Abstract
Molecular phylogenies often reveal that taxa circumscribed by phenotypical characters are not monophyletic. While re-examination of phenotypical characters often identifies the presence of characters characterizing clades, there is a growing number of studies that fail to identify diagnostic characters, especially in organismal groups lacking complex morphologies. Taxonomists then can either merge the groups or split taxa into smaller entities. Due to the nature of binomial nomenclature, this decision is of special importance at the generic level. Here we propose a new approach to choose among classification alternatives using a combination of morphology-based phylogenetic binning and a multiresponse permutation procedure to test for morphological differences among clades. We illustrate the use of this method in the tribe Thelotremateae focusing on the genus Chapsa, a group of lichenized fungi in which our phylogenetic estimate is in conflict with traditional classification and the morphological and chemical characters do not show a clear phylogenetic pattern. We generated 75 new DNA sequences of mitochondrial SSU rDNA, nuclear LSU rDNA and the protein-coding RPB2. This data set was used to infer phylogenetic estimates using maximum likelihood and Bayesian approaches. The genus Chapsa was found to be polyphyletic, forming four well-supported clades, three of which clustering into one unsupported clade, and the other, supported clade forming two supported subclades. While these clades cannot be readily separated morphologically, the combined binning/multiresponse permutation procedure showed that accepting the four clades as different genera each reflects the phenotypical pattern significantly better than accepting two genera (or five genera if splitting the first clade). Another species within the Thelotremateae, Thelotrema petractoides, a unique taxon with carbonized excipulum resembling Schizotrema, was shown to fall outside Thelotrema. Consequently, the new genera Astrochapsa, Crutarndina, Pseudochapsa, and Pseudotopeliopsis are described here and 39 new combinations are proposed.
Introduction
Molecular data have revolutionized our understanding of the evolution of organisms and have had profound impact on classifications, especially in organisms lacking complex morphologies, such as fungi [1]–[3]. Traditionally, the classification of living organisms has worked under the paradigm that taxa should be recognizable, i.e. having phenotypic features that delimit them from other taxa. However, a major challenge of the results of molecular phylogenetic studies is that lineages often do not correlate well with phenotypic features [4]–[11]. In these cases, re-examination of phenotypical characters often fails to identify diagnostic characters, especially in organismal groups lacking complex morphologies. Reasons for the absence of phenotypical differences among clades include convergent evolution, parallel but independent transformations of morphological characters in related lineages, as well as morphostasis and retention of ancestral features [12]–[16]. Hence, delimitation based on morphology alone can be difficult or even impossible. This problem has long been recognized and accepted at higher taxonomic levels, such as orders and families, which often cannot be circumscribed by phenotypic characters. Even so, their formal recognition does not appear to pose any conceptual problem, as apparent from widely accepted classifications [1], [17].
Due to the nature of binomial nomenclature introduced by Linnaeus, in which a species name is composed of the generic name and the epitheton, changes in the classification of an organism at the generic level lead to a change in the name of the organism. Thus, systematists have been reluctant to translate phylogenetic studies into classification at the generic level when monophyletic clades lack correlating phenotypical features. When a genus-level taxon is found to be poly- or paraphyletic, it can be either split or merged with another taxon to obtain monophyly. Since genera and all higher taxonomic ranks are arbitrary, both lumping or splitting would be possible and there is no a priori scientific argument to favor either solution. Such reclassifications often lead to genera that are not distinguishable by phenotypical characters, and these have been called “cryptic genera” [18]–[21] analogous to “cryptic species”, which are morphologically undistinguishable [22]–[28].
Here we propose a new, quantitative approach to choose among alternative classifications, combining the technique of morphology-based phylogenetic binning with a multi-response permutation procedure (MRPP) [29]–[31]. Phylogenetic binning [32] is a method that determines the level of congruence between phenotypical site patterns and molecular phylogenies and then applies character weights in order to improve the accuracy of the classification of taxa for which no molecular data are yet available. The advantage of this method is the individual placement of taxa in a reference tree based on closest relationship, rather than overall difference between clades. Thus, this method yields better results than simultaneous clustering or cladistic analysis of many taxa based on morphological data. MRPP compares average distances between groups based on characters (putative taxa), using data randomization to obtain evidence of statistical significance. Both methods can be combined to test alternative classification models in order to evaluate which classification best fits both the phylogenetic topology and the morphological data, under the criterion that resulting taxa should be monophyletic.
We used the tribe Thelotremateae in Graphidaceae, a family of lichenized fungi, to illustrate our approach [33], [34]. This clade includes four currently accepted genera: Chapsa, Chroodiscus, Leucodecton, and Thelotrema. Previous studies showed that Chroodiscus and Leucodecton are monophyletic [33]–[36], while Chapsa was shown to be highly polyphyletic. The core of Thelotrema was found to be monophyletic with one species having unclear phylogenetic reltionships. The genus Chapsa in its current sense is characterized morphologically by so-called chroodiscoid apothecia with widely open disc bordered by a splitting, lobulate margin, as well as the presence of lateral paraphyses, which are hyphae growing into the hymenium from the lateral margin of the fruiting body [37], [38]. However, Chapsa was found to consist of unrelated lineages [33] that fall both inside and outside the Thelotremateae, and even the Chapsa species within Thelotremateae form at least two clades, one of which having low support but including three subclades with strong support, whereas the other clade includes two supported subclades. At first glance, there are no apparent phenotypic characters that would separate these clades, since thallus morphology, ascospore type, and secondary chemistry vary widely in each clade. As a consequence, one could recognize a single genus Thelotrema for all Thelotremateae, which would, however, not do justice to the morphologically and phylogenetically well-defined clades representing Chroodiscus, Leucodecton, and Thelotrema sensu stricto. The preferred alternative would be splitting Chapsa into more than one genus, but without any obvious, supporting morphological characters the decision for either two, four, or even five genera would be arbitrary. Our approach provides statistical evidence that helps to choose among alternatives and we consider this a model case in how to tackle classifications of morphologically complex groups with clear underlying phylogenetic topologies.
Results
Phylogenetic Analyses
Seventy-five new sequences were generated for this study and aligned with 237 sequences downloaded from Genbank, most of them generated in our lab and included in a previous study (Table 1). The combined data matrix of 2482 unambiguously aligned characters with 804 characters in the nuLSU rDNA, 800 characters in mtSSU rDNA and 878 characters in RPB2 was used for phylogenetic analyses. The single gene analyses did not show any conflicts and hence the concatenated data set was analyzed. The ML tree had a likelihood value of –38,803.262 and in the B/MCMC analysis of the combined data set, the likelihood parameters in the sample had the following mean (Variance): LnL = –42,231.616 (0.17). The maximum likelihood tree did not contradict the Bayesian tree topologies and hence only the majority-rule consensus tree of the Bayesian tree sampling is shown (Fig. 1).
Table 1. Species and specimens used in the present study, with location, reference collection details, and GenBank accession numbers. Newly obtained sequenced in bold.
