Abstract
Natural killer (NK) cells are lymphocytes that play an important role in the immune response to infection and malignancy. Recent studies in mice have shown that stimulation of NK cells with cytokines or in the context of a viral infection results in memory-like properties. We hypothesized that human NK cells exhibit such memory-like properties with an enhanced recall response after cytokine preactivation. In the present study, we show that human NK cells preactivated briefly with cytokine combinations including IL-12, IL-15, and IL-18 followed by a 7- to 21-day rest have enhanced IFN-γ production after restimulation with IL-12 + IL-15, IL-12 + IL-18, or K562 leukemia cells. This memory-like phenotype was retained in proliferating NK cells. In CD56dim NK cells, the memory-like IFN-γ response was correlated with the expression of CD94, NKG2A, NKG2C, and CD69 and a lack of CD57 and KIR. Therefore, human NK cells have functional memory-like properties after cytokine activation, which provides a novel rationale for integrating preactivation with combinations of IL-12, IL-15, and IL-18 into NK cell immunotherapy strategies.
Introduction
Natural killer (NK) cells are lymphocytes important for host defense against infection and malignant tumor cells.1–5 How NK cells acquire enhanced functionality to optimally respond to target cells or inflammatory signals is an important question in NK cell biology. Traditionally, NK cells have been categorized as innate effectors because they use germline-encoded activating and inhibitory NK and cytokine receptors to orchestrate their rapid proliferative and functional (ie, IFN-γ production and cytotoxicity) responses. Recently, studies have shown that mouse NK cells exhibit memory-like properties defined by an initial activation event, a subsequent return to the resting state, followed by enhanced IFN-γ production on restimulation.6–8 Cooper et al observed that initial stimulation with the cytokines IL-12 + IL-18 results in the majority of mouse NK cells producing IFN-γ and, after 1-3 weeks, these cells exhibit memory-like NK properties with increased IFN-γ production after restimulation with cytokines or activating receptor ligation.6 Sun et al evaluated Ly49H+ NK cells after recovery from an acute infection with murine CMV (MCMV), which encodes an Ly49H ligand, and identified an enhanced IFN-γ response to Ly49H restimulation.7 In addition, von Andrian et al were the first to report that innate cells with an NK cell phenotype mediated recall responses to haptens during the delayed hypersensitivity response in Rag1−/− mice.8,9 Therefore, mouse NK cells appear to exhibit both cytokine and activating NK cell receptor–induced memory-like properties.
At present, it is unclear whether human NK cells exhibit a memory-like response. Several studies have identified persistent alterations in the NK cell compartment after viral infections, which have included human CMV (HCMV), Hantavirus, and Chikungunya virus.10–13 In addition, reactivation of HCMV in the setting of solid-organ transplantation or allogeneic hematopoietic stem cell transplantation has resulted in the induction of functional CD57+ and NKG2C+ NK cells.14,15 In the present study, we investigated whether cytokine preactivation alters the functional recall response of human NK cells after a prolonged rest period, as shown by the results of a previous study revealing murine cytokine-induced innate memory in NK cells.6 We have identified herein human cytokine-induced memory-like NK cells, and these results provide a new rationale for investigating short-term preactivation with combinations of IL-12, IL-15, and IL-18 in NK cell–based immunotherapy.
Methods
Reagents
The following anti–human mAbs were used in this study: CD56 (N901), CD3 (UCHT1), CD45 (J.33), NKG2A (Z199.1), and NKp46 (BAB281; all Beckman Coulter); CD16 (3G8), IFN-γ (B27), CD107a (H4A3), CD57 (NK-1), CD69 (FN50), CD158a (HP3E4), CD158b (CH-L), CD158e (DX9), and CD94 (HP-3D9; all BD Biosciences); NKG2C (134591; R&D Systems); and purified CD16 (3G8) and mouse IgG1 (MG1-45; both BioLegend). The following endotoxin-free recombinant human (rh) cytokines were used: rhIL-12 (PeproTech), rhIL-18 (MBL International), and rhIL-15 (CellGenix).
NK cell purification and cell culture
Human platelet apheresis donor PBMCs were obtained by ficoll centrifugation. NK cells were purified using RosetteSep (StemCell Technologies, ≥ 95% CD56+CD3−) or by flow cytometric cell sorting to ≥ 99% purity (FACSAria II cell sorter; BD Biosciences). Cells were plated at 3-5 × 106 cells/mL and preactivated for 16 hours using rhIL-12 (10 ng/mL) + rhIL-18 (50 ng/mL) + rhIL-15 (1 ng/mL) or control conditions (rhIL-15, 1ng/mL), washed 3 times to remove cytokines, and cultured in complete RPMI 1640 medium containing 10% human AB serum (Sigma-Aldrich) supplemented with rhIL-15 (1 ng/mL) to support survival, with 50% of the medium being replaced every 2-3 days with fresh cytokines. In some experiments, cells were preactivated in rhIL-12 (10 ng/mL), rhIL-15 (100 ng/mL), and IL-18 (50 ng/mL) alone or in combination to identify optimal cytokine preactivating conditions. In other experiments, the concentrations of IL-12 and IL-18 were varied during the 16-hour preactivation as indicated.
For preactivation with K562 leukemia cells, freshly purified NK cells were cocultured with irradiated (3000 rads) K562 cells for 16 hours at a 4:1 ratio either in presence of rhIL-15 (1 ng/mL) alone or in combination with rhIL-12 (10 ng/mL), rhIL-18 (50 ng/mL), or rhIL-12 (10 ng/mL) + rhIL-18 (50 ng/mL). At the end of the 16-hour preactivation NK cells underwent CD56+ selection to eliminate the leukemia cells using MACS LD columns (Miltenyi Biotec) after labeling NK cells with CD56 microbeads per manufacturer's instructions (Miltenyi Biotec). For CD16-ligation–based preactivation, flat, 48-well polystyrene plates were incubated at 37°C with purified anti-CD16 mAb or mouse IgG1 (control) for 2 hours at a 10 μg/mL concentration, followed by 3 washes with PBS. Enriched NK cells were then added at a concentration of 5 × 106/mL in a 500-μL volume to the wells for a 16-hour preactivation either in the presence of rhIL-15 (1 ng/mL) alone or in combination with rhIL-12 (10 ng/mL), rhIL-18 (50 ng/mL), or rhIL-12 (10 ng/mL) + rhIL-18 (50 ng/mL).
Functional and proliferation assays
After 7, 14, or 21 days, cells were harvested and restimulated with IL-12 + IL-15, IL-12 + IL-18 (at doses noted in the preceding section), or K562 leukemia targets (effector: target ratio, 4:1) for 6 hours in a 96-well round-bottom plate. Anti-CD107a mAb was added at the start of the functional assay, followed by brefeldin A and monensin (BD Biosciences) after 1 hour. Cells were stained for surface NK cell surface markers and intracellular IFN-γ (Cytofix/Cytoperm; BD Biosciences). For proliferation experiments, NK cells were labeled with CFSE (5μM; Sigma-Aldrich) for 5 minutes.16,17
Flow cytometric analysis
Cell staining was performed as described previously,16–18 and data were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed using Kaluza Version 1.2 (Beckman Coulter) or FlowJo Version 9.3.2 (TreeStar) software. Plots were made using GraphPad Version 5.0 software.
Statistical analysis
Statistical comparisons were performed using the Student t test. P < .05 was considered significant.
