Abstract
Eukaryotic cells use numerous endocytic pathways for nutrient uptake, protein turnover and response to the extracellular environment. While clathrin-mediated endocytosis (CME) has been extensively studied in yeast and mammalian cells, recent studies in higher eukaryotes have described multiple clathrin-independent endocytic pathways that depend upon Rho family GTPases and their effector proteins. In contrast, yeast cells have been thought to rely solely on CME. In a recent study, we used CME-defective yeast cells lacking clathrin-binding endocytic adaptor proteins in a genetic screen to identify novel factors involved in endocytosis. This approach revealed the existence of a clathrin-independent endocytic pathway involving the GTPase Rho1, which is the yeast homolog of RhoA. Further characterization of the yeast Rho1-mediated endocytic pathway suggested that the Rho1 pathway requires additional proteins that appear to play conserved roles in RhoA-dependent, clathrin-independent endocytic pathways in mammalian cells. Here, we discuss the parallels between the yeast Rho1-dependent and mammalian RhoA-dependent endocytic pathways, as well as the applications of yeast as a model for studying clathrin-independent endocytosis in higher eukaryotes.
Keywords: endocytosis, clathrin-independent, yeast, actin, Rho1 GTPase
Introduction
Endocytosis is an important process in eukaryotic cells for nutrient uptake and for regulation of cell size and plasma membrane composition. All classes of eukaryotes use endocytic pathways that ultimately result in sorting and concentration of endocytic cargos followed by membrane deformation, invagination and scission at the eventual site of internalization. Clathrin-mediated endocytosis (CME), in which clathrin coat assembly stabilizes membrane curvature, is the best-characterized endocytic pathway, and has been studied extensively in yeast and mammalian cells.1-3 These studies revealed that CME involves a large number of conserved proteins whose ordered and sequential recruitment is critical for completion of an endocytic event.
During early stages of CME, adaptor proteins serve as key connectors, linking lipids and cargo proteins with clathrin and other components of the CME machinery.4 As the clathrin-coated pit matures, actin nucleation-promoting factors mediate the formation of an Arp2/3-dependent network of branched actin filaments that provide forces to facilitate membrane invagination.1,2 The requirement for actin polymerization in CME has been a source of controversy, and likely depends on numerous factors such as cell type and membrane contacts. In mammalian cells, actin dynamics facilitate, but are generally not required for CME in unpolarized cells and at the basolateral surface of polarized epithelial cells.5 In contrast, actin is required at the apical surface of polarized cells and for internalization of clathrin-coated “plaque” structures that are larger than a canonical clathrin-coated pit (which has a diameter of approximately 200 nm).6 Unlike mammalian cells, actin polymerization is essential for endocytosis in yeast, and overcomes the high turgor pressure and membrane tension in yeast cells.1,2,7,8 The apical surface of polarized cells is also a region of high membrane tension, which may explain the similar requirement for actin at this site.
In addition to CME, numerous clathrin-independent endocytosis (CIE) mechanisms have been described. CIE pathways include internalization through phagocytosis, macropinocytosis, caveolae and the CLIC/GEEC pathway (clathrin- and dynamin-independent carrier/glycosylphosphatidylinositol-anchored protein-enriched early endosomal compartment), as well as through several poorly-characterized pathways that involve small GTPases of the Rho (RhoA, Rac1 and Cdc42) and Arf (Arf6) families.9 Similar to CME, actin dynamics can contribute to CIE pathways; thus, the known function of Rho family GTPases as regulators of actin cytoskeleton remodeling likely plays an important role during CIE.
