Abstract
Dasatinib is a highly effective second generation tyrosine kinase inhibitor approved for the treatment of imatinib-resistant or intolerant chronic myeloid leukemia and Philadelphia-positive acute lymphoblastic leukemia. This article reviews the results of phase I, II and III studies and looks at the efficacy and safety of dasatinib. This review also provides practical recommendations for the management of side effects.
Keywords: Leukemia, Myelogenous, Chronic, BCR-ABL Positive/drug therapy; Drug toxicity; Drug interactions; Gastrointestinal tract/drug effects; Drug resistance, neoplasm; Pyrimidines; Interferonalpha/administration & dosage; Piperazines/therapeutic use; Clinical Trial
Introduction
Chronic myeloid leukemia (CML) is a hematologic disorder associated with a mutual chromosomal translocation between chromosomes 9 and 22 resulting in the formation of the Philadelphia (Ph) chromosome.(1) The Ph chromosome is detected in 95% of CML patients and in 20% to 30% of adult patients with acute lymphoid leukemia (ALL).(2) This gene fusion codifies a chimeric protein, BCR-ABL, which is associated with uncontrolled tyrosine kinase ABL activity.(1)
The estimated incidence of CML is one to two cases per 100,000 inhabitants, 80% of which are diagnosed in the chronic stage and 40% of which are asymptomatic.(3) Without treatment, CML usually progresses to the accelerated phase and blast crisis, the end stage of the disease that is associated with a few months of survival.(3)
Before the development of drugs that selectively inhibit BCR-ABL kinase, such as imatinib mesylate, the therapeutic choices were hydroxyurea, interferon-alpha and cytarabine, in addition to bone marrow allografts. The International Randomized Study of Interferon versus STI-571 (IRIS) compared first line treatment with imatinib versus cytarabine and interferon-alpha.(4) A total of 1106 patients were randomized to receive imatinib (553 patients) or interferon-alpha (IFN-α) associated with low doses of cytarabine (553 patients). At 18 months of follow-up, the estimated rate of progression-free survival (PFS) for accelerated phase or blast crisis patients was 96.7% and 91.5%, respectively (p-value < 0.001). Since then, imatinib has been the first choice treatment for recently diagnosed CML.
During the eight-year follow-up of the IRIS study, 305 (55%) out of the 553 patients who received imatinib continued in the study.(5) Event-free survival at eight years was 81% and PFS for accelerated phase or blast crisis patients was 92%.
For the 45% of patients that left the study, the reasons for discontinuing treatment were related to toxicity and safety (6%), or unsatisfactory results (16%), stem cell transplantation (3%), death (3%) and related to other causes such as lack of consent renewal or withdrawal (17%). In this study, 31% of patients did not reach complete cytogenetic response (CCgR) during the first 12 months of treatment and 13% did not reach this response in five years. During the first three years, 3-7% of patients had treatment failure.
Imatinib-resistance mechanisms are of multi-factorial origin. The best known mechanism is BCR-ABL mutations preventing the effective binding of imatinib to tyrosine kinase.(6) Imatinib may be subject to absorption variations by the gastrointestinal tract as it is an orally administered drug. Imatinib is also subject to changes in the liver metabolism (individual variability of CYP3A4 concentrations) and binding to plasma proteins. Changes in the inflow and outflow of the drug in the cell, and senescence or repair mechanisms, enzymatic inactivation and apoptosis defects may also occur, in addition to development of alternative patterns of signal transduction. Resistance may be caused by the development of additional cytogenetic abnormalities.(7,8)
Dasatinib
Dasatinib has been evaluated in clinical trials (phases 1, 2 and 3) in adult patients with Ph-positive leukemias after imatinib failure or intolerance when it was shown to be effective in the chronic, accelerated and blast phases of CML. It was approved by the Federal Drug Administration (FDA) in 2006 for the treatment of CML in the three phases and also for Ph+ ALL. In Brazil, its use for all CML phases was approved by National Health Surveillance Agency (ANVISA) in March 2008 and for ALL Ph+ in April 2010.
Phase I Study
In 2006, a phase I, dose-escalation study of dasatinib was conducted in 84 patients with CML (in any phase) and ALL (Ph-positive) who were intolerant or resistant to imatinib.(9) Patients were prescribed a total of from 15 mg to 240 mg dasatinib as one daily dose or split in two. The primary objective was to evaluate the tolerability and safety of dasatinib treatment. Responses were observed in all BCR-ABL genotypes, except in the presence of the T315I mutation, which resulted in resistance to imatinib and dasatinib. The highest toxicity observed was reversible myelosuppression; non-malignant pleural effusion was also observed but at a lower rate.(9)
Phase II Studies
The Src/Abl Tyrosine kinase inhibition Activity: Research Trials (START) program included four prospective, multicenter, single arm studies (START-A, B, C and L) and one randomized study (START-R).
