Abstract
Platelets promote metastasis and angiogenesis, but their effect on tumor cell growth is uncertain. Here we report a direct proliferative effect of platelets on cancer cells both in vitro and in vivo. Incubation of platelets with ovarian cancer cells from murine (ID8 and 2C6) or human (SKOV3 and OVCAR5) origin increased cell proliferation. The proliferative effect of platelets was not dependent on direct contact with cancer cells, and preincubation of platelets with blocking antibodies against platelet adhesion molecules did not alter their effect on cancer cells. The proliferative effect of platelets was reduced by fixing platelets with paraformaldehyde, preincubating platelets with a TGF-β1–blocking antibody, or reducing expression of TGF-βR1 receptor on cancer cells with siRNA. Infusing platelets into mice with orthotopic ovarian tumors significantly increased the proliferation indices in these tumors. Ovarian cancer patients with thrombocytosis had higher tumor proliferation indices compared with patients with normal platelet counts.
Introduction
The association between malignant tumors and elevated platelet counts raises the possibility of a pathophysiologic interaction between platelets and cancer cells. Cancer cells activate and aggregate platelets.1–3 Conversely, platelets promote metastasis by protecting cancer cells against natural killer (NK) cells in the blood,4 by enhancing attachment of cancer cells to the blood vessel wall,5 by disrupting endothelial junctions,6 and by promoting angiogenesis through selective sequestering, transporting, and releasing of several growth factors.7–11 In murine models of metastasis, a deficiency in platelet adhesion molecules, such as P-selectin, glycoprotein Ibα (GPIbα), or GPIIIa, reduces the number of metastatic lesions.12–15 Recently, Labelle et al showed that TGF-β1 secreted from platelets enhances an epithelial to mesenchymal transition in cancer cells promoting metastasis via activation of TGF-β/Smad and NF-κB pathways.16
We have recently shown that reducing platelet counts decreased the size and number of tumor nodules in a murine model of orthotopic ovarian cancer.17 In the same study, we detected extravasation of platelets from tumor vasculatures and direct contact between platelets and cancer cells. In the current study, we investigated whether platelets directly affect tumor growth.
Methods
Coincubation of platelets with ovarian cancer cells
Gel-filtered murine or human platelets were isolated from whole blood.17 Twenty million resting or lysed platelets (prepared by 3 cycles of freeze-and-thaw) were incubated with cancer cells. In some experiments, platelets were isolated from C57BL/6 mice with syngeneic tumors induced 3-4 weeks after intraperitoneal injection of 1 × 106 ID8 murine ovarian cancer cells.17
A total of 50 000 murine ID8 or 2C6 and human SKOV3 or OVCA5 ovarian cancer cells were incubated with serum-free media overnight. In some experiments, cancer cells were transfected with TGF-βR1 siRNA using 2 μg siRNA and 3 μL of lipofectamine reagent (Invitrogen). Approximately 20 × 106 platelets were added to each well and incubated for 24 hours at 37°C. Murine cancer cells were incubated with murine platelets and human cancer cells with human platelets. In some experiments, different concentrations of blocking antibodies to GPIbα (Xia-B2, Emfret Analytics), P-selectin (RB40.34, BD Biosciences), TGF-β1 (N1C2, Gene Tex Inc); or eptifibatide (0.5μM, Merck); or aspirin (15 μg/mL, Sigma-Aldrich) were added to platelets before incubation with cancer cells. In control samples, appropriate buffer was added to the cells instead of platelets. To differentiate between the effect of direct contact between platelets and cells from an indirect paracrine effect, we seeded platelets either on cancer cells or on porous membranes (0.4 μm, PET membrane, BD Biosciences) separated from cells in a coculture system. To determine cell proliferation, we measured incorporation of fluorescence-conjugated EdU (5-ethynil-2′-deoxyuridine) to newly synthesized DNA according to the manufacturer's protocol (EdU-Click-it system, Invitrogen) using flow cytometry (EPICS XL 4-Color Cytometer, Beckman Coulter).
