of genetically modified CTLs with reduced TGFβ responsiveness. Transduction of CTLs with a virus encoding a DNRII154 has reached Phase I clinical trials, and recent preclinical data indicate that combining CTL therapy with TβRI-targeting SMIs may also significantly improve T cell survival and antitumour T cell cytotoxicity155. Augmenting adoptive T cell therapy with SMIs may be a particularly attractive application of TβRI SMIs as patients need not be exposed to genetically engineered T cells. Moreover, patients might only require short-term exposure to the drug for efficacy in this application, thus avoiding the side effects of long-term SMI drug exposure, such as inflammation19, cardiovascular complications126, bone and/or cartilage problems127, subphyseal hyperostosis as well as chondrocyte hypertrophy and/or hyperplasia, and reducing the risk of developing SMI drug resistance156.
Another clinical application with great promise is augmenting radiotherapy by inhibiting the TGFβ path-way10,81,157. Radiation not only physically activates latent TGFβ in vitro but also induces the biological release of this growth factor as part of a stress response158. Several groups have reported the positive role of TGFβ in supporting the DNA damage repair pathway, particularly through activation of p53 and phosphorylation of ataxia telangiectasia mutated (ATM) after radiation therapy159. Barcellos-Hoff’s group demonstrated that LY2109761 and ID11 both attenuate radiation-induced activation of p53 and ATM in breast cancer cells in vitro and in vivo, thus preventing DNA repair and accentuating the cytotoxic effect of radiation81. Even short-term dosing with TβRI inhibitors might provide a considerable therapeutic advantage in potentiating radiotherapy, with the added benefit that the local activation of pro-tumorigenic stroma and tissue fibrosis — a major complication of radiation therapy10 — may also be suppressed by these drugs. In partnership with Genzyme, this group is currently undertaking a Phase I trial of fresolimumab in combination with radiotherapy for metastatic breast cancer. Eli Lilly is also undertaking a Phase I/IIa trial to test the safety and efficacy of LY2157299 in combination with temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma157.
Myelodysplastic syndrome
Myelodysplastic syndrome (MDS) is characterized by abnormal myeloid and/or erythroid differentiation of bone marrow cells that results in various anaemias and cytopaenias. In one-third of MDS cases, a high-risk group of patients can progress to leukaemia. However, refractory cytopaenias are the major cause of morbidity and mortality in sufferers. It was recently shown that reduced expression of SMAD7, an inhibitor of TβRI, was a common and significant event observed in CD34+ myeloid progenitor cells in the bone marrow of patients with MDS160. Indeed, low levels of myeloid SMAD7 expression were seen in most patients with MDS, regardless of the risk for progression to leukaemia. Downregulation of SMAD7 expression sensitized myeloid precursors to TGFβ such that even very low levels of the ligand elicited an increase in TGFβ responsiveness, as defined by P-SMAD2 levels and enhanced immune-suppressive effect, thus providing another opportunity to utilize TGFβ inhibitors for therapeutic utility in human disease.
Treatment of primary CD34+ haematopoietic stem cells with LY2157299 suppressed the activation of TβRI by its ligand. Moreover, in a liver-specific TGFβ1-overexpressing transgenic mouse model of MDS that exhibits severe anaemia, LY2157299 decreased P-SMAD2 levels in the bone marrow and significantly increased the haematocrit of these mice. Importantly, in ten out of ten primary bone marrow cultures from patients with MDS, administration of LY2157299 significantly increased erythroid (burst-forming unit (BFU-E)) and myeloid (colony-forming unit (CFU); granulocytic monocytic) colony numbers in vitro, harbouring great promise for the treatment of patients with MDS160.
Fibrosis
IPF is a progressive, chronic and irreversible lung disease occurring in older adults, and has an unknown cause161. The main histological features of IPF are heterogeneous parenchyma, with areas of fibrosis and honeycombing alternating with areas of less-affected or normal parenchyma. IPF is characterized by a progressive reduction in lung function, with an estimated 20% survival prospect after 5 years, making it more lethal than many cancers. The progressive fibrotic reaction in IPF is associated with an epithelium-dependent fibroblast activation, in which TGFβ plays a major part16. TGFβ1, which is secreted by alveolar epithelial cells in patients with IPF, drives the process by promoting the migration, proliferation and differentiation of resident mesenchymal cells. αVβ6 integrin, which binds and activates latent TGFβ1 and TGFβ3, is highly induced following lung injury or fibrosis162. TGFβ activity163 then promotes activation and differentiation of fibroblasts into myofibroblasts, which are specialized contractile cells that cause aberrant ECM deposition, leading to the destruction of lung architecture, scarring162 and reduced lung function. TGFβ also promotes pulmonary EMT that additionally contributes to the expansion of fibroblasts and myofibroblasts164.
Pirfenidone, a novel compound that inhibits TGFβ activity in vitro, decreased the rate of decline in vital lung capacity and marginally increased progression-free survival in patients with IPF. Pooled data from two concurrent Phase III clinical trials in IPF indicated improvement in pulmonary function in the pirfenidone-treated group165. Currently, there are no US Food and Drug Administration (FDA)-approved drugs for IPF, and pirfenidone is the first such drug to be approved for IPF in Europe. Other approaches to develop TGFβ-based therapies for IPF include gene transfer of a soluble TβRII construct (as a ligand decoy), which attenuated injury and fibrosis in bleomycin-induced IPF in mice166. P144 (disitertide; Digna Biotech), a synthetic peptide that attenuates TGFβ activity and is derived from the extracellular domain of betaglycan, was also shown to reduce carbon tetrachloride-induced liver injury in mice78. P17, another Digna Biotech anti-TGFβ peptide, has been shown to be efficacious in attenuation of injury and fibrosis in bleomycin-induced IPF in mice167. Although P144 has been used clinically for skin fibrosis, drug delivery is an issue for the clinical development of P144 for IPF. However, Digna Biotech has recently partnered with Flamel Technologies to investigate the use of a proprietary drug delivery platform for this application (see the press release: ‘Flamel Technologies and Digna Biotech Announce Multiple Product Development Agreement’). Other anti-TGFβ therapies in clinical trials for IPF include the pan TGFβ-neutralizing antibody GC-1008 (Genzyme) and the αVβ6 integrin-blocking antibody STX-100 (Stromedix)168,169.
Renal fibrosis has long been thought to be driven by excess TGFβ, which results in renal scarring and, ultimately, kidney failure170. With the increasing incidence of diabetes and associated kidney damage in affluent countries, this is a clinical application of growing importance. Mice overexpressing an active form of TGFβ1 (from the liver) develop progressive liver and renal fibrosis170. Interestingly, although mice overexpressing active TGFβ1 develop progressive renal injury, latent TGFβ1 also has a protective role in renal fibrosis through negative effects on inflammation49. TGFβ1 mediates progressive renal fibrosis by stimulating the synthesis of ECM production while inhibiting its degradation49. TGFβ1 also mediates renal fibrosis by inducing the transformation of tubular epithelial cells into myofibroblasts through EMT in a similar way to the process seen in IPF171. Blockade of TGFβ1 with neutralizing TGFβ antibodies prevents or ameliorates renal fibrosis in vivo and in vitro, demonstrating the functional role of TGFβ1 in EMT and renal fibrosis. A number of therapeutic interventions that block the action of TGFβ have resulted in various degrees of improvement in kidney structure and function in preclinical studies; such interventions include TGFβ ASOs, a neutralizing anti-TGFβ antibody, a soluble TGFβ receptor, blockade of TGFβ activation by decorin172, an SMI of TGFβ receptors, delivery of the inhibitor protein SMAD7 (Ref. 173) and a THBS1-blocking peptide that interferes with TGFβ activation97. A Phase I/II trial with GC-1008, a pan-TGFβ-neutralizing antibody, exhibited encouraging efficacy in patients with focal segmental glomerulosclerosis92, and Eli Lilly is undertaking trials of its own anti-TGFβ1 monoclonal antibody, LY2382770, in diabetic kidney disease.
