Abstract
In sporadic age-related forms of Alzheimer’s disease (AD), it is unclear why amyloid-β (Aβ) peptides accumulate. Here, we show that soluble amyloid precursor protein-α (sAPP-α) decreases Aβ generation by directly associating with BACE1; thereby modulating APP processing. Whereas specifically targeting sAPP-α using antibodies enhances Aβ production, in transgenic mice with AD-like pathology, sAPP-α overexpression decreases β-amyloid plaques and soluble Aβ. In support, immunoneutralization of sAPP-α increases APP amyloidogenic processing in these mice. Given our current findings, and because a number of risk factors for sporadic AD serve to lower levels of sAPP-α in brains of AD patients, inadequate sAPP-α levels may be sufficient to polarize APP processing toward the amyloidogenic, Aβ-producing route. Therefore, restoration of sAPP-α or enhancement of its association with BACE may be viable strategies to ameliorate imbalances in APP processing that can lead to AD pathogenesis.
Introduction
Alzheimer’s disease (AD) affects approximately 30 million worldwide with 5 million individuals in the U.S. alone (2011) and is characterized by pathologic hallmarks including β-amyloid plaques composed primarily of amyloid-β (Aβ) proteins as well as neurofibrillary tangles (NFTs) formed by abnormal phosphorylated tau proteins1,2. Aβ is thought to accumulate in toxic forms and damage neurons leading to synaptic dysfunction and neurodegeneration. Widespread neuronal loss ensues resulting in severe dementia and death. Much evidence implicates generation of Aβ as a critical component of AD pathogenesis2–4. Inherited early-onset familial forms of AD result from autosomal dominant mutations in amyloid precursor protein (APP) or presenilin genes leading to a final common pathway involving excess Aβ accumulation2. Similarly, in sporadic age-related AD, risk factors for AD development, including metabolic dysfunction, cardiovascular disease, and brain injury, also appear to favor Aβ accumulation and progressive neurodegeneration5–10.
Aβ itself is produced by an alternative pathway whereby APP is cleaved first by β-site APP-converting enzyme (BACE)11. This process generates a C-terminal APP fragment (C99; β-CTF) and a large secreted N-terminal soluble fragment called sAPP-β. β-CTF is further processed by the γ-secretase complex, composed of presenilin, Aph-1, Nicastrin, and Pen-2 proteins12, generating APP intracellular domain (AICD) and Aβ peptides of various lengths; of which Aβ40 and Aβ42 are believed to be predominant neurotoxic species in AD brains13.
Under physiological conditions, Aβ is constitutively generated at relatively low levels. Hence, the majority of APP is processed by the nonamyloidogenic pathway. Here,α-secretase cleavage is performed by members of a disintegrin and metalloproteinase (ADAM) family yielding the α-secretase-generated C-terminal APP fragment (C83; α-CTF) and N-terminal portion of APP (soluble APP-α; sAPP-α)14. As α-secretase cuts APP within the Aβ region, Aβ generation is precluded. Subsequently, α-CTF is further processed by the γ-secretase complex to generate AICD and p3 peptides. Found to have neurotrophic and neuroprotective properties15, as well as, the ability to enhance long-term potentiation (LTP)16, sAPP-α is largely considered to have significant therapeutic potential17. Whereas in most familial forms of AD, where APP is “genetically hijacked” by the amyloidogenic pathway, in sporadic age-related forms of the disease, it is unclear why Aβ accumulates. Prior investigations in sporadic age-related AD, point to a number of AD risk factors including, combinations of oxidative stress, abnormal lipid metabolism, abnormal glucose metabolism, physical inactivity, and cerebral hypoperfusion; all of which are also associated with suppressed sAPP-α18–30. Furthermore, α-secretase mutations have been associated with familial late-onset AD31. These data, in combination with prior data implying that sAPP-α has a role in the autoregulation of APP processing32–34, motivated our current investigation to determine whether sAPP-α regulates APP processing.
Here, we investigated the effects of sAPP-α treatment on APP processing in Chinese hamster ovary (CHO) cells overexpressing wild-type APP (APPwt) or Swedish mutant APP (APPswe), with or without, human wild-type PS1 (PS1wt) or mutant PS1 (PS1mut). We found that purified human recombinant sAPP-α (hsAPP-α) decreases Aβ and β-CTF production, implicating β-secretase modulation. In support, immunoprecipitation analysis revealed that sAPP-α directly interacts with BACE1 and interferes with the BACE1/APP interaction, resulting decreased Aβ and β-CTF production. Moreover, in aged transgenic mice overexpressing APPswe and PS1mut (PSAPP mice), elevated central nervous system (CNS) sAPP-α mitigated β-amyloid pathology. In contrast, a specific antibody targeting sAPP-α was sufficient to drive amyloidogenic APP processing in PSAPP mice. In sum, these data suggest that inadequate levels of sAPP-α leave this model more vulnerable to AD-like pathology. This has strong clinical implications as many known AD risk factors including, oxidative stress, abnormal lipid metabolism, abnormal glucose metabolism, physical inactivity, and cerebral hypoperfusion are also associated with suppressed sAPP-α levels18–30.
Results
sAPP-α decreases Aβ species and β-CTF in vitro
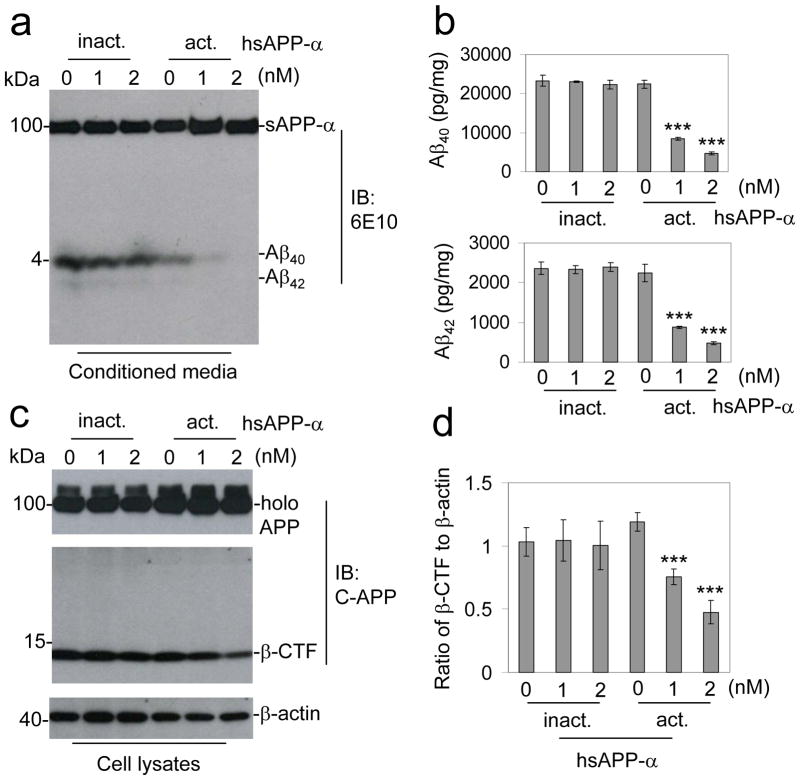
A product of non-amyloidogenic APP processing, sAPP-α exerts both neuroprotective and neurotrophic properties14,15,34–39. Owing to these properties, evidence for several mechanisms appear to be determinative, yet of particularly intrigue is evidence implicating sAPP-α itself in modulation of the parent APP molecule32–34. Thus, we endeavored to test whether sAPP-α could affect Aβ generation. To address this question, we first employed purified human recombinant sAPP-α (hsAPP-α)15,35,40,41 produced in the human embryonic kidney cell line (HEK) 293. CHO cells stably overexpressing both Swedish mutant APP and wild-type human PS1 (CHO/APPswe/PS1wt) were treated with a dose range (0 – 2 nM) of hsAPP-α for 4 h followed by examination of APP metabolites. Immunoblot (IB) analysis showed that treatment with hsAPP-α mitigates generation of both Aβ40 and Aβ42 (Fig. 1a). Heat-inactivated hsAPP-α did not affect Aβ generation. We confirmed these findings with sandwich ELISA (Fig. 1b), which showed dramatic reductions in both Aβ40 and Aβ42 levels in cells treated with hsAPP-α compared with heat-inactivated hsAPP-α. Next, in an effort to determine whether hsAPP-α could affect β-, γ-, or α-secretase APP cleavage events, we monitored APP C-terminal fragments (CTFs) by IB analysis (Fig. 1c). Densitometry analysis of these data showed that hsAPP-α dramatically decreasesβ-CTF generation compared with controls (Fig. 1d).
