Abstract
Cyclic phosphatidic acid (cPA) is a naturally occurring phospholipid mediator with a unique cyclic phosphate ring at the sn-2 and sn-3 positions of its glycerol backbone. We have previously shown that cPA significantly suppresses ischemia-induced delayed neuronal death and the accumulation of glial fibrillary acidic protein in the CA1 region of the rat hippocampus. These results indicated that the systemic administration of cPA can protect hippocampal neurons against ischemia-induced delayed neuronal cell death. In the current study, we investigated the effects of cPA on neuronal cell death caused by hypoxia in vitro and the molecular mechanisms underlying these effects. We used cobalt chloride (CoCl2) to expose cells to hypoxic conditions in vitro. Treating mouse neuroblastoma (Neuro2A) cells with CoCl2 induced nuclear DNA condensation and phosphatidylserine exposure. However, adding cPA led to the suppression of CoCl2-induced apoptosis in a cPA dose-dependent manner and attenuated the increase in the Bax/Bcl-2 ratio caused by CoCl2. Quantitative PCR analysis showed that Neuro2A cells strongly express the LPA1, LPA2, and LPA6, which are G-protein coupled receptors that can be activated by cPA. To date, LPA1 and LPA2 have been reported to exhibit antiapoptotic activity. Therefore, to assess the roles of LPA1 and LPA2 on cPA-induced neuroprotective functions, Ki16425, a selective LPA1 and LPA3 antagonist, was adopted to know the LPA1 function and siRNA was used to knockdown the expression of LPA2. On the basis of our results, we propose that cPA-induced protection of Neuro2A cells from CoCl2-induced hypoxia damage is mediated via LPA2.
Introduction
In 1992, cyclic phosphatidic acid (cPA) was originally isolated from the myxoamoebae of a true slime mold, Physarum polycephalum [1]. Later, the presence of cPA 16∶0 and 18∶1 has been found in mammalian fluids such as serum and brain tissue [2], [3]. And these are major forms of naturally occurring cPA species. Although the chemical formula of cPA is similar to that of lysophosphatidic acid (LPA), cPA has a unique structure consisting of a cyclic phosphate ring at the sn-2 and sn-3 positions of its glycerol backbone [1]. These features provide cPA with biological functions that are distinct from or oppose the functions of LPA. For example, LPA stimulates cell proliferation, cancer cell invasion, and generates pain, whereas cPA inhibits these activities [4]–[10].
Previous in vitro studies have reported that cPA 16∶0, 18∶1 and LPA 18∶1 elicit neurotrophin-like actions in embryonic hippocampal neurons [11]. We have examined the effects of cPA 18∶1 on ischemia-induced delayed neuronal death in the hippocampal CA1 region and found that the systemic administration of cPA 18∶1 has in vivo neuroprotective effects [12]. However, the mechanisms underlying these effects of cPA 18∶1 on hypoxic-ischemic brain injury have not yet been completely understood. Here, we aimed to investigate the effects of cPA 18∶1 on hypoxia-induced apoptosis and the molecular mechanism underlying these effects.
To induce hypoxic/ischemic conditions in vitro, we induced apoptosis with cobalt chloride (CoCl2), which is used as a chemical hypoxia-inducing agent for several types of neural cells [13]–[16]. Then, we investigated the possible mechanisms of LPA receptor involvement. To date, cPA has been reported to stimulate several LPA G protein-coupled receptors (GPCRs) such as LPA1–5 [6], [17]. LPA1, LPA2, and GPR87 have been shown to exhibit antiapoptotic activity [18]. cPA activates the LPA1–5 at significantly higher EC50 concentrations than LPA [6], [17]. Although cPA is a weak agonist against the LPA1, 3, 4, 5, it has a stronger efficacy against the LPA2 (140% maximal efficacy compared with LPA) [6]. Each LPA receptor exhibits a different affinity and efficacy toward cPA and LPA. In addition, each LPA receptor has a individual signaling pathway and function. Thus, cells expressing several LPA receptors might undergo a different cellular phenomenon when stimulated by cPA or LPA.
In this study, using neuroblastoma Neuro2A cells, we examined the effects of the neuroprotective functions that are specific to cPA 18∶1 and LPA 18∶1 and involve LPA receptors and also investigated the molecular mechanisms underlying these effects. Our results show that although both lipid mediators exhibit neuroprotective functions for Neuro2A, the signal pathway initiated by cPA and LPA may potentially differ.