Cardiac fibrosis is a pathological feature that is common to a number of forms of heart disease, including myocardial infarction, ischaemic, dilated and hypertrophic cardiomyopathies and congestive heart failure36. The cellular basis of cardiac fibrosis is the aberrant accumulation of collagens and other ECM proteins, which impair ventricular function and predispose to cardiac arrhythmias. Because TGFβ has pleiotropic effects in the cardiovascular system and as cardiac fibrosis is a multifactorial disease, the development of an effective therapy will require a detailed understanding of the role of the TGFβ signalling pathway in this pathogenesis. TGFβ, a potent stimulator of collagen production by cardiac fibroblasts, is induced in response to cardiovascular injury. The TGFβ-SMAD pathway activates the transcription of several key fibrotic genes, such as those encoding connective tissue growth factor (CTGF), fibronectin, collagens and plasminogen activator inhibitor 1 (PAI1)36. TGFβ reduces collagenase production and stimulates the expression of tissue inhibitor of metalloproteinases (TIMPs), resulting in an overall inhibition of ECM degradation and leading to excessive ECM accumulation. P144 has been investigated in a preclinical model of cardiac fibrosis77, and losartan can reverse fibrosis in a mouse model of hypertrophic cardiomyo pathy174; however, no drug targeting the TGFβ pathway has yet reached clinical trials for this application. A recent study demonstrated that miR-21, which is regulated by SMADs upon TGFβ activation, is consistently induced by cardiac stress. As miR-21 plays a part in tumorigenesis by promoting cell proliferation, increased expression of miR-21 might contribute to the progression of fibrotic lesions175. ASOs against miR-21 might therefore become a novel therapeutic approach for treating cardiac fibrosis.
Scleroderma
Scleroderma (progressive systemic sclerosis) is a systemic autoimmune disorder characterized by skin sclerosis, calcinosis and changes in microvasculature. Increased expression of TβRI and TβRII in sclerodermal fibroblasts suggests that increased production of type I collagen by autocrine TGFβ signalling leads to aberrant ECM deposition and scarring36. Therapeutic approaches to scleroderma have included inhibition of TGFβ activity in sclerotic tissue. Unfortunately, CAT-192, a TGFβ1-neutralizing antibody, did not show evidence of efficacy in a study on the treatment of patients with early-stage systemic scleroderma90; however, GC-1008 is now in a Phase I clinical trial for patients with diffuse systemic sclerosis. Furthermore, topical application of Digna Biotech’s P144 peptide inhibitor of TGFβ1 has shown some efficacy in reducing skin fibrosis in a Phase II clinical trial for systemic sclerosis (see the press release: ‘Flamel Technologies and Digna Biotech Announce Multiple Product Development Agreement’), with the caveat that clinical end points for quantifying skin fibrosis have not yet been standardized176.
Restenosis following coronary artery bypass and angioplasty
The development of fibromuscular intimal hyperplasia following angioplasty and coronary artery bypass surgery is a major clinical problem and can lead to coronary artery graft failure. The success of coronary artery reconstructive procedures is limited by the high incidence of restenosis secondary to intimal hyperplasia. TGFβ1 is a major player in the early development of intimal hyperplasia in arteries and peripheral vein grafts. The exact mechanism of action of TGFβ signalling in intimal hyperplasia and subsequent graft failure is unclear, but it is speculated that TGFβ1 contributes at multiple steps, including EMT, promotion of fibroblast, endothelial and vascular smooth muscle cell proliferation, increased collagen synthesis and deposition, and induction of fibrosis177. Soluble forms of the small, leucine-rich proteoglycans decorin and fibromodulin, which possess TGFβ-antagonist activity, exhibit potent intimal hyperplasia-suppressing effects in cultured human saphenous vein, offering the potential for therapeutic benefit after coronary artery bypass surgery178-180. The novel pyrrole-imidazole polyamide drug class, targeted to suppress TGFB1 gene transcription, showed efficacy in reducing neointimal hyperplasia and stimulating re-endothelialization of carotid arteries in a preclinical model of arterial injury50.
Marfan syndrome
MFS is a connective tissue disorder that affects the musculoskeletal, ocular and cardiovascular systems. It is caused by mutations in the gene encoding an ECM protein, fibrillin 1 (FBN1)181. Growing evidence suggests that FBN1 mutations perturb not only the general integrity and elasticity of tissues but also — probably more crucially — local TGFβ signalling11. Normal fibrillin 1-containing microfibrils interact with the large latent TGFβ complex (LLC) to control the release of mature, active TGFβ181. Mutated fibrillin 1 fails to sequester latent TGFβ, leading to the promiscuous activation of TGFβ11. Thus, MFS highlights the critical role of microfibrils in regulating local concentrations of TGFβ and in maintaining the homeostasis, morphogenesis and function of various organs181. Dilation of the aortic root, which leads to aortic rupture and sudden death, is a major clinical issue for patients with MFS and patients of the Loeys-Dietz spectrum who carry mutations in TGFBR1, TGFBR2 and SMAD3. A mouse model of MFS carrying a heterozygous Fbn1 mutation developed an aortic aneurism similar to that of patients with MFS. Administration of TGFβ antagonists, including a TGFβ-neutralizing antibody or the angiotensin II type 1 receptor (AT1) blocker losartan, successfully rescued both cardiovascular and non-cardiovascular manifestations of MFS12,182. As losartan is already in widespread clinical use for hypertension and has shown no adverse effects, this drug is currently being tested in Phase I/II clinical trials for MFS (ClinicalTrials.gov identifiers: NCT00429364, NCT00593710, NCT00683124, NCT00723801, NCT00763893, NCT00782327 and NCT01145612) and may plausibly reduce this life-threatening manifestation of MFS.
Postoperative scarring in ocular conditions
Trabeculectomy is a surgical procedure designed to reduce intraocular fluid pressure in patients with glaucoma. However, postoperative scarring and fibrotic blockage of the ‘filtering bleb’ that drains excess ocular fluid are serious complications of this procedure. In preclinical experiments, administration of neutralizing antibodies against human TGFβ2 (CAT-152) exhibited promising inhibition of scarring after glaucoma surgery in rabbits without having any adverse effects. Initial clinical trials with CAT-152 ameliorated scarring in patients who received trabeculectomy for intractable glaucoma88,183; however, Phase III clinical trials were unable to validate such beneficial effects184. Tranilast, another incidental TGFβ inhibitor, has also been used successfully to reduce re-occurrence of corneal fibrosis, or primary pterygium, following corneal surgery185. It is possible that topical application of more-specific TGFβ inhibitors might also be used in treating corneal haze and conjunctival scarring (Table 1).