Although CIE pathways were initially described in vertebrate cells, recent studies demonstrated the existence of at least some CIE pathways in other model organisms including Caenorhabditis elegans, Drosophila melanogaster, Dictyostelium discoideum and plant cells; thus, CIE mechanisms are present throughout evolution.10-13 Notably, CIE had not been described in fungi, including the budding yeast Saccharomyces cerevisiae. Even though clathrin is not absolutely required for endocytosis in yeast, residual function of the remaining CME machinery was thought to account for the approximately 30% level of endocytosis remaining in clathrin-deficient cells.2,14 The presumed lack of CIE in yeast has raised questions about the general relevance of yeast as a model for understanding endocytosis. However, our recent study uncovered an endocytic pathway in yeast that relies upon the GTPase Rho1 (the yeast homolog of RhoA), and functions in the absence of clathrin and other CME components, suggesting that CIE does exist in yeast.15 Here, we discuss the similarities between yeast and mammalian Rho1/RhoA-dependent CIE pathways and implications for yeast as a model system for genetic and molecular characterization of conserved CIE components. We also speculate about the functional significance of the Rho1-dependent endocytic pathway.
A Rho1-Dependent CIE Pathway in Yeast Cells
As a model for cells defective in CME, we previously developed a mutant yeast strain lacking four functionally redundant clathrin-binding endocytic adaptors: the epsins Ent1 and Ent2 and the AP180/CALM homologs Yap1801 and Yap1802.16 Quadruple ent1Δ ent2Δ yap1801Δ yap1802Δ adaptor mutant cells (termed 4Δ) require an epsin N-terminal homology (ENTH) domain from Ent1 or Ent2 for viability; the ENTH domain binds to membranes and recruits Cdc42 GTPase-activating proteins (GAPs), but is not sufficient for endocytosis.17 4Δ+ENTH1 adaptor mutant cells (ENTH1 denotes the ENTH domain of Ent1) have phenotypes including defective internalization of endocytic cargos, aberrant CME machinery dynamics and temperature-sensitive growth defects.16,18 CME is restored in 4Δ cells expressing any of the four full-length adaptor proteins, provided that an ENTH domain is present (e.g., expression of full-length Ent2 alone is viable, but full-length Yap1801 requires co-expression of an ENTH domain).
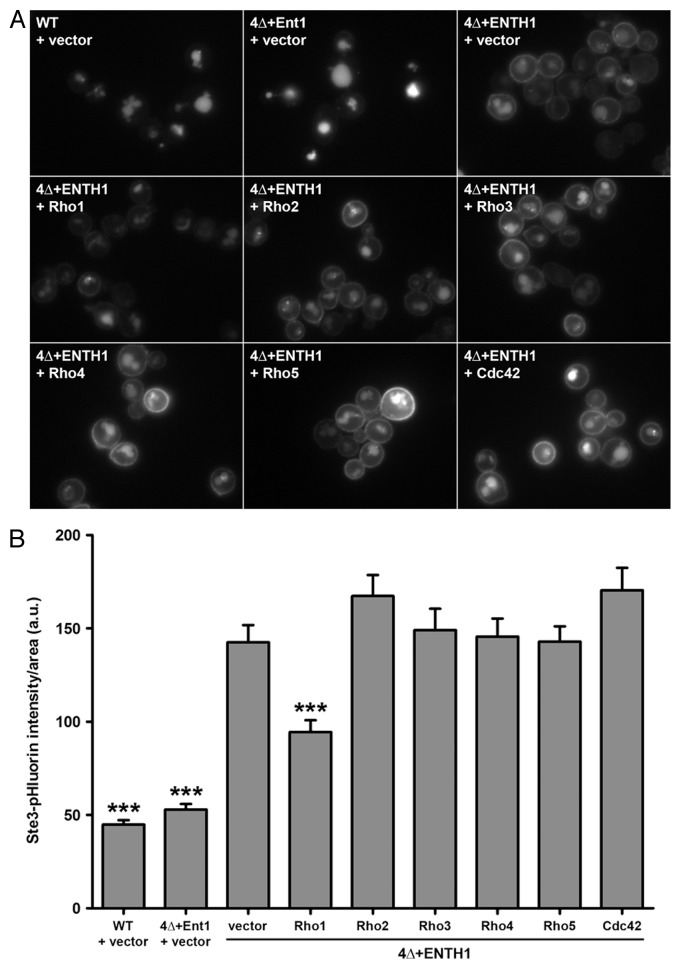