Results
Brief preactivation of human NK cells with IL-12 + IL-18 leads to memory-like IFN-γ production
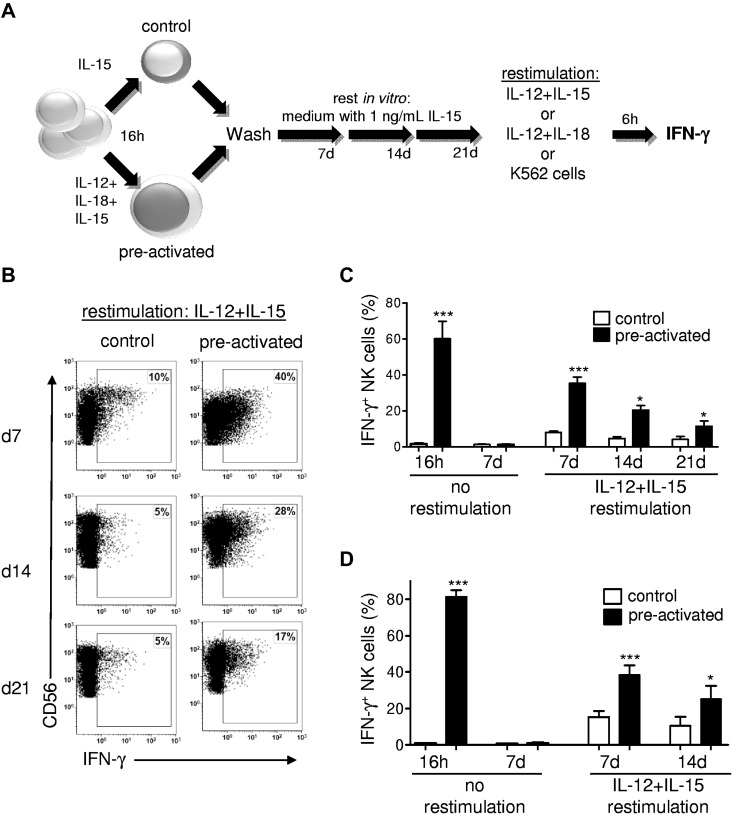
Cytokine-induced memory in NK cells manifests as an enhanced functional response after an initial preactivation and a subsequent return to a baseline functional state.6 We evaluated human NK cells for memory-like properties after IL-12 + IL-18 preactivation (Figure 1). Our experimental approach included a 16-hour preactivation, followed by a prolonged rest period in vitro with survival supported by low concentrations of IL-15 (Figure 1A). After 7, 14, or 21 days of rest in vitro, IFN-γ protein production by IL-12 + IL-18–preactivated NK cells was significant increased compared with control NK cells after restimulation with IL-12 + IL-15 (Figure 1B-C). This enhanced IFN-γ production after preactivation was confirmed to be cell intrinsic in experiments using purified NK cells (Figure 1D). To better define the NK cell functions that were enhanced by preactivation, we also evaluated NK cell surface CD107a (LAMP-1) expression triggered by K562 tumor targets as a surrogate for degranulation.19 CD107a surface expression after K562 triggering was not different between preactivated and control NK cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that preactivated NK cells did not have an enhanced ability to degranulate. However, these similar yet robust CD107a responses indicate that functional NK cells were present in both control and preactivated culture conditions. These findings provide direct evidence that, like murine NK cells, IL-12 + IL-18–preactivated human NK cells have enhanced IFN-γ production (but not degranulation) on restimulation and therefore exhibit memory-like cytokine responses.
Figure 1.
Preactivation with IL-12 + IL-18 results in human memory-like NK cells with enhanced IFN-γ production after restimulation. (A) Schema of experimental approach with IL-12 + IL-18 preactivation. In later experiments, preactivated was varied as indicated. (B-C) PBMCs (containing 13% ± 5% CD56+CD3− NK cells) were preactivated for 16 hours with control (IL-15, 1 ng/mL), or IL-12 (10 ng/mL) + IL-18 (50 ng/mL) + IL-15 (1 ng/mL). Cells were then washed 3 times to remove preactivating cytokines and cultured with low-dose IL-15 (1 ng/mL) to support survival for 7, 14, or 21 days. After each time point, cells were washed, restimulated for 6 hours with IL-12 (10 ng/mL) + IL-15 (100 ng/mL), and assayed for NK cell IFN-γ production by intracellular flow cytometry. (B) Representative bivariate flow plots from control and IL-12 + IL-18–preactivated NK cells showing IFN-γ production after IL-12 + IL-15 restimulation on days 7, 14, or 21 (gated on live CD45+CD56+CD3− NK cells). (C) Summary data shown as means ± SEM percentage of IFN-γ+ NK cells. NK cells were assessed without restimulation immediately after preactivation (16 hours; n = 6; 3 independent experiments) to confirm that NK cells were preactivated or after 7 days of rest to demonstrate a return to a resting state (n = 8; 4 independent experiments). After 7 (n = 8; 4 independent experiments), 14 (n = 4; 2 independent experiments), or 21 (n = 4; 2 independent experiments) days, NK cells were restimulated with IL-12 + IL-15 for 6 hours and assayed for IFN-γ production. (D) Purified NK cells exhibit memory-like properties. Experiments were performed as in panels A-C, but with purified NK cells without restimulation (n = 6, 3 independent experiments for 16 hours; n = 12, 4 independent experiments for 7 days) and after IL-12 + IL15 restimulation for 7 days (n = 12; 4 independent experiments) or 14 days (n = 6; 3 independent experiments). Purified NK cells were ≥ 95% CD56+CD3− with < 0.5% CD3+ T cells. *P < .05; **P < .01; ***P < .001.
Cytokine-induced memory-like NK cell IFN-γ response in CD56bright and CD56dim NK cells
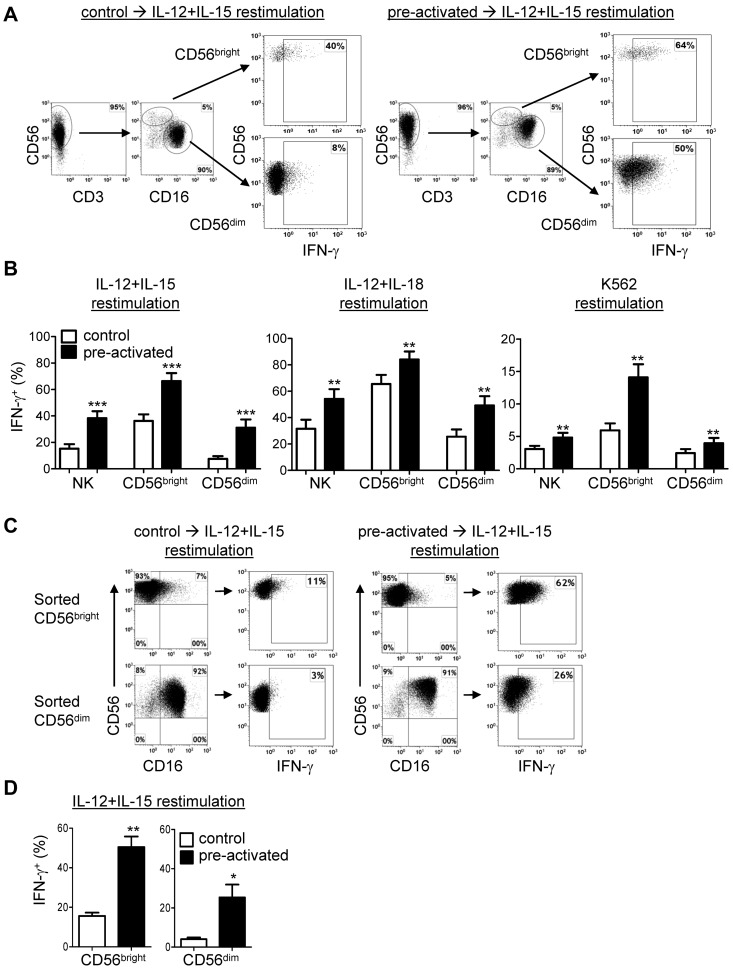
Human NK cells are divided into functionally and developmentally distinct CD56bright and CD56dim subsets.1,20 We evaluated whether both of these NK cell subsets exhibit enhanced memory-like NK cell IFN-γ production after preactivation. First, we gated on these NK cell subsets in purified NK cell cultures and observed that both CD56bright and CD56dim NK cells exhibited increased IFN-γ production on restimulation compared with controls (Figure 2A-B). This capacity for increased IFN-γ production was evident using multiple restimulation conditions: IL-12 + IL-15, IL-12 + IL-18, or K562 leukemia targets (Figure 2B). In response to K562 triggering, the increase in IFN-γ production by preactivated CD56dim NK cells was modest, with a more robust enhancement in CD56bright NK cells (Figure 2B). This is significant because freshly isolated CD56dim NK cells produce greater amounts of IFN-γ in response to activating receptors or tumor targets compared with CD56bright NK cells.1
Figure 2.