Challenges and considerations for TGFβ blockade
Individualized responses to TGFβ blockade
Many diseases being tackled with TGFβ inhibitors, including fibrosis, inflammation, autoimmunity and cancer, are complex in nature and show strong genetic predisposition owing to innate genetic variation between individuals. It is well established that there is considerable phenotypic diversity in the range of responses to reduced TGFβ signalling in vivo, which are dictated by differential inheritance of germline genetic variants. This is illustrated by the large spectrum of clinical severity and disease manifestations in individuals with mutations in TGFβ signalling pathway genes8,41,186. Moreover, in mouse models of cancer, asthma and vascular development, outcomes of reduced TGFβ1 levels are strongly influenced by interacting genetic modifier loci5,9,186,187. It is therefore most rational, economical and safe to preselect patient populations before initiating anti-TGFβ drug treatment on the basis of surrogate markers of TGFβ involvement in the disease process (such as increased TGFβ ligand and P-SMAD levels, and specific disease characteristics) and contra-indications of possible adverse side effects (such as susceptibility to inflammation or certain vascular conditions). Peripheral blood may provide non-invasive markers that might be useful in this respect, including the ability to quantitatively screen patient responses to TGFβ inhibition on the basis of measuring P-SMAD2 levels in peripheral blood mononuclear cells (PMNCs)188. Similarly, potential adverse inflammatory effects could be predicted by examining specific immunological responses of PMNCs189-192 to TGFβ inhibition ex vivo.
Patient selection for TGFβ inhibitors in oncology
The simplest biomarker for patient selection for TGFβ inhibitors in oncology is probably high circulating levels of TGFβ, as one major goal of this therapy is to reduce, but not totally ablate, TGFβ signalling193. As indicated above, non-invasive biomarkers for predicting patient responsiveness and efficacy of TGFβ inhibitors have been developed on the basis of measuring P-SMAD2 levels in PMNCs. Indeed, PMNCs can be treated with drugs ex vivo to determine, and thus predict, individual patient responses to SMIs188,193. As TGFβ inhibitors certainly act to reduce tumour metastasis, the assay of circulating tumour cell number may also be a useful indicator for therapeutic response. Moreover, as many of the pro-tumorigenic effects of TGFβ are mediated by immune system modulation, it might be possible to monitor blood cell or plasma protein profiles for patient selection and for surrogate monitoring of patient responses.
In cancer (as well as non-malignant diseases), the outcome of reduced TGFβ signalling may be highly dependent on the innate genetic background of the individual, especially when considering tumour microenvironment effects, such as immune surveillance. Elucidating specifically which genetic variants influence signalling output will not only be useful for dissecting the intricacies of this signalling pathway in vivo, but may also provide predictive markers for the outcome (desired or undesired) of TGFβ signal inhibition. However, for cancer therapeutics, patient selection may be more complex, as the response of both the tumour cell and host (tumour microenvironment and normal patient tissue) to TGFβ blockade needs to be considered. Tumour biopsy and genetic analysis (for example, loss of TGFBR2, SMAD2 or SMAD4)194,195 might predict whether the tumour retains growth sensitivity to TGFβ. Molecular and histological analyses may also contribute to the prediction of tumour responses to TGFβ inhibition. The activation of alternative intracellular signalling pathways and transcription factor profiles has been associated with the switch from tumour suppression — by TGFβ — to tumour progression. These include an increased ratio of liver-enriched inhibitory protein (LIP) to liver-enriched activating protein (LAP), which are isoforms of CCAAT/enhancer-binding protein-β (C/EBPβ), a central transcription factor that binds within the SMAD transcription factor complex to elicit TGFβ-mediated cytostatic responses196. Upregulation of SIX1 in breast cancer has also been shown to be pivotal in the growth-suppressive to tumour-progressive switch197, as has downregulation of DAB2 (Ref. 198). Any of these tumour markers, possibly in combination, may be used in the future to predict tumour responses to TGFβ inhibition.
Finally, the effects of interactions with other anticancer drugs will need to be considered, as some drugs may resurrect the growth-inhibitory arm of the TGFβ signalling pathway, which would then counter-indicate their combinatorial use with TGFβ inhibitors199. Indeed, the ability to specifically target the pro-tumorigenic versus the tumour-suppressive effects of TGFβ on the tumour cell per se will require the development of next-generation drugs focusing on these downstream pathways. In the meantime, much research still remains to be undertaken to make inroads into the area of informed patient selection for oncology applications of TGFβ inhibitors.
Drug resistance
In oncology, the development of tumour drug resistance is inevitable134,200,201. It has been documented for standard chemotherapy, pathway-targeted therapies and is even common with anti-angiogenesis inhibitors200,201. Acquired biochemical resistance of tumour cells to LY2109761 has been observed in a preclinical model of SCC and may have adverse consequences in driving a more stem cell-like phenotype156, although this remains to be tested. Carefully restricting TGFβ inhibitors to short-term or intermittent usage should avoid these complications82. Combinatorial and/or sequential treatment with complementary drugs will also be important. It is clear that oncologists will need an arsenal of different anticancer drugs to tackle cancer, in much the same way that antibiotics have been developed to combat infectious diseases.
Conclusion and future directions
In conclusion, TGFβ signalling inhibitors are generally safe and may be efficacious in several clinical applications, especially in desperate cases such as end-stage cancer or IPF. The development of these drugs may offer further therapeutic opportunities. Counterintuitively, there have been reports suggesting that inhibition of the TGFβ signalling pathway may be beneficial in autoimmune disorders, such as multiple sclerosis, through downregulation of the TH17 pathway202,203. Recent studies have also suggested that TGFβ-SMAD3 signalling regulates glucose tolerance and energy homeostasis, and that blockade of the pathway may be used for regulation of diabetes and obesity204. The outlook for anti-TGFβ signalling therapy for numerous diseases appears bright. At least four companies are well on their way in clinical drug development, and further scientific and mechanistic studies are warranted in order to optimize patient selection and drug-dosing regimens for each disease application.
Acknowledgements
The authors give thanks to J. Dotor, J. McPherson and J. Yingling for useful discussions. Work in the authors’ laboratories is funded by grants from the US National Cancer Institute, the US National Heart, Lung and Blood Institute (NHLBI), the US National Institute of Arthritis and Musculoskeletal and Skin Diseases, the US National Institute of General Medical Sciences, the Bouque Foundation and the Helen Diller Family Comprehensive Cancer Center to R.J.A., and from the NHLBI, the American Heart Association and the LeDucq Foundation to A.H. We apologize to all colleagues whose work could not be cited owing to space restrictions.
Glossary
- Epithelial-mesenchymal transition
(EMT). The transformation of a keratin-expressing epithelial cell into one with fibroblastic properties that express mesenchymal markers
- Extracellular matrix
(ECM). Matrix that supports connective tissue and is composed of proteoglycans, hyaluronic acid and fibrillar proteins secreted from the cell and rich in bound growth factors
- Fibrosis
The excess accumulation of fibroblasts and associated extracellular matrix
- Metastasis
The dissemination of tumour cells and re-establishment of tumours at a secondary site
- SMAD
Signal transduction component of the canonical transforming growth factor-β signalling pathway
- microRNA
(miRNA). Small (20-23 nucleotides long) non-coding RNA involved in post-translational regulation of gene expression. miRNAs bind to the partially complementary sequence in the 3′-untranslated region (3′-UTR) of mRNAs and negatively regulate their expression either through translational inhibition or promotion of mRNA degradation.
