Abstract
Purpose
Leptin, an adipose secreted cytokine, is implicated in mammary cancer stem cell self-renewal and tumor growth in Murine Mammary Tumor Virus (MMTV)-Wnt-1 transgenic mice. In vitro studies indicate that leptin induces expression of Cyclin D1, a cell-cycle control protein necessary for mammary tumor development. The aim of the present study was to assess Cyclin D1 expression in spontaneous tumors that develop in the MMTV-Wnt-1 transgenic mice and interrogate the in vivo effect of leptin.
Materials and methods
Cells derived from spontaneous MMTV-Wnt-1 tumors were orthotopically transplanted into wild type, leptin-deficient, and hyperleptinemic mice. After 6 weeks, the tumors were collected and formalin fixed. Immunoflurescence staining was used to assess Cyclin D1, keratin 8, α-SMA, phospho-AKT expression.
Results
Cyclin D1 is expressed exclusively in luminal keratin 8 immunoreactive tumor cells and is dependent on the adipose secreted hormone leptin. Tumor cell transplant into leptin-deficient mice resulted in approximately an 80% reduction of Cyclin D1 immunoreactivity in keratin 8 cells and this was independent of Akt activation.
Conclusions
Collectively, these data and our previous findings indicate that inhibition of leptin signaling provides an excellent therapeutic target for breast cancer. The current data indicate that in luminal mammary tumors, leptin antagonists would potentially inhibit growth in a Cyclin D1-dependent mechanism. In contrast, in basal mammary tumors, leptin antagonists would inhibit growth in an Akt-dependent manner leading to reduction in CSC self-renewal. Thus, leptin therapeutics may inhibit breast cancer via distinct mechanisms dependent on the tumor type.
Introduction
Obesity is an established risk factor for breast cancer in postmenopausal women (Di Carlo et al. 2004; Phipps et al. 2008). The molecular mechanisms underlying the relationship between obesity and breast cancer are not fully understood. Leptin is a product of human obese (ob) gene (Zhang et al. 1994), synthesized in adipose tissue, and elevated in obese individuals (Halberg et al. 2008; Fruhbeck et al. 2001). Its predominant function is in regulation of feeding behavior, metabolism, and body weight by binding to leptin receptor (LEPR) in the brain (Friedman 1998). However, leptin and its receptors are also highly expressed in multiple peripheral tissues and tumors (Park and Scherer 2011). In fact, nearly 90% of primary breast tumors express leptin and its expression is highly correlated with LEPR (Garofalo et al. 2006). Moreover, Leptin and LEPR are increased in Bloom-Richardson grade III carcinomas compared to grade I carcinomas of human breast cancers. Leptin and LEPR expression are associated with shorter time to tumor recurrence and patient death (Ishikawa et al. 2004; Garofalo et al. 2006; Miyoshi et al. 2006; Maccio et al. 2010). The mechanisms of peripheral leptin signaling in breast cancer progression remain incompletely understood. Leptin elevates estrogen levels via increased aromatization of androgens (Cirillo et al. 2008), and leptin also transactivates HER2/neu (Fiorio et al. 2008). In addition to its crosstalk with estrogen and other signaling pathways in breast cancers, leptin has been shown to directly increase tumor cell survival and proliferation in vitro through its receptor (Hu et al. 2002).
Murine mammary tumor virus (MMTV) Wnt-1 transgenic mice develop spontaneous mammary tumors that exhibit characteristics of human basal-like breast cancers. Importantly, MMTV-Wnt-1 tumors develop in a hierarchical manner from a stem cell-like cancer cell (also known as a cancer stem cell, CSC) that can give rise to multiple lineages and tumor cell populations. Using fluorescence activated cell sorting and immunohistology, the multiple cell populations have been identified and isolated including putative CSCs, myoepithelial and luminal progenitors, and mature differentiated cancer cells such as luminal and myoepithelial cells (Cho et al. 2008; Jeselsohn et al. 2010). Our published findings indicated that leptin deficiency leads to decreased levels of Akt phosphorylation and reduced Wnt-1 tumor growth (Zheng et al. 2011). Moreover, leptin deficiency significantly decreased a subpopulation of cells within Wnt-1 tumors that exhibit CSC behavior, which leads to reduction in tumorigenesis (Zheng et al. 2011).