| Species | Collection data | mtSSU acc. no. | nuLSU acc. no | RPB2 acc. no |
| Chapsa alborosella | Brazil, Lücking 31237 (F) | JX420971 | JX421438 | JX465320 |
| C. alborosella | Brazil, Lücking 31238a (F) | JX420972 | JX421439 | JX420936 |
| C. alborosella | Guatemala, Lücking 25587 (F) | JX420973 | JX421440 | JX420940 |
| C. astroidea | Thailand, Lumbsch 19750a (F) | JX420974 | JX421441 | JX420859 |
| C. astroidea | Thailand, Lücking 24011 (F) | JX465278 | JX421445 | JX420947 |
| C. astroidea | Thailand, Papong 6004 (F) | JX420975 | JX421442 | JX420865 |
| C. astroidea | Thailand, Lücking 24008 (F) | JX420978 | JX421444 | JX420945 |
| C. astroidea | Thailand, Lücking 24006 (F) | JX420977 | JX421443 | JX420943 |
| C. dilatata | Venezuela, Kalb s.n. | JX420980 | – | JX420898 |
| C. dilatata | Venezuela, Lücking 32101 (F) | JX420981 | JX421446 | JX420906 |
| C. dilatata | Venezuela, Lücking 26143 (F) | JX420982 | JX421447 | JX420949 |
| C. esslingeri | Brazil, Cáceres 6006a | JX420984 | – | JX420885 |
| C. esslingeri | Brazil, Cáceres 6006b (F) | JX465279 | – | JX420886 |
| C. esslingeri | Brazil, Cáceres s.n. (F) | JX420983 | – | JX420883 |
| C. esslingeri | Peru, Rivas Plata 107C (F) | JX420985 | – | JX420870 |
| C. esslingeri | Peru, Rivas Plata 809a (F) | JX420986 | JX465294 | JX465321 |
| C. indica | Thailand, Parnmen 018486 (RAMK) | JX465280 | JX465295 | JX465322 |
| C. laceratula | Australia, Lumbsch 19139sa (F) | JX420988 | JX421448 | JX420831 |
| C. laceratula | Australia, Lumbsch 19139sb (F) | JX420989 | JX875070 | – |
| C. aff. laceratula | Thailand, Lücking 24007 (F) | JX420969 | JX421436 | JX420944 |
| C. aff. laceratula | Thailand, Lücking 24009 (F) | JX420970 | JX421437 | JX420946 |
| C. leprocarpa | Thailand, Kalb 38794 (hb. Kalb) | JX420994 | JX421453 | JX420928 |
| C. leprocarpa | Thailand, Lumbsch 19750c (F) | JX420993 | JX421452 | JX420857 |
| C. leprocarpa | Thailand, Lücking 24004 (F) | JX420995 | JX421455 | JX420942 |
| C. leprocarpa | Philippines, Rivas Plata 1175B (F) | JX420992 | JX465296 | JX465323 |
| C. mastersonii | Fiji, Lumbsch 20500f (F) | JX420996 | JX465297 | JX465324 |
| C. mastersonii | Philippines, Rivas Plata 1111G (F) | JX420998 | JX465298 | JX420860 |
| C. mastersonii | Philippines, Rivas Plata 1200 (F) | JX420999 | JX465299 | JX420861 |
| C. meridensis | Costa Rica, Lücking 17770 (F) | EU075610 | EU075655 | JF828940 |
| C. niveocarpa | Australia, Lumbsch 19125k1 (F) | EU075568 | EU075615 | – |
| C. niveocarpa | Australia, Lumbsch 19125k2 (F) | EU675274 | – | – |
| C. niveocarpa | Australia, Lumbsch 19151p1 (F) | EU075567 | FJ708487 | – |
| C. patens | Thailand, Lücking 24003 (F) | JX421003 | JX421459 | JX420941 |
| C. patens | China, Kalb 38676 (hb. Kalb) | JX421001 | JX421458 | JX420939 |
| C. phlyctidioides | Australia, Mangold 39ze (F) | EU675275 | JX465300 | JX465326 |
| C. phlyctidioides | Fiji, Lumbsch 20500d (F) | JX421005 | JX465301 | JX465327 |
| C. phlyctidioides | Australia, Lumbsch 19100f (F) | EU075569 | JX465302 | JX465325 |
| C. platycarpa | USA, Lücking 26573 (F) | JX421007 | JX421460 | JX465328 |
| C. pseudophlyctis | Thailand, Lumbsch 19750b (F) | JX421008 | JX465303 | JX420858 |
| C. cf. pseudophlyctis | Thailand, Parnmen 018492 (RAMK) | JX465277 | JX465293 | JX465331 |
| C. pulchra | Australia, Lumbsch 19129t (F) | EU075571 | EU075619 | JX465329 |
| C. soredicarpa | Brazil, Lücking 31200 (F) | JX421011 | JX421462 | JX420935 |
| C. soredicarpa | Brazil, Lücking 31240 (F) | JX421012 | JX421463 | JX420937 |
| C. sublilacina | Mexico, Lücking RLD056 (F) | HQ639600 | JX421466 | JX420842 |
| C. thallotrema | Venezuela, Lücking 32019 (F) | JX421013 | JX467681 | JX420905 |
| C. thallotrema | Panama, Lücking 27305 (F) | JX465282 | JX465305 | JX465333 |
| C. thallotrema | Panama, Lücking s.n. (F) | JX465283 | JX465306 | JX465334 |
| Chapsa sp.1 | Thailand, Parnmen 018483 (RAMK) | JX465281 | JX465304 | JX465332 |
| Chapsa sp.2 | Fiji, Lumbsch 19815d (F) | JX421000 | JX421457 | JX465330 |
| Chroodiscus argillaceus | Australia, Lumbsch 19126a (F) | HQ639585 | JX421468 | – |
| C. australiensis | Australia, Lumbsch 5437 (F) | FJ708496 | FJ708489 | – |
| C. defectus | Thailand, Papong 5118 (F) | FJ708497 | FJ708490 | – |
| C. khaolungensis | Thailand, Papong 6071 (F) | JX465284 | – | JX465335 |
| C. parvisporus | Thailand, Papong 6069 (F) | JX465285 | JX421469 | JX420863 |
| C. verrucosus | Thailand, Papong 6070 (F) | JX465286 | JX465307 | – |
| Diploschistes cinereocaesius | AFTOL-ID 328 | DQ912306 | DQ883799 | DQ883755 |
| Leucodecton anamaliense | Venezuela, Lücking 32120 (F) | JX421077 | JX421512 | JX465336 |
| L. compunctellum | Thailand, Lumbsch 20205b (F) | JX421081 | JX421514 | – |
| L. occultum | El Salvador, Lücking 28098 (F) | HQ639611 | HQ639657 | JF828949 |
| L. occultum | USA, Mangold 50d (F) | JX421084 | – | JX420846 |
| L. phaeosporum | Australia, Mangold 22zl (F) | JF828962 | – | – |
| L. phaeosporum | USA, Mangold 50a (F) | JX465287 | JX465308 | JX465337 |
| L. subcompunctum | Australia, Lumbsch 19153p (F) | EU075576 | EU075624 | – |
| L. subcompunctum | Australia, Lumbsch 19116o (F) | EU075575 | EU075623 | – |
| Myriotrema olivaceum | Australia, Lumbsch & Mangold 19113f (F) | EU075579 | EU075627 | HM244799 |
| M. olivaceum | Fiji, Lumbsch 20520a (F) | JX465288 | JX421531 | JX465338 |
| Reimnitzia santensis | El Salvador, Lücking 28015 (F) | HQ639622 | – | JF828952 |
| Thelotrema adjectum | India, Lumbsch 19730i (F) | JX421344 | JX421642 | JX420851 |
| T. adjectum | India, Lumbsch 19737m (F) | JX421343 | JX421641 | JX420848 |
| T. adjectum | Thailand, Lumbsch 20200b (F) | JX421347 | JX421645 | JX465350 |
| T. adjectum | Thailand, Lumbsch 19756u (F) | JX465289 | JX421644 | JX420853 |
| T. aff. circumscriptum | New Zealand, Knight 61701 (F) | JX465290 | – | JX465339 |
| T. crespoae | Australia, Mangold 27v (F) | DQ384917 | FJ708493 | – |
| T. diplotrema | Australia, Mangold 31o (F) | JX421356 | – | JX420847 |
| T. diplotrema | Australia, Lumbsch 19127v (F) | EU075599 | JX421649 | JX420827 |
| T. gallowayanum | Australia, Lumbsch 19151k (F) | EU075600 | EU075653 | – |
| T. jugale | Australia, Lumbsch 19117k (F) | EU675293 | – | – |
| T. jugale | Australia, Lumbsch 19100yb (F) | JX421360 | JX465310 | JX420826 |
| T. lepadinum | Australia, Lumbsch 20004c (F) | JX421370 | JX421655 | JX420868 |
| T. lepadinum | Australia, Lumbsch 19998a (F) | JX421368 | JX465311 | JX420866 |
| T. lepadinum | Australia, Lumbsch 19997c (F) | JX421369 | JX421654 | JX420867 |
| T. lepadinum | Australia, Mangold 1b (F) | JX421362 | JX421656 | JX420837 |
| T. lepadinum | Australia, Mangold 18l (F) | JX421363 | JX421651 | JX420840 |
| T. lepadinum | Australia, Lumbsch 19983i (F) | JX421371 | JX465312 | – |
| T. lepadinum | India, Lumbsch 19744i (F) | JX421365 | JX421652 | JX420850 |
| T. lepadinum | New Zealand, Knight 61702 (F) | JX421366 | JX421653 | JX420934 |
| T. macrosporum | UK, Scotland, Lumbsch 20100c (F) | JX465291 | JX465313 | JX420890 |
| T. monosporoides | USA, Lendemer 6389 (NY) | EU075602 | EU075645 | JX465340 |