CD56bright and CD56dim NK cell subsets exhibit memory-like properties after IL-12 + IL-18 preactivation. (A-B) CD56bright and CD56dim NK subsets in purified NK cell (≥ 95% CD56+CD3−) cultures exhibit memory-like IFN-γ production. (A) Representative data from control and IL-12 + IL-18–preactivated NK cells demonstrating the NK subset analysis gating strategy and differential IFN-γ production after the 7-day rest and a 6-hour IL-12 + IL-15 restimulation. (B) Summary data demonstrating that IL-12 + IL-18–preactivated CD56bright and CD56dim NK cells exhibit a memory-like IFN-γ response after restimulation with IL-12 + IL-15, IL-12 + IL-18, or K562 leukemia cells. Purified (≥ 95% CD56+CD3−) NK cells were cultured as in Figure 1A for 7 days and restimulated with cytokines or K562 (4:1 effector: target ratio) as indicated for 6 hours and then assayed for IFN-γ. Data shown are the means ± SEM percentage of IFN-γ+ NK cells, gated on CD56bright or CD56dim subsets, with n = 12 donors (4 independent experiments) for IL-12 + IL-15 restimulation, n = 6 donors (3 independent experiments) for IL-12 + IL-18 restimulation, and n = 9 donors (4 independent experiments) for K562 restimulation. (C-D) Flow-sorted CD56bright and CD56dim NK cells cultures exhibit memory-like IFN-γ production. (C) NK cell subsets were flow sorted (≥ 99% purity) and then preactivated as per Figure 1A, rested for 7 days, restimulated (6 hours) with IL-12 + IL-15, and stained for intracellular IFN-γ. The NK cell subset phenotype was retained in each sorted population after 7 days of culture. (D) Summary data are shown as means ± SEM of the percentage of IFN-γ+ sorted CD56bright and CD56dim NK cells after 7 days of rest followed by 6 hours of restimulation with IL-12 + IL-15 (n = 6; 3 independent experiments). *P < .05; **P < .01; ***P < .001.
We next confirmed that both CD56bright and CD56dim NK cells exhibit memory-like IFN-γ production by flow sorting these subsets, preactivating as shown in Figure 1A, and assessing the IFN-γ response to IL-12 + IL-15 restimulation after 7 days of rest in low-dose IL-15. Both NK cell subsets demonstrated increased IFN-γ production after restimulation with IL-12 + IL-15 compared with controls (Figure 2C-D). Although CD56dim NK cells demonstrated a modest increase in CD56 expression, CD56bright NK cells retained their CD56/CD16 profile and did not differentiate into CD56dim NK cells in this experimental system (Figure 2C). Therefore, both CD56bright and CD56dim NK cell subsets exhibit cytokine-induced memory-like properties.
To determine the concentrations of IL-12 and IL-18 sufficient to generate human memory-like NK cells, we performed dose-response experiments that varied the concentrations of IL-12 and IL-18 used to preactivate purified NK cells (Figure 3). Both CD56bright and CD56dim NK cells were optimally preactivated with 1 ng/mL of IL-12 and 50 ng/mL of IL-18. CD56bright NK cells also had modest enhancement of restimulated IFN-γ production when preactivated with 5 ng/mL of IL-18 in concert with 1 or 10 ng/mL of IL-12.
Figure 3.
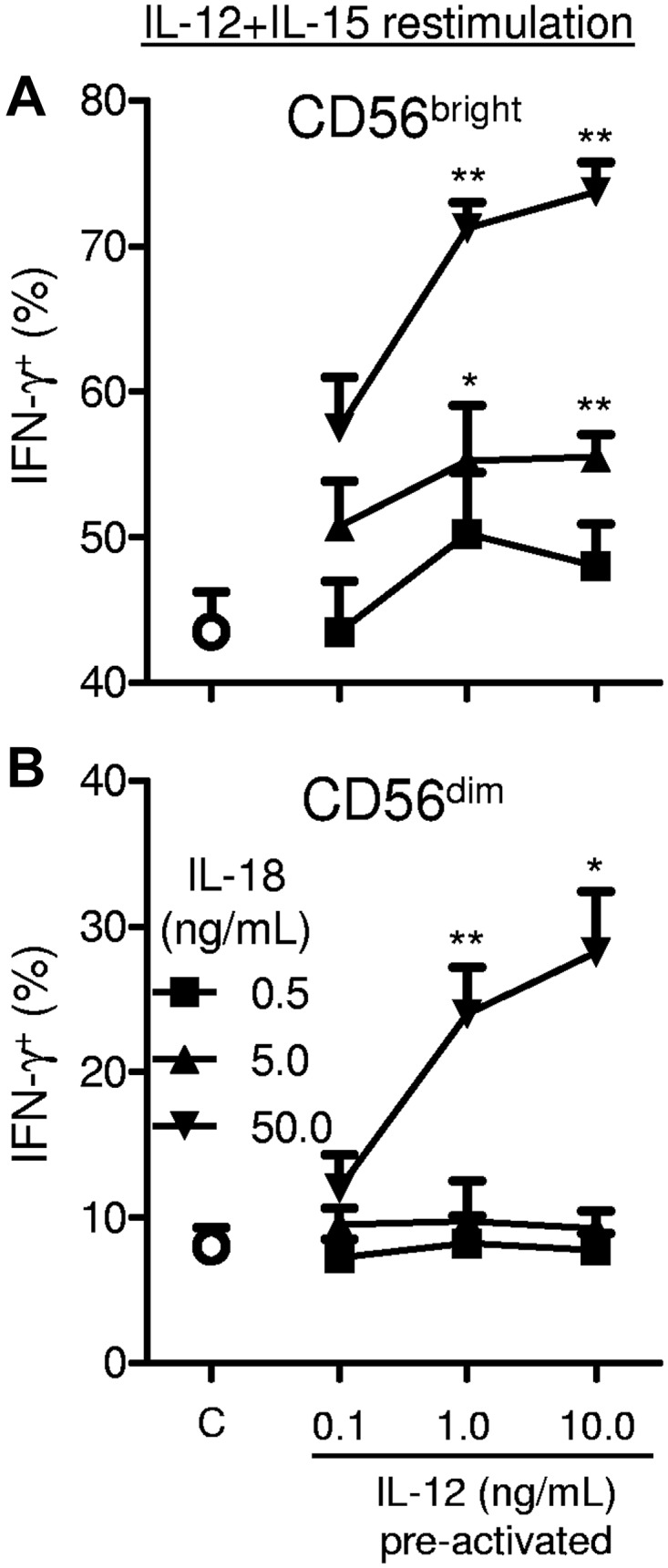