The cell cycle regulator cyclin D1 is required for mammary progenitor cell self-renewal and luminal epithelial cell differentiation (Cho et al. 2008; Jeselsohn et al. 2010). Cyclin D1 promotes the progression of the cell cycle by activating cyclin D-dependent kinase 4/6, which leads to phosphorylation of the retinoblastoma protein and in turn to progression through the G1 phase of the cell cycle. In vitro studies show that leptin induces cyclin D1 through Janus Kinase 2 (JAK2) and subsequent activation of several signaling pathways including phosphatidylinositol 3-kinase (PI3K)/Akt and the Signal Transducer and Activator of Transcription-3 (STAT3) (Gonzalez et al. 2006; Saxena et al. 2004; Saxena et al. 2007; Chen et al. 2007; Yan et al. 2012). Therefore, we hypothesized that leptin deficiency would decrease cyclin D1 expression in cancer cells and lead to loss of tumor cells. To test this hypothesis, we assessed cyclin D1 expression in MMTV-Wnt-1 tumors and investigated the effect of leptin.
Materials and Methods
Antibodies
The keratin 8 antibody (throma1) was obtained from the Developmental Studies Hybridoma Bank the University of Iowa, Department of Biology, Iowa City, IA. Keratin 6 was purchased from Covance (Princeton, NJ), α-SMA was purchased from sigma (St. Louis, MO) and cyclin D1 and p-AKT were purchased from cell signaling (Danvers, MA). All secondary antibodies including Alexa fluor 488 and 568 goat anti-rabbit, goat anti-rat were purchased from Molecular Probes (Eugene, OR).
Mice
Wild type C57BL/6J, leptin-deficient (B6. V-Lepob/J; ob/ob) and leptin receptor-deficient (B6. BKS (D)-Leprdb/J; db/db) mice were purchased from the Jackson Laboratory. The MMTV-Wnt-1 mice were kindly provided by Dr. Stephen Hursting. All mice were maintained in microisolator units and provided free access to food and water. All mouse procedures were performed under strict adherence to protocols approved by the Institute Animal Care and Use Committee at the Lerner Research Institute, Cleveland Clinic Foundation.
Transplantation of Wnt-1 mammary tumor cells
MMTV-Wnt-1 mammary tumors were minced with a scalpel and dissociated for 1h at 37°C on a rotary shaker in Medium 199 (invitrogen, Grand Island, NY) supplemented with 0.28 WUnsch units/ml liberase TH and TM (Roche, Indianapolis, IN) and 100Kunitz units/ml DNase I (Sigma-Aldrich St. Louis, MO). Sample were pipetted every 20 min while shaking. Cells were passed through 40 µm filter and centrifuged at 1000 rpm for 5 min. Cells were orthotopically transplanted (50,000 cells/mouse) into the right mammary fat pad #4 of female mice at 6 weeks of age (n=6–10). Mice were euthanized and the tumors were collected for immunofluorescence after 6 weeks.
Immunofluorescence analysis
Sections were deparaffinized by Histoclear (National Diagnostics, Atlanta, GA, USA) and rehydrated in graded alcohols. After antigen retrieval, sections were blocked with 2% goat serum in PBS for 1 h at room temperature and then incubated overnight with antibody to keratin 8, 6, α-SMA, cyclin D1 and p-AKT. The next day, sections were washed and incubated for 1 h with fluorescent secondary antibody at room temperature. Images were acquired using an epifluorescent digital microscope (Leica Microsystems, Buffalo Grove, IL).