| T. monosporum | Australia, Lumbsch 19161xb (F) | EU075596 | EU075644 | – |
| T. monosporum | Australia, Lumbsch 19158w (F) | EU075601 | EU075646 | – |
| T. nureliyum | Australia, Lumbsch 19123j (F) | JX421376 | JX421660 | JX465341 |
| T. nureliyum | Australia, Lumbsch 19100ya (F) | EU075597 | EU075647 | – |
| T. cf. nureliyum | Thailand, Lumbsch 19751f (F) | JX421408 | JX421673 | JX420854 |
| T. cf. nureliyum | Thailand, Lumbsch 19751d (F) | JX421409 | JX421674 | JX420856 |
| T. pachysporum | Tanzania, Frisch 99Tz1051 (hb. Kalb) | DQ384918 | DQ431925 | – |
| T. pachysporum | USA, Lücking 26568a (F) | JX421381 | – | JX465342 |
| T. pachysporum | Australia, Lumbsch 19162j (F) | EU675290 | – | JX420829 |
| T. petractoides | UK, Scotland, Lumbsch 20100a (F) | JX421383 | JX421664 | JX420891 |
| T. porinaceum | Australia, Lumbsch 19108d (F) | JX421384 | JX465314 | JX465343 |
| T. aff. porinaceum | Australia, Mangold 29t (F) | JX421350 | JX421646 | JX420844 |
| T. aff. porinaceum | Australia, Lumbsch 19151zb (F) | JX421349 | – | JX465344 |
| T. aff. porinaceum | Australia, Lumbsch 19156d (F) | EU675291 | JX465309 | – |
| T. porinoides | Fiji, Lumbsch 20524f (F) | JX421394 | – | JX465346 |
| T. porinoides | Fiji, Lumbsch 20532a (F) | JX421397 | JX465315 | JX465347 |
| T. porinoides | Fiji, Lumbsch 20516h (F) | JX421386 | JX465316 | JX465345 |
| T. porinoides | Philippines, Rivas Plata 2009 (F) | HQ639603 | JX421665 | – |
| T. rugatulum | Australia, Lumbsch 19082b (F) | EU075605 | JX465317 | – |
| T. subtile | USA, Lumbsch 19257b (F) | JX421402 | – | JX420836 |
| T. subtile | Japan, Ohmura 7769 (TNS) | JX421403 | JX421668 | JX420932 |
| T. aff. subtile | Australia, Mangold 3e (F) | EU675297 | DQ871013 | JX465348 |
| T. aff. subtile | Australia, Mangold 3j (F) | EU075607 | EU075651 | JX420834 |
| T. suecicum | Norway, Gaarder 4365 (BG) | JX421406 | JX421671 | – |
| T. suecicum | Norway, Gaarder 4366a (BG) | JX421407 | JX421672 | JX420832 |
| T. suecicum | Norway, Gaarder 4366b (BG) | JX465292 | JX465318 | JX465349 |
| T. aff. suecicum | Australia, Mangold Am3p (F) | JX421404 | JX421669 | JX420833 |
| T. aff. suecicum | Australia, Mangold Am6l (F) | JX421405 | JX421670 | JX420835 |
| Thelotrema sp.1 | India, Lumbsch 19729i (F) | JX421400 | JX421667 | JX420849 |
| Wirthiotrema trypaneoides | Venezuela, Lücking 32003 (F) | JX421422 | JX421681 | JX420916 |
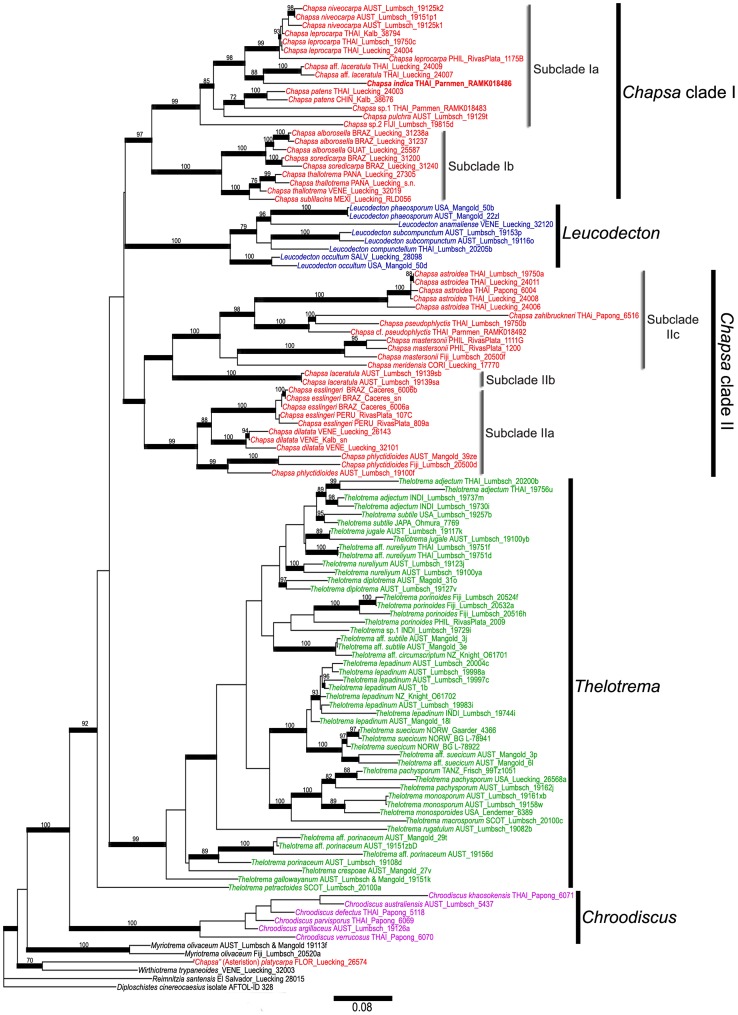
Figure 1. Bayesian 50% majority-rule consensus tree depicting relationships among genera in the tribe Thelotrematae on the basis of a concatenated data set including mtSSU rDNA, nuLSU rDNA and protein-coding RPB2.
Posterior probabilities equal or above 0.95 are indicated as bold branches. ML-bootstrap support equal or above 70% is shown as number at branches.
In the phylogenetic tree, the genus Chapsa is polyphyletic, separating into two major clades, one of which is unsupported but consists of three well-supported subclades. Distantly related species of Chapsa sensu lato also appear in other clades, such as C. platycarpa in the outgroup close to the genus Wirthiotrema. Chapsa clade I is a well-supported clade; it contains the type species, C. indica, and the morphologically similar C. leprocarpa, C. niveocarpa, C. patens, and C. pulchra, but also C. alborosella and the morphologically quite disparate C. sublilacina and relatives. The clade forms two supported subclades, one containing C. alborosella and C. sublilacina, among other species, and the other C. indica, C. leprocarpa, and C. patens, among other species. Chapsa clade II can be divided into subclades IIa, IIb, and IIc. Subclade IIa contains C. dilatata and C. phlyctidioides, which morphologically resemble species of Clade I; subclade IIb comprises the single species C. laceratula, which resembles a Topeliopsis in apothecial morphology but with well-developed, corticate thallus; and subclade IIc includes the morphologically disparate C. astroidea, C. mastersonii, and C. zahlbruckneri, among other species. Hypothesis testing using both the SH and ELW strongly rejected the monophyly of Chapsa, even if only considering the species falling within the Thelotremateae (p≤0.0001 in both tests). The Thelotrema clade is supported as a monophyletic group, but excluding Thelotrema petractoides, which falls outside the main clade as an early diverging taxon with uncertain phylogenetic relationships.
Phylogenetic Binning and Multi-response Permutation Procedure
Phylogenetic binning of the 65 described Chapsa species for which no molecular data are available suggests placement of 14 species within Clade I (Chapsa sensu stricto) and 49 species within Clade II under a 2-clade solution with ML weighting. Two species, C. chionostoma and C. microspora, are suggested to not form part of tribe Thelotremateae (Table 2; Appendix S1). MP weighting places 19 species in Clade I and 44 species in Clade II, suggesting again C. chionostoma and also C. halei as not belonging in tribe Thelotremateae. Between ML and MP weighting, the placement of 12 out of 65 species (19%) is conflictive (Table 2; Appendix S1). Using a 4-clade solution, ML weighting places 16 out of 65 species in Clade I, 24 species in Clade IIa, five species in Clade IIb, and 20 species in Clade IIc; in contrast, MP weighting places 21 out of 65 species in Clade I, 21 species in Clade IIa, seven species in Clade IIb, and 16 species in Clade IIc. The placement of 19 species (29%) is conflictive between ML and MP weighting. Under a 5-clade solution, splitting Clade I into subclades Ia (Chapsa s.str.) and Ib (alborosella-sublilacina clade), of the 16 species placed in Clade I, eight fall into Clade Ib with ML weighting and 11 with MP weighting. Six of these are identical whereas seven are conflictive between the two weighting techniques, for a total of 20 conflictive placements in the 5-clade solution (Table 2).
Table 2. Placement of species within clades based on morphological characters using phylogenetic binning method under ML and MP weighting.
| ML 2-clades | MP 2-clades | ML 4-clades | MP 4-clades | ML 5-clades | MP 5-clades | |
| Clade I | 14 | 19 | 16 | 21 | – | – |
| Subclade Ia | – | – | – | – | 8 | 10 |
| Subclade Ib | – | – | – | – | 8 | 11 |
| Clade II | 49 | 44 | – | – | – | – |
| Subclade IIa | – | – | 24 | 21 | 24 | 21 |
| Subclade IIb | – | – | 5 | 7 | 5 | 7 |
| Subclade IIc | – | – | 20 | 16 | 20 | 16 |
| Outside | 2 | 2 | 0 | 0 | 0 | 0 |
| Conflicting | 12 | 19 | 20 | |||
| Total | 65 | 65 | 65 | 65 | 65 | 65 |
The MRPP analysis resulted in non-significant or spuriously significant differences between groups for the 2-clade solution but in highly significant differences for the 4-clade solution, independent of group assignment based on ML or MP weighting and of the distance measure employed (Table 3). Group assignments based on MP weighting gave slightly better correlations than based on ML weighting, as did the Euclidean distance measure compared to a linear correlation coefficient (Table 3). This suggests that the 4-clade solution and group assignment based on MP weighting is the best fit to the data.
