Abstract
The 22q11.2 deletion syndrome (22q11DS) is a common genetic disease characterized by broad phenotypic variability. Despite the small number of studies describing hematological alterations in individuals with 22q11DS, it appears that these abnormalities are more frequent than previously imagined. Thus, the objective of our study was to report on a patient with 22q11DS presenting thrombocytopenia and large platelets and to review the literature. The patient, a 13-year-old boy, was originally evaluated due to craniofacial dysmorphia and speech delay. He also had a history of behavioral changes, neuropsychomotor delay and recurrent otitis/sinusitis. The identification of a 22q11.2 microdeletion by fluorescent in situ hybridization diagnosed the syndrome. Despite his hematological alterations, he only had a history of epistaxis and bruising of the upper and lower limbs. Assessments of the prothrombin time, thrombin time, partial thromboplastin time, bleeding time, fibrinogen levels and platelet aggregation (including the ristocetin induced platelet aggregation test) were all normal. Hematological alterations observed in 22q11DS are directly related to the genetic disorder itself (especially in respect to deletion of the GPIb gene) and secondary to some clinical findings, such as immunodeficiency. Macrothrombocytopenia is increasingly being considered a feature of the broad spectrum of 22q11DS and may potentially be a clinical marker for the syndrome.
Keywords: Blood platelets; Thrombocytopenia; Bernard-Soulier syndrome; DiGeorge syndrome; In situ hybridization; Chromosomes, Human, Pair 22
Introduction
The 22q11.2 deletion syndrome (22q11DS), also known as DiGeorge syndrome and velocardiofacial syndrome, is a common genetic disease (estimated prevalence is 1:20006000 live births) characterized by broad phenotypic variability; more than 180 clinical features have already been described. The syndrome occurs due to a deletion in region 11 of the long arm of chromosome 22 giving it an autosomal dominant pattern across generations.(1)
Despite the small number of studies describing hematological changes in individuals with 22q11DS, it appears that these abnormalities are more common than previously thought.(2,3) Thus, the aim of this study was to report on a patient with 22q11DS who presented with thrombocytopenia and large platelets and to review the literature. This work was approved by the Research Ethics Committee of the institution and consent was gained to use photos of the patient.
Case report
The patient is a 13-year-old Caucasian boy. He is the only child of young, healthy, non-consanguineous parents, with a family history of a maternal cousin who suffered from developmental delay and seizures. The patient was born by normal delivery, cephalic presentation, at term, weighing 2900 g (P10-25), measuring 47 cm (P10-25), with a head circumference of 32 cm (P3-5) and Apgar of 8/9. The only complication during pregnancy was a urinary tract infection treated with ampicillin.
The patient has a history of recurrent sinusitis and otitis. An audiometric evaluation showed a mild right hearing loss, and he was submitted to myringoplasty at 9 years old. In addition, he presented with bronchopneumonia at 3 months of life.
The patient progressed with developmental and speech delays. He attended preschool, but due to learning difficulties, he was referred to a special school. At 10 years old, his behavior was characterized by crying and shouting at night, psychomotor agitation and irritability. Consequently, he started treatment with fluoxetine and risperidone.
Evaluation by brain magnetic resonance imaging was normal. An electroencephalogram showed signs suggestive of epileptiform activity however, the mother denied any history of seizures. An ophthalmologic examination showed myopia. As the patient presented with skin peeling of the hands and feet, a diagnosis of dyshidrosis was made.
Karyotyping by GTG-Banding (400 bands) and molecular biology investigations for Fragile-X syndrome by PCR were normal. A microdeletion of 22q11.2 was identified by fluorescent in situ hybridization (FISH) using the commercially available D22S75 probe (Cytocell) thereby diagnosing 22q11DS (Figure 1).
Figure 1.
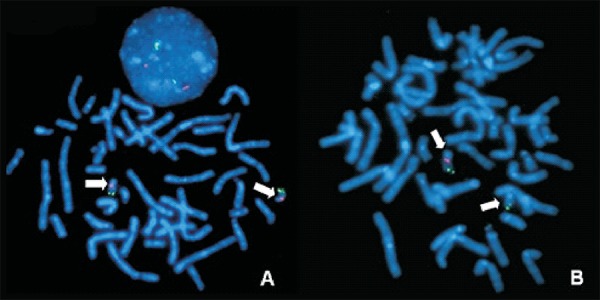
FISH - metaphase plates showing (A) the expected signs in both chromosomes 22 (normal pattern) and (B) absence of the signal corresponding to region 11.2 of the long arm (q) of chromosome 22, consistent with a 22q11.2 microdeletion (the arrows indicate the chromosomes 22). In (A) note an interphase nucleus with two red signals (normal pattern)
At 13 years old, the patient weighed 39.2 kg (P25-50), measured 153.6 cm in height (P25-50) had a head circumference of 52.3 cm (P2-50), narrow and upslanting palpebral fissures, overfolding of the ear helix (Figure 2), high arched palate, nasal voice, prognathism, an area of alopecia in the right frontal region of the scalp and a 2-cm café-au-lait spot on the right buttock.