Ki67 immunostaining
All the studies conducted on human subjects and mice were according to the protocols approved by the Institutional Review Board and Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center. These studies were conducted in accordance with the Declaration of Helsinki. Ovarian cancer tissue specimens collected from 10 patients with thrombocytosis (platelet counts > 450 000/μL) and 10 patients with normal platelets counts (< 450,000/μL) were immunostained with polyclonal Ki67 antibody (1:200, Thermo Scientific) and counterstained with hematoxylin.
To study the effect of platelets on proliferation of ovarian cancer cells in orthotopic murine model of ovarian cancer, we injected 1 × 106 SKOV3 cancer cells into the peritoneum of 20 NU/NU nude mice (The Jackson Laboratory). Ten of these mice received 6 × 108 platelets through the tail vein once a week for 4 weeks starting at the day of inoculation of cancer cells. The other 10 mice received buffer infusion with the same frequency. At the end of the 4 weeks the mice were killed and their tumors were resected and immunostained with Ki67 antibody as described in “Methods.”
Statistics
Data throughout the manuscript are presented as mean ± SD. Comparisons were made using Student t test with P < .05 considered statistically significant.
Results and discussion
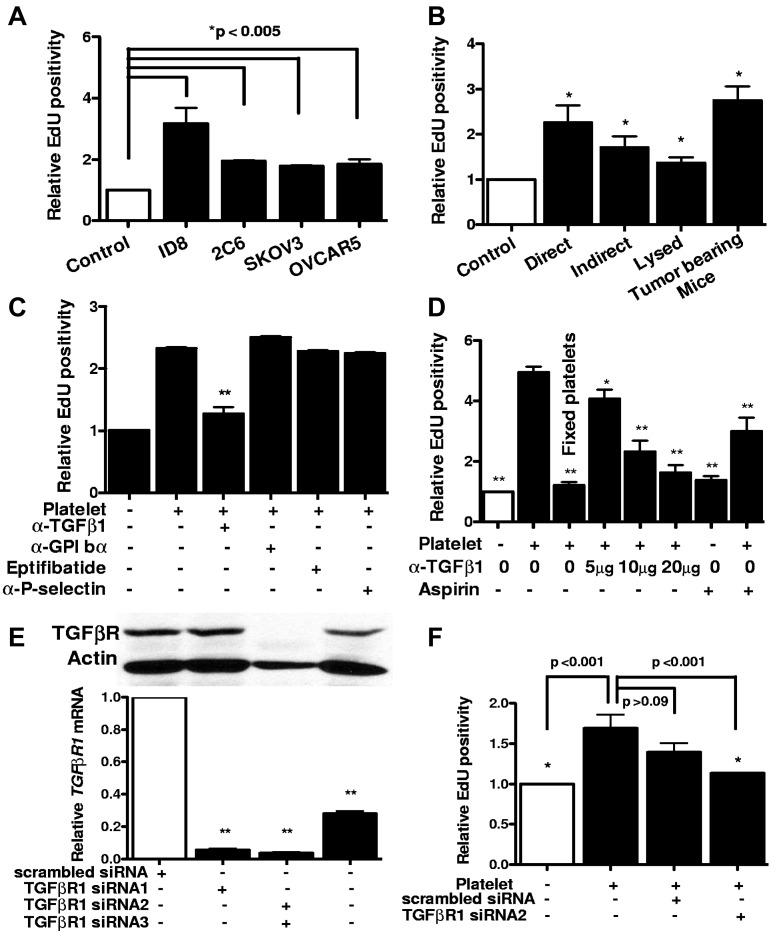
The proliferation rate of human and murine ovarian cancer cells increased significantly after coincubation with platelets (Figure 1A). This effect was platelet-specific, and red blood cells did not alter the proliferation rate (data not shown). Direct contact between platelets and cancer cells or an intact structure of platelets was not required for the proliferative response (Figure 1B). Furthermore, platelets isolated from tumor-bearing mice were similar to platelets from control mice in their proliferative effect (Figure 1B).