Results
Cyclin D1 is expressed in Keratin 8+ luminal epithelial cells of mouse MMTV-Wnt-1 mammary tumors
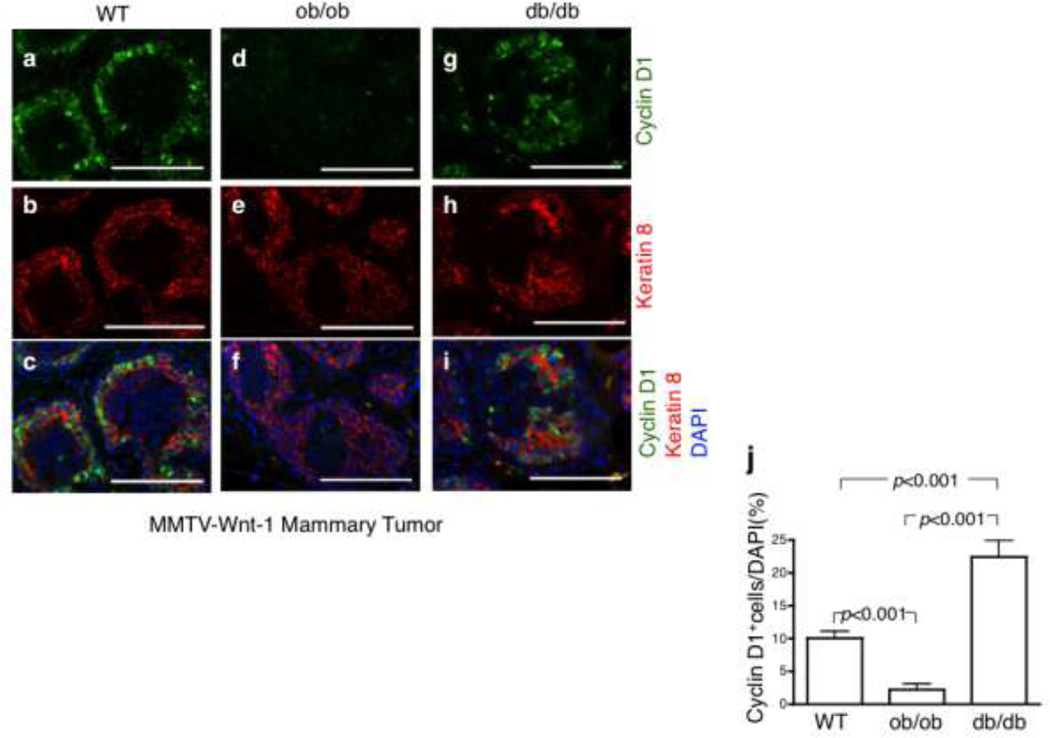
We previously used lineage selective markers and analyzed cell types of mouse MMTV-Wnt-1 tumors by immunofluorescence (IF) (Zheng et al. 2011). MMTV-Wnt-1 tumors are characteristic of heterogeneous solid tumors including multiple subtypes of cells such as luminal (Keratin 8+), myoepithelial (αSMA) and basal progenitor cells (Keratin 5+6+) (Zheng et al. 2011). In this study, we first assessed cyclin D1 localization by IF staining and found that approximately 10% of cells expressed nuclear cyclin D1 in MMTV-Wnt-1 tumors transplanted into WT mice (Fig. 1a and j). Moreover, we observed that cyclin D1 was expressed almost exclusively in K8+ luminal epithelial cells (Fig. 1b and c).
Fig 1.
The expression of cyclin D1 in mammary luminal epithelial cells and Leptin deficiency significantly inhibited expression of cyclin D1. a–i. Immunofluorescence analysis of keratin 8 (red) and cyclin D1 (green) in MMTV-Wnt-1 tumors in wild type, ob/ob and db/db mice. Nuclei were counterstained with DAPI (dark blue). Scale bar=100 mm. j. Quantification of CyclinD1 immunoreactive cells in sections of tumors from wild type, ob/ob and db/db mice. Only cells expressing nuclear cyclin D1 were analyzed. Data are presented as mean ± SEM.