Figure 1.
sAPP-α inhibitsβ-secretase cleavage of APP in vitro. CHO cells expressing APPswe and wild-type human PS1 (CHO/APPswe/PS1wt) cells were treated with active (act.) or inactive (inact.) hsAPP-α recombinant protein at 0, 1, and 2 nM as indicated for 4 h. Secreted Aβ40, 42 peptides in the cell culture medium were analyzed by (a) immunoblot (IB) and (b) Aβ ELISA analyses. The Aβ ELISA results are represented as the mean ± SD of picograms of Aβ40 or Aβ42 per milligram of total intracellular protein after hsAPP-α protein treatment. In addition, these results are representative of four independent experiments with n = 3 for each condition. (c) Cell lysates were prepared and APP CTFs were analyzed by IB using a rabbit polyclonal antibody against C-terminal APP (pAb751/770, C-APP). This β-CTF band was further confirmed by the additional IB using 6E10 antibody against Aβ1-17 peptide for sAPP-α. (d) Relative ratio (mean ± SD) of β-CTF to β-actin was calculated by densitometry analysis. The results are representative of three independent experiments with n = 3 for each condition. One-way ANOVA followed by post hoc comparison revealed significant differences between 1 or 2 and 0 nM hsAPP-α protein treatment in both of Aβ40, 42 reduction and relative ratio of β-CTF to β-actin. (***P < 0.001, Full-length APP: holo APP)
Targeting sAPP-α enhances Aβ generation
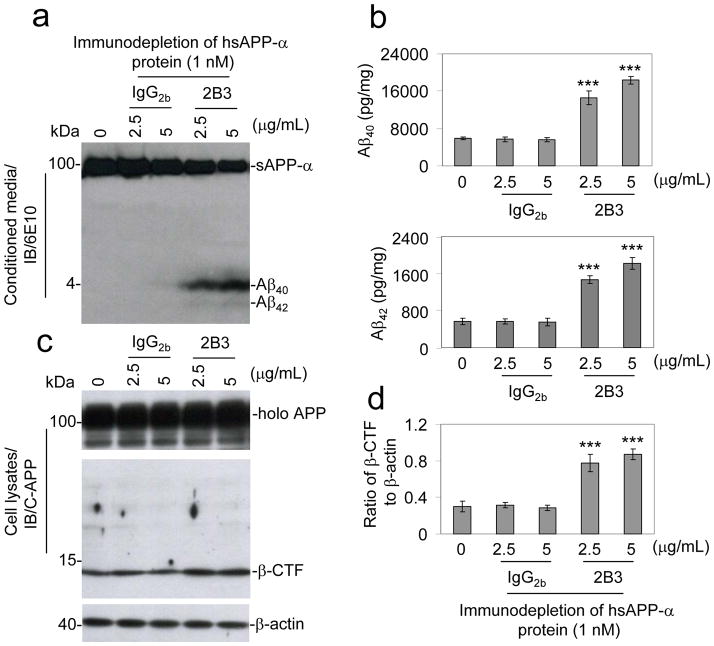
Considering our observations that hsAPP-α treatment decreases Aβ species and APP β-CTF generation, prior investigations identifying mutations in sAPP-α generating enzyme ADAM10 in familial late-onset AD31, and evidence suggesting that a number of risk factors for sporadic forms of AD may serve to lower levels of sAPP-α in brains of AD patients18–30, we hypothesized that the loss of sAPP-α would be sufficient to drive amyloidogenic APP processing. To test this hypothesis, prior to treatment of CHO cells expressing wild-type APP (CHO/APPwt cells), we first immunodepleted hsAPP-α protein with an antibody specifically against C-terminus of sAPP-α (2B3 antibody), or isotype-matched IgG2b control antibody, utilizing Protein-G Sepharose beads. After treatment, the conditioned media and cell lysates were prepared and assayed for APP metabolites. An increase in Aβ40 and Aβ42 was only observed in the cells receiving hsAPP-α preparations immunodepleted with 2B3 antibody, but not in those immunodepleted with isotype-matched IgG2b control antibody (Fig. 2a & 2b). In accordance with these findings, APP β-CTF was only increased in the cells treated with hsAPP-α immunodepleted with 2B3 antibody, but not in the cells treated with IgG2b immunodepleted hsAPP-α (Fig. 2c & 2d). These results indicated that immunodepletion of hsAPP-α using 2B3 antibody attenuates the anti-amyloidogenic effect of hsAPP-α on APP processing. As a confirmation, we have replicated this result in CHO/APPswe/PS1wt cells utilizing an antibody against Aβ1-17 peptide (6E10 antibody) (Fig. S2).
Figure 2.
