Abstract
Background
We previously demonstrated that 25-hydroxyvitamin D3 concentrations in gingival crevicular fluid are 300 times higher than those in the plasma of patients with aggressive periodontitis. Here we explored whether 25-hydroxyvitamin D3 can be synthesized by periodontal soft tissue cells. We also investigated which of the two main kinds of hydroxylases, CYP27A1 and CYP2R1, is the key 25-hydroxylase in periodontal soft tissue cells.
Methodology/Principal Findings
Primary cultures of human gingival fibroblasts and periodontal ligament cells from 5 individual donors were established. CYP27A1 mRNA, CYP2R1 mRNA and CYP27A1 protein were detected in human gingival fibroblasts and periodontal ligament cells, whereas CYP2R1 protein was not. After incubation with the 25-hydroxylase substrate vitamin D3, human gingival fibroblasts and periodontal ligament cells generated detectable 25-hydroxyvitamin D3 that resulted in the production of 1α,25-dihydroxyvitamin D3. Specific knockdown of CYP27A1 in human gingival fibroblasts and periodontal ligament cells using siRNA resulted in a significant reduction in both 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 production. Knockdown of CYP2R1 did not significantly influence 25-hydroxyvitamin D3 synthesis. Sodium butyrate did not influence significantly CYP27A1 mRNA expression; however, interleukin-1β and Porphyromonas gingivalis lipopolysaccharide strongly induced CYP27A1 mRNA expression in human gingival fibroblasts and periodontal ligament cells.
Conclusions
The activity of 25-hydroxylase was verified in human gingival fibroblasts and periodontal ligament cells, and CYP27A1 was identified as the key 25-hydroxylase in these cells.
Introduction
Vitamin D plays an important role in the regulation of bone metabolism and immunological reactions [1], [2]. In humans, vitamin D, in the form of vitamin D3, is derived from dietary sources or made from 7-dehydrocholesterol in the skin by exposure to ultraviolet rays [3], [4], [5], [6], [7]. Then, vitamin D3 is metabolized by two-step hydroxylations: first 25-hydroxylation in the liver to form 25-hydroxyvitamin D3 (25OHD3), the major circulating metabolite of vitamin D3, followed by 1,α-hydroxylation in the kidney to form 1α,25-dihydroxyvitamin D3 (1,25OH2D3), the biologically active metabolite of vitamin D3 [6], [7].
In the early years of biochemical research, a mitochondrial cytochrome P450 (CYP27A1), an important enzyme in the bile acid synthesis pathway [8], [9], was demonstrated to be 25-hydroxylase. Afterwards, Cheng et al. identified a microsomal cytochrome P450 (CYP2R1) with vitamin D 25-hydroxylase activity [10], [11]. In addition, other cytochrome P450 enzymes, such as CYP2C11, CYP2D25, CYP3A4 and CYP2J2, were all identified as vitamin D 25-hydroxylases [12], [13], [14], and the two most active 25-hydroxylases were found to be CYP27A1 and CYP2R1 [10]. It was reported that CYP27A1 was the more abundant 25-hydroxylase in the liver [10], [15]. However, mutations in human and mouse genes encoding CYP27A1 protein influenced bile acid synthesis, but had no consequence on vitamin D metabolism [15], [16], [17], [18]. Thus, the question as to which of these proteins is the key 25-hydroxylase in the liver remains controversial. In addition, it was reported that, besides the liver, there are extra-hepatic sites of 25OHD3 synthesis, including the skin [7], [19], [20], [21], prostate [22], [23], macrophages [24], [25], [26], and endothelial cells [24].
Human gingival fibroblasts (hGF) and human periodontal ligament cells (hPDLC) are two kinds of periodontal fibroblasts and are important components of periodontal soft tissues. Our previous study demonstrated that local 25OHD3 levels in gingival crevicular fluid were about 300 times higher than that in the plasma of patients with aggressive periodontitis [27], [28]. Since there is abundant 25OHD3 around periodontal soft tissues, it was hypothesized that hGF and hPDLC have 25-hydroxylase activity, and can synthesize 25OHD3. The objective of this study was to test this hypothesis.
Results
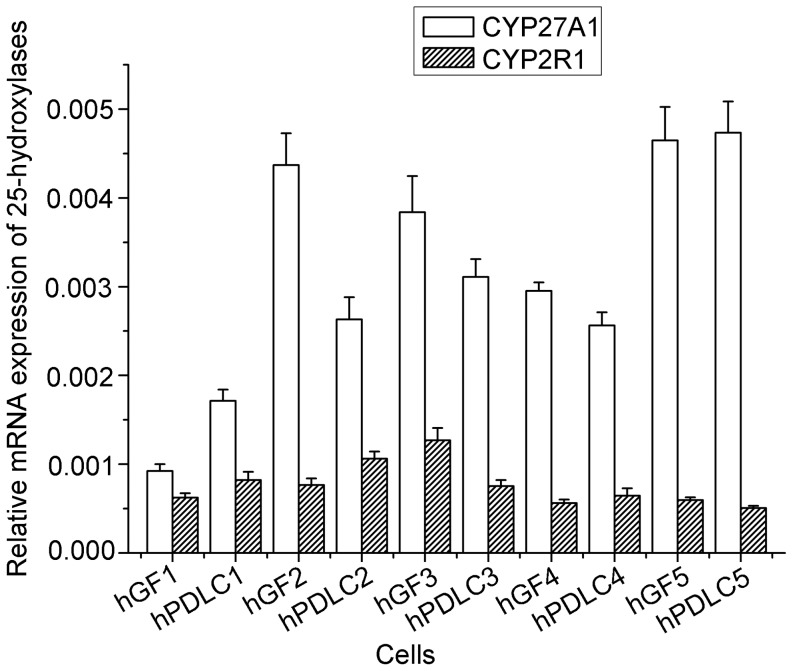
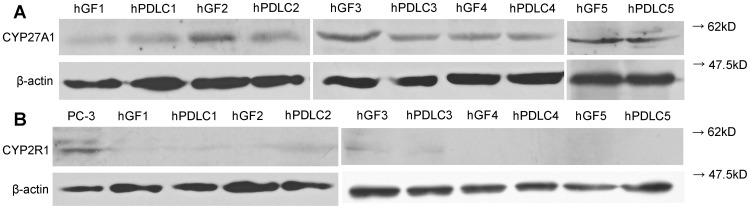
CYP27A1 and CYP2R1 mRNA were detected in all the cells of the five donors, and no significant difference was found between the mRNA levels in hGF and hPDLC (Fig. 1). CYP27A1 protein was also detected in all cells of the five donors, whereas CYP2R1 was not detected, with the premise that anti-CYP2R1 antibody was able to recognize the protein in PC-3 cells, which were used as a positive control (Fig. 2). This indicated that CYP27A1 might be the key 25-hydroxylase in hGF and hPDLC.
Figure 1. Expression of CYP27A1 and CYP2R1 mRNA in hGF and hPDLC.