In our recent study, we performed a multicopy suppressor screen in 4Δ+ENTH1 cells to identify genes whose overexpression could promote endocytosis under conditions of defective CME.15 We initially predicted three classes of suppressor genes: (1) novel clathrin-binding adaptors, (2) genes that could bypass the requirement for adaptor proteins in CME or (3) genes involved in a clathrin-independent endocytic pathway. Although we have not yet identified any genes that clearly fall into the first two categories, we identified three genes that act in a signaling cascade involved in responses to cell wall damage: the stress sensor MID2, the Rho1 guanine nucleotide exchange factor (GEF) ROM1, and the GTPase RHO1. Mid2 responds to cell wall damage by binding directly to the Rho1 GEF Rom2, and presumably also to the related GEF Rom1.19 Recruitment of Rom1/2 mediates localized activation of Rho1 to elicit cell wall repair and actin remodeling through the action of Rho1 effectors.20 The yeast genome encodes six Rho family GTPases (Rho1, Rho2, Rho3, Rho4, Rho5 and Cdc42); however, only multicopy Rho1 promoted endocytosis in 4Δ+ENTH1 cells (Fig. 1A and B). Although this finding suggests that other Rho GTPases do not contribute to endocytosis in yeast, it should be noted that overexpression may not result in sufficient levels of activation for these GTPases.
Figure 1. Specificity for Rho1 as a high-copy suppressor of endocytic defects in 4Δ+ENTH1 cells. (A) Wild-type (WT), 4Δ+Ent1 and 4Δ+ENTH1 cells expressing Ste3-GFP were transformed with empty vector or high-copy plasmids containing RHO1, RHO2, RHO3, RHO4, RHO5, or CDC42 to overexpress individual Rho GTPases as indicated. Cells were imaged by fluorescence microscopy to visualize Ste3-GFP, the a-factor mating pheromone receptor that is constitutively internalized and targeted to the vacuole in WT cells, but is partially retained at the plasma membrane when endocytosis is disrupted. (B) WT, 4Δ+Ent1 and 4Δ+ENTH1 cells expressing Ste3-pHluorin were transformed as described in panel A, and Ste3-pHluorin fluorescence intensity was quantified, with intensity values corrected for cell size. We recently developed a method for quantification of endocytosis in yeast cells using super-ecliptic pHluorin as a pH-sensitive GFP variant that becomes quenched in the acidic vacuole, allowing measurement of only fluorescently-tagged cargos that are outside of the vacuole.18 While cells with intact CME (WT and 4Δ+Ent1) are dim because Ste3-pHluorin is mainly localized in the vacuole, cells with defective CME (4Δ+ENTH1) are comparatively bright because a significant population of Ste3 is retained at the cell surface. Values are presented as mean ± SEM from a minimum of 30 cells per condition, and statistical significance was assessed by one-way ANOVA with Neuman-Keuls post hoc analysis (*** p < 0.001 compared with 4Δ+ENTH1 + vector). All strains used in this analysis were described previously.15
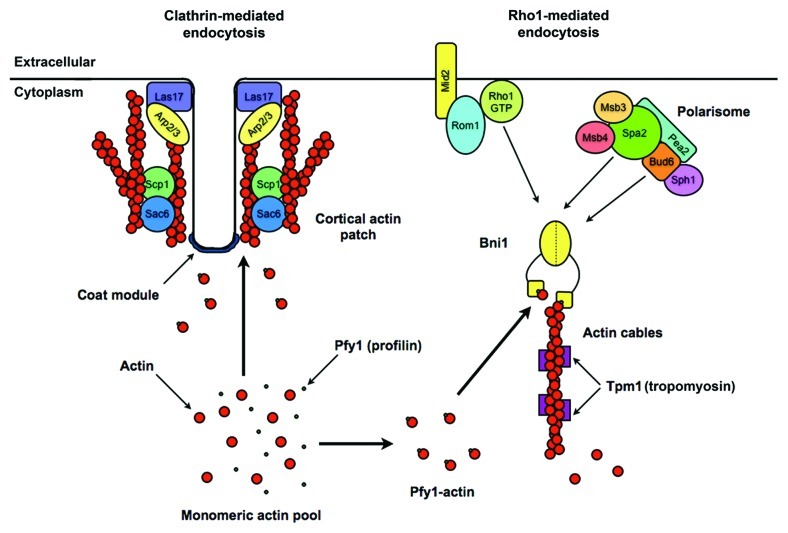