Immunodepleted sAPP-α attenuates sAPP-α effects on APP modulation in vitro. Active hsAPP-α protein at 1 nM was incubated with an anti-C-terminal sAPP-α specific 2B3 antibody or isotype-matched IgG2b control antibody at 2.5 and 5 μ/mL at 37°C for 30 min. The protein-G sepharose beads were used to pull-down sAPP-α antibody and isotype-matched IgG2b control, as well as, their associated proteins. This was followed by high-speed centrifugation at 15,000 g for 10 min, and the supernatants were collected and determined to be IgG antibody free by IB analysis using an antibody against IgG2b. These supernatants were used to treat CHO cells expressing human wild-type APP (CHO/APPwt) for 12 h. Cell cultured supernatants were collected and subjected to (a) Aβ IB analysis using 6E10 antibody and (b) the Aβ ELISA results are represented as the mean ± SD of picograms of Aβ40 or Aβ42 per milligram of total intracellular protein after the 2B3- or control IgG2b-immunodepleted hsAPP-α protein treatment. (c) Cell lysates were prepared and APP CTFs were analyzed by IB using pAb751/770 (C-APP). (d) Relative ratio (mean ± SD) of β-CTF to β-actin was calculated by densitometry analysis. For b and d, the results as presented are representative of two independent experiments with n = 3 for each condition. One-way ANOVA followed by post hoc comparison revealed significant differences between 2B3-immunodepleted hsAPP-α protein treatment at either 2.5 or 5 μg/mL and control IgG2b-immunodepleted hsAPP-α protein as measured by either Aβ levels or ratio of β-CTF to β-actin. (***P < 0.001, Full-length APP, holo APP)
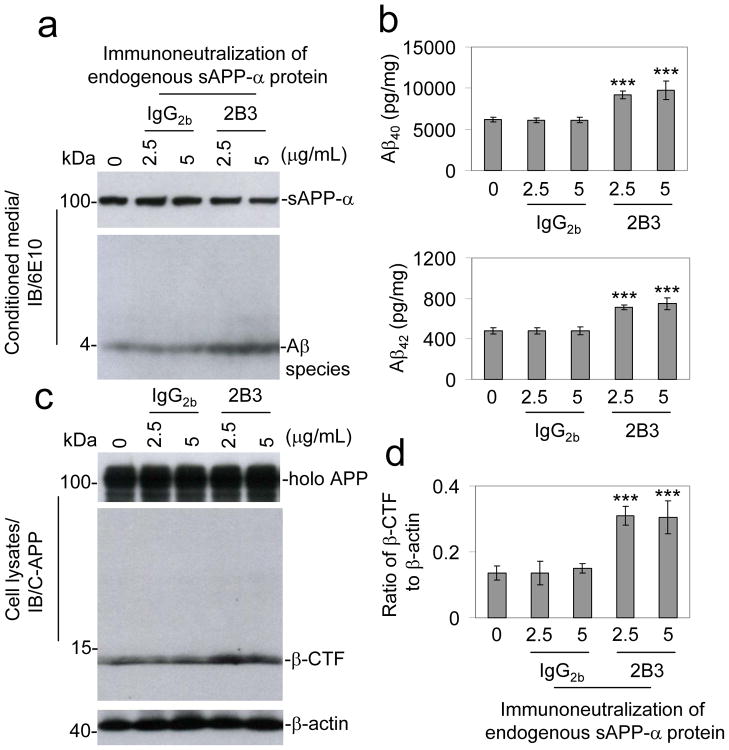
To further test our hypothesis that the loss of sAPP-α is sufficient to drive amyloidogenic APP processing, we next sought the immunoneutralization of endogenously produced sAPP-α. Separate groups of CHO/APPwt cells were treated directly with either a sAPP-α specific 2B3 antibody or isotype-matched IgG2b control antibody for 12 h. Aβ release into supernatants from these cells was analyzed by IB analysis (Fig. 3a & 3b). This analysis indicated that immunoneutralization of endogenous sAPP-α activity increases the generation of Aβ. Analysis of cell lysates showed that immunoneutralization of endogenous sAPP-α significantly enhances APP β-CTF generation (Fig. 3c) and β-CTF/β-actin ratios (Fig. 3d) compared with cells treated with isotype-matched IgG2b control antibody. In addition, we have repeated this experiment in CHO/APPswe/PS1wt cells utilizing 6E10 antibody (Fig. S3). Altogether, these data show that immunoneutralization of endogenous sAPP-α activity is sufficient to drive amyloidogenic APP processing.
Figure 3.
Immunoneutralization of endogenous sAPP-α promotes APP amyloidogenic processing. CHO/APPwt cells were treated with 2B3 or isotype-matched IgG2b control antibody for 12 h as indicated. (a) Aβ species were analyzed in conditioned media from the treated CHO/APPwt cells by IB analysis using 6E10 antibody. (b) The Aβ ELISA results are represented as the mean ± SD of picograms of Aβ40 or Aβ42 per milligram of total intracellular protein after the 2B3 antibody treatment. In addition, these results are representative of three independent experiments with n = 3 for each condition. (c) Cell lysates were prepared and APP CTFs were analyzed by IB analysis using pAb751/770 (C-APP). (d) Densitometry shows the ratio (mean ± SD) of β-CTF to β-actin. For b, d, the results as presented are representative of three independent experiments with n = 3 for each condition. One-way ANOVA followed by post hoc comparison revealed a significant difference between the 2B3 antibody at either 2.5 or 5 μg/mL and isotype-matched IgG2b control antibody treatment conditions. (***P < 0.001, Full-length APP, holo APP)
sAPP-α interacts and inhibits BACE1
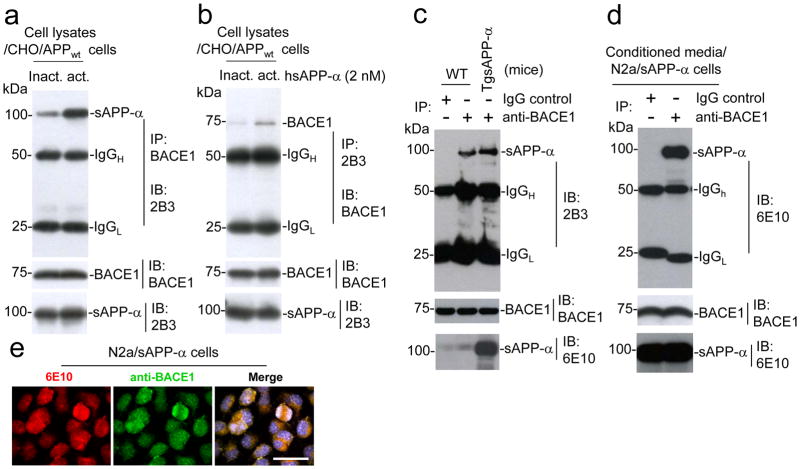
We next aimed to investigate the mechanism by which sAPP-α suppressed Aβ generation. Given the observed decreased Aβ production without accumulation of β-CTF in response to sAPP-α (Fig. 1), we hypothesized that sAPP-α could inhibit β-secretase activity in some manner, be it directly or indirectly43. BACE1 is a type I transmembrane aspartyl protease predominately responsible for processing APP into sAPP-β and β-CTF (C99) in the CNS. β-CTF is subsequently cleaved by the γ-secretase, releasing Aβ species of various lengths11. In light of recent findings indicating that a C-terminal BACE1 inhibition domain is contained within APP44, we reasoned that because sAPP-α, but not sAPP-β, also contains this domain, sAPP-α could directly or indirectly interact with BACE1 and decrease BACE1 activity. To investigate whether sAPP-α interacts with BACE1, we treated CHO/APPwt cells with hsAPP-α or heat-inactivated hsAPP-α protein and then collected cell lysates for analyses. As shown in Fig. 4a, cell lysates subjected to immunoprecipitation (IP) for BACE1 followed by IB for sAPP-α displayed strong coprecipitation of sAPP-α. Accordingly, IP for sAPP-α followed by IB for BACE1 showed strong coprecipitation of BACE1 (Fig. 4b). However, these coprecipitions were markedly reduced in heat-inactivated hsAPP-α condition.
Figure 4.
sAPP-α interacts with BACE1 in cultured cells. CHO/APPwt cells were treated with active or inactive hsAPP-α protein at 2 nM for 8 h. The cell lysates were immuoprecipitated (IP) with either (a) anti-C-terminal BACE1 (BACE1) antibody or (b) 2B3 antibody and then probed with 2B3 or BACE1 antibody. These results as presented are representative of three independent experiments with n = 3 for each condition. (c) Mouse brain homogenates prepared from TgsAPP-α mice and wild-type littermates (WT), and subjected to IP with BACE1 antibody for BACE1 followed by IB for sAPP-α using 2B3 antibody. The results as presented are representative of results obtained for 3 mice per group. (d) The conditioned media collected from cultured N2a/sAPP-α cells were subjected to IP with BACE1 antibody or isotype control IgG for BACE1 followed by IB with 6E10 antibody. As indicated below these blots, IB analyses demonstrate total protein levels of around 75 kDa BACE1, and 100 kDa sAPP-α in the different cell lysates, conditioned media or mouse brain homogenates. (e) sAPP-α strongly associates with BACE1 in N2a cell line stably transfected with human sAPP-α (N2a/sAPP-α cells). The cultured N2a/sAPP-α cells were fixed and imaged in independent channels using a confocal microscope equipped with Normarski optics. Merged images showed strong localization of both 6E10 antibody and BACE1 antibody positive N2a/sAPP-α cells (scale bar: 10 μm).
In order to confirm a physiologically relevant BACE1 and sAPP-α interaction, we performed IP on brain homogenates from TgsAPP-α mice and wild-type littermates utilizing an anti-BACE1 antibody for BACE1 and then IB with an anti-C-terminal sAPP-α specific 2B3 antibody for sAPP-α. The results displayed strong coprecipitation of sAPP-α in both TgsAPP-α mice and wild-type littermates (Fig. 4c).