Table 3. Results of the multi-response permutation procedure (MRPP) analysis.
| Euclidean | Correlation | |
| ML 2-clades | 0.0732 | 0.1697 |
| MP 2-clades | 0.0366 | 0.0785 |
| ML 4-clades | 0.0000 | 0.0000 |
| MP 4-clades | 0.0000 | 0.0000 |
| ML 5-clades | 0.0000 | 0.0000 |
| MP 5-clades | 0.0000 | 0.0000 |
p-values for significance of group distances based on morphological character matrix.
Kruskal-Wallis ANOVA indicates five characters as significantly discriminating between groups in a 2-clade solution using ML weighting and an additional three characters as marginally significant (Table 4). MP weighting results in a similar pattern but with overall fewer discriminating characters. Both the 4-clade and the 5-clade solutions suggests a much higher number of discriminating characters, again with a higher total for ML weighting. This supports the 4-clade or 5-clade solutions providing a better fit to the data than the 2-clade solution, with a slight advantage for ML over MP weighting.
Table 4. Number of individual characters discriminating between groups using phylogenetic binning method under ML and MP weighting.
| Significant (p<0.05) | Marginally significant (p<0.10) | Total | |
| ML 2-clades | 5 | 3 | 8 |
| MP 2-clades | 3 | 3 | 6 |
| ML 4-clades | 15 | 3 | 18 |
| MP 4-clades | 11 | 3 | 14 |
| ML 5-clades | 14 | 4 | 18 |
| MP 5-clades | 11 | 3 | 14 |
The best discriminating characters in the 4-clade and 5-clade solutions (Table 5) are the presence of soralia (MP weighting only), the nature of the thallus cortex, ascoma exposure and the shape of the proper and thalline margin, excipulum carbonization, ascospore number and dimensions (ML weighting only), ascospore endospore development and iodine reaction (best discriminating character under both ML and MP weighting), ascospore septation, and secondary chemistry (stictic and protocetraric acid; ML weighting only). According to these results, species of Clades Ia and IIa tend to have a loose cortex or lack a cortex altogether, whereas species of Clades Ib, IIb and IIc mostly have a dense cortex. Soralia are entirely confined to Clade I and particularly Clade Ib. Brown excipula are significantly more frequent in Clades IIa and IIc. Ascospores with amyloid endospore are particularly frequent in Clades Ib and IIa, and the latter clade also tends to have ascospores with a lower number of transverse septa than the other clades. The partial differences found in the level of character discrimination between taxon placement based on ML or MP weighting correlate with the weights determined for each character in the phylogenetic binning analysis. Characters that received high weights under an ML approach but low weights under MP include the thallus cortex, ascospore number and dimensions, and secondary chemistry, whereas the opposite was found for characters such as the presence of soralia.
Table 5. Discriminating characters as based on a Kruskal-Wallis ANOVA using the different clade solutions as grouping variables. P-values are indicated if below 0.1.
| ML-2 | MP-2 | ML-4 | MP-4 | ML-5 | MP-5 | |
| Soralia | 0.0019 | 0.0102 | 0.0002 | |||
| Oxalate crystals | 0.0129 | 0.0244 | ||||
| Cortex | 0.0744 | 0.0914 | 0.0089 | 0.0313 | ||
| Ascoma emergence | 0.0296 | 0.0567 | ||||
| Ascoma shape | 0.0962 | 0.0504 | ||||
| Ascoma aggregation | 0.0975 | 0.0544 | ||||
| Ascoma diameter | 0.0661 | 0.0980 | 0.0574 | 0.0596 | ||
| Ascoma exposure | 0.0017 | 0.0010 | 0.0043 | 0.0026 | ||
| Proper margin shape | 0.0368 | 0.0168 | 0.0057 | |||
| Proper margin striation | 0.0262 | 0.0019 | 0.0741 | 0.0049 | ||
| Proper margin split | 0.0007 | 0.0000 | 0.0544 | 0.0000 | ||
| Thallus margin shape | 0.0003 | 0.0003 | 0.0002 | |||
| Excipulum carbonization | 0.0080 | 0.0148 | 0.0123 | 0.0391 | 0.0274 | 0.0592 |
| Periphysoids presence | 0.0000 | 0.0000 | ||||
| Ascospores number | 0.0033 | 0.0080 | ||||
| Ascospores length | 0.0037 | 0.0723 | 0.0003 | 0.0008 | ||
| Ascospores width | 0.0773 | 0.0862 | 0.0077 | 0.0168 | ||
| Ascospores length-to-widthratio | 0.0006 | 0.0014 | 0.0027 | |||
| Ascospores endosporedevelopment | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||
| Ascospores iodine reaction | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||
| Ascospores transverse septa | 0.0056 | 0.0003 | 0.0919 | 0.0008 | ||
| Ascospores longitudinal septa | 0.0002 | 0.0546 | 0.0005 | 0.0272 | ||
| Chemistry stictic acid | 0.0366 | 0.0405 | ||||
| Chemistry protocetraric acid | 0.0005 | 0.0010 |
Discussion
The detection of phylogenetically defined clades lacking clearly discriminant morphological characters is not rare and particularly common in fungal groups, including lichenized species, since these organisms are composed of rather simple structures and are less differentiated than higher plants and animals [39], [40]. At higher taxonomic levels, such as family and order, this phenomenon has already found broad acceptance in fungal classifications [1], [17], [41]. Also, increasing evidence points to the frequent occurrence of cryptic species [22], [23], [25], [27], [28]. At the generic level, however, systematists have been highly reluctant to accept so-called cryptic genera, mainly because in lichenized fungi, the genus level has been the main taxonomic entity for classification purposes and herbaria collections are mostly organized using this taxonomic category. It is also commonly expected that classifications should result in the recognition of taxa, particularly at the genus level, that are phenotypically recognizable. However, increasing evidence from phylogenetic studies indicates that, while many monophyletically circumscribed genera are indeed recognizable, in other cases clades are inconsistent with morphological data. In many cases, particular morphotypes form either paraphyletic grades, with other morphotypes nested within, or are polyphyletic. Since the objective of molecular phylogenetic studies is to recognize natural groups, such para- or polyphyletic taxa cannot be maintained, except in the case of recently evolved species that have experienced the founder effect [42]–[45].
The genus Chapsa [37] had already been suspected to be not monophyletic, but the split into up to five clades within tribe Thelotremateae, and the placement of additional species outside this tribe, poses a challenge to classification, since there are no straightforward characters or a combination thereof that can be used to distinguish these lineages phenotypically. This situation is not rare in Graphidaceae and has also been found in the genera Graphis versus Allographa, Myriotrema versus Ocellis, Leucodecton versus Wirthiotrema, and Leucodecton versus Leptotrema, versus Ocellularia [15], [32], [33], [46]. There are numerous other examples of this situation among fungi and they are usually accepted if the lineages are unrelated or distantly related, but disputed in case of closer relationships, even if the underlying problem is the same. Thus, the basidiolichen genera Multiclavula and Lepidostroma include species that cannot be separated by any phenotypical character, but their very distant position among the Basidiomycota provides grounds for their taxonomic separation [47], [48]. The opposite phenomenon is also not rare: closely related lineages that are widely disparate morphologically, such as the genera Cruentotrema and Dyplolabia in Graphidaceae [15]. It is surprising that morphologically variable lineages merged into a single taxon are more readily accepted than separate, morphologically cryptic lineages, even if the underlying problem of lack of phenotypic consistency is the same.
In the case of the genus Chapsa, the situation is especially complex since up to five clades can be distinguished based on molecular phylogeny in the tribe Thelotremateae alone. Our approach shows that a 4-clade or 5-clade solution fits the data much better that a 2-clade solution, since the between-group differences are highly significant and the number of discriminating characters is much higher than in a 2-clade solution. The data do not allow to determine whether the 4-clade or 5-clade solution is the best fit (except that the latter has a slightly higher number of conflictive placements), since both are highly significant in terms of morphological discrimination and have about the same number of discriminating characters. Because of the lack of difference between the two alternatives, and since the branch leading to Clade I has high support, we opt for the more conservative solution here and maintain Clade I as a single genus, Chapsa. Our results suggest that there is a strong tendency for each of the four clades to differ in thallus cortex type, excipular carbonization, and ascospore type and septation (especially endospore development and iodine reaction), which is consistent with each clade having a long stem node and hence having evolved internal morphological variation independently. However, each clade includes a few species that morphologically would better fit in another clade. Such cases must be interpreted as either ancestral characters retained in a clade or as examples of parallel evolution. For this reason, these characters, even if statistically discriminant between genus-level clades, cannot be used to actually key out the genus-level taxa themselves.