A previous study described a role for Rho1 in endocytosis through the target of rapamycin complex 2 (TORC2), Rom2 and the β-glucan synthase subunit Fks1, which is a Rho1 effector.21 However, the TORC2-Rho1 pathway likely acts through CME, since Fks1 interacts with components of the CME machinery.22 In contrast, the Rho1 endocytic pathway uncovered in 4Δ+ENTH1 cells required the formin Bni1, which is also a Rho1 effector, as well as the Bni1-binding proteins Spa2 and Bud6 that are subunits of the polarisome complex involved in cell polarity (Fig. 2).15 Bni1 promotes incorporation of profilin-bound actin monomers into unbranched actin filaments; these filaments are bundled via the action of other proteins into cables that mediate vesicle and organelle delivery to sites of polarized growth.23,24
Figure 2. Model of clathrin-mediated and Rho1-mediated endocytic pathways in yeast. Although many proteins contribute to CME which occurs at cortical actin patches (left), the model is simplified to display proteins that are critical for functional CME but that we have found are not required for endocytosis in the presence of high-copy components of the Rho1 pathway. CME components that are not required for Rho1-mediated endocytosis include coat proteins (clathrin and the adaptors Ent1, Ent2, Yap1801 and Yap1802), the Arp2/3-activating WASp homolog Las17, and the branched actin-bundling proteins Sac6/fimbrin and Scp1/transgelin. Components of the Rho1 pathway (right) do not localize to cortical actin patches, and are thought to act independently of the CME machinery. The Rho1 pathway ultimately promotes extension of tropomyosin-stabilized unbranched actin filaments through the activity of the formin Bni1, although how membrane deformation is achieved at sites of Rho1-mediated endocytosis is currently unclear.
None of the proteins involved in Rho1-mediated endocytosis are known to localize to cortical actin patches, which are the sites of CME in yeast, suggesting that the Rho1 pathway components we are studying do not participate directly in CME. A possible exception is Mid2, which is broadly distributed across the plasma membrane, but is not concentrated in CME sites. As evidence that the Rho1 pathway does not require intact CME machinery, we found that Rho1 pathway components promoted endocytosis in several CME-deficient backgrounds other than 4Δ+ENTH1, including cells lacking clathrin heavy chain (chc1Δ), the yeast homolog of the Arp2/3-activating Wiskott-Aldrich syndrome protein WASp (las17Δ) or the branched actin-stabilizing proteins Sac6/fimbrin and Scp1/transgelin (sac6Δ scp1Δ).15 Combined with our observation that Rho1 pathway components restored endocytosis but did not correct defective protein dynamics at cortical actin patches, these data led us to propose that the Rho1 pathway is the first-known CIE mechanism in yeast.