- Myofibroblast
A contractile fibroblast that expresses smooth muscle actin and myosin, and contributes to disease progression in cancer and fibrosis
- Antisense oligonucleotides
(ASOs). Short chemically modified oligonucleotides complementary to a specific mRNA that can be used to cause specific knockdown of targeted gene expression
- Tumour-initiating cells
(TICs). The putative cancer stem cells that have the ability to maintain tumour growth, differentiate into all cell types of a heterogenous tumour, and to re-establish secondary tumours with exceedingly high efficiency.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Rosemary J. Akhurst’s homepage: http://cancer.ucsf.edu/research/akhurst-lab Akiko Hata’s homepage: http://www.cvri.ucsf.edu/Scientist/Hata.shtml ClinicalTrials.gov website: http://www.clinicaltrials.gov Flamel Technologies and Digna Biotech Announce Multiple Product Development Agreement (27 June 2011 press release): http://www.dignabiotech.com/comun/fichero.asp?id=105
References
- 1.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nature Rev. Mol. Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 2.Derynck R, Miyazono K, editors. The TGF-β Family. Cold Spring Harbor Press; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nature Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 4.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nature Rev. Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao JH, et al. Genetic variants of Tgfb1 act as context-dependent modifiers of mouse skin tumor susceptibility. Proc. Natl Acad. Sci. USA. 2006;103:8125–8130. doi: 10.1073/pnas.0602581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Wang XF. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conidi A, et al. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signaling in vivo. Cytokine Growth Factor Rev. 2011;22:287–300. doi: 10.1016/j.cytogfr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Akhurst RJ. TGFβ signaling in health and disease. Nature Genet. 2004;36:790–792. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 9.Freimuth J, et al. Epistatic interactions between Tgfb1 and genetic loci, Tgfbm2 and Tgfbm3, determine susceptibility to an asthmatic stimulus. Proc. Natl Acad. Sci. USA. doi: 10.1073/pnas.1205374109. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anscher MS, et al. Small molecular inhibitor of transforming growth factor-β protects against development of radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Neptune ER, et al. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nature Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 12.Habashi JP, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts AB, Wakefield LM. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl Acad. Sci. USA. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massague J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy L, Hill CS. Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 2011;29:140–152. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 17.Harradine KA, Akhurst RJ. Mutations of TGFβ signaling molecules in human disease. Ann. Med. 2006;38:403–414. doi: 10.1080/07853890600919911. [DOI] [PubMed] [Google Scholar]
- 18.Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF β1, β2 and β3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni AB, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson MC, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 22.Sanford LP, et al. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proetzel G, et al. Transforming growth factor-β3 is required for secondary palate fusion. Nature Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-β in a chemically defined system. J. Biol. Chem. 1994;269:26775–26782. [PubMed] [Google Scholar]
- 25.Munger JS, et al. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 26.Shi M, et al. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 28.Davis-Dusenbery BN, Hata A. Smad-mediated miRNA processing: a critical role for a conserved RNA sequence. RNA Biol. 2011;8:71–76. doi: 10.4161/rna.8.1.14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oft M, et al. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 30.Massague J, Gomis RR. The logic of TGFβ signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Massague J. How cells read TGF-β signals. Nature Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 32.Mehra A, Wrana JL. TGF-β and the Smad signal transduction pathway. Biochem. Cell Biol. 2002;80:605–622. doi: 10.1139/o02-161. [DOI] [PubMed] [Google Scholar]
- 33.Padua D, Massague J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 34.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-β signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardali E, Ten Dijke P. TGFβ signaling and cardiovascular diseases. Int. J. Biol. Sci. 2012;8:195–213. doi: 10.7150/ijbs.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nature Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 38.Coffey RJ, Jr, et al. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988;48:1596–1602. [PubMed] [Google Scholar]
- 39.Gomis RR, et al. A FoxO-Smad synexpression group in human keratinocytes. Proc. Natl Acad. Sci. USA. 2006;103:12747–12752. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel PM, Shu W, Massague J. Mad upregulation and Id2 repression accompany transforming growth factor (TGF)-β-mediated epithelial cell growth suppression. J. Biol. Chem. 2003;278:35444–35450. doi: 10.1074/jbc.M301413200. [DOI] [PubMed] [Google Scholar]
- 41.Akhurst RJ. The paradoxical TGF-β vasculopathies. Nature Genet. 2012;44:838–839. doi: 10.1038/ng.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nature Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 43.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salnikov AV, et al. Inhibition of TGF-β modulates macrophages and vessel maturation in parallel to a lowering of interstitial fluid pressure in experimental carcinoma. Lab. Invest. 2005;85:512–521. doi: 10.1038/labinvest.3700252. [DOI] [PubMed] [Google Scholar]
- 45.Willis BC, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-β1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KK, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl Acad. Sci. USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor β-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc. Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao EH, et al. A pyrrole-imidazole polyamide targeting transforming growth factor-β1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc. Res. 2009;81:797–804. doi: 10.1093/cvr/cvn355. This study evaluates the potential use of a pyrrole-imidazole polyamide that targets TGFB1 expression for preventing restenosis after the use of drug-eluting stents.
- 51.Tsai S, et al. TGF-β through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H540–H549. doi: 10.1152/ajpheart.91478.2007. This study demonstrates the role of the TGFβ-SMAD3 pathway in the development of intimal hyperplasia in response to endothelial injury.
- 52.Fu K, et al. SM16, an orally active TGFβ type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler. Thromb. Vasc. Biol. 2008;28:665–671. doi: 10.1161/ATVBAHA.107.158030. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura G, et al. δEF1 mediates TGF-β signaling in vascular smooth muscle cell differentiation. Dev. Cell. 2006;11:93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Diebold RJ, et al. Early-onset multifocal inflammation in the transforming growth factor β1-null mouse is lymphocyte mediated. Proc. Natl Acad. Sci. USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubtsov YP, Rudensky AY. TGFβ signalling in control of T-cell-mediated self-reactivity. Nature Rev. Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 56.Ramesh S, Wildey GM, Howe PH. Transforming growth factor β (TGFβ)-induced apoptosis: the rise and fall of Bim. Cell Cycle. 2009;8:11–17. doi: 10.4161/cc.8.1.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruyama T, et al. Control of the differentiation of regulatory T cells and TH17 cells by the DNA-binding inhibitor Id3. Nature Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nature Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. This study shows that T cell-specific blockade of TGFβ signalling using a dominant negative type II receptor potentiates immune eradication of tumours in mice challenged with live tumour cells.
- 59.Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-β1 and TNF-α on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–153. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- 60.Sato K, et al. TGF-β1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J. Immunol. 2000;164:2285–2295. doi: 10.4049/jimmunol.164.5.2285. [DOI] [PubMed] [Google Scholar]
- 61.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 62.Mangan PR, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 63.Gutcher I, et al. Autocrine transforming growth factor-β1 promotes in vivo TH17 cell differentiation. Immunity. 2011;34:396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Annunziato F, Romagnani S. Mouse T helper 17 phenotype: not so different than in man after all. Cytokine. 2011;56:112–115. doi: 10.1016/j.cyto.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Das J, et al. Transforming growth factor β is dispensable for the molecular orchestration of TH17 cell differentiation. J. Exp. Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghoreschi K, et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nature Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 68.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 69.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. This study shows that TGFβ blockade increases neutrophil-attracting chemokines, and ‘depolarizes’ the invading tumour-associated neutrophils to become more cytotoxic to tumour cells and to express higher levels of pro-inflammatory cytokines.