The phylogenetic binning method allows both ML and MP weighting of the morphological characters, but the underlying algorithms are different [32]. For ML weighting, the best-scoring molecular tree is compared to a set of randomized trees (e.g. 100 trees) and the weight is derived by the number of random trees in which a particular morphological character mapped on the tree receives a worse log likelihood score. If a character has a strongly consistent distribution on the best-scoring molecular tree, any randomized tree will have a lower log likelihood score for this character, and the weight will be 100%. In contrast, MP weighting is derived only from the best-scoring molecular tree, and parsimony scores are computed by mapping the morphological characters on the tree and converting the scores into weights. As a result, ML weighting will emphasize characters that are confined to particular major clades (absolute synapomorphies), whereas MP weighting will also give higher weights to characters that characterize more than one clade but are absent from others (relative synapomorphies). Which method works better depends on the context, but the slightly better MRPP results for MP weighting in the 2-clade solution in this study confirm the findings of the original paper [32] that MP weighting might give slightly more consistent results. Interestingly, the characters found here to receive higher weights under ML are ascospore number and dimensions as well as number of septa, whereas under MP their weight was zero. These characters are known to vary strongly even within clades but are usually consistent between more closely related species, which could cause the effect that randomized trees consistently give lower log likelihood scores even if the overall character distribution over the tree is near-random. It is therefore recommended to use both ML and MP weighting in combination and closely inspect taxa with conflicting placement under both approaches, but if both methods yield quantitatively similar results overall, the MP solution is preferable as done here.
In contrast to making phenotypical characters obsolete in systematics, our study underlines the importance of these data even in times where molecular data become increasingly available to reconstruct phylogenies. While phenotypical data itself should not be used in such reconstructions, they are indispensable when transforming phylogenies into classifications and, with powerful analytical methods, provide statistical evidence that can be used to compare alternative classification models based on an underlying phylogeny.
Taxonomic Conclusions
The results of our phylogenetic analysis and the combined binning/multiresponse permutation procedure support a classification accepting each of the four Chapsa clades as different genera. Also, Thelotrema petractoides was shown to be distantly related to the core genus. Consequently, the new genera Astrochapsa, Crutarndina, Pseudochapsa, and Pseudotopeliopsis are described below and 39 new combinations are proposed. We only propose new combinations for species without conflict regarding clade placement under either ML or MP weighting, whereas the conflictive species are provisionally retained in Chapsa until sequence data become available. Therefore, the number or proposed combinations is lower than the numbers indicated in Table 2 for each clade. For example, the results suggest to place 24 taxa under ML and 21 taxa under MP weighting in Pseudochapsa, but only 16 of these are identical with both approaches, and only these are recombined here. In addition, we refrained from formally recombining five taxa that did not exhibit conflict but are suspected to possibly fall outside the Thelotremateae and hence require sequencing to clarify their position. This might also apply to some of the taxa with conflicting placement, such as C. asteliae and C. lordhowensis (see Appendix S1). Two species were recombined in Pseudotopelipsis favoring the MP weighting solution.
Astrochapsa
Parnmen, Lücking & Lumbsch, gen. nov. [MycoBank MB 801540] Type species: Astrochapsa astroidea (Berk. & Broome) Parnmen, Lücking & Lumbsch.
Differing from Chapsa s.str. in the more frequently densely corticate thallus, the mostly recurved apothecial margin, and the almost exclusively subdistoseptate, non-amyloid ascospores.
Thallus usually with dense cortex, rarely with loose cortex or ecorticate. Apothecia erumpent, rounded to irregular in outline; disc exposed; margin lobulate to usually recurved. Excipulum usually brown. Ascospores septate to muriform, fusiform-ellipsoid to oblong-cylindrical, with slightly thickened septa and angular lumina (subdistoseptate), colorless or rarely brown, almost exclusively I–. Secondary chemistry: no substances or frequently stictic acid and relatives; apothecial disc sometimes pigmented.
Etymology: Derived from “astro” (Greek, starry) because of the star-like morphology of the ascomata and the genus name Chapsa.
New Combinations in Astrochapsa
Astrochapsa alstrupii
(Frisch) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801500] Bas.: Chapsa alstrupii Frisch, Biblioth. Lichenol. 92: 93 (2006).
Astrochapsa amazonica
(Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801501] Bas.: Chapsa amazonica Kalb, Herzogia 22: 24 (2009).
Astrochapsa astroidea
(Berk. & Broome) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801502] Bas.: Platygrapha astroidea Berk. & Broome, J. Linn. Soc., Bot. 14: 109 (1873); Chapsa astroidea (Berk. & Broome) Cáceres & Lücking in Cáceres, Libri Botanici 22: 51 (2007).
Astrochapsa calathiformis
(Vain.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801503] Bas.: Thelotrema calathiforme Vain., Hedwigia 46: 174 (1907); Chapsa calathiformis (Vain.) Lumbsch & Papong in Papong et al., Lichenologist 42: 136 (2010).
Astrochapsa graphidioides
(Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801504] Bas.: Chapsa graphidioides Kalb, Herzogia 22: 24 (2009).
Astrochapsa lassae
(Mangold) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801505] Bas.: Chapsa lassae Mangold in Mangold et al., Fl. Australia 57: 653 (2009).
Astrochapsa magnifica
(Berk. & Broome) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801506] Bas.: Platygrapha magnifica Berk. & Broome, J. Linn. Soc., Bot. 14: 110 (1873); Chapsa magnifica (Berk. & Broome) Rivas Plata & Mangold et al., Lichenologist 42: 183 (2010).
Astrochapsa mastersonii
(Rivas Plata, Lumbsch & Lücking) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801507] Bas.: Chapsa mastersonii Rivas Plata, Lumbsch & Lücking in Weerakoon et al., Lichenologist 44: 374 (2012).
Astrochapsa megaphlyctidioides
(Mangold) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801508] Bas.: Chapsa megaphlyctidioides Mangold in Mangold et al., Fl. Australia 57: 654 (2009).
Astrochapsa meridensis
(Kalb & Frisch) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801509] Bas.: Topeliopsis meridensis Kalb & Frisch in Frisch & Kalb, Lichenologist 38: 42 (2006); Chapsa meridensis (Kalb & Frisch) Lücking, Lumbsch & Rivas Plata in Rivas Plata et al., Lichenologist 42: 183 (2010).
Astrochapsa platycarpella
(Vain.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801510] Bas.: Thelotrema platycarpellum Vain., Proc. Amer. Acad. Arts Sci. 58: 138 (1923); Chapsa platycarpella (Vain.) Frisch, Biblioth. Lichenol. 92: 118 (2006).
Astrochapsa pseudophlyctis
(Nyl.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801511] Bas.: Graphis pseudophlyctis Nyl. in Hue, Nouv. Arch. Mus. Nat. Hist., Sér. 3, 3: 163 (1891); Chapsa pseudophlyctis (Nyl.) Frisch, Biblioth. Lichenol. 92: 120 (2006).
Astrochapsa pulvereodiscus
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801512] Bas.: Thelotrema pulvereodiscus Hale, Bull. Br. Mus. Nat. Hist., Bot. 8(3): 268 (1981); Chapsa pulvereodiscus (Hale) Rivas Plata & Mangold in Rivas Plata et al., Lichenologist 42: 183 (2010).
Astrochapsa recurva
(G. Salisb.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801513] Bas.: Thelotrema recurvum G. Salisb., Rev. Bryol. Lichénol. 38: 285 (1972); Chapsa recurva (G. Salisb.) Frisch, Biblioth. Lichenol. 95: 120 (2006).
Astrochapsa stellata
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801514] Bas.: Leptotrema stellatum Hale, Smithson. Contr. Bot. 38: 54 (1978); Chapsa stellata (Hale) Sipman in Sipman et al., Phytotaxa 55: 47 (2012).
Astrochapsa waasii
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801515] Bas.: Thelotrema waasii Hale, Bull. Br. Mus. Nat. Hist., Bot. 8(3): 270 (1981); Chapsa waasii (Hale) Sipman & Lücking in Rivas Plata et al., Lichenologist 42: 183 (2010).
Astrochapsa wolseleyana
(Weerakoon, Lumbsch & Lücking) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801516] Bas.: Chapsa wolseleyana Weerakoon, Lumbsch & Lücking in Weerakoon et al., Lichenologist 44: 377 (2012).
Astrochapsa zahlbruckneri
(Redinger) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801517] Bas.: Phaeographina zahlbruckneri Redinger, Ark. Bot. 26A(1): 93 (1934); Chapsa zahlbruckneri (Redinger) Frisch, Biblioth. Lichenol. 92: 123 (2006).
Crutarndina
Parnmen, Lücking & Lumbsch, gen. nov. [MycoBank MB 801541] Type species: Crutarndina petractoides (P.M. Jørg. & Brodo) Parnmen, Lücking & Lumbsch.
Differing from Thelotrema s.str. in having a star-like, multi-layered exciple.
Thallus ecorticate. Apothecia erumpent, rounded; disc largely obscured by exciple; margin star-like, multi-layered. Excipulum hyaline basally, carbonized apically. Ascospores transversaly septate, fusiform, with thickened septa and lens-shaped to rounded lumina (distoseptate), colorless, I+. Secondary chemistry: no substances.