Leptin regulates the expression of cyclin D1
In order to investigate the role of leptin on cyclin D1 expression, we transplanted spontaneous Wnt-1 tumors into leptin-deficient ob/ob and hyperleptinemic db/db mice. The tumors that developed were formalin fixed, and cyclin D1 expression was detected by immunofluorescence. Cyclin D1 was observed in less than 2% of cells in ob/ob mice, an 80% reduction compared to WT mice (Fig. 1d and j). In contrast, in db/db mice with high circulating leptin, cyclin D1 was expressed in greater than 20% of the tumor cells, or 2-fold higher compared to WT mice (Fig. 1g and j). Additionally, the loss of cyclin D1 in ob/ob mice did not noticeably alter histopathologic features of luminal epithelial cells or the expression of K8 (Fig. 1e and f).
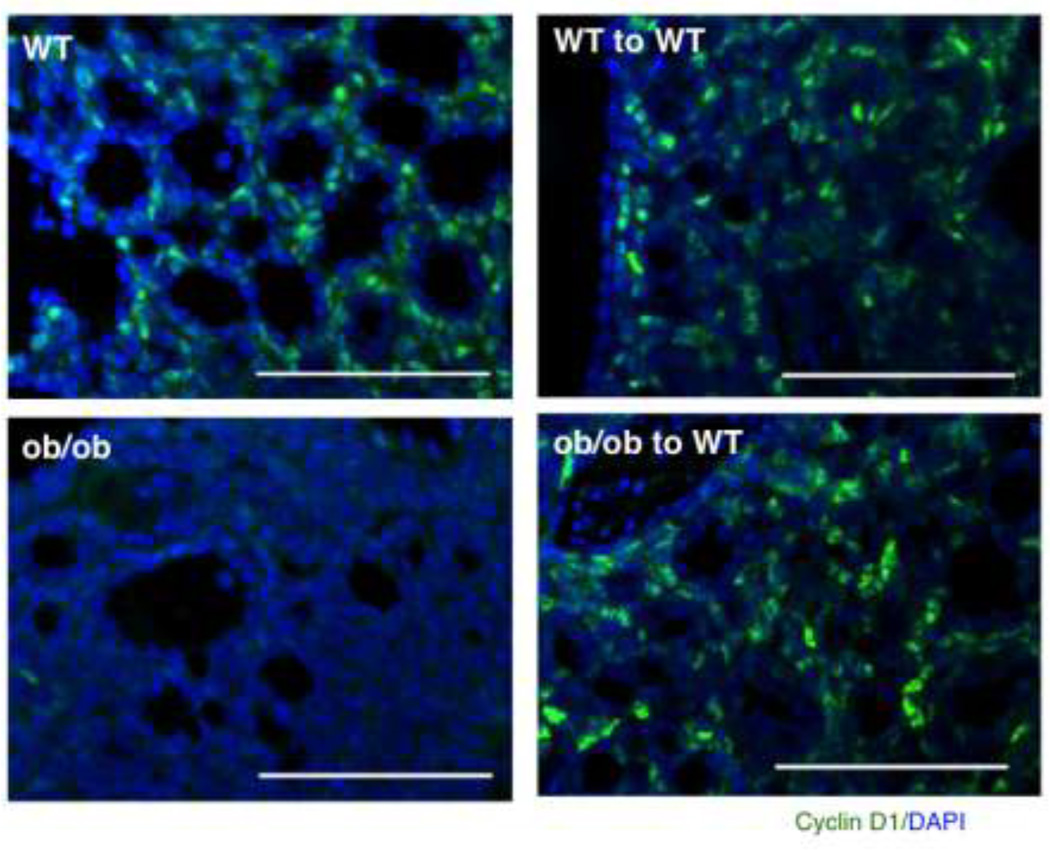
Cyclin D1 expression is rescued by serial transplantation of Wnt-1 tumor cells from ob/ob mice into wild type mice
We next transplanted tumor cells from residual tumors derived from WT or ob/ob mice into recipient WT mice. The serial transplantation of Wnt-1 tumor cells from ob/ob mice into the WT mice led to reexpression of cyclin D1 (Fig. 2). This suggests that cyclin D1 expression in MMTV-Wnt-1 mammary tumors is leptin-dependent and provides in vivo evidence for the cell studies indicating that leptin induces cyclin D1 expression.
Fig 2.