Similarities Between the Yeast Rho1 Pathway and Mammalian RhoA-Dependent Endocytic Mechanisms
Rho1 is the yeast homolog of mammalian RhoA, which is similarly involved in formin activation and formation of unbranched actin stress fibers. RHO1 is an essential gene in yeast; however, heterologous expression of human RhoA in rho1Δ cells permits growth under some conditions, suggesting that human RhoA can fulfill some functions of yeast Rho1.25 It is thus possible that Rho1-mediated endocytosis in yeast bears similarity to RhoA-dependent endocytosis in mammalian cells, and that Rho1/RhoA-dependent endocytic pathways are conserved through evolution. RhoA is implicated in CIE in mammalian cells, where it contributes to phagocytosis and to an additional poorly characterized CIE pathway (Table 1).26,27
Table 1. Comparison of shared components of the known Rho1/RhoA endocytic pathways.
| Yeast Rho1 pathway15 | RhoA-dependent phagocytosis | Mammalian RhoA-dependent CIE26 | |
|---|---|---|---|
| GTPase(s) involved |
Rho1 |
RhoA (other phagocytic pathways use Rac1 and/or Cdc42) |
RhoA |
| GEF(s) involved |
Rom1, Rom2 |
various, including Vav1 and Vav328 |
? |
| formin requirement |
Bni1 (diaphanous-related formin) |
mDia1, mDia2 (diaphanous-related formins)29-31 |
? |
| dynamin involvement |
Vps1? (vps1Δ is lethal in 4Δ+ENTH1 cells, but whether lethality is linked to endocytosis is unclear) |
Dynamin-232 |
Dynamin-1 |
| integrin involvement |
Mid2? (integrin-like, with a heavily glycosylated ectodomain and a short cytoplasmic domain that signals to the cell interior) |
β1 subunit (α4β1 and α5β1 integrins), β2 subunit (αMβ2)27,28,34 |
? |
| polarity proteins |
polarisome complex (Spa2, Bud6 and Bni1 subunits) |
? |
? |
| cargo/receptor | ? | various receptors, including integrins | interleukin 2 receptor (IL2R) |
In our characterization of the yeast Rho1 pathway, we identified additional components that share similar features with known proteins involved in both mammalian RhoA-dependent endocytic pathways, suggesting that there are parallels between the different pathways. For example, Mid2 is an integrin-like cell surface protein that recruits Rom1 and Rom2 in yeast cells, while β1 and β2 integrins recruit RhoA GEFs such as Vav1 and Vav2 during phagocytosis.27,28 Furthermore, yeast Rho1-mediated endocytosis requires actin dynamics furnished by the formin Bni1, while mammalian phagocytosis similarly relies on the formins mDia1 and mDia2; Bni1, mDia1 and mDia2 all belong to the diaphanous-related family of formins.29-31
While dynamin-2 is implicated in phagocytosis and dynamin-1 is a component of the RhoA-mediated CIE pathway, a role for the dynamin-like protein Vps1 in the yeast Rho1 pathway is less clear.26,32 Although deletion of VPS1 in 4Δ cells expressing full-length Ent1 or Ent2 is viable, vps1Δ is lethal in 4Δ+ENTH1 cells (our unpublished results). We are hesitant to conclude that the requirement for Vps1 in adaptor mutant cells is due to a role in CIE, since it is possible that the essential function of Vps1 is due to other cellular functions, including endocytosis at residual CME sites.
We also identified components of the polarisome as regulators of Rho1-mediated endocytosis in yeast. The polarisome is a protein complex involved in establishment of polarity and regulation of actin polymerization at sites of polarized growth (i.e., the growing bud and mating projection tip).33 Polarisome proteins have no clear homologs in mammalian cells, and mammalian polarity proteins are not directly implicated in RhoA-dependent endocytosis; however, professional phagocytes must be able to detect and move toward invading pathogens in order to engulf them, and this process likely requires polarization of the phagocytic machinery.
There is currently very little information about the non-phagocytic RhoA-dependent CIE pathway in mammalian cells. Aside from the requirement for RhoA and dynamin-1, the only defining feature of this pathway is that it internalizes the interleukin-2 receptor (IL2R).26 In contrast, cells use phagocytosis for uptake of larger objects, where actin polymerization drives membrane protrusions that facilitate engulfment; phagocytosis can be initiated by ligand association with a variety of cell surface receptors, including integrins.27,28 To date, we have not identified yeast cargos that preferentially internalize through the Rho1 pathway, but if we succeed, their characterization should provide clues to whether the yeast pathway more closely resembles either phagocytosis or the RhoA-dependent CIE pathway involved in IL2R uptake. In the event that the Rho1 pathway is more similar to phagocytosis, it seems likely that there will be fundamental differences between the two systems, since the rigid cell wall that constrains yeast cells would effectively prevent membrane protrusion.