Expression of CYP27B1 mRNA was detected by real-time PCR in hGF and hPDLC from all five donors (donors are numbered 1–5). The expression levels of CYP27A1 and CYP2R1 mRNA were not significantly different in the two kinds of cells. The data are presented as the mean ± SD.
Figure 2. Protein expression of CYP27A1 and CYP2R1 in hGF and hPDLC.
Protein expression of CYP27A1 was detected by Western blot in hGF and hPDLC from all five donors (donors are numbered 1–5). Protein expression of CYP2R1 was detected by Western blot in PC-3 cells, which were used as a positive control, but was not detected in hGF and hPDLC. β-actin was used as an internal control.
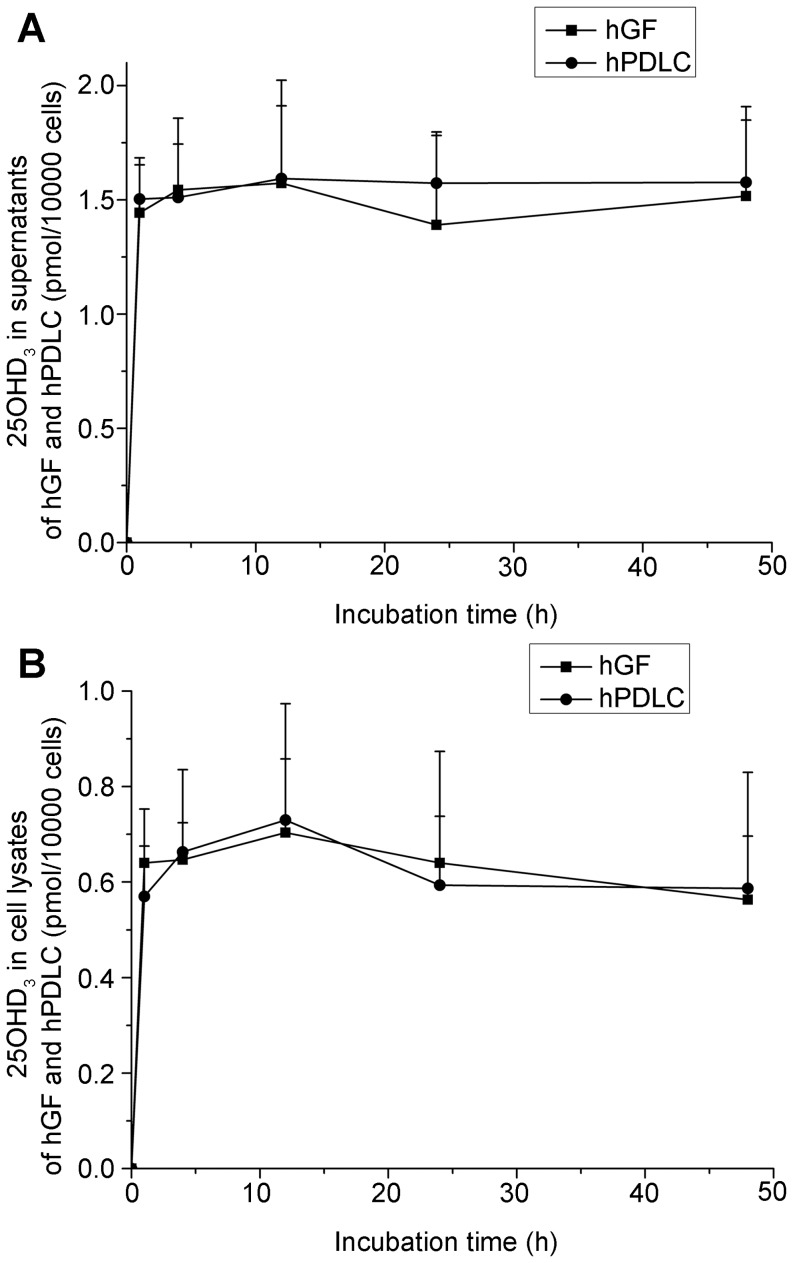
After confirming the expression of 25-hydroxylase in hGF and hPDLC, the function of 25-hydroxylase was investigated. Whereas 1000 nM vitamin D3 did not have a significant cytotoxic effect on any of the cells within 48 h, hGF and hPDLC generated 25OHD3 in response to vitamin D3 (Figs. 3A, B). The fact that extra- and intracellular 25OHD3 was generated in the presence of vitamin D3 provides direct and convincing evidence of the existence of 25-hydroxylase in hGF and hPDLC. At all time points, there was no significant difference in the levels of intracellular and extracellular 25OHD3 between the two cell types.
Figure 3. Activity of 25-hydroxylases in hGF and hPDLC.
hGF and hPDLC from donors 2, 4 and 5 were incubated with 1000 nM vitamin D3 for the times indicated, and the production of 25OHD3 was determined in supernatants(A) and cell lysates (B). After incubation, the production of 25OHD3 was detected. The amount of 25OHD3 generated was not significantly different between hGF and hPDLC. The data are presented as the mean ± SE.
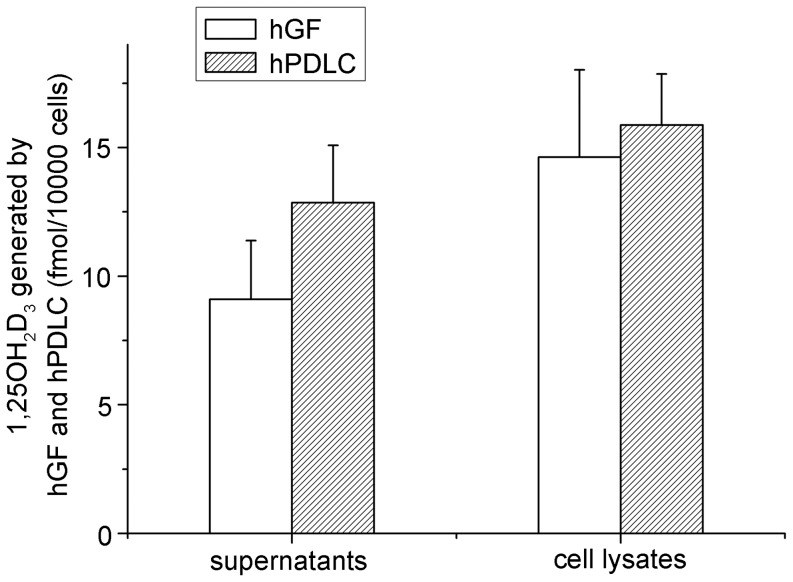
Additionally, exposure to vitamin D3 also resulted in the synthesis of 1,25OH2D3 in hGF and hPDLC (Fig. 4). The observation that hGF and hPDLC could synthesize 1,25OH2D3 when exposed to 25OHD3 [29] is further evidence of 25-hydroxylase activity in hGF and hPDLC.