Rescue of cyclin D1 expression in tumor cells injected into wild type mice. Cells arose from MMTV-Wnt-1 tumors that developed in wild type or ob/ob mice were transplanted into wild type mice. The transplantation of ob/ob tumors cells into the wild type host rescue the cyclin D1 (green) expression. Nuclei were counterstained with DAPI (dark blue). Scale bar=100 mm.
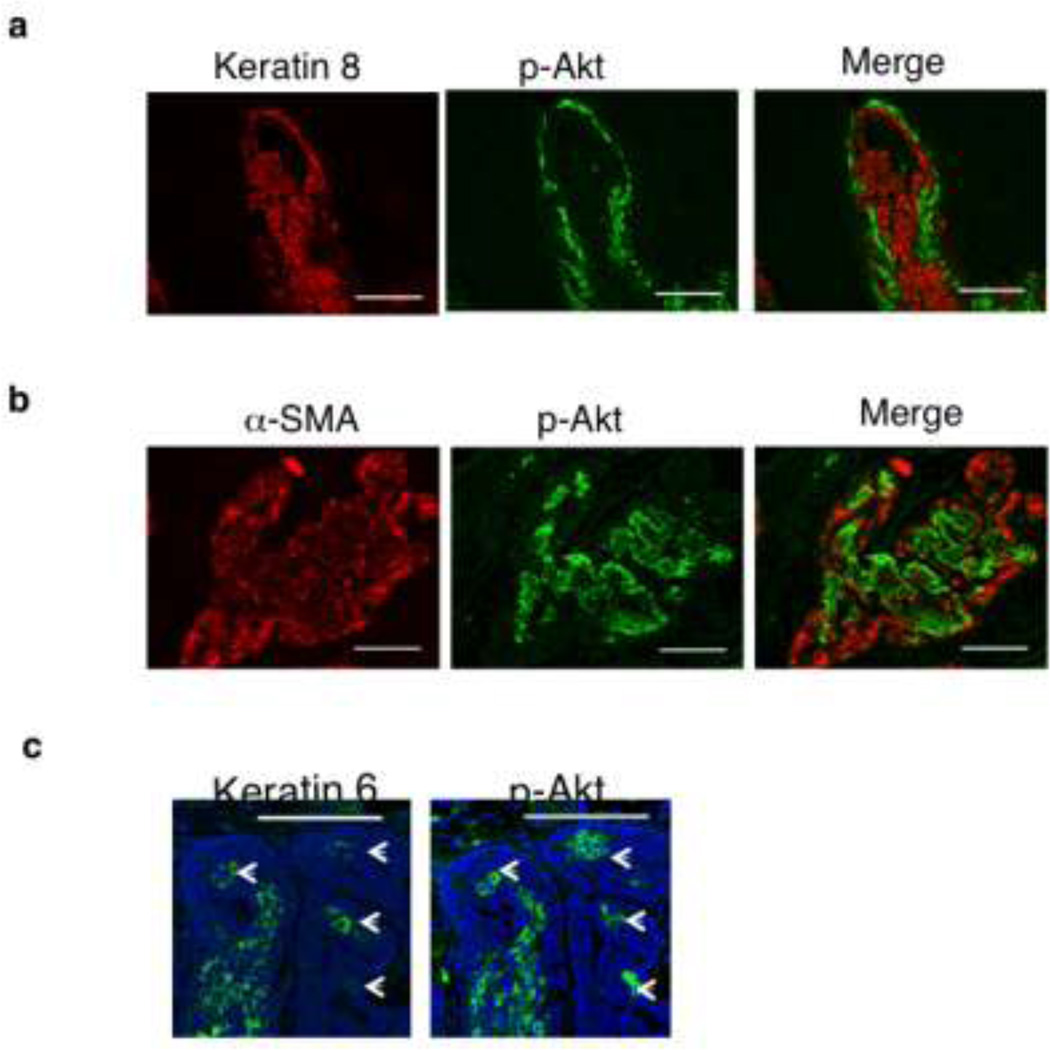
Leptin regulates cyclin D1 in an AKT-independent manner
Leptin induces cyclin D1 through JAK2 kinase and subsequent activation of several signaling pathways including Akt and STAT3 signaling. Because we previously reported that leptin activates Akt in MMTV-Wnt-1 tumors (Zheng et al. 2011) we assessed whether cyclin D1 is co-localized to the p-Akt overexpressing mammary cancer cells. Wnt-1 tumors were co-stained with p-Akt and K8 or with α-SMA (myoepithelial lineage marker). Akt phosphorylation did not colocalize with either K8+ (Fig 3a) or α-SMA+ cells (Fig 3b). Comparison of adjacent sections indicates that activated Akt is within the region in which K6 is coexpressed (Fig 3c). The data suggest that leptin-induced cyclin D1 expression is independent of Akt activation.
