Abstract
CD27+ memory B cells are reduced in the blood of patients with chronic granulomatous disease (CGD) for reasons and consequences that remain unclear. Here we confirm not only decreased CD27+ but also IgG+ B cells in the blood of CGD patients compared with healthy donors (HDs). However, among IgG+ B cells, the ratio of CD27− to CD27+ was significantly higher in CGD patients compared with HDs. Similar to conventional memory B cells, CD27−IgG+ B cells of CGD patients expressed activation markers and had undergone somatic hypermutation, albeit at levels lower than their CD27+ counterparts. Functional analyses revealed slight reductions in frequencies of total IgG but not influenza-specific memory B-cell responses, as measured by Elispot in CGD patients compared with HDs. Serum IgG levels and influenza-specific antibodies were also normal in these CGD patients. Finally, we provide evidence that influenza-specific memory B cells can be present within the CD27−IgG+ B-cell compartment. Together, these findings show that, despite reduced circulating CD27+ memory B cells, CGD patients maintain an intact humoral immunologic memory, with potential contribution from CD27− B cells.
Introduction
Chronic granulomatous disease (CGD) is an inherited immunodeficiency of the phagocyte NADPH oxidase system leading to recurrent life-threatening infections and dysregulated inflammation, causing granulomatous manifestations affecting various tissues and organs.1 CGD primarily affects innate immunity, although the adaptive arm is also dysregulated, including reduced frequencies of CD27-expressing memory B cells in the peripheral blood.2 The underlying causes and consequences of this deficiency remain uncertain, especially given that both hypergammaglobulinemia and hypogammaglobulinemia have been reported in CGD.3–5
Humoral immunologic memory is maintained by antibody-secreting plasma cells in the bone marrow and by memory B cells that reside primarily in secondary lymphoid tissues.6 CD27 is a convenient marker for identifying memory B cells in humans; however, a more accurate definition of a memory B cell is one that has undergone Ig somatic hypermutation (SHM) with/without class switching after encounter with antigen. Several studies have shown that mutated or class-switched Igs can be found among CD27− B cells.7,8 The induction of IgG-secreting cells from within the pool of CD27− B cells was first shown by Elispot, although levels were approximately 10-fold lower compared with CD27+ counterparts, and it is possible that class-switching had been induced in vitro.9 More recently, IgG+/CD27− B cells were identified and characterized by more direct methods that revealed a level of SHM that was higher than in IgG−/CD27− but lower than in IgG+/CD27+ B cells.10,11
In the present study, we investigated memory B cells in the blood of CGD patients and evaluated their functional capabilities. Our findings demonstrate that, although the overall number of peripheral blood memory B cells is reduced in CGD patients compared with controls, both CD27− and CD27+ memory B cells exist and contribute to memory responses against common pathogens, such as influenza.
Methods
Samples
Blood was obtained after informed consent per the Declaration of Helsinki in accordance with the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (protocol National Institutes of Health #05-I-0213).
Cell preparations, phenotyping, and functional analyses
PBMCs, B cells, and fractionated B-cell subsets were generated and immunophenotyped as previously described.12 The following fluorochrome-conjugated monoclonal antibodies were used to stain and sort B cells: allophycocyanin (APC) anti-CD10, APC-H7 anti-CD20, PE-Cy5 anti-CD80, and PE anti-IgG (BD Biosciences); peridinin chlorophyll protein-Cy5.5 anti-CD19 and PE-Cy7 anti-CD27 (eBioscience); FITC anti-CD21 (Beckman Coulter); FITC anti-IgA (Dako North America); and FITC anti-CD95, APC-IgM, V421 anti-CD27, PE-Cy7 anti-IgD, and PE anti-TACI (BioLegend). Cell sorting and immunophenotyping were performed on BD FACSAria II and BD FACSCanto II (BD Biosciences) flow cytometers, respectively. Analyses were performed with FlowJo Version 9.5.3 software (TreeStar). For functional assays, CGD and healthy donors (HDs) were matched for age and either time after influenza vaccination or month/year if vaccination record was not known. In vitro differentiation with polyclonal B-cell stimuli and ELISPOT were performed as previously described.12 For functional analyses of B-cell subsets, B cells were isolated by negative selection and fractionated by CD27 as previously described.12
Influenza microneutralization assay
Virus-neutralizing titers of postvaccination human sera were determined in a microneutralization assay based on the methods of the pandemic influenza reference laboratories of the Centers for Disease Control and Prevention.13 Low pathogenicity vaccine viruses were used in the assay. The X-179A virus is a 5:3 reassortant vaccine containing the HA, NA, and PB1 genes from A/California/07/2009 (H1N1pdm09) and the 5 other genes from A/PR/8/34 were donated by the high growth virus NYMC X-157. Internal controls in all assays were sheep sera generated against the corresponding strains at CBER, FDA. All individual sera were serially diluted (2-fold dilutions starting at 1:10) and were assayed against 100 TCID50 of each strain in duplicates in 96-well plates (1:1 mixtures). The titers represent the highest dilution that completely suppressed virus replication.
Ig gene sequence analysis
IgG+ B cells (CD19+/CD20+/IgD−/CD21hi/CD10−) were sorted into CD27+ and CD27− fractions. Total RNA was extracted from each subset using RNeasy Micro kit (QIAGEN) and reverse-transcribed with qScript cDNA SuperMix (Quanta BioSciences). Recombined IGH, IGK, and IGL genes were PCR-amplified as described previously using VH-leader, Vκ, and Vλ consensus forward primers with corresponding CH, Cκ, and Cλ reverse primers.14–16 PCR products were cloned into TA cloning vector (Life Technologies) and sequenced using M13 forward and reverse primers. Sequences were analyzed with IgBLAST (http://www.ncbi.nlm.nih.gov/igblast/) and JOINSOLVER (http://joinsolver.niaid.nih.gov) programs, as described previously.11,16,17
Statistics
Median values were compared by Mann-Whitney or Wilcoxon matched-pairs signed rank test where appropriate, using Prism Version 6 (GraphPad). Three-way analyses were compared simultaneously by either Kruskal-Wallis or Friedman test and, if significant, prompted appropriate pairwise comparisons.