Materials and Methods
1. Pharmacologic agents
cPA 18∶1 was chemically synthesized as previously described [19]. Bovine serum albumin (BSA; fraction V, fatty acid free) and 1-oleoyl-sn-lysophosphatidic acid (LPA 18∶1) were purchased from Sigma-Aldrich (St. Louis, MO). LPA and cPA were dissolved in PBS containing 0.1% (w/v) BSA. The inhibitor of the LPA1, 3, that is, Ki16425 (Cayman Chemicals, MI), was dissolved in DMSO and added to Neuro2A cells at a working concentration of 10 µM at 20 min prior to adding cPA or LPA.
2. Cell culture and treatment
Mouse Neuro2A cells were obtained from the Department of Biochemistry, Faculty of Medicine, the University of Tokyo. The cells were originally obtained from the American Type Culture Collection (ATCC cat. no. CCL131). The cells were cultured in Dulbecco's modified Eagle medium (DMEM; Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS; Biowest, FL), 0.2% NaHCO3, and 0.06% glutamine. Cells were grown at 37°C in a humidified incubator containing 5% CO2. For all the experiments, 1.0×106 Neuro2A cells were seeded into a 100-mm dish and incubated for 4 hours with culture media containing 10% FBS. The cells were then subjected to serum starvation for 16 hours with serum-free DMEM.
3. Cell viability
To determine cell viability, cells were seeded (4×104 cells/well) in a 96-well plate. Serum-starved Neuro2A cells were exposed to various concentrations of CoCl2 for 24 hours. The cells were then stained with 10 µM calcein-AM (Dojindo, Kumamoto, Japan) in the dark for 30 min at 37°C and washed with phosphate-buffered saline (PBS). The fluorescence intensity (em/ex, 485/530 nm) of each well was measured using a CytoFluor series 4000 fluorescence microplate reader (Applied Biosystems, Tokyo, Japan). Data were calculated as the percent cell viability compared to that of controls without CoCl2 treatment and have been presented as the mean and SE values for triplicate wells.
4. DAPI staining
Neuro2A cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. Then, the nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 10 min at room temperature. The chromatin structures of the cells were observed under a fluorescence microscope equipped with a UV combination filter (Nikon Corp., Tokyo, Japan).
5. Measurement of reactive oxygen species generation
Intracellular reactive oxygen species (ROS) generation was measured using the fluorescent probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA; Molecular Probes, Inc., OR). Serum-starved Neuro2A cells in 96-well plates were exposed to 300 µM CoCl2 and stained with 10 µM CM-H2DCFDA in the dark for 30 min. Subsequently, the cells were washed with PBS, and the fluorescence intensity (em/ex, 485/530 nm) of each well was measured using the CytoFluor series 4000 microplate reader. The data were calculated as the relative ROS induction compared to that of controls (without CoCl2 treatment, time 0) and have been presented as the mean and SE values of triplicate wells.
6. Flow cytometric analysis
Serum-starved Neuro2A cells were exposed to 300 µM CoCl2 in the presence of 10 µM cPA or LPA. After 24 hours, the extent of apoptosis in Neuro2A cells was quantified by flow cytometric analysis by using the Cell Lab Quanta™ SC MPL (Beckman Coulter, CA). Cell staining was performed with FITC-conjugated Annexin V (Trevigen, MD) or DEVD-FMK (a caspase-3 inhibitor that irreversibly binds to activated caspase-3, BioVision, CA) in accordance with the manufacturer's instructions.
7. Cell adhesion assay
Serum-starved Neuro2A cells were exposed to 300 µM CoCl2 in the presence of 10 µM cPA or LPA. After 24 hours, the non-adherent cells were removed by washing 3 times with PBS. The number of adherent cells (cells/cm2) was determined using an inverted phase-contrast microscope.
8. RNA interference
Neuro2A cells were transfected with Accell SMARTpool siRNA specific for the mouse LPA2 or Accell non-targeting siRNA (Dharmacon, Inc., CO) with Accell siRNA delivery media, according to the manufacturer's protocol. The cells were used 3 d after transfection.
9. RNA isolation and real-time PCR
Total RNA was extracted from Neuro2A cells by using the Isogen reagent (Nippongene, Toyama, Japan). It was used as a template for subsequent cDNA synthesis with oligo dT primers by using the Omniscript RT Kit (Qiagen, CA). mRNA levels were quantified using an ABI 7300 real-time PCR machine (Foster City, CA) and SYBR Premix Ex Taq II (Takara Bio Inc., Siga, Japan). Gene-specific primer sets were used as previously reported [12], [20]. To quantify the knockdown levels of LPA2 mRNA, the following 2 primer pair sets were used: primer set I, 5′-GTTGAGGTCACTCCCACGTT-3′ (F) and 5′- CGTGCCTTTCCCTAAACCTT-3′ (R); and primer set II, 5′-AGGCTGGATATGGTCATTGC-3′ (F) and 5′- GCAGCTCCAGCAGAAATGTA-3′ (R). The data were analyzed using the delta Ct method. The expression level of each LPA receptor was normalized to β-actin expression as previously described [12], [20].
