Abstract
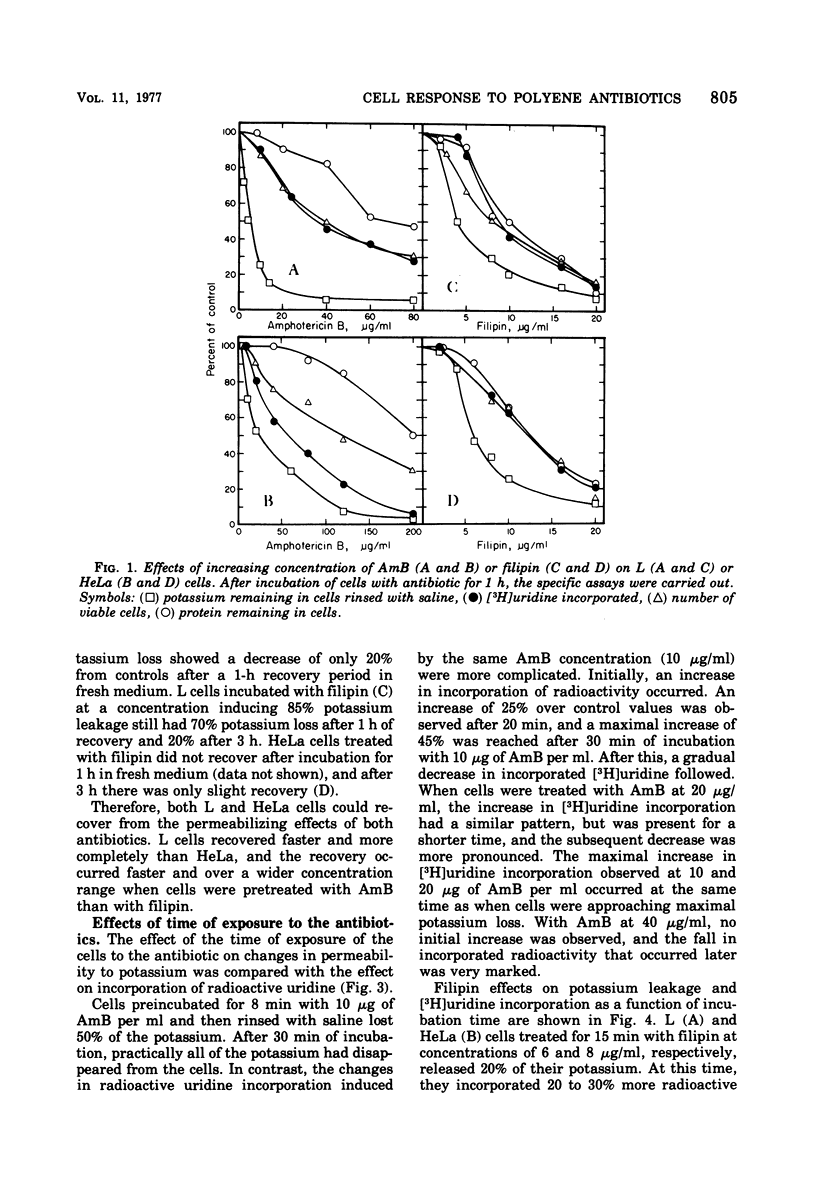
Amphotericin B (AmB) and filipin effects on L and HeLa cells were compared by monitoring drug-induced potassium leakage from cells, changes in radioactive uridine incorporation into cellular ribonucleic acid, protein leakage from cells, and cell viability. L cells were much more susceptible to both AmB and filipin than were HeLa cells, but the overall dose response was similar. For AmB, the various effects were easily separable. Potassium leakage occurred at the lowest concentrations of AmB and was reversible. Inhibition of uridine incorporation and loss of viability occurred at intermediate levels, and protein loss occurred at higher levels. In contrast, filipin was much more potent; its effects on potassium leakage were only minimally reversible, and the separation of the permeabilizing effects from complete cell lysis was possible only over a limited concentration range and for a short time.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaracco G., Cassani G. Ribonucleic acid synthesis dependent on exogenous triphosphates in nystatin-treated cells of of Kluyveromyces latis. Antimicrob Agents Chemother. 1976 May;9(5):748–753. doi: 10.1128/aac.9.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass A., Finkelstein A., Krespi V. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):100–124. doi: 10.1085/jgp.56.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent M. P., Prestegard J. H. Interaction of the polyene antibiotics with lipid bilayer vesicles containing cholesterol. Biochim Biophys Acta. 1976 Feb 19;426(1):17–30. doi: 10.1016/0005-2736(76)90425-9. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol Rev. 1973 Jun;37(2):166–196. [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Effect of polyene antibiotics on protoplasts of Neurospora crassa. J Bacteriol. 1962 Feb;83:351–358. doi: 10.1128/jb.83.2.351-358.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. V., Medoff G., Kobayashi G., Schlessinger D. Uptake of Escherichia coli DNA into HeLa cells enhanced by amphotericin B. Nature. 1974 Jul 26;250(464):323–325. doi: 10.1038/250323a0. [DOI] [PubMed] [Google Scholar]
- Kwan C. N., Medoff G., Kobayashi G. S., Schlessinger D., Raskas H. J. Potentiation of the antifungal effects of antibiotics by amphotericin B. Antimicrob Agents Chemother. 1972 Aug;2(2):61–65. doi: 10.1128/aac.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. A., Hammond S. M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem Biophys Res Commun. 1973 Sep 18;54(2):796–799. doi: 10.1016/0006-291x(73)91494-0. [DOI] [PubMed] [Google Scholar]
- Medoff G., Valeriote F., Lynch R. G., Schlessinger D., Kobayashi G. S. Synergistic effect of amphotericin B and 1,3-bis(2-chloroethyl)-1-nitrosourea against a transplantable AKR leukemia. Cancer Res. 1974 May;34(5):974–978. [PubMed] [Google Scholar]
- Norman A. W., Spielvogel A. M., Wong R. G. Polyene antibiotic - sterol interaction. Adv Lipid Res. 1976;14:127–170. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Rossomando E. F., Creme G., Maldonado B., Hesla M. A., Golub E. E. Effect of amphotericin B on growth and membrane permeability in Dictyostelium discoideum. Antimicrob Agents Chemother. 1976 Apr;9(4):618–624. doi: 10.1128/aac.9.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadomy S., Wagner G., Espinel-Ingroff E., Davis B. A. In vitro studies with combinations of 5-fluorocytosine and amphotericin B. Antimicrob Agents Chemother. 1975 Aug;8(2):117–121. doi: 10.1128/aac.8.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E. F., Holler B. W. Methylation of 45 s ribosomal RNA precursor in HeLa cells. J Mol Biol. 1967 Jan 28;23(2):149–161. doi: 10.1016/s0022-2836(67)80023-8. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]