- 70.Schlingensiepen R, et al. Intracerebral and intrathecal infusion of the TGF-β2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides. 2005;15:94–104. doi: 10.1089/oli.2005.15.94. [DOI] [PubMed] [Google Scholar]
- 71.Schlingensiepen KH, et al. Targeted tumor therapy with the TGF-β2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–139. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Jachimczak P, et al. The effect of transforming growth factor-β2-specific phosphorothioate-anti-sense oligodeoxynucleotides in reversing cellular immunosuppression in malignant glioma. J. Neurosurg. 1993;78:944–951. doi: 10.3171/jns.1993.78.6.0944. [DOI] [PubMed] [Google Scholar]
- 73.Schlingensiepen KH, et al. Transforming growth factor-β2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 2011;102:1193–1200. doi: 10.1111/j.1349-7006.2011.01917.x. [DOI] [PubMed] [Google Scholar]
- 74.Bogdahn U, et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13:132–142. doi: 10.1093/neuonc/noq142. This is the full report of a randomized, open-label, active-controlled, dose-finding Phase IIb study to evaluate the efficacy and safety of trabedersen (AP12009) administered intra-tumourally by convection-enhanced delivery in patients with recurrent or refractory high-grade glioma.
- 75.Santiago B, et al. Topical application of a peptide inhibitor of transforming growth factor-β1 ameliorates bleomycin-induced skin fibrosis. J. Invest. Dermatol. 2005;125:450–455. doi: 10.1111/j.0022-202X.2005.23859.x. This is the first evidence that the peptide inhibitor P144 may be efficacious in the treatment of skin fibrosis after topical application.
- 76.Llopiz D, et al. Peptide inhibitors of transforming growth factor-β enhance the efficacy of antitumor immunotherapy. Int. J. Cancer. 2009;125:2614–2623. doi: 10.1002/ijc.24656. [DOI] [PubMed] [Google Scholar]
- 77.Hermida N, et al. A synthetic peptide from transforming growth factor-β1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovascular Res. 2009;81:601–609. doi: 10.1093/cvr/cvn315. This study shows that P144 inhibits the TGFβ1-dependent signalling pathway and prevents myocardial fibrosis.
- 78.Ezquerro IJ, et al. A synthetic peptide from transforming growth factor β type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine. 2003;22:12–20. doi: 10.1016/s1043-4666(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 79.Nam JS, et al. An anti-transforming growth factor β antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ganapathy V, et al. Targeting the transforming growth factor-β pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer. 2010;9:122. doi: 10.1186/1476-4598-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouquet F, et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin. Cancer Res. 2011;17:6754–6765. doi: 10.1158/1078-0432.CCR-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodon Ahnert J, et al. First human dose (FHD) study of the oral transforming growth factor-β receptor I kinase inhibitor LY2157299 in patients with treatment-refractory malignant glioma. J. Clin. Oncol. 2011;29 Abstract 3011. [Google Scholar]
- 83.Schlingensiepen KH, Fischer-Blass B, Schmaus S, Ludwig S. Antisense therapeutics for tumor treatment: the TGF-β2 inhibitor AP 12009 in clinical development against malignant tumors. Recent Results Cancer Res. 2008;177:137–150. doi: 10.1007/978-3-540-71279-4_16. [DOI] [PubMed] [Google Scholar]
- 84.Oettle H, et al. Final results of a phase I/II study in patients with pancreatic cancer, malignant melanoma, and colorectal carcinoma with trabedersen. J. Clin. Oncol. 2012;30 Abstract 4034. [Google Scholar]
- 85.Nemunaitis J, et al. Phase II study of belagenpumatucel-L, a transforming growth factor β-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J. Clin. Oncol. 2006;24:4721–4730. doi: 10.1200/JCO.2005.05.5335. This paper reports the first Phase II trial of the non-viral, gene-based, allogeneic tumour cell vaccine of antisense TGFβ2-transfected NSCLC cells. This vaccine shows great promise in increasing survival rates for NSCLC.
- 86.Nemunaitis J, et al. Phase II trial of Belagenpumatucel-L, a TGF-β2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther. 2009;16:620–624. doi: 10.1038/cgt.2009.15. [DOI] [PubMed] [Google Scholar]
- 87.Bazhenova L, Carrier E, Shawler D, Fakhrai H. Long-term survival in a phase II study of belagenpumatucel-L (antisense TGFβ vaccine) in non small-cell lung cancer (NSCLC) Cancer Res. 2012;72 Abstract 5367. [Google Scholar]
- 88.Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-β2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest. Ophthalmol. Vis. Sci. 2003;44:3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 89.CAT-152 0201 Trabeculectomy Study Group CAT-152 trabeculectomy study. Ophthalmology. 2007;114:1950. doi: 10.1016/j.ophtha.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 90.Denton CP, et al. Recombinant human anti-transforming growth factor β1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:323–333. doi: 10.1002/art.22289. This report evaluates CAT-192, a recombinant human antibody that neutralizes TGFβ1, in the treatment of early-stage diffuse cutaneous systemic sclerosis and concludes that there is no evidence of efficacy.