Figure 4. 1,25OH2D3 generation by hGF and hPDLC.
hGF and hPDLC from donors 2, 4 and 5 were incubated with 1000 nM vitamin D3 for 48 h, and the production of 1,25OH2D3 was determined in supernatants and cell lysates. The amount of 1,25OH2D3 generated was not significantly different between hGF and hPDLC. The data are presented as the mean ± SE.
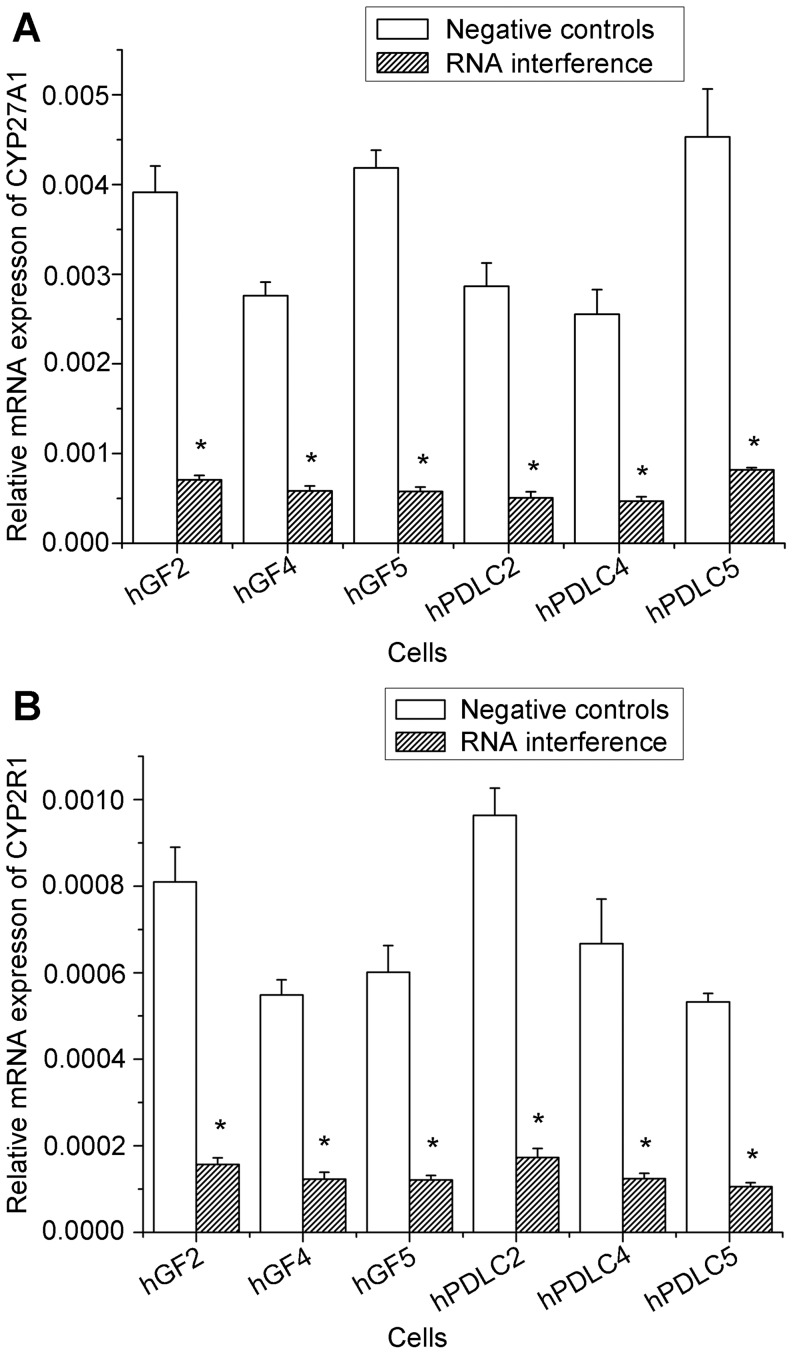
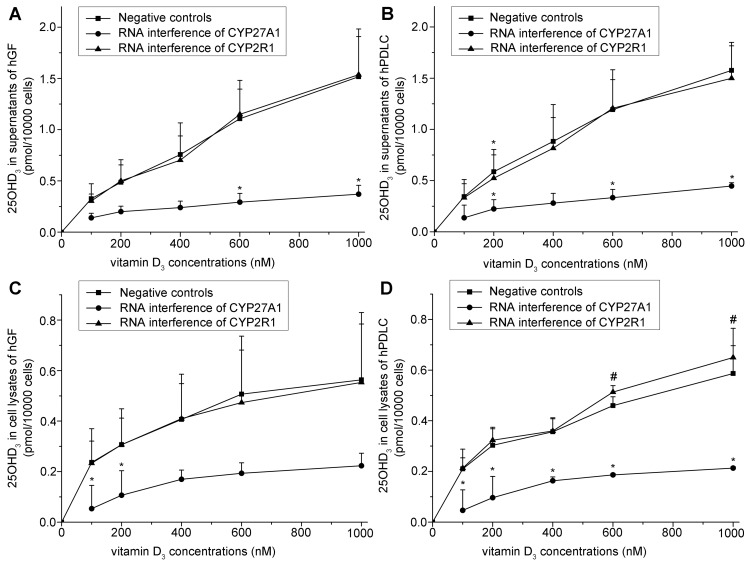
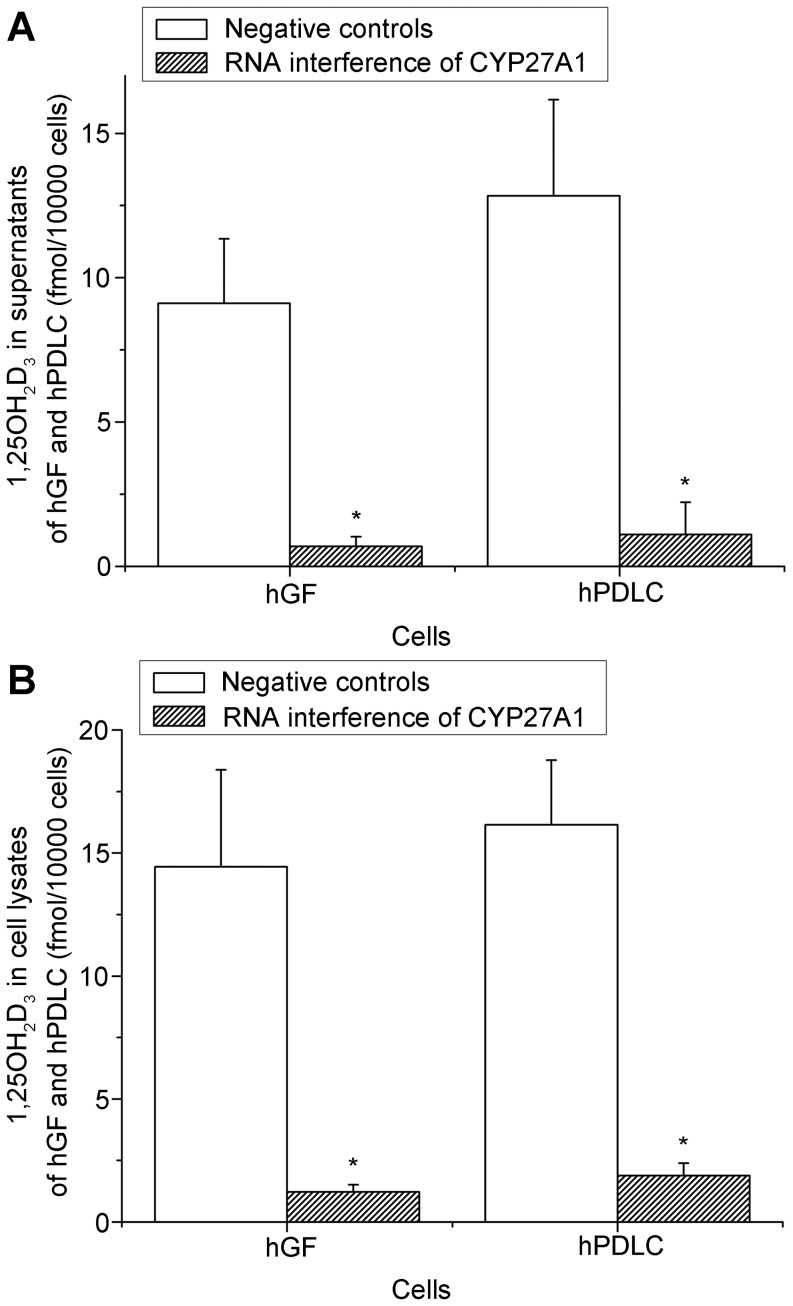
Based on the above direct evidence for 25-hydroxylase activity in hGF and hPDLC, we examined the effect of 25-hydroxylase knockdown. The efficiency of RNA interference against both CYP27A1 and CYP2R1 was both over 70% (Fig. 5). The generation of 25OHD3 increased with increasing vitamin D3 concentrations, but dropped significantly when CYP27A1 was knocked down using specific siRNA (Figs. 6A–D). However, knockdown of CYP2R1 did not significantly influence 25OHD3 generation by hGF (Figs. 6A, C), and only slightly influenced 25OHD3 generation by hPDLC (Figs. 6B, D). These results suggest that CYP27A1 might be the key 25-hydroxylase in hGF and hPDLC. In addition, knockdown of CYP27A1 resulted in a significant reduction of 1,25OH2D3 generation (Figs. 7A–B). This is additional evidence for the activity of CYP27A1 as the 25-hydroxylase in hGF and hPDLC.
Figure 5. The efficiency of RNA interference against CYP27A1 and CYP2R1.
hGF and hPDLC from donors 2, 4 and 5 were transfected with a siRNA oligonucleotide for CYP27B1, a siRNA oligonucleotide for CYP2R1, or a non-silencing control. Using real-time PCR as a measure, the efficiency of RNA interference against CYP27A1 and CYP2R1 was over 70% in hGF and hPDLC. The data are presented as the mean ± SD. * denotes difference from negative controls (p<0.05).
Figure 6. Effect of knockdown of 25-hydroxylases on 25OHD3 generation.