START-A evaluated CML patients in the accelerated phase who were resistant or intolerant to imatinib,(10) with 174 patients that received dasatinib 70 mg bid being evaluated. One 14-month follow-up study showed major hematological response (MHR) in 64% of patients (95% CI = 56.2% to 70.9%), including 45% of patients with reticulocyte hemoglobin content (RHC). Major cytogenetic response (MCgR) was observed in 39% of patients (95% CI = 31.2% to 46.2%) with 32% of patients reaching CCgR. PFS at 12 months was 66% and overall survival (OS) was 82%.(11)
In the START-B and START-L studies, 74 patients in myeloid blast crisis (MBC) and 42 patients in lymphoid blast crisis (LBC) that were resistant or intolerant to imatinib were evaluated. Dasatinib was administered at 70 mg bid. MBC patients had 31% of radiographic contrast media (RCM) and 27% of renal cell carcinoma (RCC). For LBC patients the RCM rate was 50% and the RCC rate was 43%. Growth hormone receptor (GHR) and RHC rates for MBC patients were 53% and 26%, respectively, while for LBC, those rates were 36% and 26%, respectively.(12)
START-C evaluated 387 CML patients in the chronic stage who were resistant (n = 127) or intolerant (n = 59) to imatinib.(13) Patients were given dasatinib at 70 mg bid. PFS at 15 months was 90% and OS was 96%. Complete hematological response (RHC) rates at 8 and 15.2 months were 90% and 91%, respectively. The MCgR rate at 8 and 15.2 months were 52% and 59%, respectively. The CCgR rates at 8 and 15.2 months were 39% and 49%, respectively.(13,14)
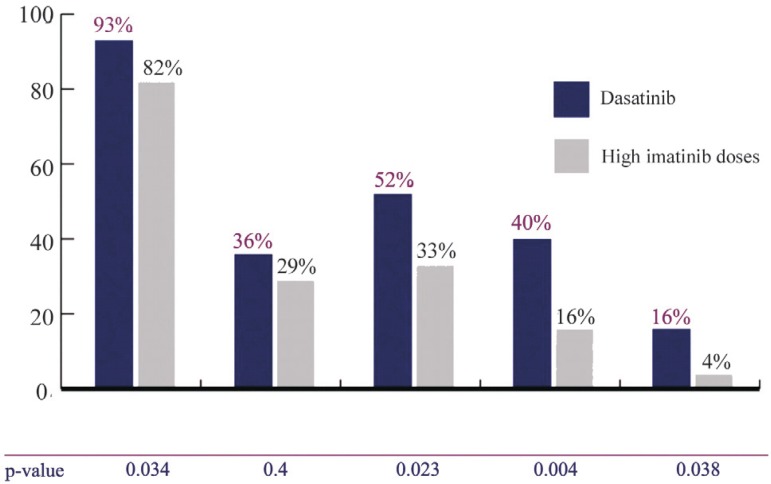
START-R was a phase II randomized study comparing dasatinib with high-doses of imatinib after failure of the latter at the usual dose.(15) Imatinib resistant CML patients in the chronic stage receiving daily doses of 400 mg or 600 mg were randomized to receive dasatinib (140 mg/day) or imatinib at a higher dose (800 mg). One hundred and fifty patients were enrolled in this study and randomized at a ratio of 2:1 (101 patients received dasatinib and 49 patients received imatinib). The primary endpoint analyzed was MCgR at 12 months and the secondary endpoints were MCgR and RHC rates at any time before crossover, MCgR and RHC duration and time to MCgR and RHC before crossover. Endpoints were also evaluated after crossover. Response rates are shown in Figure 1. The mean time to treatment failure was higher for the group receiving dasatinib with a reduction of 84% in the relative risk being observed (RR = 0.16; 95% CI = 0.1 to 0.26; p-value < 0.001). PFS also favored dasatinib, showing an 86% reduction in the relative risk (RR = 0.14; 95% CI = 0.05 to 0.4; p-value < 0.001).(15)
Figure 1.
Dasatinib response rates versus high imatinib doses
CHR = complete hematological response; MCgR = major cytogenetic response; CCgR = complete cytogenetic response; MMR = major molecular response
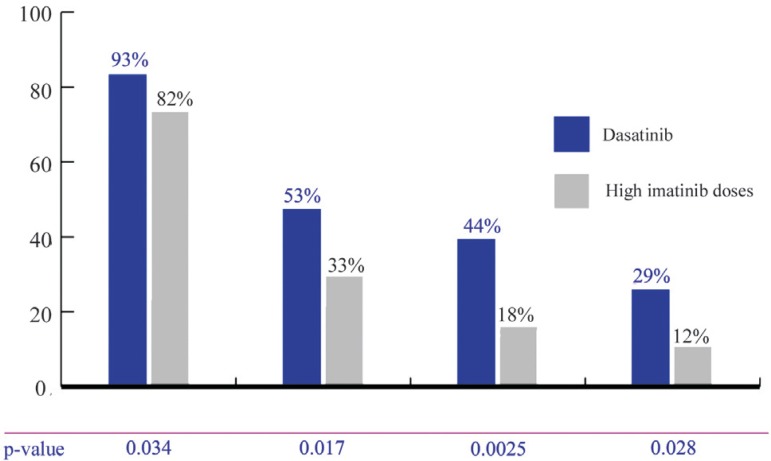
A 2-year follow-up study to this phase II study was published, showing that the dasatinib group kept the highest response rates (Figure 2) when compared to high doses of imatinib, in addition to the highest PFS (p-value = 0.0012).(16)
Figure 2.