Preactivation dose response of IL-12 and IL-18 for memory-like NK cell induction. Purified (≥ 95% CD56+CD3−) NK cells were preactivated for 16 hours with the indicated concentrations of IL-12 + IL-18 or control conditions (C; 1 ng/mL of IL-15 only). After preactivation, cells were washed as in Figure 1A and then rested for 7 days in 1 ng/mL of IL-15 before restimulation with IL-12 + IL-15 for 6 hours to assess IFN-γ production (n = 4; 2 independent experiments). Summary data are shown as means ± SEM percentage of IFN-γ+ CD56bright (A) and CD56dim (B) NK cells. Statistical comparisons are made between the indicated dose of IL-12 and IL-18 and the control cells. *P < .05; **P < .01
Phenotypic alterations in human memory-like NK cells
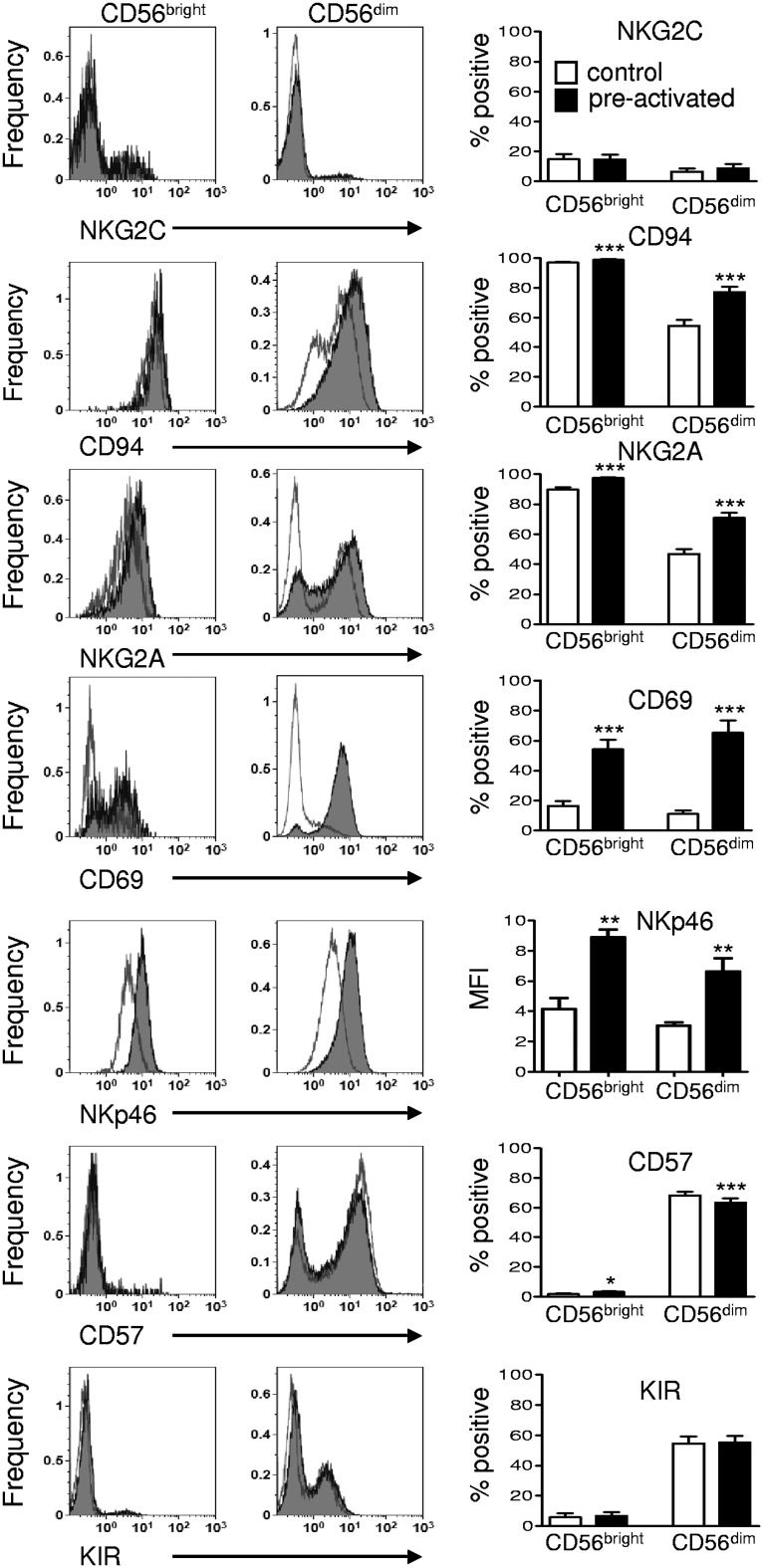
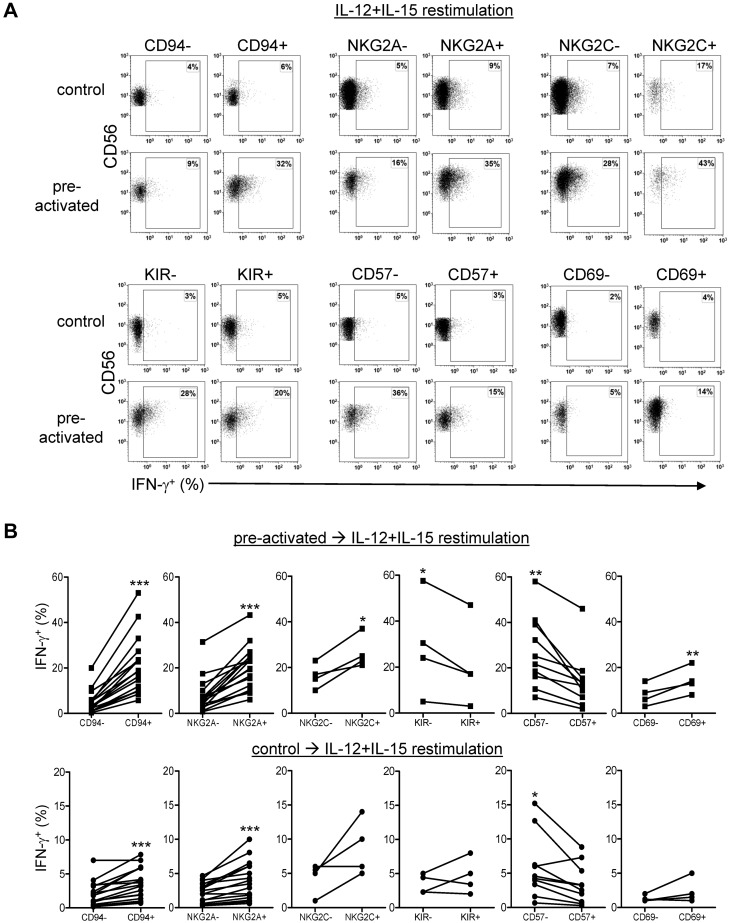
A surface marker phenotype specific to memory-like NK cells has not yet been identified in mice.6,21 Therefore, in the present study, we evaluated candidate markers that have an established correlation with human NK cell IFN-γ production.14,15,22 First, we assessed changes in cell surface expression after preactivation and 7 days of rest before any restimulation. Preactivation resulted in increased expression of CD94, NKG2A in CD56dim NK cells, and CD94 (modest), NKp46, and CD69 in CD56bright and CD56dim subsets (Figure 4). We next evaluated whether candidate surface markers discriminated preactivated NK cells with increased IFN-γ after restimulation. There was a positive correlation between IFN-γ production and the percentage of cells positive for CD94, NKG2A, NKG2C, and CD69 expression in preactivated CD56dim NK cells (Figure 5). Previously described associations of the CD94+NKG2A+ and CD57− CD56dim NK cell subsets were confirmed in control cells after IL-12 + IL-15 (Figure 5) or IL-12 + IL-18 (supplemental Figure 2) restimulation, indicating that CD56dim NK cell subset biology was preserved. We also noted a modest increase in double-positive NKG2A+NKG2C+ CD56dim NK cells in preactivated NK cells compared with control NK cells. However, these represented a small minority of IFN-γ–producing memory-like NK cells. In contrast, single-positive NKG2C+CD56dim NK cells demonstrated minimal IFN-γ production (supplemental Figure 3). In addition, preactivated CD56dim NK cells that were KIR− and CD57− were more likely to produce IFN-γ after restimulation (Figure 5). No correlation was observed between the percentage of cells positive for these markers and IFN-γ production in CD56bright NK cells, in part because many of these markers are expressed on the majority or minority of cells. To further define whether IFN-γ was correlated with the markers of interest, we also assessed the association between the expression level (mean fluorescence intensity [MFI]) of markers of interest and IFN-γ production (supplemental Figure 4). This approach confirmed the associations between CD94, NKG2A, NKG2C, and CD69 in CD56dim NK cells, and identified an association between increased MFI of CD94 or NKG2A and IFN-γ production in CD56bright NK cells. We also observed a positive correlation between increased NKp46 MFI and IFN-γ production in both NK cell subsets.