hGF and hPDLC from donors 2, 4 and 5 were treated with vitamin D3 at various concentrations indicated in the figure for 12 h after transfection with a siRNA oligonucleotide for CYP27A1, a siRNA oligonucleotide for CYP2R1, or a non-silencing control. 25OHD3 production was measured in supernatants of hGF (A), supernatants of hPDLC (B), cell lysates of hGF (C), and cell lysates of hPDLC (D). When CYP27A1 or CYP2R1 was not knocked down, the production of 25OHD3 increased with an increasing concentration of 25OHD3. When CYP27A1 was knocked down in hGF and hPDLC, the generation of 25OHD3 decreased significantly compared to when CYP27A1 was not knocked down. When CYP2R1 was knocked down in hGF (A, C), the generation of 25OHD3 was not significantly different from that when CYP2R1 was not knocked down. When CYP2R1 was knocked down in hPDLC (B, D), the generation of 25OHD3 was only slightly different at some time points from that when CYP2R1 was not knocked down. The data are presented as the mean ± SE. * hGF or hPDLC generated significantly less 25OHD3 with the same amount of added vitamin D3 when CYP27A1 or CYP2R1 was knocked down (p<0.05). # hGF or hPDLC generated significantly more 25OHD3 with the same amount of added vitamin D3 when CYP27A1 or CYP2R1 was knocked down (p<0.05).
Figure 7. The effect of 25-hydroxylase knockdown on 1,25OH2D3 generation.
hGF and hPDLC from donors 2, 4 and 5 were treated with 1000 nM vitamin D3 for 48 h after transfection with a siRNA oligonucleotide for CYP27A1 or a non-silencing control, and 1,25OH2D3 production was measured in supernatants(A) and cell lysates (B). When CYP27A1 was knocked down, the generation of 1,25OH2D3 decreased significantly compared to when CYP27A1 was not knocked down. The data are presented as the mean ± SE. * hGF or hPDLC generated significantly less 1,25OH2D3 with 1000 nM vitamin D3 when CYP27A1 was knocked down (p<0.05).