Figure 1.
Proliferative effect of platelet on ovarian cancer cells in vitro. (A) After incubation with platelets, the proliferation rate of murine (ID8 and 2C6) and human (SKOV3 and OVCAR5) ovarian cancer cells was assessed by measuring Edu incorporation. Results are normalized to Edu positivity among buffer-exposed cells. (B) Effect of manipulating platelets on proliferative response of cancer cells was investigated by either directly incubating platelets on ID8 cells (direct) or separating them using a porous membrane (indirect). Lysed platelets were prepared by repeated applications of freeze and thaw and sonication. Platelets from tumor-bearing mice were isolated from moribund C57BL/6 female mice (n = 3) by IVC venipuncture 3-4 weeks after inoculation of ID8 cells. (C) Blocking antibodies against GPIbα (5 μg/mL), P-selectin (4 μg/mL), TGF-β1 (5 μg/mL), or GPIIb-IIIa blocking agent Eptifibatide (0.5 μM) or aspirin (15 μg/mL) was added to platelets before incubation with cancer cells, and after 24 hours Edu incorporation was measured. Results were normalized to cancer cells incubated with buffer alone. (D) Increasing concentrations of TGF-β1 blocking antibody and aspirin were used for blocking platelet-induced cancer cell proliferation. All results are compared with the second bar. (E) Knockdown of TGF-βR1 gene expression after transfection of SKOV3 human ovarian cancer cells with siRNA/liposome mixture (2 μg siRNA and 3 μL lipofectamine) was investigated by measuring protein and mRNA products, using Western blotting with anti–TGF-βR1 antibody (above) and real-time RT-PCR (below), respectively. (F) ID8 cells were transfected with either TGF-βR1 siRNA or scrambled siRNA before incubation with platelets. All results are compared with the second bar. The reduction in the proliferative response induced by scrambled siRNA was not statistically significant (P > .09). Each experiment was repeated at least 3 times in triplicates. The cumulative results are summarized as bar graphs. *P < .05 (t test). **P < .01 (t test).
Blocking platelet adhesive surface proteins (GPIbα, GPIIbIIIa, and P-selectin) did not diminish proliferative effect of platelets (Figure 1C), and aspirin only partially inhibited it (Figure 1D). This effect of aspirin might result from its role in reducing platelet secretion or inhibiting COX-2 in cancer cells. Fixation of platelets with paraformaldehyde (2%) completely eliminated the enhancing effect of platelets on proliferation of cancer cells (Figure 1D). The observation that the proliferative effect of platelets did not require direct contact with cancer cells and was not inhibited by blocking adhesion receptors led us to hypothesize that platelet effect on cancer cells mediated by their release of a growth factor.
Platelets are the main source of TGF-β1 in serum. In view of a recent study attributing much of the pro-metastatic effect of platelets to TGF-β1,16 we examined the effect of blocking antibody to TGF-β1 and identified a dose-dependent inhibition of the proliferative effect of platelets on ovarian cancer cells by blocking TGF-β1 (Figure 1C-D). TGF-βR1 is the signaling component of the TGF-β1 receptor complex, and knockdown of TGF-βR1 would be expected to reduce tumor cell responses to TGF-β1. Therefore, to confirm results obtained with the TGF β1-blocking antibody, we reduced expression of TGF-βR1 receptors on ovarian cancer cells using siRNA before their incubation with platelets (Figure 1E). Consistent with results observed after treatment with the TGF-β1-blocking antibody, TGF-βR1 siRNA reduced proliferation of ovarian cancer cells exposed to platelets (Figure 1F).