10. Western blot analysis
Neuro2A cells were collected and subjected to western blot analysis to detect Bax and Bcl-2 protein expression. Proteins were separated by SDS-PAGE by using a 15% polyacrylamide gel and then transferred to an Immobilon-P Transfer Membrane (Millipore). Using anti-Bax or anti-Bcl-2 antibodies (1∶1000 dilution, Cell Signaling Technology, Inc., MA) and horseradish peroxidase-conjugated anti-rabbit IgG (1∶10,000 dilution; Kirkegaard & Perry Laboratories Inc., MD), immunodetection was performed using an enhanced chemiluminescence (ECL) system (GE Healthcare UK Ltd, Amersham Place, Little Chalfont, England).
11. Statistical analysis
All the values have been reported in terms of mean ± SE values. The data were analyzed using one-way analysis of variance (ANOVA) and subsequently with Dunnett's test. A P value less than 0.05 was considered to be statistically significant.
Results and Discussion
1. CoCl2-induced apoptosis in Neuro2A cells
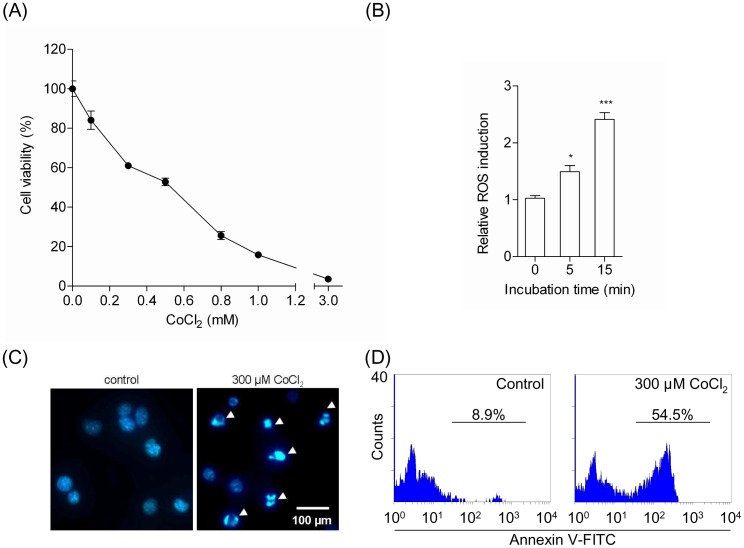
Neuro2A cells were treated with various concentrations of CoCl2. After 24 hours, exposure of Neuro2A cells to CoCl2 significantly decreased cell viability in a CoCl2 dose-dependent manner (Fig. 1A). Exposure to 300 µM CoCl2 for 24 hours resulted in 61% viable cells compared to control cells (100%). The mode of cell death, necrosis, or apoptosis was determined by DAPI staining. After exposure to CoCl2, the cells displayed apoptotic morphology characterized by the condensation of chromatin, as shown in Fig. 1B. Moreover, to assess intracellular ROS generation, we measured the oxidation of CM-H2DCFDA [13]. CoCl2 treatment has been reported to significantly increase ROS levels within 1 h of incubation [21]. We also observed that treatment of Neuro2A cells with CoCl2 for 15 min induced oxidative stress by enhancing ROS levels (Fig. 1C). Our data show that exposure of Neuro2A cells to CoCl2 rapidly increased ROS levels and might initiate apoptosis signaling. Meanwhile it was revealed that Neuro2A did not generate superoxide by treatment of CoCl2 for 0–30 min (data not shown).
Figure 1. Treatment with CoCl2 induces apoptosis in Neuro2A cells.
(A) Effects of CoCl2 on the viability of Neuro2A cells. Neuro2A cells were incubated with various concentrations of CoCl2 for 24 hours. Cell viability was estimated as described in the materials and methods section. The data represent the mean ± SE values from triplicate independent experiments. (B) Generation of reactive oxygen species (ROS) induced by CoCl2. Neuro2A cells were incubated with 300 µM CoCl2, and ROS generation was measured after 5 and 15 min. The data represent the mean ± SE values from triplicate independent experiments (*P<0.05, ***P<0.001 vs. the CoCl2-treated group). (C) Morphologic changes in the nuclei of CoCl2-treated Neuro2A cells. Neuro2A cells incubated in the absence (left) or presence (right) of 300 µM CoCl2 for 24 hours were fixed and stained with DAPI and examined by fluorescence microscopy. (D) Analysis of apoptosis-associated PS exposure on CoCl2-treated Neuro2A cells by using FITC-Annexin V. Neuro2A cells were treated with (right) or without (left) 300 µM CoCl2 for 24 hours and stained with FITC-Annexin V. The population of FITC-Annexin V-positive Neuro2A cells was quantified by flow cytometry.