START-R - Response rates after 2 follow-up years
CHR = complete hematological response; MCgR = major cytogenetic response; CCgR = complete cytogenetic response; MMR = major molecular response
An economic and effective evaluation of dasatinib in comparison with high-dose imatinib was performed.(17) Dasatinib has been associated to higher response rates, resulting in benefits regarding the number of years and quality of life.(17)
Phase III Studies
The phase III randomized study CA180-034 compared four administration regimens of dasatinib: 50 mg (bid), 70 mg (bid), 100 mg (qd) or 140 mg (qd) in 670 chronic stage CML patients who were resistant or intolerant to imatinib.(18) This study evaluated whether the incidence of treatmentrelated adverse events could have been reduced without loss of efficacy by changing the dose regimens, that is, administering the drug qd instead of bid. After a six-month follow-up, the highest cytogenetic responses were reached in 59% of patients receiving 100 mg qd and in 55% of patients receiving 70 mg bid. The number of patients experiencing grade 3-4 thrombocytopenia was significantly lower in the group treated with 100 mg qd than in the group receiving 70 mg bid (22% vs. 37%; p-value = 0.004); similarly the number of patients who discontinued treatment because of toxicity reduced (4% vs. 11%).(18) Such changes significantly reduced the incidence of pleural effusion (16% vs. 7%; p-value = 0.024). The authors concluded that treatment with a single daily dose of 100 mg is the best risk/benefit profile with higher tolerance and effectiveness maintained.(18) A three-year follow-up study, so far only published as an abstract, showed that this risk/benefit profile was sustained and that this should be the standard starting dose in chronic stage CML patients.(19)
The phase III randomized study, CA180-035, compared the single daily dose of 140 mg with the dose of 70 mg bid in accelerated phase CML patients who were resistant or intolerant to imatinib.(20) Out of 317 randomized patients, 158 received 140 mg qd and 159 patients received 70 mg bid. The major hematologic response rates (66% vs 68%) and MCgR (39% vs 43%) were similar. The estimated PFS at 24 months (51% vs. 55%) and the OS (63% vs. 72%) were not significantly different. Also, the group receiving 140 mg qd showed a lower incidence of pleural effusions than those receiving 140 mg bid (20% vs. 39%; p-value = 0.001).(20)
Safety profile
Dasatinib is usually well tolerated but is associated to reversible and manageable adverse events. Such events commonly appear at the beginning of treatment and are predominantly mild to moderate, self-limiting or resolved with supportive care, temporary interruption or dose reduction.(10,21) The management of these events is essential before continuing treatment and offering a greater chance of extended benefit.
There are no studies comparing different interventions or management practices for these adverse events in the literature. Thus, management suggestions are based on guidelines used in clinical trials and from the experience of institutions and investigators.
The classification of adverse events was based on the common terminology criteria for adverse events.(22) The most common adverse events observed during dasatinib treatment were cytopenias, fluid retention, pleural effusion, dyspnea, gastrointestinal disorders, skin rash, headache, and fatigue.
Serious adverse reactions (grades 3 and 4) were observed at the following frequencies: fluid retention (8%), pleural effusion (5%), diarrhea (3%), skin rash (1%), headache (1%), hemorrhage (6%), nausea (1%), and dyspnea (4%).(23)
A significant number of patients required at least one dose interruption, dose reduction or both because of toxicity, but just a few patients had to discontinue treatment (6% of patients in the chronic stage, 5% in the accelerated phase and 11% in the blast phase).(24)
The absence of intolerance at crossover between imatinib and dasatinib was of particular interest; there was no recurrence of adverse events associated to imatinib intolerance.(23,25) Additionally, the dasatinib tolerability profile was comparable between imatinib-resistant and intolerant patients.(25) Table 1 shows the frequency of grade 3-4 toxicities observed in dasatinib studies.
Table 1.