Fig 3.
Leptin-induced cyclin D1 expression was independent on Akt activation. a. Co-immunostained for p-AKT (green) and keratin 8 or b with α-SMA, a marker of myoepithelial lineage. c. Immunofluoresence analysis of keratin 6 and p-AKT in adjacent sections of Wnt tumors. Nuclei were counterstained with DAPI (dark blue). Scale bar=100 mm.
Discussion
We report here that cyclin D1 is expressed exclusively in luminal K8 expressing mammary tumor cells, and that cyclin D1 expression in MMTV-Wnt-1 tumors is dependent on leptin. These data suggest a role of leptin in cyclin D1 expression in vivo. The current data combined with our previous study (Zheng et al. 2011) show that therapeutics to inhibit leptin signaling may block multiple oncogenic pathways implicated in breast tumorigenesis.
Cyclin D1 is highly expressed in both mouse mammary tumors and human primary breast carcinomas (Kenny et al. 1999; Gillett et al. 1994). We observed that the normal mouse mammary tissue did not show strong nuclear cyclin D1 expression (data not shown), while cyclin D1 is highly expressed in mouse MMTV-Wnt-1 mammary tumors. The MMTV-Wnt-1 mammary tumors include multiple subtypes of cells including luminal (K8+), myoepithelial (αSMA) and basal progenitor cells (K6+). In this study, we provide direct evidence for cyclin D1 expression in luminal epithelial cells. This is in agreement with a recent study that showed cyclin D1 is required for luminal cell differentiation in the mammary gland (Jeselsohn et al. 2010). Further, cyclin D1 homozygous null mice are resistant to initiation of luminal mammary tumors induced by the neu and ras oncogenes, whereas the mice remain sensitive to MMTV-Wnt-1 induced tumorigenesis (Jeselsohn et al. 2010; Yu et al. 2001). Collectively, these data indicate that cyclin D1 marks the luminal progenitor population in mammary carcinomas and thus targeting signaling pathways that activate cyclin D1 may be an attractive therapeutic approach to inhibit luminal type mammary tumors.
Our findings indicate that leptin signaling is necessary for expression of cyclin D1 in the luminal progenitor population of MMTV-Wnt-1 mice. Leptin receptor is expressed in estrogen receptor positive breast cancer cells and luminal type tumors (Kim 2009; Ishikawa et al. 2004). This has significant ramifications and suggests that inhibiting leptin signaling would yield effective therapeutic approaches for targeting luminal mammary tumors. Indeed, the near complete inhibition in cyclin D1 expression in the transplanted MMTV-Wnt-1 tumors in leptin-deficient mice supports this hypothesis.
Leptin induces cyclin D1 through several JAK2-dependent signaling pathways. Leptin via JAK2 activates Akt to phosphorylate GSK 3b which leads to increased accumulation of β-catenin and cyclin D1 expression in the nucleus (Alt et al. 2000). In addition, leptin activates STAT3 and directly stimulates cyclin D1 activation. We recently published that activated Akt (phosphorylated Akt) is expressed in a leptin-dependent manner in MMTV-Wnt-1 tumors (Zheng et al. 2011). Our current studies show that in MMTV-Wnt-1 tumors leptin activates cyclin D1 independent of Akt. Notably, comparison of adjacent sections indicates that p-Akt is colocalized in K6+ cells that are associated with CSCs (Li et al. 2003; Stingl et al. 2006). These findings suggest that activation of Akt by leptin may play an important role in maintaining undifferentiated cancer stem/progenitor cells. Because p-Akt and K8 did not colocalize and cyclin D1 is expressed in K8+, we propose that cyclin D1 is not a downstream target of leptin-activated Akt signaling in MMTV-Wnt-1 tumors.