- 91.Lonning S, Mannick J, McPherson JM. Antibody targeting of TGF-β in cancer patients. Curr. Pharm. Biotechnol. 2011;12:2176–2189. doi: 10.2174/138920111798808392. [DOI] [PubMed] [Google Scholar]
- 92.Trachtman H, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morris JC, et al. Phase I/II study of GC1008: a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC) J. Clin. Oncol. 2008;26 doi: 10.1371/journal.pone.0090353. Abstract 9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong Z, et al. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin. Cancer Res. 2010;16:1191–1205. doi: 10.1158/1078-0432.CCR-09-1634. [DOI] [PubMed] [Google Scholar]
- 95.Van Aarsen LA, et al. Antibody-mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res. 2008;68:561–570. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- 96.Muraoka RS, et al. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J. Clin. Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am. J. Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-β signalling inhibitors for cancer therapy. Nature Rev. Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 99.Bonafoux D, Lee WC. Strategies for TGF-β modulation: a review of recent patents. Expert Opin. Ther. Pat. 2009;19:1759–1769. doi: 10.1517/13543770903397400. [DOI] [PubMed] [Google Scholar]
- 100.Saunier EF, Akhurst RJ. TGFβ inhibition for cancer therapy. Curr. Cancer Drug Targets. 2006;6:519–532. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 101.Akhurst RJ. Large- and small-molecule inhibitors of transforming growth factor-beta signaling. Curr. Opin. Investig. Drugs. 2006;7:513–521. [PubMed] [Google Scholar]
- 102.Washio H, et al. Transcriptional inhibition of hypertrophic scars by a gene silencer, pyrrole-imidazole polyamide, targeting the TGF-β1 promoter. J. Invest. Dermatol. 2011;131:1987–1995. doi: 10.1038/jid.2011.150. [DOI] [PubMed] [Google Scholar]
- 103.Chen M, et al. Pretranscriptional regulation of Tgf-β1 by PI polyamide prevents scarring and accelerates wound healing of the cornea after exposure to alkali. Mol. Ther. 2010;18:519–527. doi: 10.1038/mt.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen HY, et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60:590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kapoor S. Smad7 gene transfer therapy: therapeutic applications beyond colonic fibrosis. Eur. J. Clin. Invest. 2008;38:876–877. doi: 10.1111/j.1365-2362.2008.02038.x. author reply 878-880. [DOI] [PubMed] [Google Scholar]
- 106.Sheridan C. Gene therapy finds its niche. Nature Biotech. 2011;29:121–128. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- 107.Foster AE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J. Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rainusso N, et al. Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther. 2012;19:212–217. doi: 10.1038/cgt.2011.83. [DOI] [PubMed] [Google Scholar]
- 109.Nakazawa Y, et al. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol. Ther. 2011;19:2133–2143. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahmed N, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin. Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed N, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 112.Laverty HG, et al. Effects of avotermin (transforming growth factor β3) in a clinically relevant pig model of long, full-thickness incisional wounds. J. Cutan. Med. Surg. 2010;14:223–232. doi: 10.2310/7750.2010.09069. [DOI] [PubMed] [Google Scholar]
- 113.Cohn RD, et al. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nature Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Podowski M, et al. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. J. Clin. Invest. 2012;122:229–240. doi: 10.1172/JCI46215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lanz TV, et al. Angiotensin II sustains brain inflammation in mice via TGF-β. J. Clin. Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou H, Latham CW, Zander DS, Margolin SB, Visner GA. Pirfenidone inhibits obliterative airway disease in mouse tracheal allografts. J. Heart Lung Transplant. 2005;24:1577–1585. doi: 10.1016/j.healun.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 117.Taniguchi H, et al. The clinical significance of 5% change in vital capacity in patients with idiopathic pulmonary fibrosis: extended analysis of the pirfenidone trial. Respir. Res. 2011;12:93. doi: 10.1186/1465-9921-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 119.Grainger DJ, et al. The serum concentration of active transforming growth factor-β is severely depressed in advanced atherosclerosis. Nature Med. 1995;1:74–79. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- 120.Cui W, et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 121.Bierie B, Moses HL. Gain or loss of TGFβ signaling in mammary carcinoma cells can promote metastasis. Cell Cycle. 2009;8:3319–3327. doi: 10.4161/cc.8.20.9727. [DOI] [PubMed] [Google Scholar]
- 122.Bierie B, et al. Abrogation of TGF-β signaling enhances chemokine production and correlates with prognosis in human breast cancer. J. Clin. Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larsson J, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akhurst RJ. TGF-β antagonists: why suppress a tumor suppressor? J. Clin. Invest. 2002;109:1533–1536. doi: 10.1172/JCI15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang YA, et al. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J. Clin. Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. This is the first demonstration that long-term exposure to a TGFβ antagonist in mice does not manifest any major adverse effects.
- 126.Anderton MJ, et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol. Pathol. 2011;39:916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 127.Frazier K, et al. Inhibition of ALK5 signaling induces physeal dysplasia in rats. Toxicol. Pathol. 2007;35:284–295. doi: 10.1080/01926230701198469. [DOI] [PubMed] [Google Scholar]
- 128.Goudie DR, et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nature Genet. 2011;43:365–369. doi: 10.1038/ng.780. [DOI] [PubMed] [Google Scholar]
- 129.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arnault JP, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J. Clin. Oncol. 2009;27:e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 131.Esser AC, Abril A, Fayne S, Doyle JA. Acute development of multiple keratoacanthomas and squamous cell carcinomas after treatment with infliximab. J. Am. Acad. Dermatol. 2004;50:S75–S77. doi: 10.1016/j.jaad.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 132.Su F, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arnault JP, et al. Skin tumors induced by sorafenib; paradoxic RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin. Cancer Res. 2012;18:263–272. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]
- 134.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shipitsin M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 136.Ikushima H, et al. Autocrine TGF-β signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 137.Anido J, et al. TGF-β receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. This is a demonstration of the targeting of tumour-initiating cells by TGFβ blockade in a mouse model of glioblastoma.
- 138.Penuelas S, et al. TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 139.Naka K, et al. TGF-β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. This is a demonstration of the targeting of leukaemia-initiating cells by TGFβ blockade in a mouse model of chronic myeloid leukaemia.
- 140.Bragado P, et al. Microenvironmental signals dictate disseminated tumor cells (DTCs) fate through regulation of TGFβII and p38α. Cancer Res. 2012;72(Suppl. 1) Abstract 5234. [Google Scholar]
- 141.Biswas T, Yang J, Zhao L, Sun LZ. Development of murine models of breast cancer metastasis for the evaluation of the efficacy of TGF-β inhibitors as therapeutic agents. Cancer Res. 2012;72(Suppl. 1) Abstract 1366. [Google Scholar]
- 142.Ohmori T, Yang JL, Price JO, Arteaga CL. Blockade of tumor cell transforming growth factor-βs enhances cell cycle progression and sensitizes human breast carcinoma cells to cytotoxic chemotherapy. Exp. Cell Res. 1998;245:350–359. doi: 10.1006/excr.1998.4261. [DOI] [PubMed] [Google Scholar]
- 143.Ehata S, et al. Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells. Oncogene. 2011;30:1693–1705. doi: 10.1038/onc.2010.546. [DOI] [PubMed] [Google Scholar]
- 144.Tang B, et al. Transforming growth factor-β can suppress tumorigenesis through effects on the putative cancer stem or early progenitor cell and committed progeny in a breast cancer xenograft model. Cancer Res. 2007;67:8643–8652. doi: 10.1158/0008-5472.CAN-07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hayashi T, et al. Transforming growth factor β receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin. Cancer Res. 2004;10:7540–7546. doi: 10.1158/1078-0432.CCR-04-0632. This is the first report of a chemical inhibitor of TβRI showing antitumour effects via activity on the tumour microenvironment in a mouse model of multiple myeloma.
- 147.Bandyopadhyay A, et al. Doxorubicin in combination with a small TGFβ inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PLoS ONE. 2010;5:e10365. doi: 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takeuchi K, et al. TGF-β inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PLoS ONE. 2010;5:e9870. doi: 10.1371/journal.pone.0009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yin JJ, et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shinto O, et al. Combination effect of a TGF-β receptor kinase inhibitor with 5-FU analog S1 on lymph node metastasis of scirrhous gastric cancer in mice. Cancer Sci. 2010;101:1846–1852. doi: 10.1111/j.1349-7006.2010.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kano MR, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling. Proc. Natl Acad. Sci. USA. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J. Immunother. 2012;35:299–308. doi: 10.1097/CJI.0b013e3182518e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang L, et al. Immunotherapy for human renal cell carcinoma by adoptive transfer of autologous transforming growth factor β-insensitive CD8+ T cells. Clin. Cancer Res. 2010;16:164–173. doi: 10.1158/1078-0432.CCR-09-1758. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Q, et al. Blockade of transforming growth factor-β signaling in tumor-reactive CD8+ T cells activates the antitumor immune response cycle. Mol. Cancer Ther. 2006;5:1733–1743. doi: 10.1158/1535-7163.MCT-06-0109. [DOI] [PubMed] [Google Scholar]
- 155.Wallace A, et al. Transforming growth factor-β receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers. Clin. Cancer Res. 2008;14:3966–3974. doi: 10.1158/1078-0432.CCR-08-0356. This is the first demonstration of the use of a chemical TGFβ receptor inhibitor augmenting adoptive T cell therapy via its immunomodulatory effects.