Rates of most commonly reported adverse events during dasatinib studies
| Variable | Chronic stage | Accelerated phase | MBC | LBC | Ph+ALL | |
| Regimen and Dose | 100 mg qd | 70 mg bid | 70 mg bid | |||
| n | 165 | 655 | 174 | 109 | 48 | 46 |
| Study | CA-180-034 | CA-180-034 and START-C | START-A | START-B | START - L | START - L |
| G3/4 Cytopenia (%) | ||||||
| Neutropenia | 34 | 43 - 61 | 76 | 80 | 81 | 78 |
| Thrombocytopenia | 22 | 38 - 56 | 82 | 82 | 88 | 78 |
| Leukopenia | 17 | 23 - 27 | 59 | 61 | 71 | 65 |
| Anemia | 10 | 17 - 21 | 69 | 69 | 50 | NR |
| Fever (%) | ||||||
| All Grades | 4 | 10 - 14 | 24 | 20 | 17 | 22 |
| G3/4 | < 1 | 0 - 1 | 4 | 5 | 2 | 2 |
| Pleural Effusion (%) | ||||||
| All Grades | 10 | 17- 27 | 27 | 36 | 13 | 24 |
| G3/4 | 2 | 2 - 6 | 5 | 15 | 6 | 7 |
| Peripheral Edema (%) | ||||||
| All Grades | 10 | 10 - 18 | 22 | 18 | 13 | 13 |
| G3/4 | 0 | 0 | < 1 | 0 | 0 | 0 |
| Dyspnea (%) | ||||||
| All Grades | 13 | 14 - 30 | 21 | 21 | 13 | NR |
| G3/4 | 2 | 4 - 5 | 4 | 6 | 2 | NR |
| Diarrhea (%) | ||||||
| All Grades | 23 | 25 - 37 | 52 | 39 | 33 | 33 |
| G3/4 | < 1 | 2 - 4 | 8 | 7 | 2 | 9 |
| Nausea (%) | ||||||
| All Grades | 18 | 24 - 27 | 28 | 19 | 25 | 22 |
| G3/4 | < 1 | 0 - 1 | < 1 | 4 | 0 | 0 |
| Vomiting (%) | ||||||
| All Grades | 7 | 9 - 11 | 20 | 22 | 25 | 11 |
| G3/4 | < 1 | 0 - 1 | 2 | 3 | 2 | 0 |
| Headache (%) | ||||||
| All Grades | 32 | 25 - 32 | 29 | 10 | 17 | NR |
| G3/4 | < 1 | 1 - 2 | < 1 | 2 | 2 | NR |
| Fatigue (%) | ||||||
| All Grades | 21 | 17-31 | 26 | 18 | 27 | NR |
| G3/4 | 2 | 2 - 4 | 4 | 2 | 4 | NR |
| Skin Rash (%) | ||||||
| All Grades | 13 | 16 - 26 | 21 | 14 | 17 | 15 |
| G3/4 | 1 | 0 - 1 | 1 | 0 | 4 | 2 |
MBC = myeloid blast crisis; LBC = lymphoid blast crisis; ALL = acute lymphoblastic Leukemia; n = number of patients; NR = not reported
Adverse event management
Cytopenias
Cytopenias usually occur during the first two treatment months and are more common in advanced CML patients and Ph-positive ALL patients compared to chronic stage CML patients.(23) Advanced disease patients may have cytopenias related to the disease.(23) Patients should be monitored frequently at the beginning of treatment and a weekly 3-hydroxy-3-methylglutaryl (HMG) test is recommended during the first two months of treatment. Afterwards, monthly or as per clinical indication tests should be performed.(23)
a) Anemia
Grade 3/4 anemia (Hb < 80 g/L) was observed in 10% of chronic stage CML patients treated with dasatinib at 100 mg qd; the highest incidence occurred at 70 mg bid (17% to 21%).(14)
Grade 3/4 anemia was more frequent in the advanced stage CML, and occurred in up to 50-69% of patients.(12) Supportive treatment (packed red blood cell transfusion) or withdrawal of treatment until Hb levels are ≥ 80 g/L is recommended for grade 3/4 anemia. After recurrent episodes, dose reduction or discontinuation should be considered. Recent guidelines do not recommend the use of erythropoietin or darbepoietin in patients with myeloid hematologic neoplasms.(26)
b) Neutropenia and febrile neutropenia
Despite of the high frequency of neutropenia, only 5% of patients treated with dasatinib experienced febrile neutropenia.(2) The use of granulocyte colony stimulating factors may help in neutropenia management.(26,27)
Table 2 shows neutropenia management in chronic and advanced stages of CML, while Table 3 shows the treatment for dasatinib-related febrile neutropenia.(2,28)
Table 2.
Management of dasatinib-related neutropenia
| Neutropenia (Grade 3 and 4) | Chronic stage CML (100 mg/day dose ) | Advanced CML or Ph+ ALL (140 mg/day dose) | Supportive treatment |
| First episode | Withdraw treatment until ANC≥ 1000/mm3 | Evaluate bone marrow to check if cytopenia is related to leukemia. If so, consider dose escalation up to 180 mg/day. If not, withdraw dasatinib until ANC ≥ 1000/mm3 and resume at the original dose | Prophylaxis with antibiotics or filgrastim |
| If recovery within 7 days, resume at the original dose; if recovery after 7 days, resume at a lower dose (level 1 *) | |||
| Second episode | Withdraw treatment and resume at a lower dose (level 1*) or consider treatment discontinuation | Withdraw treatment and resume at a lower dose (level 1*) | Consider filgrastim |
| Third episode | Treatment discontinuation | Withdraw treatment, and reduce dose to one level lower and consider treatment discontinuation |
*Dose reduction: chronic stage, 100 mg/day ? 80 mg (level 1); advanced stage, 140 mg/day ? 100 mg/day (level 1) ? 80 mg/day (level 2)
CML = chronic myeloid leukemia; ALL = acute lymphoblastic Leukemia; ANC - Absolute Neutrophil Count
Table 3.