Results
Phenotypic analyses of memory B cells in CGD and HD
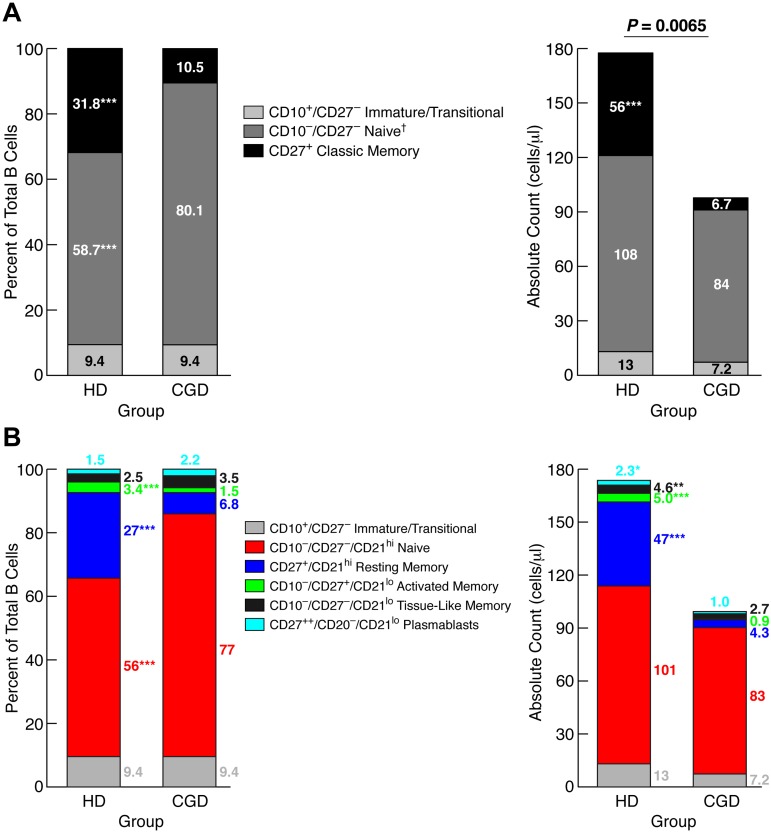
Reduced frequencies of CD27+ memory B cells were previously reported in the blood of CGD patients.2 Given more recent evidence of additional memory B-cell subsets,11,18 we revisited the question of paucity of circulating memory B cells in CGD patients. Comparison of 28 CGD patients to sex- and age-matched HDs revealed a significantly lower CD19+ B-cell count in the CGD group (Table 1). This difference was the result, at least in part, of immune-suppressive therapies in the CGD group because exclusion of CGD patients taking doses of prednisone > 0.1 mg/kg per day or methotrexate from the analysis reduced the difference to nonsignificant (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Three immunophenotypic subsets were studied in each group: immature/transitional (CD10+/CD27−), naive (CD10−/CD27−), and classic memory (CD27+) B cells. Immature/transitional B-cell counts and percentages (of total B cells) were similar between the 2 groups; however, classic memory B-cell counts and percentages were significantly higher in HDs, whereas naive B-cell frequencies were significantly higher in CGD, although the total counts for this subset were not different (Figure 1A). These data confirm that CGD is associated with reduced CD27+ memory B cells, but the normal counts for nonmemory B cells suggest that this particular reduction is not the result of B-cell lymphopenia.
Table 1.
Participant profiles
| Patient no. | Sex | Age, y | Mutated gene | IgG, mg/dL | WBC, ×103/μL | % lymphocytes | % B cells | B-cell count/μL | Immune-modulating medication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 19 | gp91phox | 1120 | 4.9 | 15.0 | 12.1 | 89 | Prednisone < 0.1‡ |
| 2 | M | 29 | gp91phox | 1210 | 3.9 | 22.9 | 14.0 | 123 | — |
| 3 | M | 28 | p47phox | 1720 | 8.0 | 12.5 | 7.3 | 72 | — |
| 4 | M | 21 | p47phox | 951 | 24.7 | 9.3 | 3.4 | 77 | Prednisone 0.3 |
| 5 | M | 21 | gp91phox | 638 | 5.0 | 17.4 | 24.0 | 209 | Prednisone < 0.1 |
| 6 | M | 25 | gp91phox | 735 | 3.6 | 1.0 | 10.3 | 4 | Prednisone < 0.1 |
| 7 | M | 20 | gp91phox | 1120 | 11.0 | 17.5 | 9.2 | 177 | Prednisone < 0.1; Humira |
| 8 | M | 23 | gp91phox | 893 | 5.5 | 11.0 | 4.7 | 28 | Prednisone 0.2 |
| 9 | M | 23 | gp91phox | 1340 | 4.4 | 32.0 | 21.1 | 295 | Humira |
| 10 | M | 30 | p47phox | 1240 | 8.0 | 17.4 | 3.6 | 49 | Prednisone < 0.1 |
| 11 | M | 28 | gp91phox | 1250 | 5.6 | 34.1 | 1.6 | 31 | Humira |
| 12 | F | 38 | p47phox | 836 | 6.5 | 2.8 | 3.8 | 7 | Prednisone 0.5 |
| 13 | M | 22 | p47phox | 1230 | 9.1 | 12.3 | 6.8 | 76 | Prednisone < 0.1; Humira |
| 14 | M | 22 | gp91phox | 1360 | 6.9 | 31.1 | 20.0 | 429 | — |
| 15 | M | 40 | gp91phox | 1690 | 9.9 | 15.4 | 14.5 | 221 | Prednisone < 0.1 |
| 16 | M | 22 | gp91phox | 1690 | 7.9 | 24.5 | 5.7 | 109 | Prednisone < 0.1 |
| 17 | M | 23 | gp91phox | 1350 | 4.5 | 27.4 | 14.2 | 176 | Prednisone < 0.1 |
| 18 | M | 42 | p47phox | 1280 | 5.9 | 46.0 | 7.0 | 190 | Prednisone < 0.1 |
| 19 | M | 18 | p22phox | 1110 | 6.4 | 13.1 | 3.4 | 28 | Prednisone 0.2 |