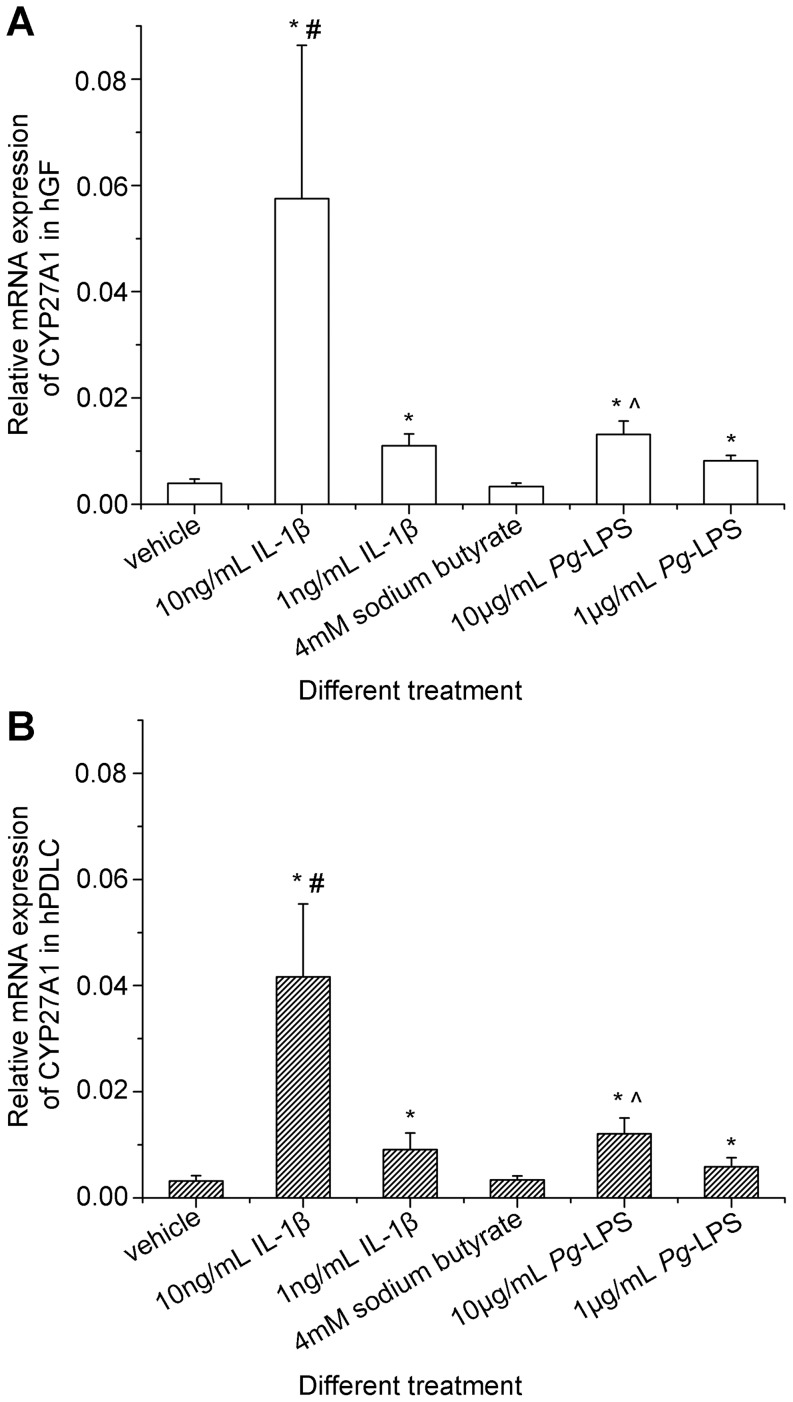
After the comprehensive confirmation of 25-hydroxylase activity in hGF and hPDLC, and the verification of CYP27A1 as the key 25-hydroxylase, the regulation of CYP27A1 in hGF and hPDLC was investigated. Interleukin-1β (IL-1β) and Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) strongly induced CYP27A1 expression (Fig. 8). Additionally, dose-dependent increases in expression of CYP27A1 mRNA in hGF and hPDLC following incubation with IL-1β or Pg-LPS were demonstrated (Fig. 8). By contrast, sodium butyrate did not influence significantly CYP27A1 mRNA expression in hGF and hPDLC (Fig. 8). In addition, no significant differences between hGF and hPDLC were observed in the regulation of CYP27A1.
Figure 8. Preliminary investigation of CYP27A1 regulation by inflammatory stimuli in hGF and hPDLC.
hGF and hPDLC from donors 2, 3, 4 and 5 were stimulated with different treatments indicated in the figure for 24 h, and CYP27A1 mRNA expression was determined by real-time PCR. IL-1β and Pg-LPS significantly up-regulated CYP27A1 mRNA expression and the higher dose of IL-1β or Pg-LPS raised higher CYP27A1 mRNA up-regulation in both hGF and hPDLC. Sodium butyrate did not significantly influence CYP27A1 mRNA expression. Additionally, the characteristics of CYP27A1 regulation in hGF and hPDLC were not significantly different. The data are presented as the mean ± SE. * CYP27A1 mRNA expression was significantly different from that of the vehicle group (p<0.05). # CYP27A1 mRNA expression was significantly different from that of the 1 ng/mL IL-1β group (p<0.05). ∧ CYP27A1 mRNA expression was significantly different from that of the 1 µg/mL Pg-LPS group (p<0.05). IL-1ß : interleukin-1β. Pg-LPS: Porphyromonas gingivalis lipopolysaccharide.
Discussion
In the present study, our hypothesis that hGF and hPDLC have 25-hydroxylase activity, and that they can synthesize 25OHD3 was verified. Therefore, the origin of high 25OHD3 concentrations in gingival crevicular fluid [27], [28] might be hGF and hPDLC. Having demonstrated 1α-hydroxylase activity in hGF and hPDLC [29], we could consider that the conversion of vitamin D3 to 1,25OH2D3 in hGF and hPDLC consisted of two steps:
from vitamin D3 to 25OHD3, under the action of 25-hydroxylase CYP27A1;
from 25OHD3 to 1,25OH2D3, under the action of 1α-hydroxylase CYP27B1. This two-step conversion is similar to that observed in human keratinocytes [7], [19], [30], [31], [32]. In addition, Slominski et al. reported an alternate pathway of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) [33], [34], [35], [36]. P450scc activity in hGF and hPDLC is worth further investigation in our future study.
We can then calculate and compare the amount of 1,25OH2D3 synthesized from 1000 nM vitamin D3 and from 1000 nM 25OHD3. According to the present study, the amount of 1,25OH2D3 generated would be:
In hGF exposed to 1000 nM vitamin D3 for 48 h, 9 fmol/10000 cells in supernatants +14 fmol/10000 cells in cell lysates = 23 fmol/10000 cells (Fig. 4).
In hPDLC exposed to 1000 nM vitamin D3 for 48 h, 13 fmol/10000 cells in supernatants +16 fmol/10000 cells in cell lysates = 29 fmol/10000 cells (Fig. 4). According to our previous study [29], the amount of 1,25OH2D3 generated would be the following:
In hGF exposed to 1000 nM 25OHD3 for 48 h, 5 fmol/10000 cells in supernatants +13 fmol/10000 cells in cell lysates = 18 fmol/10000 cells.
In hPDLC exposed to 1000 nM 25OHD3 for 48 h, 13 fmol/10000 cells in supernatants +14 fmol/10000 cells in cell lysates = 27 fmol/10000 cells. It is interesting that 1000 nM vitamin D3 could induce hGF and hPDLC to generate even more 1,25OH2D3 than 1000 nM 25OHD3. Particular attention should be paid to the observation that after 1000 nM vitamin D3 treatment for 48 h, the 25OHD3 concentration in the cell supernatants of hGF and hPDLC were only about 45 nM–64 nM and 30 nM–50 nM respectively, much lower than the added 1000 nM vitamin D3. So, why was less 25OHD3 converted to more 1,25OH2D3? One reason might be that after vitamin D3 treatment, 25OHD3 is found not only in the supernatant, but also in the cell lysates, allowing intracellular 25OHD3 to act directly as substrate of 1α-hydroxylase. On the other hand, exogenous 25OHD3 should enter the cells before eliciting a response. Thus, the direct availability at the site of action might be of great importance.