Speculations on the Significance of Rho1-Mediated Endocytosis in Yeast Cells
Studies of endocytosis in yeast have underscored the importance of CME as the principal route of entry; thus, the discovery of a second, clathrin-independent endocytic pathway raises questions about the relative contributions of CME vs. CIE in yeast. All currently known endocytic cargos in yeast are internalized through the CME pathway, where adaptor proteins recognize specific peptide sequences or ubiquitin modifications on the cytosolic domain of cargos. It is possible that a minor population of these cargos normally enters through the Rho1 pathway; however, we have not observed any obvious defects in cargo internalization upon deletion of components of the Rho1 pathway. Instead, a comprehensive study of potential endocytic cargo proteins in cells lacking key components of the CME and Rho1 pathways might reveal whether specific cargos show preference for either of the two pathways.
As opposed to differences in cargo specificity for the CME and Rho1 pathways, it is possible that the Rho1 pathway becomes activated under specific growth conditions or during specific stages of the cell cycle. Mid2, Rom1 and Rho1 are involved in responding to cell wall damage,19,20 suggesting that cell wall stress or changes in osmotic conditions could trigger endocytosis via the Rho1 pathway. Indeed, providing 4Δ+ENTH1 cells with osmotic support improved endocytosis in a Rho1 pathway-dependent manner.15 Repair of damaged cell wall and/or plasma membrane might necessitate engulfment of large portions of the cell surface in a manner resembling phagocytosis in other cell types.
It is worth noting that many Rho1 pathway components (Mid2, Rom2, Rho1, Bni1, Spa2 and Bud6) localize to sites of cell growth, such as the tip of growing buds, the bud neck during cytokinesis, and to the mating projection tip. These sites correspond to regions of rapid membrane and cell wall remodeling, and endocytosis may be necessary to promote protein recycling during budding, cell division and mating. Thus, known sites of rapid cell growth or membrane remodeling are particularly interesting as candidate sites for Rho1-mediated endocytosis.
Conclusions and Perspectives
The discovery of a Rho1-dependent, clathrin-independent endocytic pathway in yeast provides a useful tool for studying and characterizing similar pathways in other cell types. As a model system, yeast allows powerful and rapid genetic characterization of molecular pathways, many of which are conserved through evolution. The combination of candidate and screening approaches will allow us to identify additional components and cargos of the Rho1-dependent endocytic machinery, and findings from yeast studies will allow a more complete characterization of RhoA-dependent CIE pathways in mammalian cells.
Acknowledgments
We would like to acknowledge the many excellent studies related to this topic that we were unable to discuss or cite due to space constraints. We are grateful to Dr Hay-Oak Park (Ohio State University) for kindly providing the high-copy Rho5 plasmid used in Figure 1. This work was supported by grants to B.W. from the National Institutes of Health (RO1 GM60979) and the National Science Foundation (MCB 1024818).