| 20 | M | 41 | gp91phox | 2000 | 6.6 | 17.2 | 12.7 | 145 | — |
| 21 | M | 20 | gp91phox | 774 | 6.0 | 5.4 | 12.7 | 41 | Prednisone < 0.1 |
| 22 | F | 32 | p47phox | 1740 | 6.3 | 24.8 | 16.0 | 250 | — |
| 23 | F | 28 | p47phox | 1390 | 6.7 | 18.9 | 10.9 | 138 | Methotrexate |
| 24 | M | 21 | p47phox | 1660 | 4.8 | 18.4 | 1.8 | 16 | Prednisone 0.3 |
| 25 | M | 19 | gp91phox | 737 | 6.1 | 20.5 | 4.0 | 50 | Prednisone < 0.1 |
| 26 | M | 41 | gp91phox | 2070 | 9.2 | 9.0 | 24.5 | 202 | Prednisone 1.0 |
| 27 | M | 25 | gp91phox | 1170 | 8.2 | 9.9 | 13.0 | 106 | — |
| 28 | M | 25 | p47phox | 1830 | 4.3 | 33.0 | 28.5 | 404 | — |
| Median | 25M/3F | 24 | 1245 | 6.3 | 17.4† | 10.6 | 107† | — | |
| Range | 18-42 | 638-2070 | 3.6-24.7 | 1.0-46.0 | 1.6-28.5 | 3.5-429 | — | ||
| Controls (N = 21) | |||||||||
| Median | 16M/5F | 27 | 5.8 | 28.6† | 9.9 | 186† | — | ||
| Range | 22-42 | 642-1730* | 3.7-10.9 | 20.6-47.1 | 4.3-16.0 | 57-386 | — |
— indicates not applicable or no medication.
Normal range in clinic.
Significant difference between groups.
Prednisone expressed in milligrams/kilogram per day.
Figure 1.
Reduced memory B cells in blood of CGD patients. PBMCs of CGD patients and HDs were stained with antibodies against CD3, CD10, CD19, CD21, and CD27 for FACS analysis. Gating on CD19+/CD3− cells established the B-cell population. The mean percentages of each B-cell subset, based on a (A) 3- or (B) 5-subset definition, were calculated within the B-cell population (left panel), and white blood cell counts and percent lymphocyte from complete blood counts were used to calculate absolute counts for each subset (right panel). *P < .05. **P < .001. ***P < .0001. Horizontal bars represent medians. †Subset also includes tissue-like (CD21lo/CD27−) memory B cells, but these B cells represented < 3% of total B cells for both groups.
Several CD27− memory B-cell subsets have been identified, albeit at low frequencies in HDs.18 Based on a five-subset definition of mature (CD10−) B-cell subsets,12 increased resting (CD21hi/CD27+), activated (CD21lo/CD27+), tissue-like (CD21lo/CD27−) memory, and plasmablast (CD20−/CD21lo/CD27++) but not naive (CD21hi/CD27−) B-cell counts were observed in HDs compared with CGD patients (Figure 1B). These data further demonstrate the paucity of memory and terminally differentiated B cells in CGD patients; however, there was no increase in CD21lo subsets that have been reported in other B-cell immunodeficiencies.19,20
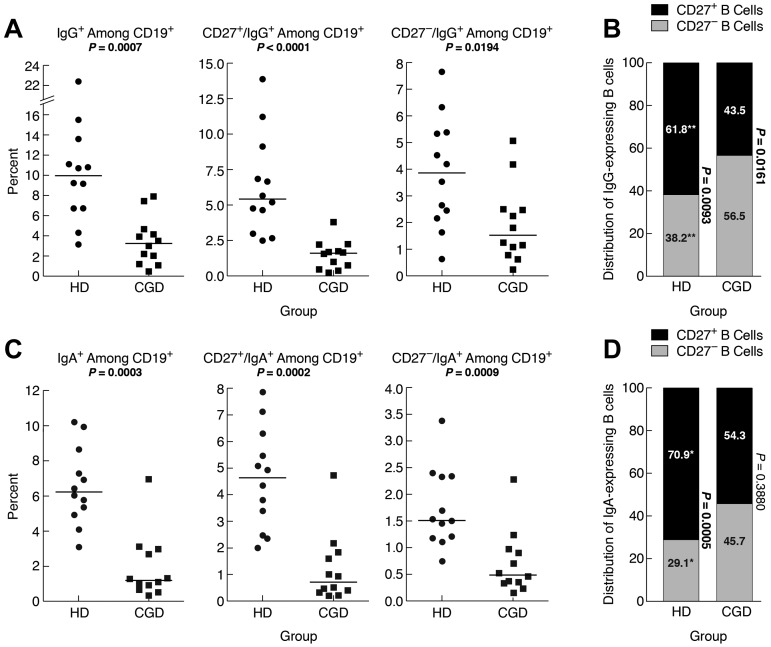
IgG-expressing memory CD27− B cells have been reported in the blood of HDs.10 Here, the frequency of IgG+ B cells was significantly higher in HDs compared with CGD patients, and this difference extended to CD27+ and CD27− B cells (Figure 2A). However, among IgG+ B cells, there was a significantly higher frequency of CD27− compared with CD27+ B cells in CGD patients, whereas the reverse was seen in HDs, and these frequencies were significantly different between the 2 groups (Figure 2B). Thus, although frequencies of CD27+ and IgG+ memory B cells are reduced in CGD patients, a significant proportion of IgG+ B cells belong to the CD27− B-cell compartment.