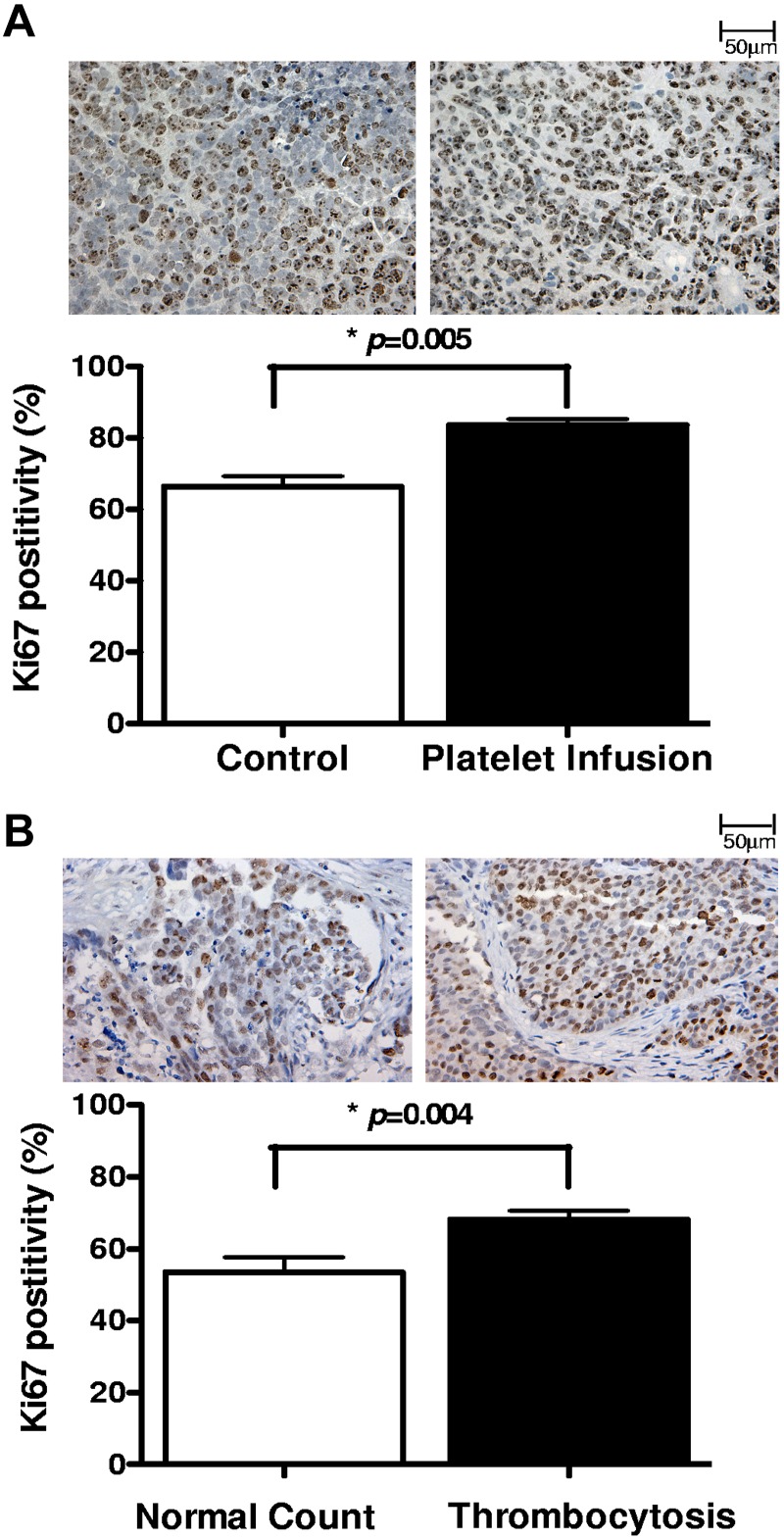
To study the effect of platelets in vivo, we injected syngeneic platelets to mice with orthotopic ovarian cancer on a weekly basis for 4 weeks and measured the proliferation index in the resected tumors using Ki67 staining. We found that mice receiving platelet infusion had a significantly higher percentage of Ki67 positivity compared with control tumor-bearing mice (83.5% vs 66.3%, respectively; P < .0005; Figure 2A). To extend these findings to human disease, we next measured proliferation indices in 20 tumor specimens collected from patients with ovarian cancer. Ten of these patients had elevated platelet counts with an average count of 635 (× 103/μL) and a range of 480-844 (× 103/μL), and 10 had normal platelet counts with an average of 284 (× 103/μL) and a range of 232-391 (× 103/μL). Thrombocytosis was associated with a higher percentage of Ki67 positivity in tumors (68% vs 57%, respectively; P = .03; Figure 2B).