- 156.Connolly EC, et al. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long term TβRI/II kinase inhibition with LY2109761. Cancer Res. 2011;71:1–11. doi: 10.1158/0008-5472.CAN-10-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang M, et al. Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–7167. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 158.Barcellos-Hoff MH, Akhurst RJ. Transforming growth factor-β in breast cancer: too much, too late. Breast Cancer Res. 2009;11:202. doi: 10.1186/bcr2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kirshner J, et al. Inhibition of transforming growth factor-β1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 2006;66:10861–10869. doi: 10.1158/0008-5472.CAN-06-2565. [DOI] [PubMed] [Google Scholar]
- 160.Zhou L, et al. Reduced SMAD7 leads to overactivation of TGF-β signaling in MDS that can be reversed by a specific inhibitor of TGF-β receptor I kinase. Cancer Res. 2011;71:955–963. doi: 10.1158/0008-5472.CAN-10-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cerri S, Spagnolo P, Luppi F, Richeldi L. Management of idiopathic pulmonary fibrosis. Clin. Chest Med. 2012;33:85–94. doi: 10.1016/j.ccm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 162.Sheppard D. Transforming growth factor β: a central modulator of pulmonary and airway inflammation and fibrosis. Proc. Am. Thorac. Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Katsumoto TR, Violette SM, Sheppard D. Blocking TGFβ via inhibition of the αvβ6 integrin: a possible therapy for systemic sclerosis interstitial lung disease. Int. J. Rheumatol. 2011;2011:208219. doi: 10.1155/2011/208219. This report summarizes the inhibition of αVβ6 integrin as an attractive therapeutic option for systemic sclerosis and interstitial lung disease mediated by TGFβ.
- 164.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu. Rev. Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 165.Azuma A. Pirfenidone treatment of idiopathic pulmonary fibrosis. Ther. Adv. Respir. Dis. 2012;6:107–114. doi: 10.1177/1753465812436663. This report summarizes the outcome of a number of clinical trials of pirfenidone, which is the first antifibrotic agent to be approved for the treatment of IPF.
- 166.Yamada M, et al. Gene transfer of soluble transforming growth factor type II receptor by in vivo electroporation attenuates lung injury and fibrosis. J. Clin. Pathol. 2007;60:916–920. doi: 10.1136/jcp.2006.039396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arribillaga L, et al. Therapeutic effect of a peptide inhibitor of TGF-β on pulmonary fibrosis. Cytokine. 2011;53:327–333. doi: 10.1016/j.cyto.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 168.Crunkhorn S. Deal watch: Biogen acquires Stromedix to pursue novel fibrosis therapy. Nature Rev. Drug Discov. 2012;11:260. doi: 10.1038/nrd3708. [DOI] [PubMed] [Google Scholar]
- 169.Allison M. Stromedix acquisition signals growing interest in fibrosis. Nature Biotech. 2012;30:375–376. doi: 10.1038/nbt0512-375. [DOI] [PubMed] [Google Scholar]
- 170.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 171.Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis — evidence for and against. Int. J. Exp. Pathol. 2011;92:143–150. doi: 10.1111/j.1365-2613.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alan C, Kocoglu H, Altintas R, Alici B, Resit Ersay A. Protective effect of decorin on acute ischaemia-reperfusion injury in the rat kidney. Arch. Med. Sci. 2011;7:211–216. doi: 10.5114/aoms.2011.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Terada Y, et al. Gene transfer of Smad7 using electroporation of adenovirus prevents renal fibrosis in post-obstructed kidney. Kidney Int. 2002;61:S94–S98. doi: 10.1046/j.1523-1755.2002.0610s1094.x. This report indicates that genetic transfer of Smad7 prevents unilateral ureteral obstruction-induced renal fibrosis, suggesting that SMAD7 may be applicable for the treatment of renal fibrosis.
- 174.Lim DS, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van Rooij E, Olson EN. Searching for miR-acles in cardiac fibrosis. Circ. Res. 2009;104:138–140. doi: 10.1161/CIRCRESAHA.108.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Merkel PA, et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 2008;59:699–705. doi: 10.1002/art.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McCaffrey TA. TGF-β signaling in atherosclerosis and restenosis. Front. Biosci. (Schol. Ed.) 2009;1:236–245. doi: 10.2741/s23. [DOI] [PubMed] [Google Scholar]
- 178.Friedl R, et al. Intimal hyperplasia and expression of transforming growth factor-β1 in saphenous veins and internal mammary arteries before coronary artery surgery. Ann. Thorac. Surg. 2004;78:1312–1318. doi: 10.1016/j.athoracsur.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 179.Ranjzad P, Salem HK, Kingston PA. Adenovirus-mediated gene transfer of fibromodulin inhibits neointimal hyperplasia in an organ culture model of human saphenous vein graft disease. Gene Ther. 2009;16:1154–1162. doi: 10.1038/gt.2009.63. [DOI] [PubMed] [Google Scholar]
- 180.Kapur NK, et al. Inhibition of transforming growth factor-β restores endothelial thromboresistance in vein grafts. J. Vasc. Surg. 2011;54:1117–1123. doi: 10.1016/j.jvs.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ramirez F, Dietz HC. Marfan syndrome: from molecular pathogenesis to clinical treatment. Curr. Opin. Genet. Dev. 2007;17:252–258. doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 182.Holm TM, et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. This report demonstrates a significant contribution of the non-canonical TGFβ signalling pathway to aortic aneurysm progression in MFS.
- 183.Siriwardena D, et al. Human antitransforming growth factor β2 monoclonal antibody — a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 2002;109:427–431. doi: 10.1016/s0161-6420(01)00997-6. [DOI] [PubMed] [Google Scholar]
- 184.Khaw P, et al. A phase III study of subconjunctival human anti-transforming growth factor β2 monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114:1822–1830. doi: 10.1016/j.ophtha.2007.03.050. This study reports the results from a Phase III study examining the efficacy of CAT-152, a monoclonal antibody to TGFβ2, in preventing the progression of fibrosis in patients undergoing first-time trabeculectomy. The study found no difference between CAT-152 and placebo in preventing the failure of trabeculectomy.