Finally, we generated a stable N2a cell line overexpressing hsAPP-α (N2a/sAPP-α). Cell supernatants collected from these cells were subjected to IP for BACE1 and then IB for sAPP-α. They displayed strong coprecipitation of sAPP-α compared with isotype IgGcontrol (Fig. 4d). Moreover, we performed immunofluorescence staining on these cells and visualized both human sAPP-α and BACE1, and observed considerable co-localization of sAPP-α and BACE1 in these cells (Fig. 4e). These findings indicate that sAPP-α interferes with amyloidogenic APP processing through direct or directly interaction with BACE1. In addition, this experiment was repeated in CHO/APPswe/PS1wt cells. Again, a strong interaction of BACE1 with sAPP-α was further confirmed in both conditioned media (Fig. S4a) and cell lysates (Fig. S4b).
We next wondered whether BACE1/APP interaction was affected by sAPP-α treatment. Remarkably, the cell lysates prepared from CHO/APPswe/PS1wt cells that had been treated with sAPP-α, followed by IP for BACE1 and IB for C-terminal APP showed a dramatic reduction in coprecipitation of full-length APP; an effect not observed with heat-inactivated sAPP-α (Fig. S4b). Moreover, confirming our earlier CTF interpretation, decreased β-CTF correlated with high BACE1/sAPP-α and low BACE1/APP-interacting conditions created by sAPP-α treatment (Fig. S4b). These results further support our hypothesis that sAPP-α interacts and inhibits BACE1 activity in terms of APP processing.
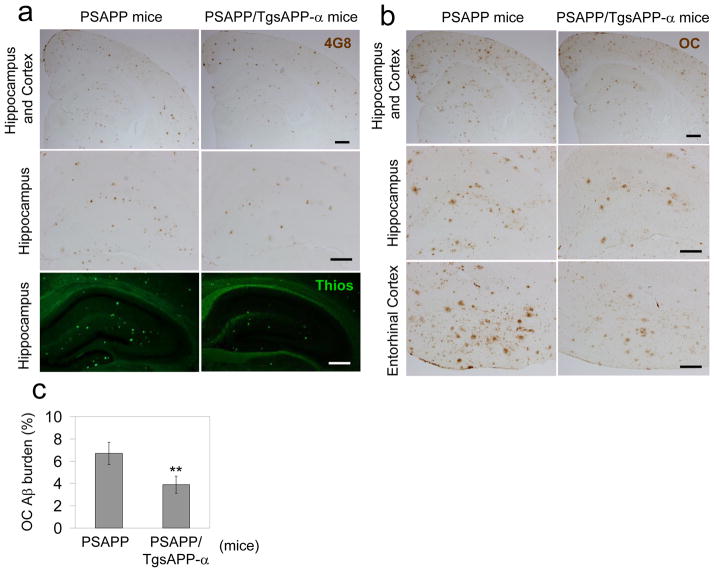
sAPP-α suppresses β-amyloid pathology in AD mice
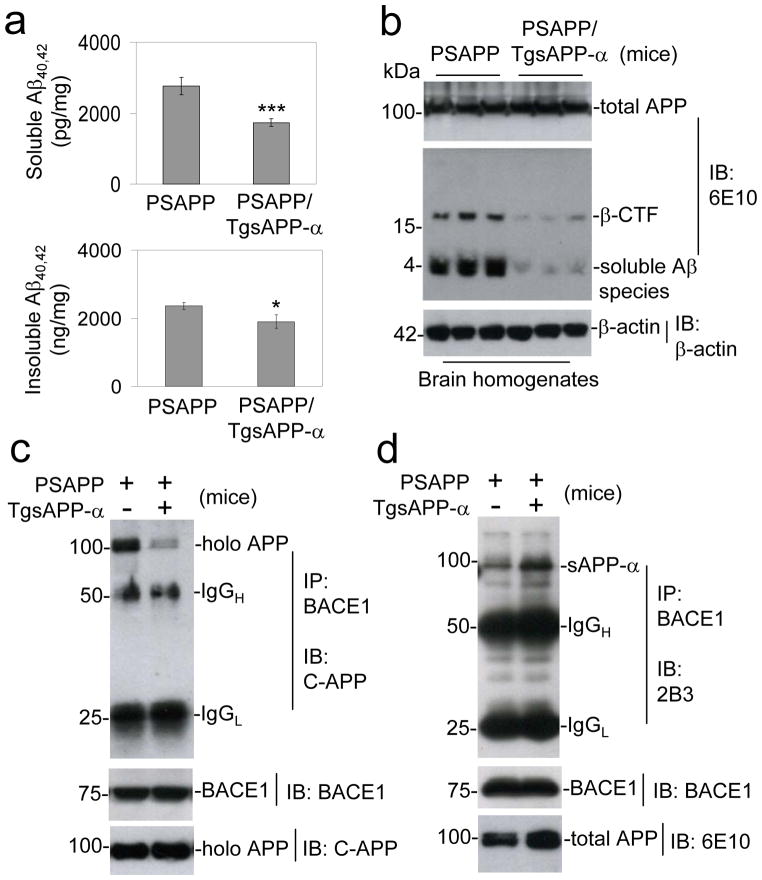
To determine whether sAPP-α reduces amyloidogenic APP processing in vivo, we generated transgenic mice overexpressing human sAPP-α (TgsAPP-α mice) in CNS tissues, and crossed them with the double transgenic APPswe/PSEN1ΔE9 (PSAPP) AD mouse model. Female triple transgenic and littermate control mice were aged to 8 months, euthanized, and AD-like pathology was characterized. Overexpression of sAPP-α substantially decreases cortical and hippocampal β-amyloid plaques, as well as Aβ oligomeric forms visualized by immunohistochemistry (4G8 and an anti-Aβ oligomer antibody OC), and reduces fibrillar Aβ species visualized by Thioflavin-S staining (Fig. 5a–c). Correspondingly, brain extracts prepared from these same mice were analyzed for soluble and insoluble Aβ40, 42 by ELISA (Fig. 6a) and IB utilizing 6E10 antibody (Fig. 6b). sAPP-α overexpression also significantly decreases Aβ40 and Aβ42, both soluble and insoluble Aβ levels, compared with PSAPP littermate controls. In order to test whether sAPP-α suppresses BACE1-mediated APP processing in vivo, we further analyzed APP CTF expression (Fig. 6b). Overexpression of sAPP-α results in dramatic inhibition of β-CTF.