After comprehensive verification of 25-hydroxylase activity and the demonstration of CYP27A1 as the key 25-hydroxylase in hGF and hPDLC, the regulation of CYP27A1 in these cells was preliminarily investigated. IL-1β in gingival crevicular fluids of patients with periodontitis decreases significantly after initial periodontal therapy, indicating that IL-1β is associated with periodontitis [28]. Porphyromonas gingivalis is an important pathogen of periodontitis and butyrate is one of its metabolites [37]. It was demonstrated that the butyrate concentrations in gingival crevicular fluids of patients with periodontitis are significantly higher than those of healthy controls, and that butyrate concentrations in gingival crevicular fluids are significantly correlated with periodontal inflammation [38], [39]. To investigate the regulation of CYP27A1 in hGF and hPDLC, IL-1β, Pg-LPS and sodium butyrate were chosen for the present study. It should be considered, however, that although stimuli with periodontal characteristics were used to simulate a periodontitis-like condition, this does not properly model the chronic disease situation in vivo, and can only help to investigate the regulation of CYP27A1 in hGF and hPDLC. The NF-κB activator, IL-1β, was demonstrated to be a potent up-regulator of CYP27A1 mRNA in hGF and hPDLC (Fig. 8). Pg-LPS could also up-regulate significantly the expression of CYP27A1 mRNA, whereas sodium butyrate could not. It was reported that Pg-LPS is the ligand of Toll-like receptor 2 (TLR2) and TLR4 [40], [41] and that both hGF and hPDLC expressed TLR2 and TLR4 [42]. Upon ligand binding, TLR2 or TLR4-mediated signaling could activate signal transduction, leading to NF-κB activation [43], [44]. Thus, NF-κB might be involved in the regulation of CYP27A1 expression, an observation that warrants further investigation.
Each donor supplied both hGF and hPDLC in the present study. Although hGF and hPDLC are two different kinds of cells, they shared many features in 25-hydroxylase expression, activity and regulation, and only subtle differences were detected. As shown in Fig. 6, when CYP2R1 was knocked down, 25OHD3 generation by hGF was not changed significantly, whereas 25OHD3 generation by hPDLC was affected slightly. However, the difference did not affect our conclusion that CYP27A1 might be the key 25-hydroxylase in hGF and hPDLC.
Since 1,25OH2D3 may enhance the antibacterial defense of human gingival epithelial cells [45] and hGF and hPDLC could synthesize 1,25OH2D3 with 25OHD3 [29], the confirmation of 25-hydroxylase activity in hGF and hPDLC implies that these cells could generate 25OHD3 as a substrate for 1,25OH2D3. From this perspective, 25-hydroxylase activity in hGF and hPDLC may be involved in the innate immune defense of the oral cavity. Recently, it was reported that oral calcium and vitamin D supplementation have a positive effect on periodontal health [46], [47]. However, topical application of vitamin D has not been reported. Since hGF and hPDLC have the ability to synthesize 25OHD3 and then to synthesize 1,25OH2D3, the topical application of vitamin D3 might fulfill the function of 1,25OH2D3. Thus, our data suggest a potential benefit of topical application of vitamin D3 in periodontal therapy.
In conclusion, hGF and hPDLC were identified as new extra-hepatic sites of 25OHD3 synthesis for the first time, and CYP27A1 might be the key 25-hydroxylase in these cells.
Materials and Methods
Ethics Statement
The study protocol was approved by the institutional review board of Peking University School and Hospital of Stomatology (PKUSSIRB-2011007) and written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Cell Culture
Primary culture of hGF and hPDLC was carried out according to our previous methods [29]. In brief, hPDLC were obtained from extracted third molars of 5 young healthy volunteers, and hGF was isolated from the gingiva of the same 5 donors. The periodontal ligament tissues attached to the middle third of the roots were curetted gently by a surgical scalpel, minced and placed in 24-well plates. Gingivae were also minced and transferred into 24-well plates. Tissue explants were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; PAA, Coelbe, Germany), 100 U/mL penicillin G and 100 µg/mL streptomycin. Cultures were maintained in a humidified atmosphere of 5% (v/v) CO2 at 37°C. After reaching 80% confluence, hGF and hPDLC were digested with a mixture of 0.25% (w/v) trypsin and 0.02% (w/v) EDTA, and subcultured at a 1∶3 ratio. DMEM without phenol red (Sigma, St. Louis, MO, USA), 10% (v/v) dextran-coated, charcoal-stripped FBS (DCC-FBS; TBD, Tianjin, China) and hGF and hPDLC of passage 4 were used in all the following experiments. All experiments were conducted in triplicate.
The prostate cancer cell line, PC-3 (American Type Culture Collection, Rockville, MD, USA), was cultured in RPMI 1640 (Gibco, Gaithersburg, MD, USA) supplemented with 10% (v/v) FBS (FBS; PAA, Coelbe, Germany) in a humidified atmosphere of 5% CO2 at 37°C and was used when the cells were in the logarithmic phase and reached 80% confluence.
Cytotoxicity Test of Vitamin D3
hGF and hPDLC of three donors were used in the cytotoxicity test. hGF and hPDLC in their logarithmic growth phase were plated into 96-well plates at a density of 3000 cells/well in DMEM with 10% DCC-FBS, and the medium was replaced by DMEM without DCC-FBS after 24 h. After another 24 h, the medium was changed to DMEM with 10% DCC-FBS, and supplemented with 1000 nM vitamin D3 or vehicle, respectively. The cytotoxicity test was carried out according to the Cell Counting Kit-8 protocol (CCK-8; Dojindo, Kumamoto, Japan). At hours 0, 24 and 48, cells were incubated with CCK-8 for the last 3 h of the culture period, after which the optical density values (OD values) were detected at 490 nm with a microplate reader (Bio-Rad Model 550, Hercules, CA, USA).
Detection of 25-hydroxylase Expression
hGF and hPDLC from all five donors were seeded into six-well plates at a density of 5000 cm−2 in DMEM supplemented with 10% DCC-FBS. Four days later, a portion of the cells were harvested using Trizol agent (Dongsheng Biotech, Guangzhou, China). RNA was extracted using Trizol according to the manufacturer’s instructions, and was reverse transcribed to cDNA using a reverse transcription kit (Bio-Rad, Hercules, CA, USA). Real-time PCR reactions were accomplished using SYBR® Premix Ex Taq™ II (TaKaRa Biotechnology, Dalian, China) in an ABI 7500 real-time Thermocycler (Applied Biosystems, Foster City, CA, USA). The data were analyzed using the SDS software, according to the manufacturer’s instructions.
Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was used as an internal control. Data were presented as relative mRNA levels calculated by the equation 2−ΔCt (ΔCt = Ct of target gene minus Ct of GAPDH) [48]. The primers used are listed in Table 1.