Figure 4.
Cell surface marker changes after preactivation and induction of human memory-like NK cells. Purified NK cells (≥ 95% CD56+CD3−) were cultured for 7 days as in Figure 1A and assayed without restimulation for CD94, NKG2A, NKG2C, NKp46, CD69, KIR, and CD57. Representative flow histograms are shown in pre-gated CD56bright and CD56dim NK cell subsets, overlaying control cells (open lines) and preactivated cells(gray filled lines). Summary data are shown as means ± SEM percentage of positive or MFI for the marker indicated in CD56bright and CD56dim NK cells. CD56dim NK cells had significantly increased expression of CD94 and NKG2A 7 days after preactivation compared with controls. Both CD56dim and CD56bright NK cells had increased CD69 and NKp46 surface expression 7 days after preactivation (n = 4-6; 2 independent experiments) *P < .05; **P < .01; ***P < .001.
Figure 5.
Enhanced IFN-γ production by human memory-like NK cells is associated with a CD94+NKG2A+CD57−KIR−CD69+ phenotype. Purified NK cells (≥ 95% CD56+CD3−) were cultured as in Figure 1A for 7 days and assessed for IFN-γ in concert with the indicated cell surface marker after 6 hours of IL-12 + IL-15 restimulation. (A) Representative bivariate flow plots from control and IL-12 + IL-18–preactivated NK cells showing correlation of IFN-γ production (all were restimulated with IL-12 + IL-15) in populations positive or negative for CD94, NKG2A, NKG2C, KIR, CD57, and CD69. (B) Because CD56bright NK cells had > 95% or < 10% expression of CD94, NKG2A, KIR, and CD57 (Figure 6), IFN-γ correlative data are shown gated on CD56dim NK cells. There were a greater percentage of IFN-γ+ NK cells in the CD94+, NKG2A+, NKG2C+, and CD69+ fractions of CD56dim NK cells. In contrast, there were a significantly greater percentage of IFN-γ+ NK cells in CD57− and KIR− CD56dim NK cells. There were no significant correlations of NKG2C or CD69 expression with IFN-γ production in CD56bright NK cells (not shown). Statistical comparisons are between marker positive and negative preactivated or control NK cell association with IFN-γ. A larger number of donors were assessed for CD94, NKG2A, and CD57 correlations to assess the more subtle differences between marker positive and negative control cells (n = 4-17 donors; 2-6 independent experiments). *P < .05; **P < .01; ***P < .001.
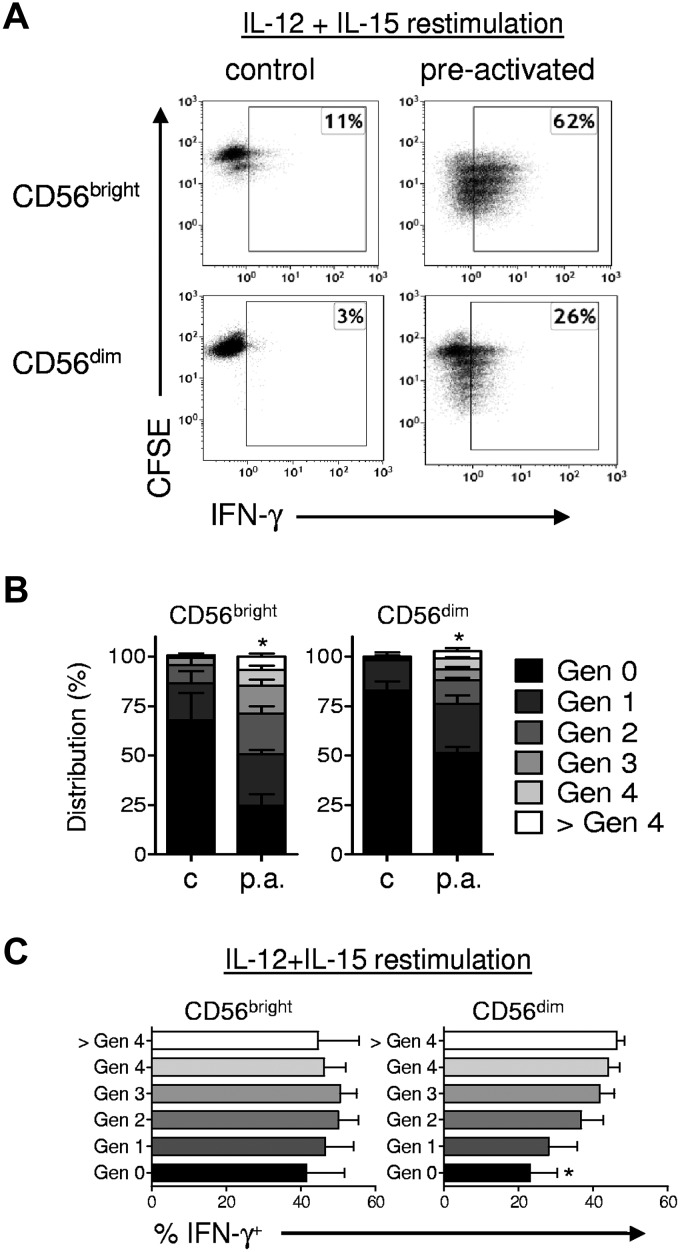
Human memory-like NK cells preserve enhanced IFN-γ production after cell division
Murine memory-like NK cells proliferate and pass on their enhanced IFN-γ production to daughter cells.6 We therefore next evaluated whether human NK cell subsets proliferated after IL-12 + IL-18 activation and observed that a higher proportion of preactivated NK cells underwent cell division compared with controls. This was evident both in flow-sorted NK cell subsets (Figure 6A-B) and when gating on CD56bright and CD56dim NK cell subsets within bulk PBMC cultures (supplemental Figure 5). The degree of proliferation in CD56dim NK cells after IL-12 + IL-18 stimulation is remarkable because this subset has previously been shown to exhibit less proliferation to standard mitogenic stimuli compared with CD56bright NK cells.20 After 7 days, the percentage of CD56+CD3− NK cells within PBMCs was modestly increased in preactivated (14% ± 3%) compared with control NK cells (11% ± 2%; P < .05), whereas the absolute numbers were similar (2.1 × 105 vs 2.4 × 105; P = .4). In addition, the percentages of CD56bright (preactivated 18% ± 4% vs control 19% ± 4%; P = .49) and CD56dim cells (preactivated 82% ± 4% vs control 81 ± 4%; P = .49) were similar. Further, we observed no significant differentiation of flow-sorted CD56bright NK cells, with 6% ± 2% CD16+ before culture and 10% ± 5% CD16+ after preactivation and 7 days of rest (P = .13; n = 6; 3 independent experiments; Figure 2C). Therefore, because flow-sorted CD56bright NK cells retained their phenotype in this system without evidence of differentiation into CD56dimCD16bright NK cells, it is unlikely that CD56dim NK cells present after 7 days in preactivated PBMC cultures resulted from differentiation of CD56bright precursors. Moreover, preactivated CD56bright and CD56dim NK cells that underwent > 2 cell divisions had similar degrees of enhanced IFN-γ production, indicating heritable memory-like properties, similar to the murine memory-like NK cells.6 These data indicate that IL-12 + IL-18–preactivated human NK cells undergo cell division and pass on the enhanced capacity to produce IFN-γ to their cellular progeny.