- 185.Fukuda K, Chikama T, Takahashi M, Nishida T. Long-term follow-up after lamellar keratoplasty in a patient with bilateral idiopathic corneal keloid. Cornea. 2011;30:1491–1494. doi: 10.1097/ICO.0b013e31822018f2. [DOI] [PubMed] [Google Scholar]
- 186.Benzinou M, et al. Mouse and human strategies identify PTPN14 as a modifier of angiogenesis and hereditary haemorrhagic telangiectasia. Nature Commun. 2012;3:616. doi: 10.1038/ncomms1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bonyadi M, et al. Mapping of a major genetic modifier of embryonic lethality in TGFβ1 knockout mice. Nature Genet. 1997;15:207–211. doi: 10.1038/ng0297-207. [DOI] [PubMed] [Google Scholar]
- 188.Farrington DL, et al. Development and validation of a phosphorylated SMAD ex vivo stimulation assay. Biomarkers. 2007;12:313–330. doi: 10.1080/13547500601162441. [DOI] [PubMed] [Google Scholar]
- 189.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McClymont SA, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.You S, et al. Adaptive TGF-β-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc. Natl Acad. Sci. USA. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Clay TM, Hobeika AC, Mosca PJ, Lyerly HK, Morse MA. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin. Cancer Res. 2001;7:1127–1135. [PubMed] [Google Scholar]
- 193.Baselga J, et al. TGF-β signalling-related markers in cancer patients with bone metastasis. Biomarkers. 2008;13:217–236. doi: 10.1080/13547500701676019. [DOI] [PubMed] [Google Scholar]
- 194.Bornstein S, et al. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J. Clin. Invest. 2009;119:3408–3419. doi: 10.1172/JCI38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu SL, et al. Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gomis RR, Alarcon C, Nadal C, Van Poznak C, Massague J. C/EBPβ at the core of the TGFβ cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell. 2006;10:203–214. doi: 10.1016/j.ccr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 197.Micalizzi DS, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-β signaling. J. Clin. Invest. 2009;119:2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hannigan A, et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-β from a tumor suppressor to a tumor promoter. J. Clin. Invest. 2010;120:2842–2857. doi: 10.1172/JCI36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Law BK, et al. Rapamycin potentiates transforming growth factor β-induced growth arrest in nontransformed, oncogene-transformed, and human cancer cells. Mol. Cell. Biol. 2002;22:8184–8198. doi: 10.1128/MCB.22.23.8184-8198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr. Opin. Genet. Dev. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 201.Corcoran RB, et al. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci. Signal. 2010;3:ra84. doi: 10.1126/scisignal.2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Acharya M, et al. αv Integrin expression by DCs is required for TH17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Melton AC, et al. Expression of αvβ8 integrin on dendritic cells regulates TH17 cell development and experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 206.Keller B, et al. Interaction of TGFβ and BMP signaling pathways during chondrogenesis. PLoS ONE. 2011;6:e16421. doi: 10.1371/journal.pone.0016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Montero JA, Lorda-Diez CI, Ganan Y, Macias D, Hurle JM. Activin/TGFβ and BMP crosstalk determines digit chondrogenesis. Dev. Biol. 2008;321:343–356. doi: 10.1016/j.ydbio.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 208.Goumans MJ, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol. Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. This study demonstrates that TGFβ regulates the activation state of the endothelium via two opposing type I receptors, ALK1 and TβRI (also known as ALK5).
- 209.Hau P, et al. Inhibition of TGF-2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–212. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 210.Chamberlain MC. Convection-enhanced delivery of a transforming growth factor-β2 inhibitor trabedersen for recurrent high-grade gliomas: efficacy real or imagined?, in reference to Bogdahn et al. (Neuro-Oncology 2011;13:132-142) Neuro Oncol. 2011;13:558–559. doi: 10.1093/neuonc/nor048. author reply 561-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wick W, Weller M. Trabedersen to target transforming growth factor-β: when the journey is not the reward, in reference to Bogdahn et al. (Neuro-Oncology 2011;13:132-142) Neuro Oncol. 2011;13:559–560. doi: 10.1093/neuonc/nor046. author reply 561-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roldan Urgoiti GB, Singh AD, Easaw JC. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J. Neurooncol. 2012;108:173–177. doi: 10.1007/s11060-012-0826-3. [DOI] [PubMed] [Google Scholar]
- 213.Azaro A, et al. The oral transforming growth factor-β (TGF-β) receptor I kinase inhibitor LY2157299 plus lomustine in patients with treatment-refractory malignant glioma: the first human dose study. J. Clin. Oncol. 2012;30 Abstract 2042. [Google Scholar]
- 214.Occleston NL, et al. Discovery and development of avotermin (recombinant human transforming growth factor β3): a new class of prophylactic therapeutic for the improvement of scarring. Wound Repair Regen. 2011;19(Suppl. 1):S38–S48. doi: 10.1111/j.1524-475X.2011.00711.x. [DOI] [PubMed] [Google Scholar]
- 215.Platten M, et al. N-[3,4-dimethoxycinnamoyl]-anthranilic acid (tranilast) inhibits transforming growth factor-β relesase and reduces migration and invasiveness of human malignant glioma cells. Int. J. Cancer. 2001;93:53–61. doi: 10.1002/ijc.1289. [DOI] [PubMed] [Google Scholar]
- 216.Isaji M, et al. Inhibitory effects of tranilast on the proliferation and functions of human pterygium-derived fibroblasts. Cornea. 2000;19:364–368. doi: 10.1097/00003226-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 217.Schlingensiepen KH, et al. The TGF-β1 antisense oligonucleotide AP 11014 for the treatment of non-small cell lung, colorectal and prostate cancer: preclinical studies. J. Clin. Oncol. 2004;22 Abstract 3132. [Google Scholar]
- 218.Nam JS, et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor β in a mouse model of breast cancer. Cancer Res. 2006;66:6327–6335. doi: 10.1158/0008-5472.CAN-06-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Melisi D, et al. LY2109761, a novel transforming growth factor β receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol. Cancer Ther. 2008;7:829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-β up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–1566. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 221.Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor β receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology. 2009;50:1140–1151. doi: 10.1002/hep.23118. [DOI] [PubMed] [Google Scholar]
- 222.Zhang B, Halder SK, Zhang S, Datta PK. Targeting transforming growth factor-β signaling in liver metastasis of colon cancer. Cancer Lett. 2009;277:114–120. doi: 10.1016/j.canlet.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 224.Hjelmeland MD, et al. SB-431542, a small molecule transforming growth factor-β-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol. Cancer Ther. 2004;3:737–745. [PubMed] [Google Scholar]
- 225.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 226.Uhl M, et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. This is the first report of the use of a chemical inhibitor of TβRI showing efficacy in reduced tumour growth and invasion as well as increased immunogenicity in a mouse model of glioblastoma.
- 227.Ge R, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-β type I receptor kinase in vivo. Clin. Cancer Res. 2006;12:4315–4330. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 228.Suzuki E, et al. A novel small-molecule inhibitor of transforming growth factor β type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67:2351–2359. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- 229.Kim S, et al. Systemic blockade of transforming growth factor-β signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68:10247–10256. doi: 10.1158/0008-5472.CAN-08-1494. This is the first demonstration of the use of a chemical TGFβ receptor inhibitor, acting via its immunomodulatory effects, to enhance the efficacy of an adenovirus expressing IFNβ-based immunotherapy.
- 230.Gellibert F, et al. Discovery of 4-{4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]pyridin-2-yl}-N-(tetrahydro-2H-pyran-4-yl)benzamide (GW788388): a potent, selective, and orally active transforming growth factor-β type I receptor inhibitor. J. Med. Chem. 2006;49:2210–2221. doi: 10.1021/jm0509905. [DOI] [PubMed] [Google Scholar]
- 231.Petersen M, et al. Oral administration of GW788388, an inhibitor of TGF-β type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73:705–715. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 232.Tan SM, Zhang Y, Connelly KA, Gilbert RE, Kelly DJ. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1415–H1425. doi: 10.1152/ajpheart.01048.2009. [DOI] [PubMed] [Google Scholar]