Figure 2.
Reduced IgG and IgA memory B cells in blood of CGD patients. PBMCs of samples used in the functional analyses in Figure 3 were stained for expression of (A) IgG or (C) IgA among CD19+ (left panel), CD27+ (middle panel), and CD27− (right panel) B cells. (B) IgG- and (D) IgA-expressing B cells among PBMCs in panels A and C were divided into CD27+ and CD27− compartments and shown as means for each group. The P values were determined and shown as follows for stacked data. *P < .05. **P < .001. ***P < .0001. (B,D) The numerical P values refer to the comparison between CD27− and CD27+ B cells within each group.
IgA-expressing CD27− B cells have also been identified in the blood of HDs.11 Here, IgA-expressing B cells in HDs represented ∼ 6% of all B cells (Figure 2C), and 71% of these IgA-expressing B cells coexpressed CD27 (Figure 2D), compared with 62% for IgG-expressing B cells (Figure 2B). In CGD patients, IgA-expressing B cells represented 1.2% of all B cells, significantly less than in HDs (Figure 2C). Furthermore, unlike in HDs, the IgA expression among CD27− and CD27+ B cells was not significantly different in the CGD group (Figure 2D). This is also in contrast to the higher proportion in CGD patients of IgG-expressing B cells within the CD27− B-cell compartment (compare Figure 2B and D). Thus, very few B cells in the blood of CGD patients express IgA; and among these, CD27 expression is not a distinguishing feature.
Finally, we considered whether the type of mutation in NADPH oxidase carried by the CGD patients influenced the B-cell profiles described in Figures 1 and 2. As shown in Table 1, the most common mutation was in the NADPH oxidase subunit gp91phox (17 of 28 patients; or 61%), followed by p47phox (10 of 28 patients; or 36%), and 1 patient had a mutation in p22phox. When profiles of patients with the gp91phox mutation were compared with those with the p47phox mutation, we found the percent but not absolute counts of classic memory (CD27+), including both resting and activated memory B cells, to be significantly lower in the gp91phox group (supplemental Figure 2). When the 2 CGD groups were each compared with HDs, frequencies of classic and resting memory B cells remained significantly lower for both CGD groups, whereas frequencies of activated memory B cells were significantly different between HDs and the gp91phox but not p47phox mutation CGD group (supplemental Figure 2). No other properties were found to be significantly different, although there were only 3 p47phox CGD patients in the analyses relating to IgG and IgA expressing B cells shown in Figure 2. Thus, the gp91phox mutation appears to be associated with a lower frequency of CD27-expressing memory B cells, although the relevance of this difference is unclear given that CD27+ B-cell counts were not affected by the type of mutation.
Expression of activation markers on memory B cells of CGD patients
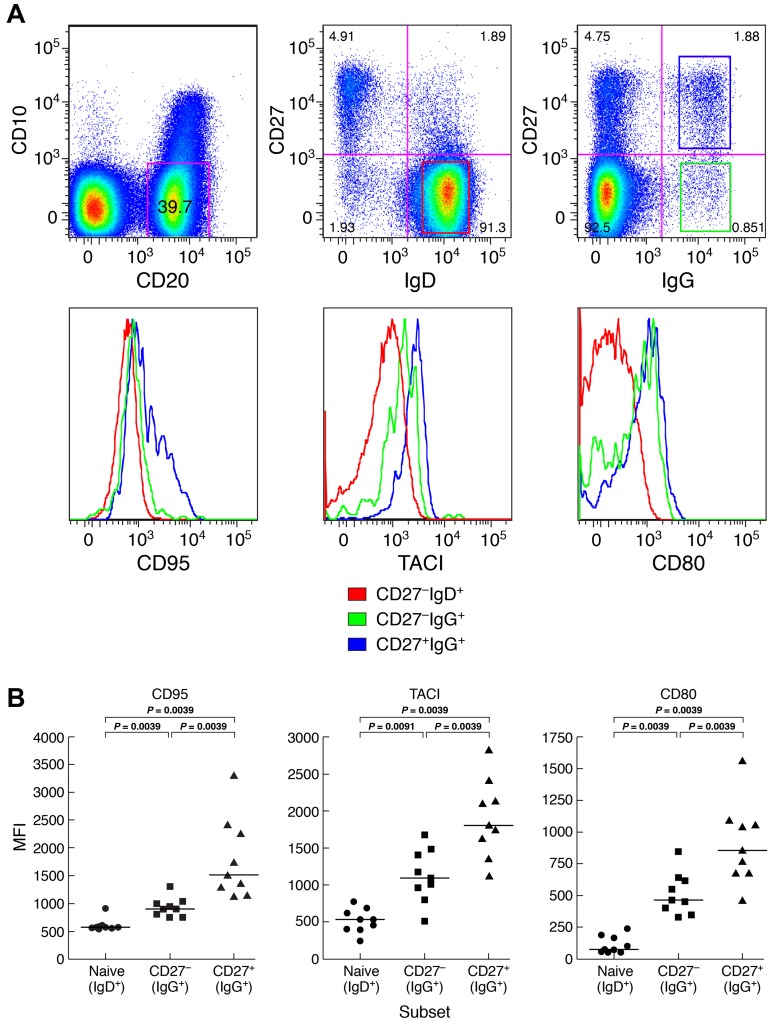
In addition to Ig class-switching, memory B cells can also be distinguished from their naive counterparts by an increased expression of certain activation markers, including members of the B7 (CD80/CD86) and TNF receptor (TACI/CD95) superfamilies.21–23 In a recent study of several memory B-cell subsets in HDs, Ig class-switched CD27+ B cells expressed the highest levels of activation markers and Ig class-switched CD27− B cells expressed levels of activation markers that were intermediate between their CD27+ counterpart and naive B cells.11 Here, we observed a similar pattern for CGD patients; expression levels of activation markers CD80, CD95, and TACI were lowest on naive, intermediate on CD27−IgG+, and highest on CD27+IgG+ B cells (Figure 3).