Figure 6.
Memory-like NK cells proliferate and retain enhanced IFN-γ production after cell division. Purified NK cells were labeled with CFSE to track cell division, sorted into CD56bright and CD56dim NK subsets (≥ 99% purity), and preactivated and rested as per Figure 1A. After 7 days, cells were restimulated with IL-12 + IL-15 and analyzed simultaneously for IFN-γ and CFSE to track cell divisions. (A) Representative bivariate flow plots of CD56bright and CD56dim NK cells demonstrating that preactivation results in both increased cell division (CFSE dilution) and enhanced IFN-γ production. (B) Summary results showing the increased proliferation 7 days after preactivation (p.a.) compared with control (c) in both CD56bright and CD56dim NK cell subsets. (C) Summary results are shown as the means ± SEM percentage of IFN-γ+ by NK cell generation (n = 4 donors, 2 independent experiments). Similar findings were observed in purified NK cells that were then gated on CD56bright and CD56dim NK cells (n = 6 donors; 2 independent experiments; supplemental Figure 5). *P < .05; **P < .01; ***P < .001.
Minimal changes in cytokine receptor expression, STAT signaling, and IFN-γ mRNA abundance in human memory-like NK cells
Cytokine receptor expression and/or signaling changes are potential mechanisms that may result in enhanced recall to restimulation with IL-12, IL-18, and IL-15 by preactivated NK cells. Recent work in mice has implicated IL-12 signaling as being central to the generation of memory NK cells during an MCMV infection.23 In the present study, we investigated preactivated and control NK cells for alterations in the expression of IL-12 and IL-18 receptor components at the protein and mRNA levels (supplemental Figures 6 and 7). In CD56bright and CD56dim NK cells, there were minimal or no differences in these receptors between preactivated and control conditions. Furthermore, there was no significant difference in the phosphorylation of STAT4 or STAT3, suggesting that alteration of downstream pSTAT signaling was not a predominant mechanism responsible for enhanced IFN-γ production by preactivated memory-like NK cells (supplemental Figures 6C and 8).
We next evaluated IFN-γ mRNA abundance in control versus preactivated NK cells because increased basal or restimulated IFN-γ mRNA levels could result in the increased IFN-γ protein produced by human memory-like NK cells (supplemental Figure 9). We detected no difference in the relative abundance of IFN-γ mRNA in control versus preactivated NK cells after 7 days of rest without restimulation, similar to murine memory-like NK cells.6 Although we did observe a substantial increase in IFN-γ mRNA in IL-12 + IL-15–restimulated cells after 6 hours, there was no difference in the magnitude of this induction comparing preactivated and control NK cells from the same subject. This suggests that the predominant mechanism responsible for enhanced memory-like NK cell function in humans occurs at the posttranscriptional and/or posttranslational level.
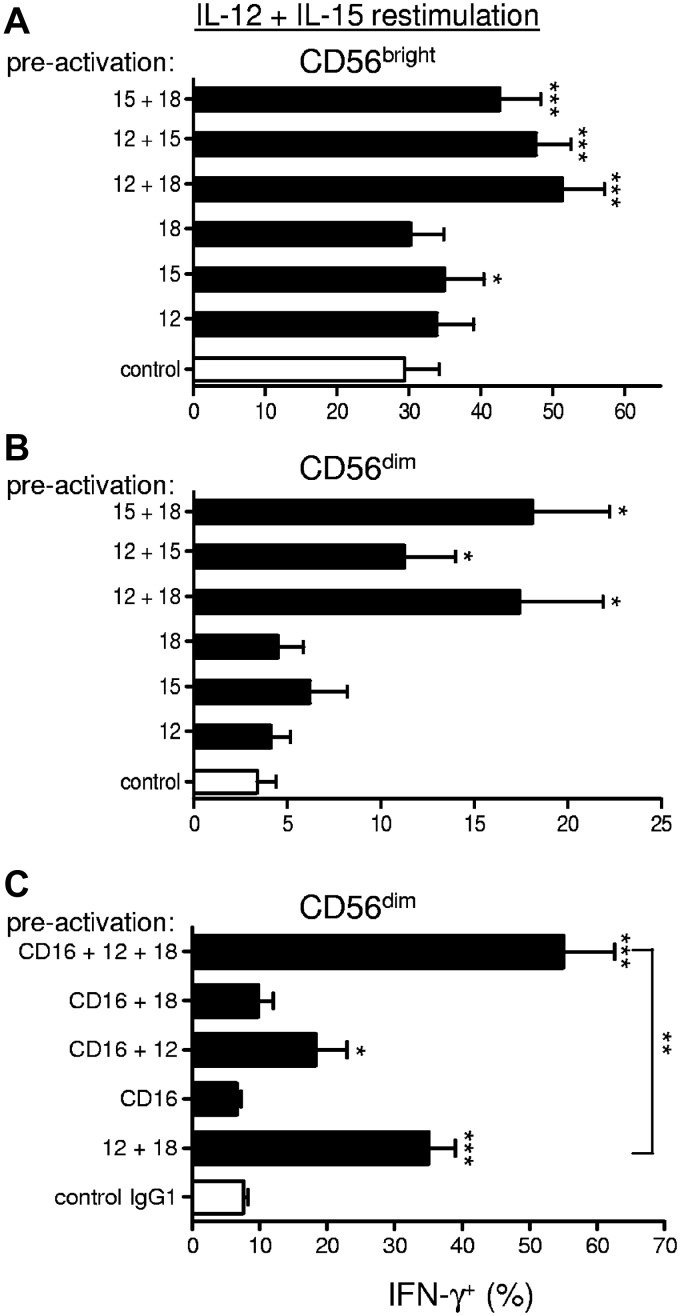
Additional cytokine and activating receptor combinations are sufficient to preactivate human NK cells for enhanced memory-like IFN-γ production
Our study initially focused on the capability of IL-12 + IL-18 to preactivate human NK cell memory-like properties based on findings in mice.6 To define additional signals that lead to memory-like NK cell IFN-γ production, we also evaluated IL-12, IL-15, and IL-18 alone and in all combinations and found that memory-like enhanced function requires combined cytokine preactivation (Figure 7A-B). Novel preactivating combinations that led to enhanced IFN-γ production on restimulation in addition to IL-12 + IL-18 included IL-15 + IL-18 and IL-15 + IL-12, suggesting that no one single cytokine was requisite and multiple combinations were sufficient to induce human memory-like NK cell differentiation.