Table 1. Primer sequences used for PCR or real-time PCR.
| Target genes | Forward primer (5′ →3′) | Reverse primer (5′ →3′) | Products (bp) |
| CYP27A1 | GCTCTTGGAGCAAGTGATG | AGCATCCGTATAGAGCGC | 196 |
| CYP2R1 | TTGGAGGCATATCAACTGTGGT | CTCGGCCATATCTGGAATTGAG | 153 |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | 226 |
PC-3 cells and the remaining hGF and hPDLC were harvested using lysis buffer [20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% (v/v) Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate and 2 mM Na3VO4 supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany)] [29] for Western blotting. The protein concentration was determined using the Bicinchoninic Acid Protein Assay Kit (Applygen, Beijing, China). Twenty micrograms of total protein from each sample were loaded onto a gel comprising a 5% (w/v) stacking gel and a 10% (w/v) running gel. At the end of the electrophoresis, samples were transferred onto nitrocellulose blotting membranes (Hybond™; Amersham Pharmacia, Little Chalfont, UK). Blots were probed with a goat polyclonal antibody to CYP27A1 (diluted 1∶200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), a mouse polyclonal antibody to CYP2R1 (diluted 1∶500; ABCAM, Cambridge, UK) or a mouse monoclonal antibody to β-actin (diluted 1∶1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, blots were incubated with horseradish peroxidase-linked secondary antibody. The secondary antibodies against sheep (Kirkegaard & Perry Laboratories, Inc., Maryland, USA) and mouse (Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China) IgG were both diluted 1∶2500. Antigen-antibody complexes were detected using the Enhanced Chemiluminescence reagent (Applygen, Beijing, China).
Detection of 25OHD3 Production
Cells from 3 donors were treated with 1000 nM vitamin D3 (Sigma, St. Louis, MO, USA) for 1, 4, 12, 24 or 48 h, after which supernatants were collected, and the cells were scraped in PBS containing 0.2% Triton X-100 and stored at −80°C. Prior to use, cell lysates were sonicated on ice in a sonifier cell disrupter for 2×15 s. The levels of 25OHD3 in cell supernatants and cell lysates were detected using a 25OHD3 radioimmunoassay kit (DiaSorin, Stillwater, MN, USA) with a sensitivity of 1.5 ng/mL.
Detection of 1,25OH2D3 Production
Cells from 3 donors were treated with 1000 nM vitamin D3 (Sigma, St. Louis, MO, USA) for 48 h and then supernatants were collected and cells were scraped in PBS containing 0.2% Triton X-100 and stored at −80°C. Prior to use, cell lysates were sonicated on ice in a sonifier cell disrupter for 2×15 s. The levels of 1,25OH2D3 in cell supernatants and cell lysates were determined using a 1,25OH2D3 radioimmunoassay kit (DiaSorin, Stillwater, MN, USA). The sensitivity of the assay was 2.0 pg/mL.
RNA Interference of 25-hydroxylase
To confirm the dependence of vitamin D3 conversion to 25OHD3 on 25-hydroxylase, the highly specific technique of RNA interference was utilized. Cells were seeded at a density of 15000 cm−2 in six-well plates. Eight hours later, the cells were transfected with either CYP27A1 siRNA (10 nM) or CYP2R1 siRNA (10 nM), or a non-silencing control siRNA using Hiperfect™ transfection reagent (Qiagen, Duesseldorf, Germany), according to the manufacturer's instructions. The target sequence of CYP27A1 siRNA was 5′- CACGCTGACATGGGCCCTGTA -3′, the target sequence of CYP2R1 siRNA was 5′- TGGGTTGATCACAGACGATTA -3′, and the non-silencing control was a non-homologous, scrambled sequence equivalent.
Sixty hours after transfection, cells were harvested, RNA and cDNA were obtained, and real-time PCR was performed as described earlier to test the effect of RNAi.
After confirming the effect of RNAi, 25OHD3 production after RNAi was determined. Cells were first transfected with CYP27A1 siRNA (10 nM) or CYP2R1 siRNA (10 nM), or non-silencing control siRNA. Twelve hours after transfection, these cells were treated with 100 nM, 200 nM, 400 nM, 600 nM or 1000 nM vitamin D3 (Sigma, St. Louis, MO, USA) for another 12 h. Then, the 25OHD3 concentrations in the cell supernatants and cell lysates were determined as described earlier.
Some other cells were first transfected with CYP27A1 siRNA (10 nM), or non-silencing control siRNA, and 12 h after transfection, these cells were treated with 1000 nM vitamin D3 (Sigma, St. Louis, MO, USA) for another 48 h. Then, the 1,25OH2D3 concentrations in the cell supernatants and cell lysates were detected as described earlier.
Regulation of CYP27A1 in hGF and hPDLC
Cells from four donors were seeded into six-well plates at a density of 5000 cm−2 in DMEM supplemented with 10% DCC-FBS. Four days later, cells were incubated with IL-1β (PeproTech, London, UK; 1 ng/mL and 10 ng/mL), Pg-LPS (Invivogen, San Diego, CA, USA; 1 µg/mL and 10 µg/mL) or sodium butyrate (SCRC, Shanghai, China; 4 mM) for 24 h. Then mRNA expression was detected by real-time PCR as described previously.
Statistical Methods
The Shapiro-Wilk test was used to determinate the distribution of the variants. The paired samples t-test was used to compare differences of the mRNA expression levels of CYP27A1 and CYP2R1 between hGF and hPDLC, differences of 25OHD3 generation by hGF and hPDLC, and the effect of RNA interference. Comparison of 25OHD3 generation with and without knockdown of 25-hydroxylase, and 1,25OH2D3 generation with and without knockdown of CYP27A1 were also performed using a paired samples t-test. The impact of stimulation on CYP27A1 mRNA expression was analyzed using a paired-samples t-test, and the difference between CYP27A1 regulation in hGF and hPDLC was analyzed using a Wilcoxon test.