Figure 3.
Distinct profiles of activation marker expression on memory CD27−IgG+ B cells of CGD patients. (A) Representative and (B) comparisons of mean fluorescence intensity (MFI) profiles of expression for activation markers CD80, TACI, and CD95 were obtained from PBMCs of 9 CGD patients. Only persons with very low frequencies of CD21lo B cells were included. Gating on CD20+/CD10− cells established the B-cell population, and expression of IgD, IgG, and CD27 was used to establish naive (IgD+CD27−), as well as IgG+ CD27− and CD27+ memory B cells. Horizontal bars represent medians.
SHM analyses of memory B cells
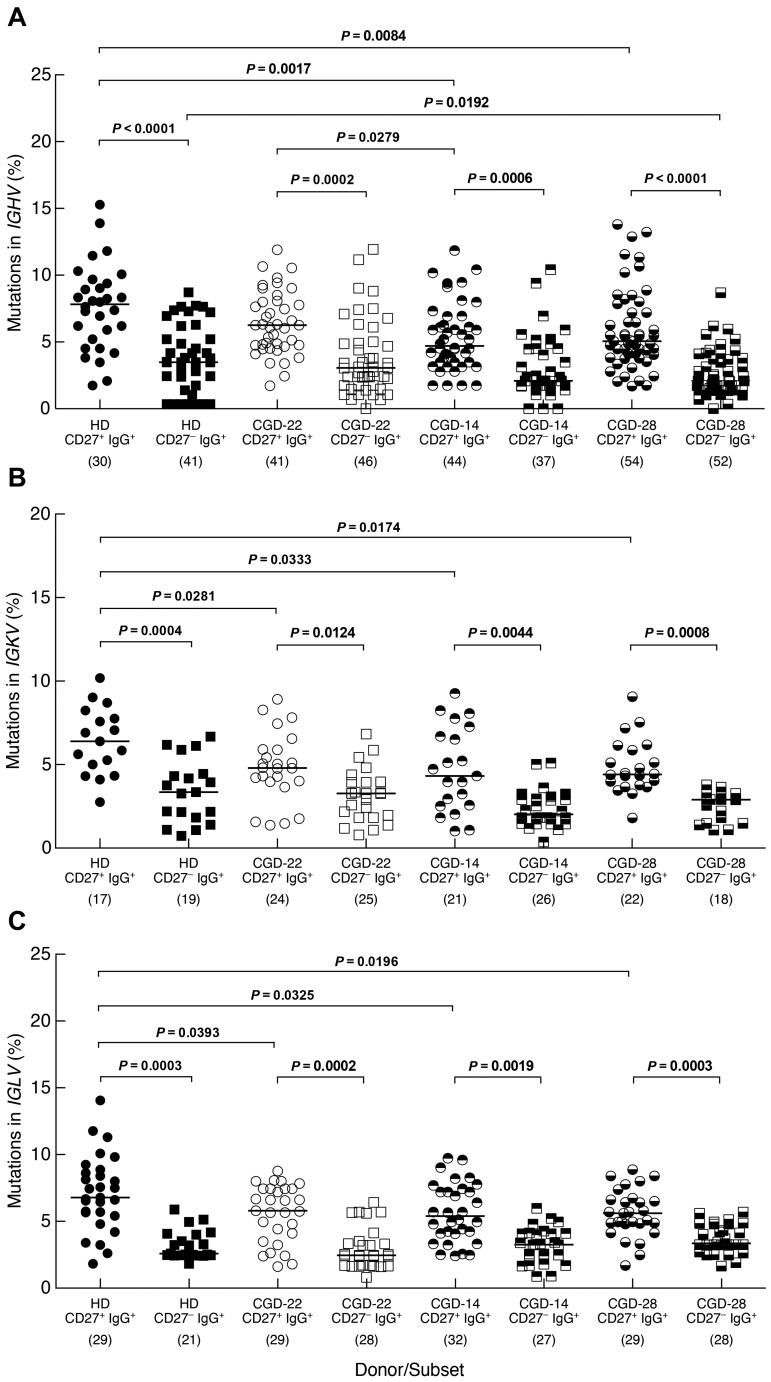
The expression of activation markers on CD27−IgG+ B cells of CGD patients further suggested that these Ig class-switched cells were memory B cells. To formally address their memory status, we investigated levels of SHM in 3 CGD patients and one HD. Similar to findings of a recent study,11 frequencies of SHM in the IGHV genes were significantly lower among CD27−IgG+ B cells of HDs compared with their CD27+ counterpart (Figure 4A). Similar differences were observed for the IGHV genes of the 3 CGD patients. In addition, lower SHM frequencies were found in the CD27+ subset of 2 CGD patients (CGD-14 and CGD-28) compared with HDs, although this could be the result of donor-to-donor variability considering that patient CGD-22 also had a higher mutation frequency in the CD27+ subset compared with patient CGD-14. In the CD27− subset, one CGD patient (CGD-28) had a significantly lower SHM frequency compared with HDs (Figure 4A). Features, including length of IGH-CDR3, the ratio of replacement to silent mutations and charge, were also evaluated but not found to be different either between the 2 IgG+ subsets or among the 4 persons studied (data not shown). SHM in the IgG light chain genes was also assessed; differences in frequencies similar to those in the IGHV genes were found between CD27−IgG+ and CD27+IgG+ B cells for IGKV and IGLV genes of CGD patients and HDs (Figure 4B-C). In addition, whereas SHM frequencies of both light chains were significantly lower in the CD27+ subsets of the 3 CGD patients compared with HDs, there were no differences between CGD and HD for the CD27− subset. Thus, both the phenotypic and genotypic analyses of CD27−IgG+ B cells suggest that these cells have encountered antigen; and although this subset appears distinct from CD27+IgG+ memory B cells, there are few differences regarding SHM within this subset between CGD and HD.