Figure 7.
Additional preactivation conditions lead to enhanced memory-like NK cell IFN-γ production. (A-B) Purified (≥ 95% CD56+CD3−) NK cells were stimulated for 16 hours with either low-dose IL-15 (1 ng/mL, control), IL-12 (10 ng/mL), high-dose IL-15 (100 ng/mL), IL-18 (50 ng/mL), or the indicated IL-12/IL-15/IL-18 combinations. After 16 hours of activation, the cells were washed as in Figure 1A and then rested for 7 days before restimulation with IL-12 + IL-15 for 6 hours to assess IFN-γ production. In all conditions in which high-dose IL-15 was not used, low-dose IL-15 was included to support survival during preactivation. These data show that preactivation with all combinations of IL-12, IL-15, and IL-18 lead to memory-like NK cell IFN-γ production on restimulation (n = 4; 2 independent experiments). (C) In a separate set of donors, purified (≥ 95% CD56+CD3−) NK cells were preactivated for 16 hours by cross-linking CD16 with plate-bound anti-CD16 mAb (or control mouse IgG1) or CD16 cross-linking in combination with IL-12 (10 ng/mL), IL-18 (50 ng/mL), or IL-12 (10 ng/mL) + IL-18 (50 ng/mL). The preactivated cells were washed as in Figure 1A, rested for 7 days, and then restimulated with IL-12 + IL-15 for 6 hours to assess IFN-γ production. Summary data are shown as means ± SEM percentage of IFN-γ+ CD56dim NK cells. These data show that CD16 cross-linking in combination with cytokines (IL-12 and IL-12 + IL-18) results in enhanced IFN-γ production on restimulation (n = 6; 3 independent experiments). Statistical comparisons are with control conditions, except in panel C as indicated. *P < .05; **P < .01; ***P < .001.
In addition to cytokines, we also evaluated preactivation through NK-activating receptors by coculturing with K562 leukemia cells or cross-linking CD16 (FcγRIIIa). K562 preactivation alone for 16 hours was unable to induce enhanced IFN-γ production after 7 days of rest and IL-12 + IL-15 restimulation in both CD56bright (48% ± 8.2% vs 52% ± 8.6%; P = .33) and CD56dim (11.25% ± 2.6% vs 10.8% ± 3.4%; P = .76) NK cells. Similarly, K562 preactivation in combination with IL-12, IL-18, or IL-12 + IL-18 for 16 hours did not lead to enhanced IFN-γ production after 7 days of rest and IL-12 + IL-15 restimulation (data not shown). Cross-linking CD16 alone also failed to enhance memory-like IFN-γ production after IL-12 + IL-15 restimulation (Figure 7C). In contrast, combining CD16 cross-linking with IL-12 or IL-12 + IL-18 resulted in a significant enhancement in IFN-γ production compared with each signal alone, suggesting that CD16 cross-linking could costimulate memory-like NK cell induction during preactivation.
Discussion
In the present study, we identified human NK cells that exhibit enhanced IFN-γ production after short-term preactivation with IL-12 + IL-18, followed by 1-3 weeks of in vitro rest and subsequent restimulation with cytokines (IL-12 + IL-15 and IL-12 + IL-18) or K562 leukemia tumor targets. This novel finding in human NK cells is similar to previous reports of IL-12 + IL-18–induced memory-like NK cells in mice,6 and we therefore refer to these comparable preactivated human NK cells as “memory-like.” In addition, we identified additional combined signals, including IL-15 + IL-18, IL-12 + IL-15, and CD16 ligation + IL-12, as preactivating conditions sufficient to induce memory-like NK cells with an enhanced capacity to produce IFN-γ. However, preactivation with exposure to a human leukemia cell line (K562) failed to result in memory-like NK cells. Furthermore, the 2 predominant human NK cell subsets, CD56bright and CD56dim, both exhibited memory-like NK cell IFN-γ production in response to several different restimulation conditions. Preactivation led to proliferation, and memory-like NK cells retained their enhanced capacity to produce IFN-γ after extensive cell division. In human memory-like NK cells, increased IFN-γ production was associated with expression of CD94, NKG2A, NKp46, and CD69 and a lack of KIR and CD57. Therefore, human NK cells can acquire memory-like properties after a brief initial activation.
Memory attributes of NK cells have been reported in several studies using different approaches and model systems.6–8 Initial reports from von Andrian et al demonstrated that mice lacking T and B lymphocytes exhibited delayed-type hypersensitivity recall responses to haptens that were mediated by NK cells that homed to the liver.8 Follow-up studies reported similar hepatic “adaptive” NK cell responses to structurally distinct antigens, which depended on the chemokine receptor CXCR6.9 Although these adaptive NK cell responses were Rag-1 and Rag-2 independent, for such diverse antigen-specific receptor responses to occur, alternative recombined receptors could be involved. Direct experimental assessment of this delayed-type hypersensitivity NK memory would be challenging in humans. Subsequently, Yokoyama et al and Lanier et al reported murine NK cell responses with memory properties after cytokine activation6 or MCMV infection,7 respectively. In those studies, “memory-like” or “memory” NK cells were defined by an initial preactivation, resolution of the inflammatory response, and later an enhanced recall response after restimulation either through cytokine or activating NK receptors. MCMV-induced NK cell memory responses were recently reported to be critically dependent on proinflammatory cytokines induced early during infection (eg, IL-12),23 suggesting that the mechanisms inducing these 2 types of NK memory may be similar and dependent on cytokine activation. The results of the present study support this assertion and identify several combined cytokine signals sufficient to induce human cytokine-induced memory-like NK cells. In our human system of in vitro rest, the percentages of IFN-γ+ memory-like NK cells waned over weeks, likely because of suboptimal long-term support of primary NK cells in vitro. This potential explanation is supported by findings in mice that memory-like NK cell responses persist for at least 1 month in vivo after adoptive transfer.6,21 Currently, we are evaluating in vivo xenograft approaches in NOD-SCID-γc−/− mice to assess whether preactivated memory-like NK cells have enhanced survival and/or function in this setting compared with controls. Such human NK cell xenograft models (with or without human leukemia) will provide a useful preclinical tool with which to evaluate the utility of NK cell preactivation. Therefore, the human memory-like NK cells reported herein are similar to murine cytokine-induced memory-like NK cells and may share preactivation signals in common with murine memory NK cells that differentiate after MCMV infection.