Statistical analyses were accomplished using the SPSS 11.5 software package (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Funding Statement
This research was funded by National Natural Science Foundations of China (http://www.nsfc.gov.cn/Portal0/default152.htm) (30772420, 81100749) and Beijing Natural Science Foundation (http://www.bjkw.gov.cn/n1143/n1240/n1465/n2276/index.html) (7102164). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Christakos S, Dhawan P, Liu Y, Peng X, Porta A (2003) New insights into the mechanisms of vitamin D action. J Cell Biochem 88: 695–705. [DOI] [PubMed] [Google Scholar]
- 2. von EM, Kongsbak M, Schjerling P, Olgaard K, Odum N, et al. (2010) Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 11: 344–349. [DOI] [PubMed] [Google Scholar]
- 3. Holick MF, Clark MB (1978) The photobiogenesis and metabolism of vitamin D. Fed Proc. 37: 2567–2574. [PubMed] [Google Scholar]
- 4. Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JJ, et al. (1980) Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 210: 203–205. [DOI] [PubMed] [Google Scholar]
- 5. Holick MF (1981) The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol 77: 51–58. [DOI] [PubMed] [Google Scholar]
- 6. Jones G, Strugnell SA, DeLuca HF (1998) Current understanding of the molecular actions of vitamin D. Physiol Rev. 78: 1193–1231. [DOI] [PubMed] [Google Scholar]
- 7. Bikle DD (2011) Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 347: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cali JJ, Russell DW (1991) Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem 266: 7774–7778. [PubMed] [Google Scholar]
- 9. Okuda K, Usui E, Ohyama Y (1995) Recent progress in enzymology and molecular biology of enzymes involved in vitamin D metabolism. J Lipid Res 36: 1641–1652. [PubMed] [Google Scholar]
- 10. Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW (2003) De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem 278: 38084–38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (2004) Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 101: 7711–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamasaki T, Izumi S, Ide H, Ohyama Y (2004) Identification of a novel rat microsomal vitamin D3 25-hydroxylase. J Biol Chem 279: 22848–22856. [DOI] [PubMed] [Google Scholar]
- 13. Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH (2004) CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res 19: 680–688. [DOI] [PubMed] [Google Scholar]
- 14. Aiba I, Yamasaki T, Shinki T, Izumi S, Yamamoto K, et al. (2006) Characterization of rat and human CYP2J enzymes as Vitamin D 25-hydroxylases. Steroids 71: 849–856. [DOI] [PubMed] [Google Scholar]
- 15. Bjorkhem I, Holmberg I (1978) Assay and properties of a mitochondrial 25-hydroxylase active on vitamine D3. J Biol Chem 253: 842–849. [PubMed] [Google Scholar]
- 16. Skrede S, Bjorkhem I, Kvittingen EA, Buchmann MS, Lie SO, et al. (1986) Demonstration of 26-hydroxylation of C27-steroids in human skin fibroblasts, and a deficiency of this activity in cerebrotendinous xanthomatosis. J Clin Invest 78: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cali JJ, Hsieh CL, Francke U, Russell DW (1991) Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 266: 7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 18. Rosen H, Reshef A, Maeda N, Lippoldt A, Shpizen S, et al. (1998) Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J Biol Chem 273: 14805–14812. [DOI] [PubMed] [Google Scholar]
- 19. Lehmann B, Pietzsch J, Kampf A, Meurer M (1998) Human keratinocyte line HaCaT metabolizes 1alpha-hydroxyvitamin D3 and vitamin D3 to 1alpha,25-dihydroxyvitamin D3 (calcitriol). J Dermatol Sci 18: 118–127. [DOI] [PubMed] [Google Scholar]
- 20. Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I (2001) Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids 66: 399–408. [DOI] [PubMed] [Google Scholar]
- 21. Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 22. Flanagan JN, Young MV, Persons KS, Wang L, Mathieu JS, et al. (2006) Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 26: 2567–2572. [PubMed] [Google Scholar]
- 23. Tang W, Norlin M, Wikvall K (2007) Regulation of human CYP27A1 by estrogens and androgens in HepG2 and prostate cells. Arch Biochem Biophys 462: 13–20. [DOI] [PubMed] [Google Scholar]
- 24. Bjorkhem I, Andersson O, Diczfalusy U, Sevastik B, Xiu RJ, et al. (1994) Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc Natl Acad Sci U S A 91: 8592–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quinn CM, Jessup W, Wong J, Kritharides L, Brown AJ (2005) Expression and regulation of sterol 27-hydroxylase (CYP27A1) in human macrophages: a role for RXR and PPARgamma ligands. Biochem J 385: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansson M, Wikvall K, Babiker A (2005) Regulation of sterol 27-hydroxylase in human monocyte-derived macrophages: up-regulation by transforming growth factor beta1. Biochim Biophys Acta 1687: 44–51. [DOI] [PubMed] [Google Scholar]
- 27. Liu K, Meng H, Tang X, Xu L, Zhang L, et al. (2009) Elevated plasma calcifediol is associated with aggressive periodontitis. J Periodontol 80: 1114–1120. [DOI] [PubMed] [Google Scholar]
- 28. Liu K, Meng H, Lu R, Xu L, Zhang L, et al. (2010) Initial periodontal therapy reduced systemic and local 25-hydroxy vitamin D(3) and interleukin-1beta in patients with aggressive periodontitis. J Periodontol 81: 260–266. [DOI] [PubMed] [Google Scholar]
- 29. Liu K, Meng H, Hou J (2012) Characterization of the autocrine/paracrine function of vitamin d in human gingival fibroblasts and periodontal ligament cells. PLoS One 7: e39878 doi:10.1371/journal.pone.0039878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bikle DD, Nemanic MK, Gee E, Elias P (1986) 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest 78: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bikle DD, Nemanic MK, Whitney JO, Elias PW (1986) Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry 25: 1545–1548. [DOI] [PubMed] [Google Scholar]
- 32. Lehmann B, Rudolph T, Pietzsch J, Meurer M (2000) Conversion of vitamin D3 to 1alpha,25-dihydroxyvitamin D3 in human skin equivalents. Exp Dermatol 9: 97–103. [DOI] [PubMed] [Google Scholar]
- 33. Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, et al. (2004) A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271: 4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, et al. (2005) The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 272: 4080–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, et al. (2008) Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J 275: 2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, et al. (2010) Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One 5: e9907 doi:10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurita-Ochiai T, Ochiai K, Suzuki N, Otsuka K, Fukushima K (2002) Human gingival fibroblasts rescue butyric acid-induced T-cell apoptosis. Infect Immun 70: 2361–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li QQ, Meng HX, Gao XJ, Wang ZH (2005) Analysis of volatile fatty acids in gingival crevicular fluid of patients with chronic periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi 40: 208–210. [PubMed] [Google Scholar]
- 39. Lu RF, Meng HX, Gao XJ, Feng L, Xu L (2008) Analysis of short chain fatty acids in gingival crevicular fluid of patients with aggressive periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi 43: 664–667. [PubMed] [Google Scholar]
- 40. Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, et al. (2004) Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun 72: 5041–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kocgozlu L, Elkaim R, Tenenbaum H, Werner S (2009) Variable cell responses to P. gingivalis lipopolysaccharide. J Dent Res 88: 741–745. [DOI] [PubMed] [Google Scholar]
- 42. Hatakeyama J, Tamai R, Sugiyama A, Akashi S, Sugawara S, et al. (2003) Contrasting responses of human gingival and periodontal ligament fibroblasts to bacterial cell-surface components through the CD14/Toll-like receptor system. Oral Microbiol Immunol 18: 14–23. [DOI] [PubMed] [Google Scholar]
- 43. Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680. [DOI] [PubMed] [Google Scholar]
- 44. Mahanonda R, Pichyangkul S (2007) Toll-like receptors and their role in periodontal health and disease. Periodontol 2000 43: 41–55. [DOI] [PubMed] [Google Scholar]
- 45. McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, et al. (2011) Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun 79: 2250–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, et al. (2011) One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 82: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, et al. (2011) The impact of vitamin D status on periodontal surgery outcomes. J Dent Res 90: 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]