Figure 4.
Distinct SHM profiles for memory CD27−IgG+ and CD27+IgG+ B cells. The CD19+/CD3− cells of PBMCs from one HD and 3 CGD patients were sorted into CD27−IgG+ and CD27+IgG+ fractions and mutation frequencies were calculated for (A) IGHV, (B) IGKV, and (C) IGLV genes of each fraction. The number of clones sequenced from each subset/donor is shown in parentheses below the graphs. Numbers in the CGD identifiers below the graphs correspond to the patients studied (Table 1). Horizontal bars represent medians.
Functional attributes of memory B cells
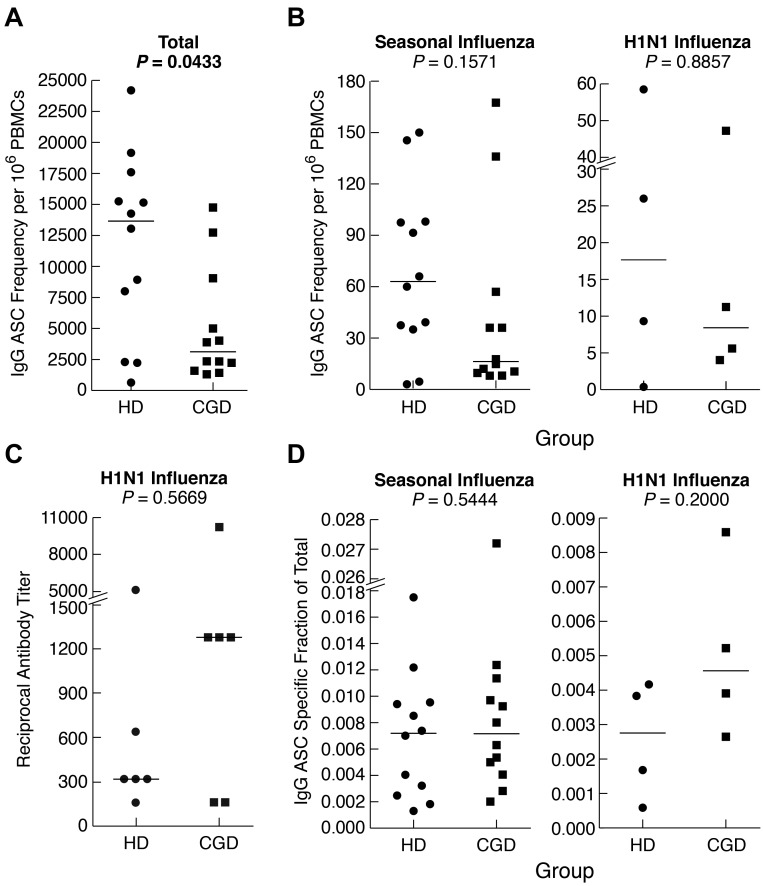
Finally, we measured memory B-cell function after in vitro differentiation into antibody-secreting cells (ASCs). Consistent with the paucity of immunophenotyped memory B cells, the frequency of IgG-ASCs was significantly lower among PBMCs of CGD patients compared with HDs (Figure 5A). However, the difference was not as pronounced as in the phenotypic analyses and was not significant (P = .06) when ASCs were reported per B cell (supplemental Figure 3A). This is consistent with normal serologic IgG levels observed in the patients studied here (Table 1) and in 82% (88 of 107) of our larger CGD cohort, many of whom have maintained a narrow stable range of serum IgG levels > 10 years (data not shown). Furthermore, frequencies of seasonal influenza-specific ASCs were not significantly different between the 2 groups, whether reported per PBMC (Figure 5B) or B cell (supplemental Figure 3B). Six persons in each group had received the H1N1–2009/10 vaccine, and cells were available on 4 persons at 2 months after vaccination. As shown in Figure 5B and supplemental Figure 3C, the memory B-cell responses were similar in both groups. In addition, there was no difference in H1N1-specific serum antibody neutralizing titers between the 2 groups (Figure 5C). Finally, when the antigen-specific responses were reported as a fraction of the total IgG response, there was no difference between the 2 groups for both seasonal and H1N1 vaccines (Figure 5D). Collectively, these data indicate that, despite slightly reduced total IgG ASC frequencies in CGD patients, their memory response to a specific antigen, namely, influenza, was normal.
Figure 5.
Reduced total but normal antigen-specific antibodies and IgG memory B-cell responses in CGD patients. PBMCs or CD27-fractionated B cells were cultured for 4 days with SAC and CpG and evaluated for IgG ASC frequencies by Elispot using various antigens to capture ASCs and detection with antihuman IgG. (A) Total IgG ASC frequencies were measured by coating wells with antihuman λ and κ light chain antibodies. (B) IgG ASC frequencies specific for seasonal (left panel) or H1N1 (right panel) were measured by coating wells with respective influenza vaccine preparations. (C) H1N1 influenza-specific serum antibody titers were measured by microneutralization assay. (D) Influenza-specific ASCs were calculated and reported as a fraction of the total ASC response shown in panel A. Horizontal bars represent medians.