Figure 5.
sAPP-α reduces β-amyloid pathology in a mouse model of AD. (a) Mouse brain sections from PSAPP and PSAPP/TgsAPP-α mice were stained with anti-Aβ17-24 antibody [4G8: brown, up (scale bar: 50 μm) and middle panels (scale bar: 50 μm)] or Thioflavin S [ThioS: green signal, bottom panel (scale bar: 50 μm)] at 8 months of age. (b) Mouse brain sections from PSAPP and PSAPP/TgsAPP-α mice were stained with Aβ OC antibody (brown signal). (c) Percentage of Aβ OC antibody immunoreactive plaques from hippocampus and cortex (mean ± SD; n = 5 females per group) was quantified by image analysis. A t test c, for independent samples revealed significances between groups. (**P < 0.01)
Figure 6.
sAPP-α inhibits amyloidogenic APP processing in vivo. Mouse brain homogenates were prepared from 8-month-old PSAPP and PSAPP/TgsAPP-α mice. (a) Total soluble and insoluble Aβ40, 42 were biochemical assessed in brain homogenates by ELISA and (b) total soluble Aβ species from brain homogenates were analyzed by IB using 6E10 antibody. For (a), data are presented as picograms (soluble Aβ40, 42) or nanograms (insoluble Aβ40, 42) per milligrams of protein (mean ± SD; n = 5 females per group). Mouse brain homogenates (200 μg) per each mouse were immunoprecipitated (IP) with (c) BACE1 antibody for BACE1, followed by IB analysis with pAb751/770 (C-APP) for full-length APP (holo APP); (d) with an anti-C-terminal BACE1 antibody for BACE1, followed by IB for sAPP-α with 2B3 antibody. As indicated below this figure, IB analyses demonstrate total protein levels of around 100 kDa total APP by 6E10 antibody and 75 kDa BACE1 in the different mouse brain homogenates. All results as presented are representative of results obtained for 5 mice per group. (*P < 0.05, ***P < 0.001)
Finally, we determined whether sAPP-α could interfere with BACE1/APP association in vivo through analysis of these same brain extracts utilizing IP for BACE1 followed by IB for C-terminal APP. Here, our findings clearly indicate a dramatic reduction in coprecipitation of full-length (holo) APP with BACE1 in PSAPP/sAPP-α mice compared with PSAPP littermate controls (Fig. 6c). Most importantly, IB utilizing an anti-C-terminal sAPP-α specific 2B3 antibody for sAPP-α reveals a significant increase in coprecipitation of sAPP-α with BACE1 in PSAPP/sAPP-α mice compared with PSAPP littermates. These data altogether indicate that sAPP-α interferes with amyloidogenic APP processing through association with BACE1 by preventing association and amyloidogenic proteolysis of APP (Fig. 6d).
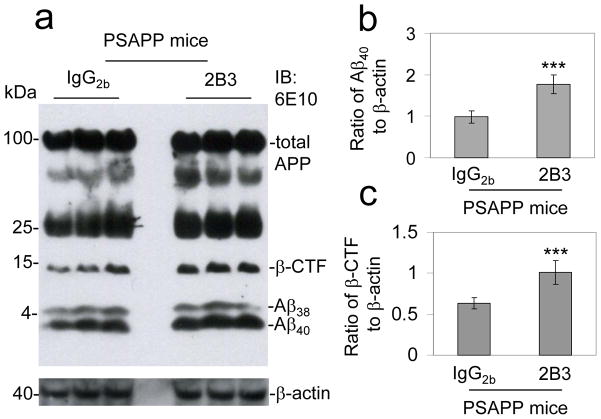
Immunoneutralization of sAPP-α enhances Aβ generation in vivo
The above findings suggest that sAPP-α is able to decrease amyloidogenic APP processing in vivo. We hypothesized based on our in vitro and in vivo findings that the inverse was also true, specifically that loss of sAPP-α function could drive amyloidogenic APP processing. Consistent with this hypothesis, 8 month-old female PSAPP mice treated intracerebroventricular (i.c.v.) with a sAPP-α specific 2B3 antibody for 48 h displayed enhanced amyloidogenic APP processing compared with control (isotype-matched IgG2b control antibody. Specifically, immunoneutralization of sAPP-α increases soluble Aβ40, 42 levels by IB analysis (Fig. 7a & 7b) compared with PSAPP mice receiving i.c.v. isotype-matched IgG2b control antibody. In accordance with these findings β-CTF generation was enhanced with sAPP-α immunoneutralization (Fig. 7c). In addition, our preliminary study also showed that PSAPP mice i.c.v. treated with 6E10 exhibit an increase in soluble Aβ and enhancement of β-CTF generation. Altogether, these data show that sAPP-α immunoneutralization enhances amyloidogenic APP processing.
Figure 7.
Antibody targeting of sAPP-α promotes amyloidogenic APP processing in vivo. PSAPP mice (3 female mice per group) at 8 months of age were intracerebroventricular (i.c.v.) injected with 2B3 antibody or isotype-matched IgG2b control antibody at 5 μg/mL and sacrificed at 48 h after the injection. (a) Mouse brain homogenates were prepared from these mice and subjected to IB analysis for APP processing. IB using 6E10 antibody shows total APP and three bands corresponding to β-CTF and soluble Aβ species 38 and 40. Densitometry analysis shows the ratios of Aβ40 to β-actin (b) and β-CTF to β-actin (c). A t test revealed significant differences in either ratio of Aβ40 to β-actin or β-CTF to β-actin between 2B3 antibody- and control IgG2b antibody-i.c.v. injected PSAPP mice. (***P < 0.001)
Discussion
Our findings suggest that sAPP-α can promote non-amyloidogenic APP processing. Our initial indication of this was evidenced in cells overexpressing APPswe and PS1wt (CHO/APPswe/PS1wt cells) where exogenous hsAPP-α inhibited Aβ40, 42 and β-CTF production (Fig. 1); a pattern indicative of suppression of β-secretase APP cleavage (Fig. 1c & 1d).
Underlying this, we found a physical association between sAPP-α with BACE1 in vitro (Fig. 4a & 4b), and in both TgsAPP-α mice and wild-type littermates and at endogenous levels (Fig. 4c); suggesting a physiologically relevant phenomenon. Indeed, immunoprecipitaton (IP) with an anti-BACE1 antibody and immunoblot (IB) with an anti-C-terminal sAPP-α specific 2B3 antibody exhibited strong co-IP of sAPP-α in both TgsAPP-α mice and wild-type littermates (Fig. 4b). These results strongly support a physiologically relevant BACE1 and sAPP-α interaction at endogenous levels. Furthermore, others have already shown that APP itself contains a unique C-terminal sequence found to decrease BACE1 activity44 lending further credence to our data and conclusions as this region is also present in sAPP-α but not in sAPP-β. In further support, BACE1 has been shown to be secreted in conditioned media45–48 and thus binding of sAPP-α to BACE1 (Fig. 4d & 4e) could occur in either the extracellular compartment, at the membrane, or presumably during endocytosis.
Reduced interaction of BACE1 with full-length APP would be expected if sAPP-α could interfere with their association. Indeed, we found that PSAPP mice overexpressing sAPP-α show decreased BACE1 and full-length APP interaction (Fig. 6c), whereas PSAPP mice exhibited the opposite (Fig. 6c & 6d). Alternatively, suppression of sAPP-α function via antibody immunodepletion of exogenous (Fig. 2), and immunoneutralization of endogenously generated sAPP-α (Fig. 3) enhanced Aβ production. These findings demonstrate for the first time, that sAPP-α may be involved in a positive feedback loop whereby it promotes its own production by inhibiting BACE1’s ability to interact with and proteolyze full-length APP at physiological levels.
To confirm this physiological relevance, we conducted the co-IP experiments both in conditioned media and brain homogenates. Specifically, we performed co-IP of CHO/APPwt cell lysates utilizing the specific sAPP-α antibody (2B3) that neither recognizes Aβ nor full-length APP. These co-IP data suggested that sAPP-α interacts with BACE1 in some manner in these cell culture systems and this interaction is associated with decreased Aβ generation. However, these data do not completely rule out the possibility that intermediate factors are required for inhibition of BACE1’s association and proteolysis of APP. In light of the attenuation of sAPP-α’s Aβ lowering effects by immunoneutralization utilizing 2B3 antibody (Fig. 3), as well as, similar findings with utilizing an antibody against Aβ1-17 peptide (6E10 antibody) (Fig. S3), intermediate factors presumably still present in this immunoneutralization paradigm did not appear to mitigate the effects of directly targeting sAPP-α.