Figure 2.
Proliferative effect of platelet on ovarian cancer cells in vivo. The proliferation index was measured in (A) tumors resected from tumor-bearing mice infused with buffer (control; n = 10) or platelets (n = 10) and (B) surgical specimens from ovarian cancer patients with normal platelet counts (n = 10) or thrombocytosis (n = 10), by Ki67 immunostaining. From each tissue block 5 sections were prepared, and from each section 5 fields were counted at original magnification ×200 of a light microscope by 2 different observers as independent blinded assessments. The ratio of positive Ki67 nuclear staining to total number of nuclei was measured for each field. The cumulative results are summarized as bar graphs, and a representative Ki67 staining is shown above each column.
In conclusion, we observed that platelets increase the proliferation of ovarian cancer cells in vitro and in vivo. The in vitro effect was not mediated by platelet GPIbα, GPIIbIIIa, or P-selectin; was partially dependent on platelet signaling through COX-1; and was dependent on released TGF-β1 binding to tumor cell TGF-β1R. These results corroborate that platelet-derived TGF-β1 is important for platelet-tumor cell cross-talk16 and extend our understanding of mechanisms by which platelets contribute to tumor progression.
Acknowledgments
This work was supported by Baylor College of Medicine University of Texas MD Anderson Cancer Centers (Collaborative Multidisciplinary Research Grant, A.K.S. and V.A.-K.; P50 CA083639 and U54 CA151668, A.K.S; T32 training grant CA101642, J.B.-M., R.S., and B.Z.) and Foundation for Women's Cancer/Ovarian Cancer National Alliance Ovarian Cancer Research Grant (J.B.-M.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S.C. and J.B.-M. designed and performed experiments and analyzed and interpreted data; H.G.V., R.S., and B.Z. performed experiments; M.H.K. interpreted data; A.K.S. designed experiments and interpreted data; and V.A-K. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vahid Afshar-Kharghan, Department of Benign Hematology, The University of Texas MD Anderson Cancer Center, Unit 1464, 1515 Holcombe Bd, Houston, TX 77030; e-mail: vakharghan@mdanderson.org.
References
- 1.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3(7):425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362–2367. doi: 10.1161/ATVBAHA.110.207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan K, Crowley D, Smyth P, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. 2011;6(10):e26125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300. [PubMed] [Google Scholar]
- 5.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81(4):1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L434–L442. doi: 10.1152/ajplung.00075.2003. [DOI] [PubMed] [Google Scholar]
- 7.Tsopanoglou NE, Maragoudakis ME. On the mechanism of thrombin-induced angiogenesis: potentiation of vascular endothelial growth factor activity on endothelial cells by up-regulation of its receptors. J Biol Chem. 1999;274(34):23969–23976. doi: 10.1074/jbc.274.34.23969. [DOI] [PubMed] [Google Scholar]
- 8.Klement GL, Yip TT, Cassiola F, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94(2):663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wartiovaara U, Salven P, Mikkola H, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80(1):171–175. [PubMed] [Google Scholar]
- 11.Kisucka J, Butterfield CE, Duda DG, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A. 2006;103(4):855–860. doi: 10.1073/pnas.0510412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95(16):9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A. 2002;99(4):2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Zuka M, Liu J, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104(21):9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakewell SJ, Nestor P, Prasad S, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100(24):14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]