Etymology: Named after the distinguished British lichenologist Peter Crittenden (Nottingham) with whom SP and HTL collected material of the genus sequenced here on a field trip organized by the British Lichen Society. The name Crittenden is derived from the old British and Welsh and means “the cot on the lower hill”; derived from “cru” (cot); “tarn” (lower), and “dun” or “din” (hill).
New Combination in Crutarndina
Crutarndina petractoides
(P.M. Jørg. & Brodo) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801518] Bas.: Thelotrema petractoides P.M. Jørg. & Brodo, in Purvis et al., Biblioth. Lichenol. 58: 352 (1995).
Pseudochapsa
Parnmen, Lücking & Lumbsch, gen. nov. [MycoBank MB 801542]. Type species: Pseudochapsa dilatata (Müll. Arg.) Parnmen, Lücking & Lumbsch.
Differing from Chapsa s.str. in the usually brown excipulum and the almost exclusively distoseptate, amyloid ascospores.
Thallus usually with loose cortex or ecorticate, very rarely with dense cortex. Apothecia erumpent, rounded to irregular in outline; disc exposed; margin usually fissured to lobulate, rarely recurved. Excipulum usually brown. Ascospores septate to muriform, fusiform-ellipsoid to oblong-cylindrical, mostly with thickened septa and lens-shaped to rounded lumina (distoseptate), colorless or very rarely brown, almost exclusively I+ violet-blue (amyloid). Secondary chemistry: no substances or frequently stictic acid and relatives; apothecial disc rarely pigmented.
Etymology: Derived from “pseudo” (Greek, false) and the genus name Chapsa.
New Combinations in Pseudochapsa
Pseudochapsa albomaculata
(Sipman) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801519] Bas.: Thelotrema albomaculatum Sipman, Trop. Bryol. 5: 89 (1992); Chapsa albomaculata (Sipman) Sipman & Lücking in Rivas Plata et al., Lichenologist 42: 183 (2010).
Pseudochapsa crispata (Müll. Arg.)
Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801520] Bas.: Ocellularia crispata Müll. Arg., J. Linn. Soc. London 30: 452 (1895); Chapsa crispata (Müll. Arg.) Mangold in Mangold et al., Fl. Australia 57: 653 (2009) [non Chapsa crispata (Müll. Arg.) Rivas Plata & Mangold in Rivas Plata et al., Lichenologist 42: 182 (2010); comb. superfl.].
Pseudochapsa dilatata (Kalb)
Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801521] Bas.: Ocellularia dilatata Müll. Arg., J. Linn. Soc., London 30: 452 (1895); Chapsa dilatata (Müll. Arg.) Kalb, Biblioth. Lichenol. 99: 140 (2009).
Pseudochapsa esslingeri (Hale)
Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801522] Bas.: Ocellularia esslingeri Hale, Smithson. Contr. Bot. 38: 20 (1978); Chapsa esslingeri (Hale) Sipman in Sipman et al., Phytotaxa 55: 36 (2012).
Pseudochapsa hypoconstictica
(Rivas Plata & Lücking) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801523] Bas.: Chapsa hypoconstictica Rivas Plata & Lücking, Fung. Div. (in press).
Pseudochapsa isidiifera
(Frisch & Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801524] Bas.: Chapsa isidiifera Frisch & Kalb, Biblioth. Lichenol. 99: 136 (2009).
Pseudochapsa kalbii
(Frisch) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801525] Bas.: Chapsa kalbii Frisch, Biblioth. Lichenol. 92: 103 (2006).
Pseudochapsa lueckingii
(Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801526] Bas.: Chapsa lueckingii Kalb, Herzogia 22: 25 (2009).
Pseudochapsa phlyctidea
(Nyl.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801527] Bas.: Thelotrema phlyctideum Nyl., Ann. Sci. Nat., Bot., Sér. 4, 11: 222 (1859).
Pseudochapsa phlyctidioides
(Müll. Arg.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801528] Bas.: Ocellularia phlyctidioides Müll. Arg., Hedwigia 32: 130 (1893); Chapsa phlyctidioides (Müll. Arg.) Mangold, Aust. Syst. Bot. 21: 221 (2008).
Pseudochapsa pseudoexanthismocarpa
(Patw. & C. R. Kulk.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801529] Bas.: Ocellularia pseudoexanthismocarpa Patw. & C.R. Kulk., Norw. J. Bot. 24: 130 (1977); Chapsa pseudoexanthismocarpa (Patw. & C. R. Kulk.) Rivas Plata & Lücking in Rivas Plata et al., Lichenologist 42: 183 (2010).
Pseudochapsa pseudoschizostoma
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801530] Bas.: Ocellularia pseudoschizostoma Hale, Smithson. Contr. Bot. 38: 28 (1978); Chapsa pseudoschizostoma (Hale) Sipman in Sipman et al., Phytotaxa 55: 46 (2012).
Pseudochapsa rhizophorae
(Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801531] Bas.: Chapsa rhizophorae Kalb, Herzogia 22: 28 (2009).
Pseudochapsa rivas-platae
(Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801532] Bas.: Chapsa rivas-platae Kalb, Herzogia 22: 29 (2009).
Pseudochapsa sipmanii
(Frisch & Kalb) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801533] Bas.: Chapsa sipmanii Frisch & Kalb, Biblioth. Lichenol. 99: 138 (2009).
Pseudochapsa subpatens
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801534] Bas.: Thelotrema subpatens Hale, Bull. Br. Mus. Nat. Hist., Bot. 8: 269 (1981); Chapsa subpatens (Hale) Mangold in Mangold et al., Fl. Australia 57: 654 (2009).
Pseudotopeliopsis
Parnmen, Lücking & Lumbsch, gen. nov. [MycoBank MB 801543] Type species: Pseudotopeliopsis laceratula (Hale) Parnmen, Lücking & Lumbsch.
Differing from Chapsa s.str. in the densely corticate thallus and the Topeliopsis-like apothecia with striate excipulum filling the disc.
Thallus usually with dense cortex, rarely with loose cortex. Apothecia erumpent, rounded to irregular in outline; disc pore-like; margin fissured lobulate, in concentric layers covering the disc. Excipulum hyaline to brown. Ascospores septate to muriform, fusiform-ellipsoid to oblong-cylindrical, with slightly thickened septa and angular lumina (subdistoseptate), colorless to brown, I–. Secondary chemistry: no substances.
Etymology: Derived from “pseudo” (Greek, false) and the genus name Topeliopsis.
New Combinations in Pseudotopeliopsis
Pseudotopeliopsis aggregate
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801535] Bas.: Phaeotrema aggregatum Hale, Smithson. Contr. Bot. 16: 29 (1974); Chapsa aggregata (Hale) Sipman & Lücking in Rivas Plata et al., Lichenologist 42: 182 (2010).
Pseudotopeliopsis laceratula
(Müll. Arg.) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801536] Bas.: Thelotrema laceratulum Müll. Arg., Flora 70: 399 (1887); Chapsa laceratula (Müll. Arg.) Rivas Plata & Lücking in Rivas Plata et al., Lichenologist 42: 183 (2010).
Pseudotopeliopsis scabiocarpa
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801537] Bas.: Chapsa scabiocarpa Rivas Plata & Lücking, Fung. Div. (in press).
Pseudotopeliopsis scabiomarginata
(Hale) Parnmen, Lücking & Lumbsch, comb. nov. [MycoBank MB 801538] Bas.: Thelotrema scabiomarginatum Hale, Bull. Br. Mus. Nat. Hist., Bot. 8: 269 (1981); Chapsa scabiomarginata (Hale) Rivas Plata & Lücking in Rivas Plata et al., Lichenologist 42: 183 (2010).
Materials and Methods
Taxon Sampling and Molecular Methods
We assembled a three-locus data set consisting of mtSSU rDNA, nuLSU rDNA, and the protein-coding genes RPB2. The taxon sampling contained 60 species focusing on the tribe Thelotremateae (Table 1). The outgroup taxa were chosen based on previous phylogenetic results [33]. New sequences were generated for this study using the Sigma REDExtract-N-Amp Plant PCR Kit (St. Louis, Missouri, SA) for DNA isolation following the manufacturer’s instructions, except that 40 µL of extraction buffer and 40 µL dilution buffer were used. DNA dilutions (5x) were used in PCR reactions of the genes coding for the nuLSU, mtSSU and RPB2, respectively.Primers for amplification were: (a) for nuLSU: AL2R [35], and nu-LSU-1125-3′ ( = LR6) [49], (b) for mtSSU: mr-SSU1 and Mr-SSU3R [50], and (c) for RPB2: fRPB2-7cF and fRPB2-11aR [51]. The cycle sequencing conditions were as follows: 96°C for 1 minute, followed by 25 cycles of 96°C for 10 seconds, 50°C for 5 seconds and 60°C for 4 minutes. Samples were precipitated and sequenced using Applied Biosystems 3730 DNA Analyzer (Foster City, California, U.S.A.). Sequence fragments obtained were assembled with SeqMan 4.03 (DNASTAR) and manually adjusted.