The possibility of the immunodepletion antibody, 6E10, interfering with either Aβ generation or its measurement by ELISA exists, but at an extremely low likelihood given that we found similar results with the most specific sAPP-α 2B3 antibody by both IP and IB analyses (Fig. 2). In this regard, one may also speculate that contaminating sAPP-β may be present and confounding our data. However, this is also likely not occurring because this peptide would not be removed through immunodepletion of sAPP-α with the specific 2B3 antibody and thus suppression of Aβ peptides should still be observed; yet the results support the opposite conclusion (Fig. 2 & Fig. S2).
The possibility of another receptor, in addition or alternative to BACE1, being responsible for sAPP-α’s Aβ lowering effects exists since APP and its metabolites, sAPPs are known to exhibit multimodal effects and multiple binding partners. Importantly, however, the immunoneutralization results using sAPP-α specific antibody (Fig. 3) suggested that no regions, other than the region at or near the target epitope at the C-terminal of sAPP-α, appear required for sAPP-α’s anti-amyloidogenic effects. Further, the regions bound by the immunoneutralization antibodies are regions of sAPP-α characterized to have a unique BACE1 inhibition sequence44. In contrast IgG2b immunoneutralization or immunodepletion of endogenous or exogenous sAPP-α, respectively, yielded no changes in APP processing essentially ruling out the possibility of off-target antibody effects in our model systems. Importantly, 2B3 antibody characterization (Fig. S1) indicated no detectable binding to endogenous murine APP, Aβ, and CTF species, further confirming specificity of this antibody. Prior reports, in combination with these data presented here, altogether supported our hypothesis that sAPP-α directly mediates suppression of Aβ generation.
Based on our findings that sAPP-α binds and interferes with BACE1-mediated APP processing, suppression of sAPP-α likely results in BACE1 disinhibition. These results implicate decreased sAPP-α as a potential pathologic determinant in AD pathogenesis. These properties of sAPP-α appeared to depend in part on the secondary and tertiary structure of sAPP-α given that heat-inactivated sAPP-α lost its ability to suppress Aβ production or interfere with BACE1/APP interaction (Fig. S4). Additionally, the possibility that sAPP-α is binding to and interfering with full-length APP32–34 or β-CTF rather than BACE1 exists but in our model systems is unlikely given negative IP/IB data suggesting that sAPP-α did not interact well with full-length APP or β-CTF (Fig. S4c & S4d).
Perhaps most importantly, in PSAPP mouse model (human mutant APPK595N/M596L [APPswe] and PS1ΔE9 overexpressing mice), sAPP-α overexpression led to reduced levels of detergent soluble and insoluble Aβ (Fig. 6a), decreased β-CTF production (Fig. 6b), and ameliorated brain β-amyloid plaques (Fig. 5), as well as, in our cell systems where sAPP-α bound BACE1 and reduced BACE1/APP binding indicating that in vivo sAPP-α can regulate APP processing (Fig. 6c & 6d). Finally, i.c.v. administration of an anti-C-terminal sAPP-α specific 2B3 antibody in PSAPP mice resulted in exacerbation of amyloidogenic APP processing compared with i.c.v. injection using isotype-matched IgG2b control antibody (Fig. 7).
The results of the present study implicated that inadequate sAPP-α levels are likely sufficient to polarize APP processing toward the amyloidogenic, Aβ producing route due to dishinhibition of BACE1. β-secretase cleavage is considered to be the rate-limiting step in the generation of Aβ. While direct inhibition of BACE shows promise for the treatment of AD, other BACE substrates, including the proteins involved in control of myelination49–55, could be affected by non-selectively inhibiting BACE1 activity.
In sum, we suggested a novel mechanism of APP autoregulation via sAPP-α, which appears to attenuate Aβ formation under the physiological condition. If this endogenous sAPP-α-mediated BACE1 inhibition is disturbed, pathologic Aβ generation likely responsible for subsets of sporadic AD may ensue. We have confirmed this mechanism through multiple modalities both in vitro and in three mouse models whereby there is a physical interaction between sAPP-α and BACE1; an event that in AD patients may precede the generation of toxic Aβ species. Development of selective BACE modulators, interventions to restore sAPP-α’s BACE modulation functions, or sAPP-α mimetics, could hold therapeutic value as AD prophylactics or therapeutics.
Methods
Reagents and antibodies
Human HEK293 expressed human sAPP-α (hsAPP-α) was generated and purified as previously described15,35,40,41. Briefly, hsAPP-α was purified through successive FPLC steps with heparin-sepharose and TSK-gel DEAE-SPW columns. The identity of hsAPP-α was confirmed by immunoblot (IB), and apparent homogeneity was evidenced by silver staining after SDS-PAGE gel electrophoresis, and subsequently tested negative for the presence of endotoxins. Heat inactivation was conducted at 100°C for 5 min and confirmation of the apparent homogeneity of heat-inactivated hsAPP-α was demonstrated by Coomassie Blue visualization after gel electrophoresis and IB for sAPP-α (Fig. S1a). For cell treatments and i.c.v. injections, sterile, azide-free and low endotoxin antibodies were used, including anti-C-terminal human sAPP-α specific antibody (2B3, 100 μg/mL; IBL, Minneapolis, MN), Aβ1-17 antibody (6E10, 1 mg/mL; Covance, Emeryville, CA) and isotype-matched control IgG antibodies (1 mg/mL; BioLegend, San Diego, CA). This sAPP-α specific 2B3 antibody was further characterized in our in vitro and in vivo systems indicating that this antibody neither recognizes Aβ nor full-length APP (Fig. S1b–f). Other antibodies include: mouse monoclonal BACE1 antibody (1 mg/mL; Millipore, Billerica, MA); rabbit polyclonal BACE1 antibody and APP C-terminal antibody (500 μg/mL; EMD Biosciences, La Jolla, CA); APP N-terminus antibody (100 μg/mL, Millopore); Aβ17-24 antibody (1 mg/mL; 4G8, Covance) and Aβ1-12 antibody (BAM10, 500μg/mL; Sigma-Aldrich, St. Louis, MO); β-actin antibody (100 μg/mL; Sigma-Aldrich); rabbit anti-APP C-terminus polyclonal antibody (500 μg/mL; pAb369) generously provided by Dr. Sam Gandy; rabbit anti-APP C-terminus polyclonal antibody (pAb751/770, 100 μg/mL; Calbiochem); rabbit polyclonal oligomer/conformational (OC) antibody (500 μg/mL) was provided by Dr. Suhail Rasool and Dr. Charles G. Glabe at University of California.
Cell Culture
Chinese hamster ovary cells engineered to express wild-type human APP (CHO/APPwt), or Swedish mutant human APP (APPswe) and wild-type human PS1 (CHO/APPswe/PS1wt) were kindly provided by Dr. Stefanie Hahn and Dr. Sascha Weggen, (University of Heinrich Heine, Dusseldorf, Germany). SH-SY5Y cells expressing APPswe and wild-type human PS1 (SH/APPswe/PS1wt) was a gift from Dr. Wataru Araki (National Institute of Neuroscience, Tokyo, Japan). These cells were cultured as described previously56,57 with minor modifications.