The current data combined with our previous study (Zheng et al. 2011) indicate that inhibition of leptin signaling provides an excellent therapeutic target for breast cancer. Leptin can stimulate Akt in undifferentiated cancer stem/progenitor cells and in parallel regulate cyclin D1 expression in the luminal progenitor tumor cells. We propose that leptin therapeutics may inhibit breast cancer by more then one mechanism. In luminal mammary tumors, leptin antagonists would potentially inhibit growth via a cyclin D1-dependent mechanism. In basal mammary cancer, leptin antagonists would inhibit growth via an Akt-dependent manner leading to reduced CSC self-renewal.
Acknowledgements
The research was supported by development grants from the NIH-NCI Transdisciplinary Research in Energetics and Cancer (U54 CA116867, Nathan A. Berger, PI) and the Scott Hamilton CARES Foundation to OR. The authors would like to thank Dr. Lyuba Varticovski, National Cancer Institute, who developed the Wnt-1 tumor cell suspension, Lauren Malone (Hursting Laboratory) for technical assistance, and Judy Drazba and the Lerner Research Institute Imaging Core for assistance in immunohistology.
Contributor Information
Qiao Zheng, Email: zhengq@ccf.org, Department of Cell Biology, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
Stephen D. Hursting, Department of Nutritional Sciences, University of Texas, Austin, TX, USA Department of Carcinogenesis, University of Texas MD Anderson Cancer Center, Smithville, TX, USA.
Ofer Reizes, Department of Cell Biology, Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH, USA.
References
- Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14(24):3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, Guo IC. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and downregulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14(2):513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26(2):364–371. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: An overview. J Cell Biochem. 2008;105(4):956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- Di Carlo C, Tommaselli GA, Sammartino A, Bifulco G, Nasti A, Nappi C. Serum leptin levels and body composition in postmenopausal women: effects of hormone therapy. Menopause. 2004;11(4):466–473. doi: 10.1097/01.gme.0000109313.11228.2b. [DOI] [PubMed] [Google Scholar]
- Fiorio E, Mercanti A, Terrasi M, Micciolo R, Remo A, Auriemma A, Molino A, Parolin V, Di Stefano B, Bonetti F, Giordano A, Cetto GL, Surmacz E. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8:305. doi: 10.1186/1471-2407-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56(2 Pt 2):s38–s46. doi: 10.1111/j.1753-4887.1998.tb01685.x. discussion s54-75. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280(6):E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54(7):1812–1817. [PubMed] [Google Scholar]
- Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281(36):26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37(3):753–768. x–xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94(22):1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10(13):4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, Hinds PW. Cyclin D1 Kinase Activity is Required for the Self-renewal of Mammary Stem and Progenitor Cells that Are Targets of MMTV-ErbB2 Tumorigenesis. Cancer Cell. 2010;17(1):65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, Sutherland RL, Robertson JF. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5(8):2069–2076. [PubMed] [Google Scholar]
- Kim HS. Leptin and leptin receptor expression in breast cancer. Cancer Res Treat. 2009;41(3):155–163. doi: 10.4143/crt.2009.41.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100(26):15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Massa D, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med. 2010 doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118(6):1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- Park J, Scherer PE. Leptin and cancer: from cancer stem cells to metastasis. Endocr Relat Cancer. 2011;18(4):C25–C29. doi: 10.1530/ERC-11-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2078–2086. doi: 10.1158/1055-9965.EPI-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004;18(13):1612–1614. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Vertino PM, Anania FA, Sharma D. leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282(18):13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced Epithelial-Mesenchymal Transition in Breast Cancer Cells Requires beta-Catenin Activation via Akt/GSK3- and MTA1/Wnt1 Protein-dependent Pathways. J Biol Chem. 2012;287(11):8598–8612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411(6841):1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18(4):491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]