Sequences Alignments and Phylogenetic Analyses
Alignments were done using Geneious Pro 5.5.2 [52]. Ambiguously aligned portions were removed manually. The single-locus and concatenated alignments were analyzed by maximum likelihood (ML) and a Bayesian approach (B/MCMC). To test for potential conflict, ML bootstrap analyses were performed on the individual data sets, and 75% bootstrap consensus trees were examined for conflict [2].
The ML analysis of the concatenated alignment was performed with the program RAxML-HPC2 (version 7.3.1) on XSEDE [53] using the default rapid hill-climbing algorithm. The model of nucleotide substitution chosen was GTRGAMMA. The data set was partitioned into five parts (mtSSU, nuLSU and each codon position of RPB2), so each gene partition was treated as an independent data set. Rapid bootstrap estimates were carried out for 1000 pseudoreplicates [54].
The B/MCMC analysis was conducted on the concatenated data set using MrBAYES 3.1.2 [55], with the same substitution model as in the ML analysis. A run with 10,000,000 generations, starting with a random tree and employing four simultaneous chains, was executed. No molecular clock was assumed. Heating of chains was set to 0.2. Posterior probabilities were approximated by sampling trees using a variant of Markov Chain Monte Carlo (MCMC) method. To avoid autocorrelation, only every 1000th tree was sampled. The first 4,000 generations were discarded as burn in. We used AWTY [56] to compare splits frequencies in the different runs and to plot cumulative split frequencies to ensure that stationarity was reached. Of the remaining19992 trees (9996 from each of the parallel runs) a majority rule consensus tree with average branch lengths was calculated using the sumt option of MrBAYES. Posterior probabilities were obtained for each clade. Clades with bootstrap support above 70% under ML and posterior probabilities ≥0.95 were considered as strongly supported. Phylogenetic trees were visualized using the program Treeview [57].
Anatomical and Chemical Studies
Anatomical studies were conducted using standard light microscopy on hand-cut sections mounted in water. Secondary lichen substances were identified by thin-layer chromatography (TLC) and high performance thin-layer chromatography (HPTLC) according to standard methods [58], [59].
Hypothesis Testing
Our phylogenetic analyses revealed that the genus Chapsa did not form a monophyletic group. Thus we tested whether our data are sufficient to reject monophyly of this genus. For the hypothesis testing, we used two different methods: (1) Shimodaira- Hasegawa (SH) test [60] and (2) expected likelihood weight (ELW) test [61]. The SH and ELW test were performed using Tree-PUZZLE v.5.2 [62] with the concatenated dataset, comparing the best tree agreeing with the null hypotheses, and the unconstrained ML tree. These trees were inferred in Tree-PUZZLE using the GTR+I+G nucleotide substitution model.
Morphology-based Phylogenetic Binning
Since the molecular data set corresponding to the genus Chapsa included 21 species, but the entire genus considered here comprises 86 accepted species, molecular data were unavailable for 65 species or about 75% of all currently accepted species. In this case, the phylogenetic binning method provides a statistical approach for a predictive classification of species, by weighting the morphological characters based on their distribution on the phylogenetic tree of the sequenced species and then placing each additional species known from morphological characters only separately in the tree and testing alternative placements by means of bootstrapping [32]. The weighting can be applied using both an MP and an ML approach. In this case, we applied the binning method for three alternative solutions: a 2-genus, a 4-genus, and a 5-genus solution. We and used both MP and ML weighting, to generate six possible alternative classifications of Chapsa based on both molecular and morphological data: ML-2, MP-2, ML-4, MP-4, ML-5, and MP5.
Multi-response Permutation Procedure
A multiresponse permutation procedure is a simple and effective tool to test for differences between groups of entities, in this case the groups obtained by the four alternative classifications obtained from the molecular phylogeny and subsequent binning method [63]. Since the morphological data matrix included only binary and ordered multistate characters, both Euclidean distances and linear correlation coefficients between each element within and between each group were computed. Within- and between group distances were then compared and statistical significance was tested by random data permutation using random shuffling of group partitions [63]. If within-group distances are smaller than expected by chance, it supports the recognition of a group as taxon, since such a result is evidence for partially independent phenotypic evolution. The analysis was performed in PC-Ord 5.03 [63]. For each individual character, we also employed Kruskal-Wallis ANOVA using each of the alternative clade solutions as grouping variable, to test whether the character discriminates between the resulting groups; this analysis was done in STATISTICA™ 6.0.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix http://www.mycobank.org/MB/. The online version of this work is archived and available from the following digital repositories: PubMed Central, LOCKSS.
Supporting Information
Clade placement of taxa according to molecular phylogenetic analysis and phylogenetic binning according to the different classification solutions using 2, 4, or 5 clades under either ML or MP weighting.
(DOC)
Acknowledgments
All new sequences were generated in the Pritzker Laboratory for Molecular Systematics and evolution at The Field Museum (Chicago). We wish to thank Marcela Cáceres (Recife), Allison Knight (Auckland), Eimy Rivas Plata (Durham, NC), Khwanruan Papong (Maharasakam), Andreas Frisch (Stockholm), Klaus Kalb (Neumarkt/Opf.), Armin Mangold (Berlin), and Tor Tønsberg (Bergen) for providing material that was used in this study.
Funding Statement
Funding provided by National Science Foundation (NSF), DEB-1025861 to The Field Museum; PI HTL, CoPI RL, “ATM – Assembling a taxonomic monograph: The lichen family Graphidaceae”. Website: http://www.nsf.gov/awardsearch/showAward.do?AwardNumber=1025861. Collection of material and sequencing work for this study was supported by four NSF grants: TICOLICHEN (DEB-0206125 to The Field Museum; PI RL), Phylogeny and Taxonomy of Ostropalean Fungi, with Emphasis on the Lichen-forming Thelotremataceae (DEB-0516116 to The Field Museum; PI HTL; Co-PI RL), Neotropical Epiphytic Microlichens - An Innovative Inventory of a Highly Diverse yet Little Known Group of Symbiotic Organisms (DEB-0715660 to The Field Museum; PI RL), and ATM - Assembling a taxonomic monograph: The lichen family Graphidaceae (DEB-1025861 to The Field Museum; PI HTL, CoPI RL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. (2007) A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547. [DOI] [PubMed] [Google Scholar]
- 2. Lutzoni F, Kauff F, Cox C, McLaughlin D, Celio G, et al. (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. American Journal of Botany 91: 1446–1480. [DOI] [PubMed] [Google Scholar]
- 3. McLaughlin DJ, Hibbett DS, Lutzoni F, Spatafora JW, Vilgalys R (2009) The search for the fungal tree of life. Trends in Microbiology 17: 488–497. [DOI] [PubMed] [Google Scholar]
- 4. Hillis DM (1987) Molecular versus morphological approaches to systematics. Annual Review of Ecology and Systematics 18: 23–42. [Google Scholar]
- 5. Thomas RH, Hunt JA (1993) Phylogenetic relationships in Drosophila: a conflict between molecular and morphological data. Molecular Biology and Evolution 10: 362–374. [DOI] [PubMed] [Google Scholar]
- 6. Wiens JJ, Hollingsworth BD (2000) War of the iguanas: Conflicting phylogenies, long branch attraction, and disparate rates of molecular and morphological evolution in iguanid lizards. Systematic Biology 49: 69–85. [DOI] [PubMed] [Google Scholar]
- 7. Lee MSY (2001) Uninformative characters and apparent conflict between molecules and morphology. Molecular Biology and Evolution 18: 676–680. [DOI] [PubMed] [Google Scholar]
- 8. Wiens JJ (2004) The role of morphological data in phylogeny reconstruction. Systematic Biology 53: 653–661. [DOI] [PubMed] [Google Scholar]
- 9. Blanco O, Crespo A, Elix JA, Hawksworth DL, Lumbsch HT (2004) A molecular phylogeny and a new classification of parmelioid lichens containing Xanthoparmelia-type lichenan (Ascomycota: Lecanorales). Taxon 53: 959–975. [Google Scholar]
- 10. Hibbett DS (2007) After the gold rush, or before the flood? Evolutionary morphology of mushroom-forming fungi (Agaricomycetes) in the early 21st century. Mycological Research 111: 1001–1018. [DOI] [PubMed] [Google Scholar]
- 11. Lumbsch HT, Huhndorf SM (2007) Whatever happened to the pyrenomycetes and loculoascomycetes? Mycological Research 111: 1064–1074. [DOI] [PubMed] [Google Scholar]
- 12. Moore J, Willmer P (1997) Convergent evolution in invertebrates. Biological Reviews 72: 1–60. [DOI] [PubMed] [Google Scholar]
- 13.Stearns S, Hoekstra R (2005) Evolution: An Introduction. Oxford: Oxford University Press.