Mice
Transgenic mice overexpressing hsAPP-α were generated at H. Lee Moffitt Cancer Center (Tampa, FL) by pronuclear injection utilizing standard methods. Briefly, the pcDNA3.1 plasmid containing hsAPP-α based on the predicted α-secretase cleavage of the 695 amino acid isoform of APP (hsAPP-α695) predominant in human CNS was obtained (Dr. Steven Barger, University of Arkansas). To facilitate CNS expression of hsAPP-α, hsAPP-α695 was cut from the pcDNA3.1 plasmid and cloned into the prion promoter driven MoPrP.Xho vector58,59. The resulting construct analyzed for proper insertion and alignment by PCR. After this confirmation, the final construct was linearized to remove the plasmid backbone prior to injection into oocytes. Next, sAPP-α transgenic mice were generated by injection of gel-purified DNA into C57BL/6J x C3H/HeJ F2 embryos. Several lines of transgenic mice were generated. The line with the highest level of sAPP-α protein expression by IB was selected and maintained by heterozygous backcrossing on the C57BL6/J mouse strain for at least 12 generations prior to experimental use. Genotyping of TgsAPP-α mice was conducted by quantitative real-time PCR using the following sequences of primers: hsAPP-α forward, 5′-GCCTGGACGATCTCCAGC-3′; hsAPP-α reverse, 5′-TGGCCCGGTGTTAGCACTGGC-3′; β-actin forward, 5-AGCTTGCTGTATTCCCCTCCATCGTG-3′; β-actin reverse, 5′-AATTCGGATGGCTACGTACATGGCTG-3′. Human mutant APPK595N/M596L (APPswe) and PS1ΔE9 overexpressing (PSAPP) mice were purchased (The Jackson Laboratory, Bar Harbor, ME). Triple transgenic PSAPP/TgsAPP-α mice and littermates were generated and genotyped as described above and previously59. Animals were housed and maintained at University of South Florida (USF) and all experiments were in compliance with protocols approved by USF Institutional Animal Care and Use Committee.
Tissue preparation
Mice were euthanized with isofluorane anesthesia and then transcardially perfused with ice-cold phosphate buffered saline (PBS). Brains were rapidly isolated and one hemisphere was frozen immediately in liquid nitrogen and stored at −80°C. For molecular analysis brain hemispheres were sonicated in RIPA buffer (Cell Signaling Technology, Danvers, MA) and centrifuged at 14,000 rpm for 1 h at 4°C. Supernatant was transferred to a new tube for soluble Aβ analysis and pellet was used for insoluble Aβ extraction as described previously60. The other hemisphere was placed in 4% paraformaldehyde (PFA) in PBS for cryostat sectioning. 25 μm free floating coronal sections were collected and stored in PBS with 100 mM sodium azide in 24-well plates at 4°C.
Immunoblot and immunoprecipitation
Cells were washed with ice cold PBS for 3 times, then lysed with cell lysis buffer (Cell Signaling Technology) and kept at −80°C until use. Aβ species secreted from cells and from brain homogenates were analyzed by Aβ immunoblot (IB) analysis60. Briefly, 10% bicine/tris gel containing 8 M urea was used for separation of proteins from brain homogenates or CHO cell supernatants. Proteins were then transferred to 0.2 μm pore-size nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was boiled in PBS for 3 – 5 min before blocking to enhance sensitivity. 6E10 was used for detection of Aβ. 6E10 and BAM10 were used for detection of Aβ. 4 – 20% precast polyacrylamide gels (Bio-Rad, Richmond, CA) were used for APP C-terminal antibody and 16.5% Tris-tricine gels were used for pAb369 and pAb751/770. 10% Tris-SDS gels were used for all other antibodies detection. Densitometry analysis was done as previously described using a FluorS Multiimager with Quantity One™ software60. Immunoprecitipation (IP) was performed by first incubating condition media or brain homogenates with appropriate antibodies and Protein-G Sepharose beads (1:10, Sigma-Aldrich) overnight with gentle rocking at 4°C. Following 3 washes with 1 X cell lysis buffer, samples were subjected to IB as described above.
ELISA
Aβ40, 42 species from cell systems and brain homogenates were detected by the Aβ40, 42 ELISA kits (Invitrogen, Carlsbad, CA). The manufacturer’s instructions were strictly followed. In addition, for the 6E10 immunodepletion experiments, we have established an alternative procedure to measure Aβ40, 42 levels in the cultured media. Briefly, rabbit polyclonal anti-Aβ32 –40 antibody (Abcam, Cambridge, MA) (5 μg/mL in PBS) was used for coating the 96-well plate at 4°C with overnight incubation. Following 3 washes (PBS with 0.05% Tween-20), blocking buffer (1% BSA in PBS) was applied for 2 h at ambient temperature. Next, the plate was washed 4 times and samples (cell cultured media) were applied to the plate for overnight incubation at 4 °C. After washing, 6E10 (2 μg/mL) was added to the plate for 2 h incubation at ambient temperature to bind Aβ. Finally, following 4 washes, HRP-conjugated goat anti-mouse IgG (0.5μg/mL) (Cell Signaling Technology) was used for detection and developed with TMB substrate-chromogen (Dako, Carpinteria, CA) then stopped with 2 N sulfuric acid and read spectrophotometrically at 450 nm. The Aβ40, 42 levels are represented as relative fold OD reading mean (± SD) over control.
Immunohistochemistry (IH)
Brain sections from PSAPP and PSAPP/sAPP-α mice were stained with 4G8 or OC antibody. Aβ burden was determined by quantitative image analysis as described previously60. Briefly, images of five sections (150 μm apart) through each anatomic region of interest were captured and a threshold optical density was obtained that discriminated staining form background. Manual editing of each field was used to eliminate artifacts. Data are reported as percentage of immunolabeled area captured (positive pixels divided by total pixels captured). This was performed by a single examiner (T.M.) blinded to sample identities.
Intracerebroventricular injection of sAPP-α targeting antibodies
At 8 months of age (3 female mice per group) were anesthetized using isoflurane (chamber induction at 4 – 5% isoflurane, intubation and maintenance at 1 – 2%). After reflexes were checked to ensure that mice were unconscious, they were positioned on a stereotaxic frame (Stoelting Lab Standard™, Wood Dale, IL) with ear-bars positioned and jaws fixed to a biting plate. The axis coordinates were taken from a mouse brain atlas, and a 5-mm sterile plastic guide cannula (Plastic One, Roanoke, VA) was implanted into the left lateral ventricle delimited from the stereotaxic coordinates (coordinates relative to bregma: −0.6 mm anterior/posterior, +1.2 mm medial/lateral, and −3.0 mm dorsal/ventral) using the stereotaxic device (Stoelting Lab Standard) and an attached probe holder. Antibodies targeting regions of sAPP-α characterized to contain a unique BACE1 inhibition sequence44 were i.c.v. injected with anti-C-terminal sAPP-α specific antibody (2B3), anti-Aβ1-17 antibody (6E10) or isotype-matched IgG control antibodies (5 μg/mouse administered at the rate of 1 μL/min using a Hamilton syringe modified with a solder stop to prevent over insertion of the needle). The wounds were closed with 1 – 2 staples and mice were all observed until anesthesia had cleared. Forty eight hours after the i.c.v. injections animals were sacrificed with isofluorane and brain tissues collected. All dissected brain tissues were rapidly frozen for analysis.
Statistical analysis
All data were normally distributed; therefore, in instances of single mean comparisons, Levene’s test for equality of variances followed by t-test for independent samples was used to assess significance. In instances of multiple mean comparisons, analysis of variance (ANOVA) was used, followed by post-hoc comparison using Bonferonni’s method. Alpha levels were set at 0.05 for all analyses. The statistical package for the social sciences release 10.0.5 (SPSS, Chicago, IL) was used for all data analysis.
Supplementary Material
Acknowledgments
This work was supported by the NIH/NIA [R01AG032432 and R42AG031586 (J.T.)], a Veterans Affairs Merit grant (J.T.), and the National Institute on Aging Intramural Research Program. Demian Obregonand Huayan Hou contributed to this work equally. We would like to thank Terrence Town and Song Min for helpful discussion.