Glossary
Abbreviations:
- CIE
clathrin-independent endocytosis
- CLIC/GEEC
clathrin- and dynamin-independent carrier/glycosylphosphatidylinositol-anchored protein-enriched early endosomal compartment
- CME
clathrin-mediated endocytosis
- ENTH
epsin N-terminal homology
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- IL2R
interleukin-2 receptor
- TORC2
target of rapamycin complex 2
- WASp
Wiskott-Aldrich syndrome protein
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/21631
References
- 1.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–87. doi: 10.1016/S0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 2.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–20. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reider A, Wendland B. Endocytic adaptors--social networking at the plasma membrane. J Cell Sci. 2011;124:1613–22. doi: 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13:1124–31. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saffarian S, Cocucci E, Kirchhausen T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009;7:e1000191. doi: 10.1371/journal.pbio.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 8.Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol. 2009;11:1039–42. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 10.Cardelli J. Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic. 2001;2:311–20. doi: 10.1034/j.1600-0854.2001.002005311.x. [DOI] [PubMed] [Google Scholar]
- 11.Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell. 2007;18:4387–96. doi: 10.1091/mbc.E07-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windler SL, Bilder D. Endocytic internalization routes required for delta/notch signaling. Curr Biol. 2010;20:538–43. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandmann V, Homann U. Clathrin-independent endocytosis contributes to uptake of glucose into BY-2 protoplasts. Plant J. 2011;70:578–84. doi: 10.1111/j.1365-313X.2011.04892.x. [DOI] [PubMed] [Google Scholar]
- 14.Chu DS, Pishvaee B, Payne GS. The light chain subunit is required for clathrin function in Saccharomyces cerevisiae. J Biol Chem. 1996;271:33123–30. doi: 10.1074/jbc.271.51.33123. [DOI] [PubMed] [Google Scholar]
- 15.Prosser DC, Drivas TG, Maldonado-Báez L, Wendland B. Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin. J Cell Biol. 2011;195:657–71. doi: 10.1083/jcb.201104045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldonado-Báez L, Dores MR, Perkins EM, Drivas TG, Hicke L, Wendland B. Interaction between Epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis. Mol Biol Cell. 2008;19:2936–48. doi: 10.1091/mbc.E07-10-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar RC, Longhi SA, Shaw JD, Yeh LY, Kim S, Schön A, et al. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc Natl Acad Sci U S A. 2006;103:4116–21. doi: 10.1073/pnas.0510513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prosser DC, Whitworth K, Wendland B. Quantitative analysis of endocytosis with cytoplasmic pHluorin chimeras. Traffic. 2010;11:1141–50. doi: 10.1111/j.1600-0854.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philip B, Levin DE. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol Cell Biol. 2001;21:271–80. doi: 10.1128/MCB.21.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–91. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.deHart AK, Schnell JD, Allen DA, Tsai JY, Hicke L. Receptor internalization in yeast requires the Tor2-Rho1 signaling pathway. Mol Biol Cell. 2003;14:4676–84. doi: 10.1091/mbc.E03-05-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavin AC, Bösche M, Krause R, Grandi P, Marzioch M, Bauer A, et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature. 2002;415:141–7. doi: 10.1038/415141a. [DOI] [PubMed] [Google Scholar]
- 23.Sagot I, Klee SK, Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat Cell Biol. 2002;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- 24.Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat Cell Biol. 2002;4:260–9. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- 25.Qadota H, Anraku Y, Botstein D, Ohya Y. Conditional lethality of a yeast strain expressing human RHOA in place of RHO1. Proc Natl Acad Sci U S A. 1994;91:9317–21. doi: 10.1073/pnas.91.20.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–71. doi: 10.1016/S1097-2765(01)00212-X. [DOI] [PubMed] [Google Scholar]
- 27.Werner E, Kheradmand F, Isberg RR, Werb Z. Phagocytosis mediated by Yersinia invasin induces collagenase-1 expression in rabbit synovial fibroblasts through a proinflammatory cascade. J Cell Sci. 2001;114:3333–43. doi: 10.1242/jcs.114.18.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, et al. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–82. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol. 2006;174:701–13. doi: 10.1083/jcb.200605006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colucci-Guyon E, Niedergang F, Wallar BJ, Peng J, Alberts AS, Chavrier P. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr Biol. 2005;15:2007–12. doi: 10.1016/j.cub.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven MA, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999;190:1849–56. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheu YJ, Santos B, Fortin N, Costigan C, Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol Cell Biol. 1998;18:4053–69. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci. 2008;121:1773–83. doi: 10.1242/jcs.018036. [DOI] [PubMed] [Google Scholar]