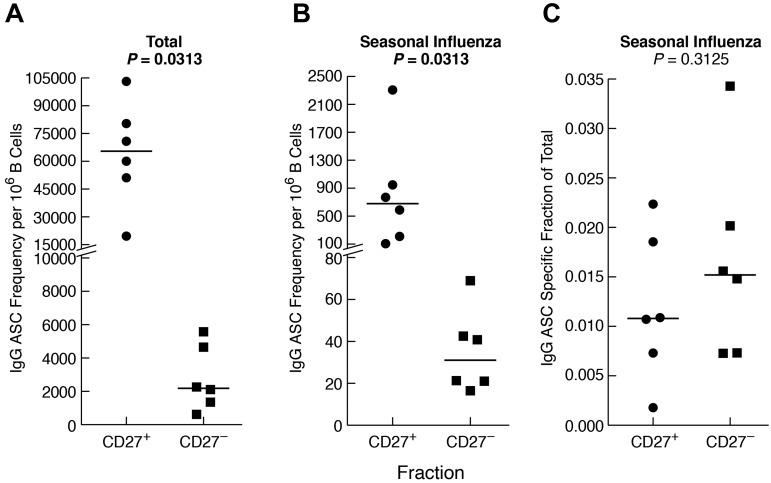
Given the comparatively high frequency of IgG+ B cells that were CD27− in CGD patients and, to a lesser extent, HDs (Figure 2B), we considered the contribution of CD27− B cells to the influenza-specific IgG memory response (Figure 5B). Sufficient quantities of cells to perform B-cell isolation and separation into CD27− and CD27+ fractions could only be obtained from HDs. As anticipated, total (Figure 6A), as well as influenza-specific IgG (Figure 6B), ASC frequencies were higher in the CD27+ compared with the CD27− B-cell fraction. Nonetheless, influenza-specific IgG-ASCs were detected in the CD27− fraction, and the influenza-specific relative to total IgG response was similar for the 2 compartments (Figure 6C). In vitro induced IgG class-switching was minimal, as evidenced by the absence of influenza-specific IgG-ASCs in the CD27− fraction after preculture IgG depletion (data not shown). Furthermore, contamination of the CD27− fraction with CD27+ cells was excluded by immunophenotyping and by a markedly lower ratio of IgA/IgG ASCs in the CD27− compared with the CD27+ fraction (data not shown). Collectively, these data indicate that influenza-specific IgG+ B cells are present among nonclassic CD27− memory B cells.
Figure 6.
Influenza-specific IgG memory B-cell response is present among CD27− B cells. B cells were isolated from the PBMCs of 6 HDs and fractionated by CD27 using magnetic bead-based positive and negative selection. The fractions were cultured for 4 days, and IgG-ASCs were measured for each fraction as follows: (A) total, (B) seasonal influenza-specific, and (C) seasonal influenza-specific as a fraction of total. Horizontal bars represent medians.
Discussion
In this study, we investigated phenotypic and functional characteristics of memory B cells in the blood of CGD patients and compared them with those of HDs. Consistent with a previous report,2 we found significantly fewer memory B cells in the blood of CGD patients compared with HDs. However, there was no evidence of a corresponding functional B-cell defect in CGD patients as they had normal IgG antibody and memory B-cell responses to influenza, a common pathogen that typically induces humoral immunity with CD4+ T-cell help.24 Furthermore, we found evidence that nonconventional CD27− memory B cells, which were over-represented in CGD patients, may contribute to influenza-specific B-cell responses. This could have contributed to the observation that CGD patients had normal overall antibody responses to influenza.
Previous studies of HDs have shown the presence of Ig class-switched B cells having undergone SHM among CD27− B cells.10,11 In addition, the capacity to undergo terminal differentiation and secrete IgG antibodies in vitro has been previously reported among CD27− B cells, although in vitro class-switching was not formally excluded and few antigen-specific B cells, in this case anthrax, were detected within this subset.9 Here, we found evidence of influenza-specific responses within the CD27− memory B-cell compartment of HDs. Whether influenza and anthrax elicit similar memory B-cell pathways remains to be determined; however, distinct memory B-cell pathways have been proposed to explain the distinct B-cell subsets.25 In this regard, it has been suggested that CD27+IgG+ B cells have high SHM frequencies because they define B cells that have accumulated mutations through repeated germinal center reactions.11 Such a phenomenon has been put forth to explain the highly mutated antibodies that have been cloned from HIV-infected persons,26,27 as well as persons who were infected with the pandemic H1N1 influenza virus.28 Although this would suggest that the influenza-specific response within the CD27−IgG+ B-cell subset would not be as effective as from its CD27+ counterpart, it is also thought that new specificities originating from the recruitment of naive B cells against influenza and other pathogens are also important for protection.24,25 Furthermore, the wide spectrum of SHM frequencies reported here suggest heterogeneity within subsets that could reflect a wide range of antigenic specificities. Of note, the influenza-specific antibodies of CGD patients were as effective in neutralization as those of the HDs studied (Figure 5C); furthermore, surface plasmon resonance analyses also revealed similar binding affinities for the 2 groups studied (H.G. and S.K., unpublished findings, July 2012). Although the relationship between memory B cells and the plasma cells from which serum antibodies originate remains unclear, there is evidence for similar antigenic specificities between these 2 compartments.27,29 Finally, given the genotypic and phenotypic similarities between CGD and HD CD27−IgG+ B cells described here and for HDs elsewhere,11 there is reason to believe that these similarities may extend to functional properties as well. Therefore, as shown in the HDs, CD27−IgG+ B cells may contribute to pathogen-specific B-cell responses in CGD, even though difficulties in isolating a rare population (IgG+ B cells) within the CD27− fraction (typically ≥ 95% B cells in CGD are CD27−) limited our ability to directly verify the contribution of CD27−IgG+ B cells to the influenza-specific response of these patients.