- 14.Barton NH, Briggs DEG, Eisen JA, Goldstein DB, Patel NH (2007) Evolution. Cold Spring: Harbor Laboratory Press.
- 15. Rivas Plata E, Lumbsch HT (2011) Parallel evolution and phenotypic divergence in lichenized fungi: a case study in the lichen-forming fungal family Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales). Molecular Phylogenetics and Evolution 61: 45–63. [DOI] [PubMed] [Google Scholar]
- 16.Futuyama DJ (2005) Evolution. Sunderland: Sinauer Associates.
- 17. Lumbsch HT, Huhndorf SM (2010) Myconet Volume 14. Part One. Outline of Ascomycota - 2009. Fieldiana (Life and Earth Sciences) 1: 1–42. [Google Scholar]
- 18. Zalar P, de Hoog GS, Schroers HJ, Frank JM, Gunde-Cimerman N (2005) Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology 87: 311–328. [DOI] [PubMed] [Google Scholar]
- 19. Vilnet AA, Milyutina IA, Konstantinova NA, Ignatov MS, Troitsky AV (2007) Phylogeny of the genus Lophozia (Dumort.) Dumort. s. str. inferred from nuclear and chloroplast sequences ITS1–2 and TRNL-F. Russian Journal of Genetics 43: 1306–1313. [PubMed] [Google Scholar]
- 20. Fucikova K, Rada JC, Lewis LA (2011) The tangled taxonomic history of Dictyococcus, Bracteacoccus and Pseudomuriella (Chlorophyceae, Chlorophyta) and their distinction based on a phylogenetic perspective. Phycologia 50: 422–429. [Google Scholar]
- 21. Fucikova K, Lewis LA (2011) Unravelling the taxonomic knot of Bracteacoccus, Dictyococcus, Pseudomuriella, and Chromochloris (Chlorophyceae, Chlorophyta): A case of cryptic genera. Journal of Phycology 47: S39–S39. [Google Scholar]
- 22. Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W (2004) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator . Proceedings of the National Academy of Sciences of the United States of America 101: 14812–14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, et al. (2007) Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22: 148–155. [DOI] [PubMed] [Google Scholar]
- 24. Burns JM, Janzen DH, Hajibabaei M, Hallwachs W, Hebert PDN (2008) DNA barcodes and cryptic species of skipper butterflies in the genus Perichares in Area de Conservacion Guanacaste, Costa Rica. Proceedings of the National Academy of Sciences of the United States of America 105: 6350–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crespo A, Perez-Ortega S (2009) Cryptic species and species pairs in lichens: A discussion on the relationship between molecular phylogenies and morphological characters. Anales del Jardín Botánico de Madrid 66: 71–81. [Google Scholar]
- 26. Seifert B (2009) Cryptic species in ants (Hymenoptera: Formicidae) revisited: we need a change in the alpha-taxonomic approach. Myrmecological News 12: 149–166. [Google Scholar]
- 27. Trontelj P, Fiser C (2009) Cryptic species diversity should not be trivialised. Systematics and Biodiversity 7: 1–3. [Google Scholar]
- 28. Crespo A, Lumbsch HT (2010) Cryptic species in lichen-forming fungi. IMA Fungus 1: 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Sickle J (1997) Using mean similarity dendrograms to evaluate classifications. Journal of Agricultural, Biological, and Environmental Statistics 2: 370–388. [Google Scholar]
- 30.Mielke PW, Berry KJ (2001) Permutation Methods: A Distance Function Approach. Berlin: Springer Verlag.
- 31.McCune B, Grace JB (2002) Analysis of Ecological Communities. Gleneden Beach, Oregon, USA: MjM Software Design.
- 32. Berger SA, Stamatakis A, Lücking R (2011) Morphology-based phylogenetic binning of the lichen genera Graphis and Allographa (Ascomycota: Graphidaceae) using molecular site weight calibration. Taxon 60: 1450–1457. [Google Scholar]
- 33.Rivas Plata E, Parnmen S, Staiger B, Mangold A, Frisch A, et al.. (2012) A molecular phylogeny of Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales) including 437 species. MycoKeys in press.
- 34. Rivas-Plata E, Lücking R, Lumbsch HT (2012) A new classification for the lichen family Graphidaceae s.lat. (Ascomycota: Lecanoromycetes: Ostropales). Fungal Diversity 52: 107–121. [Google Scholar]
- 35. Mangold A, Martin MP, Lücking R, Lumbsch HT (2008) Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota : Ostropales). Taxon 57: 476–486. [Google Scholar]
- 36. Papong K, Corush J, Mangold A, Lucking R, Lumbsch HT (2009) Phylogenetic position of the foliicolous genus Chroodiscus (Ostropales, Ascomycota) inferred from nuclear and mitochondrial ribosomal DNA sequences. Fungal Diversity 38: 147–153. [Google Scholar]
- 37. Frisch A, Kalb K, Grube M (2006) Contributions towards a new systematics of the lichen family Thelotremataceae. Bibliotheca Lichenologica 92: 1–539. [Google Scholar]
- 38. Rivas Plata E, Lücking R, Sipman HJM, Mangold A, Lumbsch HT (2010) A world-wide key to the thelotremoid Graphidaceae, excluding the Ocellularia-Myriotrema-Stegobolus clade. Lichenologist 42: 139–185. [Google Scholar]
- 39.Alexopoulos CJ, Mims CW, Blackwell M (1996) Introductory Mycology. New York: John Wiley & Sons.
- 40.Jahns HM, Ott S (1994) Thallic mycelial and cytological characters in ascomycete systematics. In: Hawksworth DL, editor. Ascomycete Systematics Problems and Perspectives in the Nineties: NATO Advanced Science Institutes Series, Plenum Press, New York. 57–62.
- 41.Kirk PM, Cannon PF, David JC, Stalpers JAe (2009) Ainsworth & Bisby’s Dictionary of the Fungi. 10th edition: CAB International, Wallingford, Oxon. 655 p.
- 42. Lumbsch HT, Leavitt SD (2011) Goodbye morphology? A paradigm shift in the delimitation of species in lichenized fungi Fungal Diversity 50: 59–72. [Google Scholar]
- 43.Mayr E (1963) Animal species and evolution. Cambridge, MA: Harvard University Press.
- 44. Wang HY, Lumbsch HT, Guo SY, Huang MR, Wei JC (2010) Ascomycetes have faster evolutionary rates and larger species diversity than basidiomycetes. Science China, Life Sciences 53: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 45. Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: Frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology Evolution and Systematics 34: 397–423. [Google Scholar]
- 46.Rivas Plata E (2011) Historical biogeography, ecology and systematics of the family Graphidaceae (Lichenized Ascomycota: Ostropales). Chicago: University of Illinois at Chicago. 238 p.
- 47. Ertz D, Lawrey JD, Sikaroodi M, Gillevet PM, Fischer E, et al. (2008) A new lineage of lichenized basidiomycetes inferred from a two-gene phylogeny: The Lepidostromataceae with three species from the tropics. American Journal of Botany 95: 1548–1556. [DOI] [PubMed] [Google Scholar]
- 48. Hodkinson BP, Uehling JK, Smith ME (2012) Lepidostroma vilgalysii, a new basidiolichen from the New World. Mycological Progress 11: 827–833. [Google Scholar]
- 49. Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zoller S, Scheidegger C, Sperisen C (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31: 511–516. [Google Scholar]
- 51. Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- 52.Drummond A, Ashton B, Buxton S, Cheung M, Cooper A, et al. (2011) Geneious v5.4. Available from http://www.geneious.com/.
- 53. Stamatakis A (2006) RAxML-VI-HPC: Maximum Likelihood-based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 54. Stamatakis A, Hoover P, Rougemont J (2008) A Rapid Bootstrap Algorithm for the RAxML Web Servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- 55. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 56. Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2007) AWTY (Are We There Yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583. [DOI] [PubMed] [Google Scholar]
- 57. Page RDM (1996) Treeview: an application to display phylogenetic trees on personal computers. Computer Applied Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- 58. Arup U, Ekman S, Lindblom L, Mattsson J-E (1993) High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist 25: 61–71. [Google Scholar]
- 59.Orange A, James PW, White FJ (2001) Microchemical Methods for the Identification of Lichens: British Lichen Society. 101 p.
- 60. Shimodaira H, Hasegawa M (2001) CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17: 1246–1247. [DOI] [PubMed] [Google Scholar]
- 61. Strimmer K, Rambaut A (2002) Inferring confidence sets of possibly misspecified gene trees. Proceedings of the Royal Society of London Series B, Biological Sciences 269: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schmidt HA, Strimmer K, Vingron M, von Haeseler A (2002) TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18: 502–504. [DOI] [PubMed] [Google Scholar]
- 63.McCune B, Mefford MJ (1999) PC-ORD. Multivariate Analysis of Ecological Data, Version 4.0. Gleneden Beach, OR: MjM Software Design. 237 p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clade placement of taxa according to molecular phylogenetic analysis and phylogenetic binning according to the different classification solutions using 2, 4, or 5 clades under either ML or MP weighting.
(DOC)