Footnotes
Contributions
D.O. performed experiments, assisted in the design of the study, analyzed the data and drafted the manuscript. H.H. developed TgsAPP-α mice and generated PSAPP/TgsAPP-α mice, and performed IB, IP, and IH analyses, and ELISA, and contributed to data analysis. J.D. performed i.c.v., IB and ELISA experiments and helped with technical issues. B.G. assisted in the design of the study, manuscript composition and editing. J.T. performed IB, IP and ELISA experiments. D.D. helped with technical issues. M.S. helped with technical issues. Y.Z. performed IH experiment and assisted with technical issues. T.M. supervised IH technical issues, contributed to IH experiment, data analysis, and the manuscript editing. M.M. supervised protein isolation and purification, assisted in the design of the study, and manuscript composition and editing. J.T. designed and supervised the study, analyzed the data and assisted in the composition and editing of manuscript. All of the authors discussed the results and commented on the final version of the manuscript.
Competing financial interests
The authors declare no competing financial interest.
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Walsh DM, et al. The role of cell-derived oligomers of Aβ in Alzheimer’s disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- 4.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocca WA, Amaducci LA, Schoenberg BS. Epidemiology of clinically diagnosed Alzheimer’s disease. Ann Neurol. 1986;19:415–424. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- 6.Sparks DL, et al. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 7.Kamboh MI, Ferrell RE, DeKosky ST. Genetic association studies between Alzheimer’s disease and two polymorphisms in the low density lipoprotein receptor-related protein gene. Neurosci Lett. 1998;244:65–68. doi: 10.1016/s0304-3940(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 8.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 9.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 10.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassar R, et al. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 12.Kimberly WT, et al. γ-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano S, et al. Expression of β-amyloid precursor protein-CD3γ chimeras to demonstrate the selective generation of amyloid β(1-40) and amyloid β(1-42) peptides within secretory and endocytic compartments. J Biol Chem. 1999;274:32295–32300. doi: 10.1074/jbc.274.45.32295. [DOI] [PubMed] [Google Scholar]
- 14.Postina R, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattson MP, et al. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 16.Ishida A, Furukawa K, Keller JN, Mattson MP. Secreted form of β-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 18.Racchi M, et al. Secretory processing of amyloid precursor protein is inhibited by increase in cellular cholesterol content. Biochem J. 1997;322:893–898. doi: 10.1042/bj3220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahrle S, et al. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 20.Webster NJ, Green KN, Peers C, Vaughan PF. Altered processing of amyloid precursor protein in the human neuroblastoma SH-SY5Y by chronic hypoxia. J Neurochem. 2002;83:1262–1271. doi: 10.1046/j.1471-4159.2002.01236.x. [DOI] [PubMed] [Google Scholar]
- 21.Abad-Rodriguez J, et al. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol. 2004;167:953–960. doi: 10.1083/jcb.200404149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster NJ, Green KN, Settle VJ, Peers C, Vaughan PF. Altered processing of the amyloid precursor protein and decreased expression of ADAM 10 by chronic hypoxia in SH-SY5Y: no role for the stress-activated JNK and p38 signalling pathways. Brain Res Mol Brain Res. 2004;130:161–169. doi: 10.1016/j.molbrainres.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Tamagno E, et al. β-Site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- 24.Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid β-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J Neurosci. 2005;25:11165–11174. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auerbach ID, Vinters HV. Effects of anoxia and hypoxia on amyloid precursor protein processing in cerebral microvascular smooth muscle cells. J Neuropathol Exp Neurol. 2006;65:610–620. doi: 10.1097/00005072-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Westphal CH, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen C, et al. Hydrogen peroxide promotes Aβ production through JNK-dependent activation of γ-secretase. J Biol Chem. 2008;283:17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamagno E, et al. Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiroz-Baez R, Rojas E, Arias C. Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of α-, β- and γ-secretase expression. Neurochem Int. 2009;55:662–670. doi: 10.1016/j.neuint.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses β-amyloid production by activating the α-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Kim M, et al. Potential late-onset Alzheimer’s disease-associated mutations in the ADAM10 gene attenuated α-secretase activity. Hum Mol Genet. 2009;18:3987–3996. doi: 10.1093/hmg/ddp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaden D, et al. Homophilic interactions of the amyloid precursor protein (APP) ectodomain are regulated by the loop region and affect beta-secretase cleavage of APP. J Biol Chem. 2008;283:7271–7279. doi: 10.1074/jbc.M708046200. [DOI] [PubMed] [Google Scholar]
- 33.Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin β1. Neural Dev. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem. 2009;284:15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted β-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa K, et al. Increased activity-regulating and neuroprotective efficacy of α-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 37.Meziane H, et al. Memory-enhancing effects of secreted forms of the β-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci U S A. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein TD, et al. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2008;29:554–565. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP. Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of β-amyloid precursor. J Neurochem. 1995;64:2087–2096. doi: 10.1046/j.1471-4159.1995.64052087.x. [DOI] [PubMed] [Google Scholar]
- 41.Oltersdorf T, et al. The secreted form of the Alzheimer’s amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989;341:144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- 42.Milne JC, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussain I, et al. Oral administration of a potent and selective non-peptidic BACE-1 inhibitor decreases β-cleavage of amyloid precursor protein and amyloid-β production in vivo. J Neurochem. 2007;100:802–809. doi: 10.1111/j.1471-4159.2006.04260.x. [DOI] [PubMed] [Google Scholar]
- 44.Na CH, Jeon SH, Zhang G, Olson GL, Chae CB. Inhibition of amyloid β-peptide production by blockage of β-secretase cleavage site of amyloid precursor protein. J Neurochem. 2007;101:1583–1595. doi: 10.1111/j.1471-4159.2006.04441.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmeister A, et al. BACE1 is a newly discovered protein secreted by the pancreas which cleaves enteropeptidase in vitro. JOP. 2009;10:501–506. [PubMed] [Google Scholar]
- 46.Benjannet S, et al. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem. 2001;276:10879–10887. doi: 10.1074/jbc.M009899200. [DOI] [PubMed] [Google Scholar]
- 47.Yan R, Han P, Miao H, Greengard P, Xu H. The transmembrane domain of the Alzheimer’s beta-secretase (BACE1) determines its late Golgi localization and access to beta-amyloid precursor protein (APP) substrate. J Biol Chem. 2001;276:36788–36796. doi: 10.1074/jbc.M104350200. [DOI] [PubMed] [Google Scholar]
- 48.Hussain I, et al. Characterization of the ectodomain shedding of the beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) J Biol Chem. 2003;278:36264–36268. doi: 10.1074/jbc.M304186200. [DOI] [PubMed] [Google Scholar]
- 49.Hu X, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitazume S, et al. Alzheimer’s β-secretase, β-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci U S A. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichtenthaler SF, et al. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J Biol Chem. 2003;278:48713–48719. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- 52.von Arnim CAF, et al. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 53.Wong HK, et al. β subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 54.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 55.Willem M, et al. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 56.Hahn S, et al. Presenilin-1 but not amyloid precursor protein mutations present in mouse models of Alzheimer’s disease attenuate the response of cultured cells to gamma-secretase modulators regardless of their potency and structure. J Neurochem. 2011;116:385–395. doi: 10.1111/j.1471-4159.2010.07118.x. [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Araki W, Akiyama H, Tabira T. Amino-truncated amyloid β-peptide (Aβ5-40/42) produced from caspase-cleaved amyloid precursor protein is deposited in Alzheimer’s disease brain. FASEB J. 2004;18:1755–1757. doi: 10.1096/fj.03-1070fje. [DOI] [PubMed] [Google Scholar]
- 58.Borchelt DR, et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 59.Bailey AR, et al. Aberrant T-lymphocyte development and function in mice overexpressing human soluble amyloid precursor protein-α: implications for autism. FASEB J. 2011 Nov 15; doi: 10.1096/fj.11-195438. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Y, et al. CD45 deficiency drives amyloid-β peptide oligomers and neuronal loss in Alzheimer’s disease mice. J Neurosci. 2011;31:1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.