Figure 2.
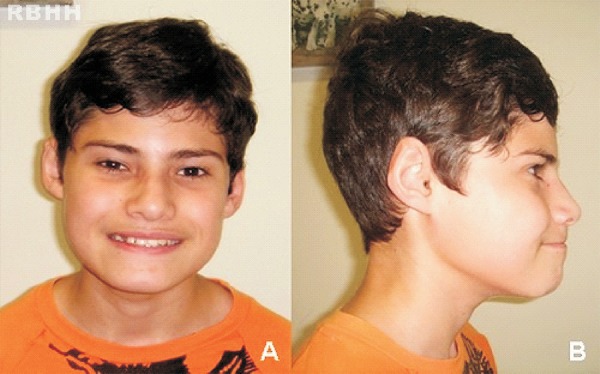
Craniofacial characteristics of the patient. Note that these are subtle with the dysmorphisms that most call attention being the narrow, slightly upslanting palpebral fissures and overfolding of the ear helix
The patient had a history of recurrent urinary tract infections but abdominal ultrasound was normal. A cardiac evaluation showed a systolic murmur, and echocardiography showed a slight dilatation of the aorta. The red blood and white blood cell counts, as well as thyroid function tests and measurement of calcium and immunoglobulin levels were normal.
Interestingly, the blood tests always showed thrombocytopenia (platelet counts ranged from 128,000 to 175,000/µL with the reference range being 200,000-400,000/µL) and large platelets. The patient presented with episodes of epistaxis requiring cauterization, besides bruises on the arms and legs. The prothrombin time, thrombin time, partial thromboplastin time, bleeding time, levels of fibrinogen and the platelet aggregation test were all normal (Table 1).
Table 1.
Clinical findings presented by the patient compared to frequencies described in the literature for 22q11DS
| Clinical features | Patient | 22q11DS* |
| % | ||
| Neuropsychomotor delay | + | 68-96 |
| Speech delay | + | 11-92 |
| Seizures | - | 21-40 |
| Psychiatric disorder | + | 9-58 |
| Congenital heart defect | - | 26-75 |
| Hearing loss | + | 28-39 |
| Cleft palate | - | 9-31 |
| Immunodeficiency | - | 40-95 |
| Hypocalcemia | - | 15-64 |
| Renal disorders | - | 2-36 |
| Thrombocytopenia | + | 12-28 |
* Rosa et al. (1)
Discussion
Macrothrombocytopenia has been a finding frequently observed in patients with 22q11DS. However, it has not been correlated with the presence of conotruncal cardiac defects or with immunological findings, both of which are common in the syndrome. It occurs because the vast majority of patients with 22q11DS (> 90%) are heterozygous for a deletion of the GPIb gene, and so are heterozygotes for Bernard-Soulier syndrome (BSS), a rare autosomal recessive coagulation disorder.(3-6) This finding is also consistent with the observation that macrothrombocytopenia is described even in patients with heterozygous mutations of the GPIb gene.(5) This gene encodes a subunit of the platelet GPIb-IX-V receptor, which is critical for platelet adhesion and important for thrombin aggregation and activation.(5,6)
Patients with 22q11DS present with a significant drop in platelet count, while the average size and volume of platelets is increased, but not as markedly as in BSS. In some cases of 22q11DS, platelet aggregation may also be reduced due to the GPIb-dependent agonist, ristocetin, and there may be a reduced response to the thrombin receptor-activating peptide(6) as seen in BSS. However, in general, patients with 22q11DS do not manifest an increased tendency of bleeding. The degree of thrombocytopenia, which is more pronounced in BSS, is considered the most important predictor for the risk of bleeding.(7)
Thus, although there are generally no important clinical implications some studies have suggested that thrombocytopenia and increased platelet size (especially > 10 fL) might be used as true indicators in the clinical diagnosis of 22q11DS.(7-9) This would facilitate the identification of patients to be tested for 22q11DS using the FISH technique.