Management of dasatinib - related febrile neutropenia
| Febrile neutropenia (Grade 3 and 4) | Conduct | Supportive treatment |
| First episode | Withdraw treatment until ANC ≥ 1000/mm3 and temperature < 38°C and resume at a lower dose (level 1*) | Filgrastim, antibiotic therapy |
| Second episode | Withdraw treatment and reduce dosage (level 2*) or consider treatment discontinuation |
*Dose reduction: chronic stage, 100 mg/day → 80 mg (level 1); advanced stage, 140 mg/day → 100 mg/day (level 1) → 80 mg/day (level 2);
ANC - absolute neutrophil count
c) Thrombocytopenia
Platelet transfusion is recommended usually when levels are lower than 10 to 20 x 109/L. Preliminary data suggest that oprevelkin (interleukin 11) may help in the management of thrombocytopenia.(27)
Table 4 shows management of dasatinib-related thrombocytopenia.(2,28)
Table 4.
Management of dasatinib-related thrombocytopenia
| Thrombocytopenia (Grade 3 / 4) | Chronic stage CML (100 mg/day dose) | Advanced CML or Ph+ ALL (140 mg/day dose) | Supportive treatment |
| First episode | Withdraw treatment until platelets > 50 x 109/L | Evaluate bone marrow to check if cytopenia is related to leukemia. If so, consider dose escalation up to 180 mg/day. If not, withdraw dasatinib until platelets ≥ 20 x 109/L and resume at the original dose | Platelet transfusion and consider the use of oprevelkin |
| If recovery within 7 days, resume at the original dose; if recovery after 7 days, resume at a lower dose (level 1*) | |||
| Second episode | Withdraw treatment and resume at a lower dose (level 1*) or consider treatment discontinuation | Withdraw treatment and resume at a lower dose (level 1 *) | Consider oprevelkin |
| Third episode | Consider treatment discontinuation | Withdraw treatment, reduce the dose one additional level and consider treatment discontinuation |
*Dose reduction: chronic stage, 100 mg/day → 80 mg (level 1); advanced phase, 140 mg/day → 100 mg/day (level 1) → 80 mg/QD (level 2)
CML = chronic myeloid leukemia; ALL = acute lymphoblastic leukemia;
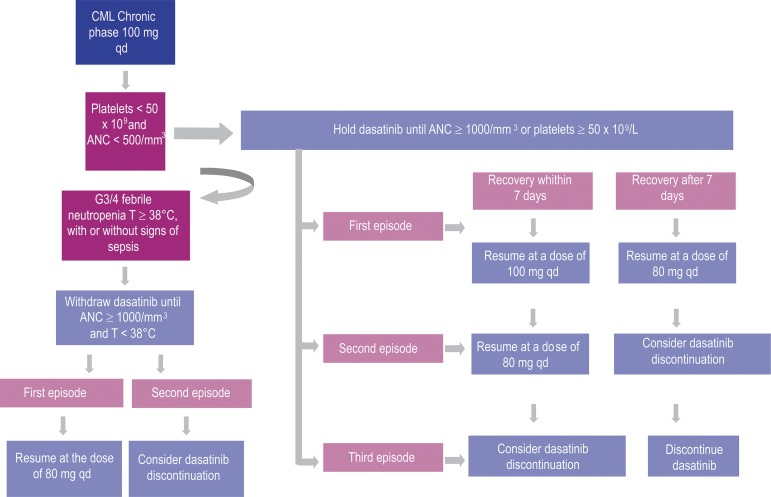
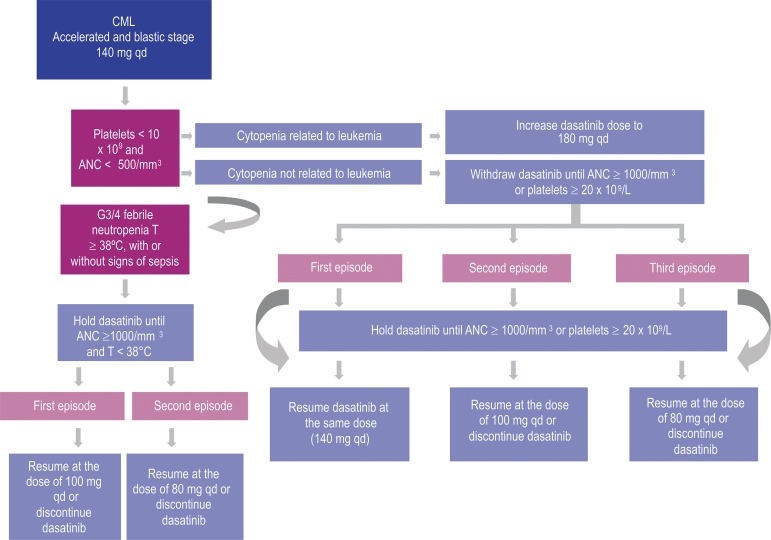
Flowcharts 1 and 2 (Figures 3 and 4).
Figure 3.
Dasatinib dose adjustment – neutropenia, febrile neutropenia and thrombocytopenia (G3/4) in the treatment of CML (chronic phase)(2,28)
Figure 4.