Human NK cells differentiate from CD56bright (stage IV) in secondary lymphoid organs into CD56dim (stage V) NK cells.1,24 Further, CD56dim NK cells have been categorized into less mature (NKG2A+CD94+CD57−KIR−) and more differentiated (CD57+KIR+NKG2A−CD94−) subsets.22,25–27 Human cytokine-induced memory-like NK cells demonstrated increased expression of CD94, NKG2A, NKp46 and CD69 compared with control NK cells from the same donor. In addition, enhanced IFN-γ production was also associated with a less differentiated phenotype in CD56dim NK cells, which were CD94+NKG2A+ but CD57−KIR−. Currently, the functional contribution of these surface molecules to enhanced IFN-γ production is unknown; however, they do provide a starting point for narrowing the immunophenotype of memory-like NK cells and their alterations in human health and disease. In addition, these data suggest that the enhanced memory-like IFN-γ production is enriched in less mature CD56dim NK cells, precisely those subsets that have been shown to respond better to IL-12 + IL-18. In our experiments initiated with sorted CD56bright NK cells, we did not observe differentiation of CD56bright NK cells into CD56dim NK cells. At the cell-population level, CD56dim NK cells contained stable percentages of KIR+CD57+ cells, but we did observe a modest increase in the percentage of cells expressing CD94, NKG2A, and NKG2C. This may represent a relative enrichment and/or survival of less differentiated CD56dim NK cells in this experimental system. In donors assessed for NKG2C, we observed small but clearly positive NKG2C+ NK cell populations in freshly isolated, control, and preactivated cells from the same donor. Preactivation appeared to modestly increase the percentage of NKG2C+NKG2A+ CD56dim NK cells compared with controls; however, the overall contribution by NKG2C+ NK cells to IFN-γ production was minor. Therefore, it does not appear that cytokine-induced human memory-like NK cell IFN-γ production is strongly associated with NKG2C expression. This contrasts with recent studies showing enhanced IFN-γ production by NKG2C+CD57+ NK cells in patients after Hantavirus infection or CMV reactivation after solid-organ or hematopoietic stem cell transplantation.12,14,15 It is important to note the technical and experimental challenge of formally identifying NK cell memory in the context of viral infections in humans, which would require demonstration that the same NK cells were activated by viral infection/reactivation, returned to a baseline state, and then exhibited enhanced function on restimulation. It is possible that the enhanced NK cell functionality observed in chronic viral infection is because of persistent activation by virus and/or cytokine responses that persist in the host.17 These data suggest that human memory-like NK cells, arising in the context of cytokine preactivation, are distinct from NK cells with enhanced function “imprinted” in the context of viral infection in humans.
Similar to mice, the mechanism underlying enhanced functionality of human memory-like NK cells has not yet been elucidated. One potential mechanism for enhanced recall IFN-γ after restimulation is augmentation of cytokine receptor expression and/or downstream signaling on preactivation. We focused on the IL-12 pathway because: (1) IL-12 + IL-18 was sufficient to induce mouse memory-like NK cell generation, (2) recent data in memory NK cells during MCMV identified IL-12 as a requisite signal during that model viral infection, and (3) our own restimulation conditions extensively used IL-12. Although we did observe a modest increase in IL-12R expression at the protein level, this failed to result in differential phospho-STAT4 signaling after restimulation. Because IFN-γ was the primary enhanced NK cell function in this setting, we next assessed IFN-γ mRNA in memory-like NK cells. Similar to mice,6 the basal level of IFN-γ mRNA in preactivated NK cells remained essentially identical to the level expressed in control NK cells from the same donor at 7 days. This indicates that the increased IFN-γ protein produced by memory-like NK cells is not the consequence of an increase in the pool of available IFN-γ mRNA transcript. Furthermore, although we did observe a robust increase in IFN-γ mRNA in both control and preactivated NK cells after stimulation with IL-12 + IL-15, there was no difference in the amount of mRNA between the 2 conditions. Therefore, the increased IFN-γ protein production in memory-like NK cells after reactivation is not because of higher levels of transcription of the IFN-γ gene, which indicates that the mechanism responsible likely lies downstream of mRNA transcription. Several posttranscriptional and posttranslational mechanisms may be proposed to account for the increased IFN-γ protein production in memory-like NK cells. Increases in protein production may be accomplished via introduction of a positive regulatory event, such as enhanced translation or protein stabilization. Alternatively, increased IFN-γ protein production could be accomplished by removal or interruption of negative regulators. Although the posttranscriptional regulation of the IFN-γ mRNA is complex,28 miRNAs have recently been implicated in the control of IFN-γ,16,29 and one potential mechanism is altered expression of miRNAs (that basally inhibit IFN-γ translation) after preactivation and subsequent memory-like NK cell generation. These hypotheses warrant future investigation.
Adoptive NK cell therapies are currently under investigation in leukemia and other cancers, with promising proof-of-principle results. Currently, clinical strategies to prepare NK cell products use no preactivation, overnight IL-2 stimulation, or extensive culture and expansion ex vivo.30,31 The efficacy of these approaches is restricted by short-term persistence and limited survival and/or function after adoptive transfer of the NK cells into a patient. In contrast to recent findings by Gill et al suggesting that proliferation resulted in lower NK cell functionality by mouse NK cells,32 human and mouse6 memory-like NK cells have enhanced functional capacity after extensive proliferation, further supporting their potential utility in the immunotherapy setting. Because only brief ex vivo cytokine activation of NK cells would be required to generate memory-like human NK cells, this approach could be readily translated into future clinical trials of adoptive NK cell therapy. Further study of cytokine-preactivated NK cells, for example, in human NK cell xenograft models, will be required to provide clear preclinical data supporting this approach.
Supplementary Material
Acknowledgments
The authors thank the Siteman Cancer Center Flow Cytometry Core (funded by National Institutes of Health [NIH] grant P30 CA091842) and the Washington University Pathology & Immunology Flow Cytometry Core.
This work was supported by NIH T32 HL708836 (to R.P.S.), an American Society of Hematology Trainee Award (to J.M.C.), K08AI085030 (NIH), Children's Discovery Institute (to M.A.C.), K08HL093299 (NIH), the American Society of Hematology Foundation, the Howard Hughes Medical Institute, Division of Oncology Translational Oncology Group, and the Edward Mallinckrodt Jr Foundation (to T.A.F.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.R., M.A.C., and T.A.F. conceived and designed the study; R.R., S.E.S., J.W.L., J.M.C., and C.R.K. collected and assembled the data; R.R., J.M.C., and T.A.F. wrote the manuscript; and all authors analyzed and interpreted the data and edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Todd A. Fehniger, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: tfehnige@wustl.edu.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson HA, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101(08):27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8(4):259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9(5):473–475. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- 6.Cooper MA, Elliott JM, Keyel PA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Leary JG, Goodarzi M, Drayton DL, Yu H, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 9.Paust S, Gill HS, Wang B-Z, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumá M, Angulo A, Vilches C, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 11.Gumá M, Budt M, Sáez A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107(9):3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 12.Björkström NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208(1):13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petitdemange C, Becquart P, Wauquier N, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathogens. 2011;7(9):e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Verges S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57+NKG2C high natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108(36) doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan RP, Leong JW, Schneider SE, et al. MicroRNA deficient NK cells exhibit decreased survival but enhanced function. J Immunol. 2012;188(7):3019–3030. doi: 10.4049/jimmunol.1102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White DW, Keppel CR, Schneider SE, et al. Latent herpesvirus infection arms NK cells. Blood. 2010;115(22):4377–4383. doi: 10.1182/blood-2009-09-245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin MG, Welch JS, Uy GL, et al. Limited engraftment of low-risk myelodysplastic syndrome cells in nod/scid gamma-c chain knockout mice. Leukemia. 2010;24(9):1662–1664. doi: 10.1038/leu.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1-2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 21.Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Reports. 2009;10(10):1103–1110. doi: 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Mao HC, Wei M, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human nk-cell subsets. Blood. 2010;115(2):274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JC, Madera S, Bezman N a, et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209(5):947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freud AG, Caligiuri M a. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Vergès S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ nk-cell subset. Blood. 2010;116(19):3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Béziat V, Descours B, Parizot C, Debré P, Vieillard V. Nk cell terminal differentiation: correlated stepwise decrease of nkg2a and acquisition of kirs. PloS One. 2010;5(8):e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of cd56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 28.Young HR. Regulation of interferon-gamma gene expression. J Interferon Cytokine Res. 1996;16(8):563–568. doi: 10.1089/jir.1996.16.563. [DOI] [PubMed] [Google Scholar]
- 29.Ma F, Xu S, Liu X, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 30.Ljunggren H-G, Malmberg K-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7(5):329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 31.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 32.Gill S, Vasey AE, De Souza A, et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood. 2012;119(24):5758–5768. doi: 10.1182/blood-2012-03-415364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.