In conclusion, CGD patients mount adequate antigen-specific antibody and memory B-cell responses, as evidenced by the normal frequencies of influenza-specific IgG memory responses and serum antibodies. One explanation for an intact humoral immunologic memory compartment despite profoundly reduced CD27+ B-cell counts is the presence of IgG+ B cells among CD27− B cells. Whether induction of CD27− and CD27+ memory B cells after encounter with antigen occurs via distinct pathways or whether CD27 expression is modulated differently in CGD patients remains unknown. Clinical manifestations and oxidative deficiencies may also alter B-cell differentiation in CGD patients and/or the traffic of B cells between blood and tissues. Regardless of these unknowns, our findings suggest that nonclassic memory B cells probably contribute to host immunologic memory and may be more important in certain disease settings, such as CGD.
Supplementary Material
Acknowledgments
The authors thank the patients and healthy volunteers for their participation and the National Institute of Allergy and Infectious Diseases (NIAID) clinic teams for help in patient care and blood sample collection.
This work was supported by the Intramural Research Program of NIAID, National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.M. and S.S.D.R. designed the study and wrote the manuscript; S.S.D.R. and H.L.M. provided clinical care; J.H. and H.G. designed the assays; B.H.S., J.Y.K., J.G.P., C.M.B., W.W., J.M., L.R.K., S.K., and L.K. performed the experiments, analyzed the data, and reviewed the manuscript; M.G. and B.E.M. collected samples and analyzed data; T.-W.C. contributed samples and advice and reviewed the manuscript; and A.S.F. and H.L.M. helped design the study and edit the manuscript.
Conflict-of-interest disclosure: All authors are US government employees and declare no competing financial interests.
Correspondence: Suk See De Ravin, Laboratory of Host Defenses, NIAID, NIH, 10/CRC/5W-3816, 10 Center Dr, Bethesda, MD 20892; e-mail: sderavin@niaid.nih.gov; and Susan Moir, Laboratory of Immunoregulation, NIAID, NIH, 10/6A02, 10 Center Dr, Bethesda, MD 20892; e-mail: smoir@niaid.nih.gov.
References
- 1.Kang EM, Marciano BE, DeRavin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127(6):1319–1326. doi: 10.1016/j.jaci.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleesing JJ, Souto-Carneiro MM, Savage WJ, et al. Patients with chronic granulomatous disease have a reduced peripheral blood memory B cell compartment. J Immunol. 2006;176(11):7096–7103. doi: 10.4049/jimmunol.176.11.7096. [DOI] [PubMed] [Google Scholar]
- 3.Carnide EG, Jacob CA, Castro AM, Pastorino AC. Clinical and laboratory aspects of chronic granulomatous disease in description of eighteen patients. Pediatr Allergy Immunol. 2005;16(1):5–9. doi: 10.1111/j.1399-3038.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 4.Seger RA. Modern management of chronic granulomatous disease. Br J Haematol. 2008;140(3):255–266. doi: 10.1111/j.1365-2141.2007.06880.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanoglu D, Ozgur TT, Ayvaz D, Koker MY, Sanal O. Chronic granulomatous disease presenting with hypogammaglobulinemia. J Investig Allergol Clin Immunol. 2011;21(4):310–312. [PubMed] [Google Scholar]
- 6.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–150. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 7.Jackson SM, Wilson PC, James JA, Capra JD. Human B cell subsets. Adv Immunol. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- 8.Tangye SG, Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009;39(8):2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 9.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1):111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177(6):3728–3736. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- 11.Berkowska MA, Driessen GJ, Bikos V, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118(8):2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moir S, Buckner CM, Ho J, et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116(25):5571–5579. doi: 10.1182/blood-2010-05-285528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 14.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329(1):112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meffre E, Milili M, Blanco-Betancourt C, Antunes H, Nussenzweig MC, Schiff C. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J Clin Invest. 2001;108(6):879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meffre E, Davis E, Schiff C, et al. Circulating human B cells that express surrogate light chains and edited receptors. Nat Immunol. 2000;1(3):207–213. doi: 10.1038/79739. [DOI] [PubMed] [Google Scholar]
- 17.Souto-Carneiro MM, Longo NS, Russ DE, Sun HW, Lipsky PE. Characterization of the human Ig heavy chain antigen binding complementarity determining region 3 using a newly developed software algorithm, JOINSOLVER. J Immunol. 2004;172(11):6790–6802. doi: 10.4049/jimmunol.172.11.6790. [DOI] [PubMed] [Google Scholar]
- 18.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010;162(2):271–279. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YJ, de Bouteiller O, Arpin C, et al. Normal human IgD+IgM- germinal center B cells can express up to 80 mutations in the variable region of their IgD transcripts. Immunity. 1996;4(6):603–613. doi: 10.1016/s1074-7613(00)80486-0. [DOI] [PubMed] [Google Scholar]
- 22.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182(2):890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 23.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179(11):7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 24.Dormitzer PR, Galli G, Castellino F, et al. Influenza vaccine immunology. Immunol Rev. 2011;239(1):167–177. doi: 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 25.Good-Jacobson KL, Tarlinton DM. Multiple routes to B-cell memory. Int Immunol. 2012;24(7):403–408. doi: 10.1093/intimm/dxs050. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Zhou T, Zhu J, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109(23):9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.