Dasatinib dose adjustment – neutropenia, febrile neutropenia and thrombocytopenia (G3/4) in the treatment of CML (accelerated and blastic phases) and Ph-positive acute lymphoblastic leukemia(2,28)
Pleural effusion and other events related to fluid retention
The onset of pleural effusion depends on the dose and number of daily dosages of dasatinib.(2) In the dose optimization study, CA180-034, dose adjustment from 70 mg (bid) to 100 mg (qd) significantly reduced the incidence of pleural effusion from 16% to 7% (p-value = 0.024).(18) Risk factors for developing pleural effusion and fluid retention must be considered to identify the most susceptible patients, namely: advanced age, advanced disease stage, heart disease, hypertension, hypercholesterolemia, autoimmune disease and history of skin rash during treatment with imatinib or dasatinib.(29,30) Cytologic testing of pleural effusion shows exsudate present in 78% of cases, with a predominance of lymphocytes (90%) and absence of neoplastic cells.(30)
The mechanism explaining the appearance of pleural effusion is still not clear and may be multi-factorial.(21) Patients must be counseled to recognize the symptoms, such as dry cough, shallow breathing and shortness of breath early. The degree of dyspnea is correlated with the extent of pleural effusion.(21) The diagnosis must be confirmed by an imaging scan (chest X-ray).
The severity of pleural effusion must be determined and the treatment must be interrupted until the event grade is < 1.(21) After improvement, treatment should be resumed at a lower dose: in chronic stage, 80 mg qd and in accelerated and blast stages, 80 to 100 mg qd. If an improvement is not noticed within seven days, supportive treatment with diuretics and steroids (e.g., prednisone 20 mg/day for 3 days) must be initiated.(21,26) Table 5 shows the management of dasatinibrelated pleural effusion.
Table 5.
Management of dasatinib-related pleural effusion
| Pleural effusion | Definition National Cancer Institute Common Toxicity Criteria (NCICTC) | Recommendation |
| Grade 1 | Absence of symptoms | Monitoring by chest X-ray |
| Grade 2 | Symptomatic requiring diuretics or ≤ 2 therapeutic thoracentesis | First episode: withdraw treatment until effusion has decreased to grade 1 or lower, supportive treatment (use steroids, diuretics, thoracentesis if large volume or significant symptoms). |
| Second episode: withdraw treatment and resume at a lower dose (level 1*) and supportive treatment | ||
| Third episode: withdraw treatment and reduce the dose one additional level or consider treatment discontinuation | ||
| Grade 3 | O2 supplement required, > 2 thoracentesis required, thoracic draining or pleurodesis | First episode: withdraw treatment until effusion has decreased to grade 1 or lower, supportive treatment (steroids, diuretics, thoracentesis, thoracic draining, pleurodesis) |
| Second episode: withdraw treatment and reduce the dose one additional level or consider treatment discontinuation | ||
| Grade 4 | Life-threatening, hemodynamic instability, requiring mechanical ventilation | Discontinue treatment |
*Dose reduction: chronic stage, 100 mg/day → 80 mg (level 1); advanced phase, 140 mg/day → 100 mg/day (level 1) → 80 mg/day (level 2)
Peripheral edema was reported in 10 to 22% of patients and was usually grade 1 and 2.(2) Other events such as pericardial effusion, pulmonary edema, pulmonary hypertension, ascites and anasarca were not frequent (< 3% in all grades and < 1% grades 3 and 4).(2) Some studies have shown that 29% of patients with pleural effusion also experienced pericardial effusion.(21) The management of fluid retention is similar to that of pleural effusion, including temporary interruption of treatment, dose reduction and administration of diuretics.(2)
Bleeding
Epistaxis occurred in 11% of patients, while fatal bleeding of the central nervous system occurred in patients in blast crisis when they had grade 4 thrombocytopenia.(24) Patients in advanced stage may have thrombocytopenia and other cytopenias related to the disease but not to the treatment.(23)
Heart changes
Prolongation of the QT interval is a rare, although serious, adverse event that needs to be monitored by routine electrocardiograms.(23) The overall incidence of congestive heart failure or cardiac dysfunction related to dasatinib is 2%.(2) No sudden deaths were observed with dasatinib.(2)
All patients must be evaluated for the risk of prolongation of the QT interval, including the existence of hypokalemia, hypomagnesemia and prolonged QT syndrome and the prescription of concomitant drugs that may aggravate the condition, such as amiodarone, methadone, erythromycin, haloperidol, etc.(23) Before starting dasatinib treatment, hypokalemia and hypomagnesemia must be corrected.(2)
In the event of cardiotoxicity, medication must be withdrawn until the event is resolved. To resume the drug, the dose must be reduced by two levels, or treatment must be interrupted.(2)
Gastrointestinal disorders
In the studies conducted, 31% of patients experienced diarrhea, 22% had nausea and 13% had vomiting, usually of mild to moderate severity.(2) The treatment of such events with antidiarrheal and antiemetic medications is recommended. In the event of grade 3/4 symptoms, consider interrupting treatment or reducing the dose if the condition does not improve with supportive treatment.(2) Table 6 shows management of dasatinib-related gastrointestinal events.