Our patient was not diagnosed with BSS as his bleeding and prothrombin times as well as the ristocetin platelet aggregation test were normal. Moreover, despite the history of epistaxis and bruising, he did not have episodes of major bleeding. However, cases of BSS, although rare, have been described in individuals with 22q11DS. In these, there is the combination of haploinsufficiency of the GPIb gene in the deficient chromosome 22 due to a microdeletion and an allelic mutation of the intact chromosome 22.(10,11)
Reports of patients with immune thrombocytopenia, with or without autoimmune hemolytic anemia, that is, Evans syndrome, have also been published.(3,12-14) It is estimated that immune thrombocytopenic purpura is about 200 times more common in individuals with 22q11DS than in the general population;(3,6) it is believed that this is mainly related to immunodeficiency, a common finding in 22q11DS (40-95% of patients).(3) Akar & Adekile also reported a patient simultaneously presenting large platelets, platelet dysfunction and immune thrombocytopenia.(3)
Saito et al. described a patient who, besides presenting large platelets and thrombocytopenia, had leukocyte inclusion bodies. This finding suggests the presence of the May-Hegglin anomaly or Sebastian syndrome.(15) Interestingly, the gene associated to these conditions and to Fechtner syndrome encodes the non-muscle myosin heavy chain 9 (MYH9) which is located on 22q11. Saito et al. suggest that 22q11DS patients should be better investigated not only in respect to the platelet size but also to the presence of these leukocyte inclusion bodies in order to determine whether they belong to the group of hematologic abnormalities associated with the syndrome.(15)
Thus, hematological changes observed in 22q11DS are directly related to the genetic disorder itself and secondary to some clinical findings, such as immunodeficiency. Macrothrombocytopenia is increasingly being considered a feature of the broad spectrum of 22q11DS and may potentially be a clinical marker for the syndrome. We believe that, as stated by Latger-Cannard et al.,(2) 22q11DS, due to its prevalence, should be considered a possible etiology of hereditary disorders of large platelets.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1.Rosa RM, Zen PG, Roman T, Graziadio C, Paskulin GA.Síndrome de deleção 22q11.2: compreendendo o CATCH22 Rev Paul Pediatr 200927 (2): 211-220 [Google Scholar]
- 2.Latger-Cannard V, Bensoussan D, Grégoire MJ, Marcon F, Cloez JL, Leheup B, et al. Frequency of thrombocytopenia and large platelets correlates neither with conotruncal cardiac anomalies nor immunological features in the chromosome 22q11.2 deletion syndrome Eur J Pediatr 2004163 (6): 327-328 [DOI] [PubMed] [Google Scholar]
- 3.Akar NA, Adekile AD.Chromosome 22q11.2 deletion presenting with immune-mediated cytopenias, macrothrombocytopenia and platelet dysfunction Med Princ Pract 200716 (4): 318-320 [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Kosaka K, Kimura M, Imamura S, Yamada O, Iwai K, et al. Thrombocytopenia in patients with 22q11.2 deletion syndrome and its association with glycoprotein Ib-beta Genet Med 20035 (2): 113-119 [DOI] [PubMed] [Google Scholar]
- 5.Lawrence S, McDonald-McGinn DM, Zackai E, Sullivan KE.Thrombocytopenia in patients with chromosome 22q11.2 deletion syndrome J Pediatr 2003143 (2): 277-278 [DOI] [PubMed] [Google Scholar]
- 6.Liang HP, Morel-Kopp MC, Curtin J, Wilson M, Hewson J, Chen W, et al. Heterozygous loss of platelet glycoprotein (GP) Ib-V-IX variably affects platelet function in velocardiofacial syndrome (VCFS) patients Thromb Haemost 200798 (6): 1298-1308 [PubMed] [Google Scholar]
- 7.Van Geet C, Devriendt K, Eyskens B, Vermylen J, Hoylaerts MF.Velocardiofacial syndrome patients with a heterozygous chromosome 22q11 deletion have giant platelets Pediatr Res 199844 (4): 607-611 [DOI] [PubMed] [Google Scholar]
- 8.Lazier K, Chow EW, AbdelMalik P, Scutt LE, Weksbergs R, Bassett AS.Low platelet count in a 22q11 deletion syndrome subtype of schizophrenia Schizophr Res 200150 (3): 177-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naqvi N, Davidson SJ, Wong D, Cullinan P, Roughton M, Doughty VL, et al. Predicting 22q11.2 deletion syndrome: A novel method using the routine full blood count. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.02.027. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Budarf ML, Konkle BA, Ludlow LB, Michaud D, Li M, Yamashiro DJ, et al. Identification of a patient with Bernard-Soulier syndrome and a deletion in the DiGeorge/velo-cardio-facial chromosomal region in 22q11.2 Hum Mol Genet 19954 (4): 763-766 [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa M, Okuno M, Okamoto N, Fujino H, Kato H.Bernard-Soulier syndrome associated with 22q11.2 microdeletion Am J Med Genet 200199 (4): 286-288 [DOI] [PubMed] [Google Scholar]
- 12.DePiero AD, Lourie EM, Berman BW, Robin NH, Zinn AB, Hostoffer RW.Recurrent immune cytopenias in two patients with DiGeorge/velocardiofacial syndrome J Pediatr 1997131 (3): 484-486 [DOI] [PubMed] [Google Scholar]
- 13.Lévy A, Michel G, Lemerrer M, Philip N.Idiopathic thrombocytopenic purpura in two mothers of children with DiGeorge sequence: a new component manifestation of deletion 22q11? Am J Med Genet 199769 (4): 356-359 [DOI] [PubMed] [Google Scholar]
- 14.Kratz CP, Niehues T, Lyding S, Heusch A, Janssen G, Göbel U.Evans syndrome in a patient with chromosome 22q11.2 deletion syndrome: a case report Pediatr Hematol Oncol 200320 (2): 167-172 [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Ishikawa T, Ito Y, Shimizu H.Hematological abnormalities in a patient with a 22q11.2 deletion Brain Dev 200426 (5): 342-344 [DOI] [PubMed] [Google Scholar]