Table 6.
Management of dasatinib-related gastrointestinal events
| Adverse event | Incidence | Recommendation |
| Nausea | 22% (all grades); 1% (grades 3/4) | Grade 3: withdraw treatment until grade 1 toxicity is reached; antiemetic drugs, hydration and electrolytic replacement as needed |
| Grade 4: withdraw treatment until grade 1 toxicity is reached; resume the drug at lower dose; antiemetic drugs, hydration and electrolytic replacement as needed. | ||
| Diarrhea | 31% (all grades); 3% (grades 3/4) | Grade 3: withdraw treatment; support therapy with antidiarrheal drugs, hydration and electrolytic replacement as needed. |
| Grade 4: withdraw treatment until grade 1 toxicity is reached; resume the drug at a lower dose; antidiarrheal drugs, hydration and electrolytic replacement as needed. | ||
| Gastrointestinal bleeding | 3% (all grades); 1% (grades 3/4) | Interrupt treatment; transfusions as needed; resume treatment at a lower dose but with caution |
Skin rash
This event was seen in 22% of treated patients, but only 1% was grade 3/4.(2) Dasatinib-related skin rash is resolved with drug interruption and, differently from allergic rash, may not recur with the resumption of the drug, mainly if the dose is reduced.(2)
Mild events may be treated with topical corticosteroids or antihistaminic drugs. In most severe cases, drug interruption and rapid courses of systemic corticosteroids are recommended; wait for resolution before resuming treatment at a lower dose.(2)
Laboratory changes
Grade 3/4 chemical changes require drug interruption with resumption of treatment at a lower dose when grade < 1 is reached.(2) Grade 3 and 4 hypophosphatemia was seen in 10% of CML patients in chronic stage and 12-20% of advanced CML patients or Ph+ ALL patients; a good response was attained with oral supplementation.(2) The incidence of grade 3 and 4 hypocalcemia was 2% in chronic stage CML patients and 7-11% in advanced CML or Ph+ ALL patients; this was successfully managed with oral calcium supplementation.(2) Changes in transaminases and bilirubin were rare (1% to 7%).(2)
Other adverse events
Other adverse events may be observed, such as headache in 24% of cases and fatigue in 21%.(2) Headache is usually manageable with common analgesics and rarely requires treatment adjustments.(2) Fever occurred in 39% of patients.(24)
Dose adjustment, treatment withdraw and discontinuation
In chronic stage CML patients, a daily single dose of dasatinib 100 mg has been associated with lower need to decrease dose (33% vs. 57%), treatment withdrawal (58% vs. 71%) and treatment discontinuation (22% vs. 32%) compared to a 70 mg dose bid.(2)
Conclusion
The introduction of tyrosine kinase inhibitors resulted in a significant change in the treatment of CML because of their superiority when compared to other therapies. The constant increase of effective therapeutic choices represents a need of adequate monitoring to choose the best secondline treatment and the introduction of such therapy at the most appropriate moment.
There is enough evidence in the literature showing the efficacy of dasatinib in the treatment of patients with CML or Ph+ALL who are resistant or intolerant to imatinib.
Dasatinib tolerability is satisfactory and most adverse events that occurred with dasatinib were mild to moderate and reversible and manageable by supportive care or dose adjustments and administration.
Rates of treatment discontinuation due to toxicity are low, and there is no evidence of cross intolerance with imatinib. Therefore, dasatinib is an effective and well tolerated therapeutic choice as a second-line treatment for CML.
Funding Statement
Financial support: This article was written with the support of Bristol-Myers Squibb
References
- 1.Sawyers CL.Chronic myeloid leukemia N Engl J Med 1999340 (17): 1330-1340Comment in: N Engl J Med. 1999;341(10):765. [DOI] [PubMed] [Google Scholar]
- 2.Khoury HJ, Guilhot F, Hughes TP, Kim DW, Cortes JE.Dasatinib treatment for Philadelphia chromosome-positive leukemias: practical considerations Cancer 2009115 (7): 1381-1394 [DOI] [PubMed] [Google Scholar]
- 3.Redaelli A, Bell C, Casagrande J, Stephens J, Botteman M, Laskin B, et al. Clinical and epidemiologic burden of chronic myelogenous leukemia Expert Rev Anticancer Ther 20044 (1): 85-96 [DOI] [PubMed] [Google Scholar]
- 4.O'Brien SG, Deininger MW.Imatinib in patients with newly diagnosed chronic-phase myeloid leukemia Semin Hematol 200340 (2): Suppl 226-30 [DOI] [PubMed] [Google Scholar]
- 5.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ, IRIS Investigators Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia N Engl J Med 2003348 (11): 994-1004Comment in: Curr Hematol Rep. 2004;3(1):37-8. N Engl J Med. 2003;348 (11):1048-50. Clin Lab Haematol. 2005;27(6):416-7 [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Talpaz M, Giles F, OBrien S, Cortes J.New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance Ann Intern Med 2006145 ((12)): 913-923 [DOI] [PubMed] [Google Scholar]
- 7.Apperley JF.Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia Lancet Oncol 20078 (11): 1018-1029 [DOI] [PubMed] [Google Scholar]
- 8.Apperley JF.Part II: management of resistance to imatinib in chronic myeloid leukaemia Lancet Oncol 20078 (12): 1116-1128 [DOI] [PubMed] [Google Scholar]
- 9.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosomepositive leukemias N Engl J Med 2006354 (24): 2531-2541Comment in: N Engl J Med. 2006;354(24):2594-6. N Engl J Med. 2006;355(10):1062-3; author reply 1063-4. N Engl J Med. 2006; 355(10):1062; author reply 1063-4 [DOI] [PubMed] [Google Scholar]
- 10.Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or intolerant chronic myeloid leukemia in accelerated phase Blood 2007109 (10): 4143-4150 [DOI] [PubMed] [Google Scholar]
- 11.Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial J Clin Oncol 200927 (21): 3472-3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes J, Kim DW, Raffoux E, Martinelli G, Ritchie E, Roy L, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase Leukemia 200822 (12): 2176-2183 [DOI] [PubMed] [Google Scholar]
- 13.Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy Blood 2007109 (6): 2303-2309Erratum in: Blood. 2007;110(5):1438 [DOI] [PubMed] [Google Scholar]
- 14.Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib Leukemia 200822 (6): 1200-1206 [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, et al. Dasatinib or high-dose imatinib for chronicphase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial Blood 2007109 (12): 5143-5150 [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Pasquini R, Levy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronicphase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer 2009115 (18): 4136-4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor MJ, Scuffham PA.Pharmacoeconomic benefits of dasatinib in the treatment of imatinib-resistant patients with chronic myelogenous leukemia Expert Rev Pharmacoecon Outcomes Res 20099 (2): 117-121 [DOI] [PubMed] [Google Scholar]
- 18.Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia J Clin Oncol 200826 (19): 3204-3212Comment in: Nat Clin Pract Oncol. 2009;6(2):68-9 [DOI] [PubMed] [Google Scholar]
- 19.Stone RM, Kim DW, Kantarjian HM, Rousselot P, Hochhaus A, Dorlhiac-Llacer PE, et al. Dasatinib dose-optimization study in chronic phase chronic myeloid leukemia (CML-CP): three-year follow-up with dasatinib 100 mg once daily and landmark analysis of cytogenetic response and progression-free survival (PFS) [abstract] Paper presented at: 51º American Society of Hematology Annual Meeting2009 Dec 5-8New OrleansJ Clin Oncol 200927 (15s) 7007.[cited 2010 Jan 12] Available from: http://www.asco.org/ascov2/Meetings/Abstracts?& vmview=abst_ detail_view& confID=65& abstractID=33899 [Google Scholar]
- 20.Kantarjian H, Cortes J, Kim DW, Dorlhiac-Llacer P, Pasquini R, DiPersio J, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up Blood 2009113 (25): 6322-6329Comment in: Nat Rev Clin Oncol. 2009;6(12):680-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masiello D, Gorospe G 3rd, Yang AS. The occurrence and management of fluid retention associated with TKI therapy in CML, with a focus on dasatinib. J Hematol Oncol. 2009;2:46. doi: 10.1186/1756-8722-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [Internet]Washington: National Institutes of Health; 2006[cited 2010 Apr 30] Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_ applications/docs/ctcaev3.pdf [Google Scholar]
- 23.Wong SF. New dosing schedules of dasatinib for CML and adverse event management. J Hematol Oncol. 2009;2:10. doi: 10.1186/1756-8722-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate Clin Cancer Res 200814 (2): 352-359 [DOI] [PubMed] [Google Scholar]
- 25.Cortes J, Rousselot P, Kim DW, Ritchie E, Hamerschlak N, Coutre S, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis Blood 2007109 (8): 3207-3213 [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. NCCN Practice Guidelines in Oncology 22010[ database on the Internet] Washington: NACCN; 2010; [cited 2010 May 16] Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [Google Scholar]
- 27.Quintas-Cardama A, Kantarjian H, O'Brien S, Garcia-Manero G, Rios MB, Talpaz M, et al. Granulocyte-colony-stimulating factor(filgrastim) may overcome imatinib-induced neutropenia in patients with chronic-phase chronic myelogenous leukemia Cancer 2004100 (12): 2592-2597Comment in: Cancer. 2005;103 (1):210-11 [DOI] [PubMed] [Google Scholar]
- 28.Sprycel [ Internet]São Paulo: Bristol-Myers Squibb; [cited 2010 Apr 30] Available from: http://www4.anvisa.gov.br/base/visadoc/BM/BM[32257-1-0].PDF [Google Scholar]
- 29.de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immunemediated pathogenesis Br J Haematol 2008141 (5): 745-747 [DOI] [PubMed] [Google Scholar]
- 30.Quintas-Cardama A, Kantarjian H, O'Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure J Clin Oncol 200725 (25): 3908-3914 [DOI] [PubMed] [Google Scholar]