Flow cytometric analysis with FITC-Annexin V was used to analyze the rate of apoptosis induced by CoCl2 (Fig. 1D). Representative data show that exposure to 300 µM CoCl2 for 24 hours resulted in 54.5% FITC-Annexin V-positive Neuro2A cells in the entire cell population. On the other hand, no exposure to CoCl2 for 24 hours resulted in only 8.9% FITC-Annexin V-positive Neuro2A cells in the entire cell population. These results suggest that stimulation by 300 µM CoCl2 for 24 hours induced apoptosis in Neuro2A cells. Therefore, these conditions were used to induce apoptosis in Neuro2A cells in all subsequent experiments.
2. cPA protected Neuro2A cells against CoCl2-induced apoptosis
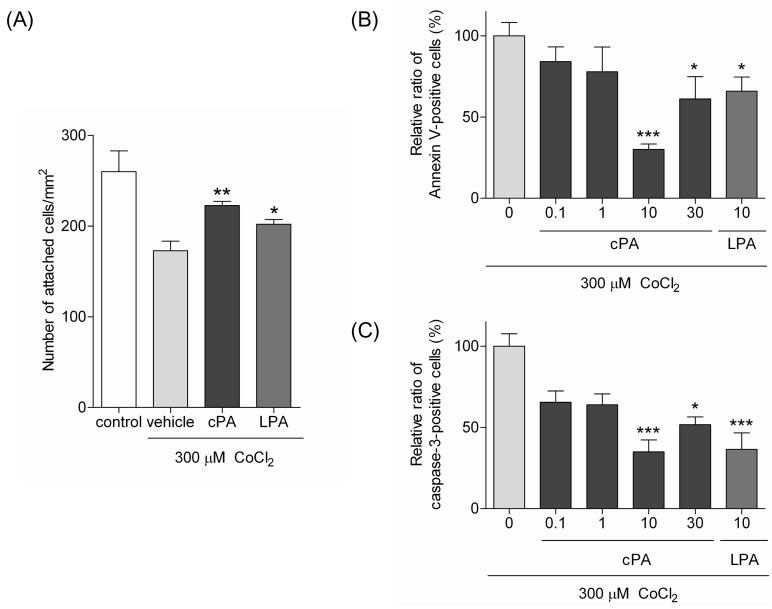
To examine the effects of cPA on CoCl2-induced apoptosis, Neuro2A cells were treated with CoCl2 in the presence or absence of cPA. Twenty-four hours later, the number of adherent cells (cells/cm2) was counted (Fig. 2A). At a concentration of 10 µM, cPA was observed to inhibit CoCl2-induced cell detachment. Although LPA is less potent than cPA, it also inhibited cell detachment. These results suggest that cPA and LPA could potentially attenuate CoCl2-induced Neuro2A cytotoxicity.
Figure 2. cPA protects against CoCl2-induced apoptosis in Neuro2A cells.
(A) The effects of cPA and LPA on a number of adhesive Neuro2A cells treated with CoCl2. Neuro2A cells were incubated with 300 µM CoCl2 in the presence of 10 µM cPA or LPA for 24 hours, and the number of cells attached to the surface of dishes was determined (*P<0.05, **P<0.01 vs. the CoCl2-treated group). (B & C) Analysis of the percent of FITC-Annexin V- and FITC-DEVD-FMK-positive Neuro2A cells after CoCl2 treatment by flow cytometry. Neuro2A cells were incubated with 300 µM CoCl2 and various concentrations of either cPA or LPA for 24 hours. Cells were subsequently stained with either FITC-Annexin V (B) or FITC-DEVD-FMK, an activated caspase-3 inhibitor (C) and subjected to flow cytometric analysis. The data represent the mean ± SE values from triplicate independent experiments (*P<0.05, **P<0.01, ***P<0.001 vs. the CoCl2-treated group).
We then investigated the effects of cPA and LPA on CoCl2-induced apoptosis by measuring exposure of phosphatidylserine (PS) and activation of caspase-3. Exposure of PS on the surface of the cell membrane is related to the occurrence of early stages of apoptotic cell death and can be detected using Annexin V (PS-binding protein). Flow cytometric analysis with FITC-Annexin V showed that cPA-treatment significantly decreased the number of FITC-Annexin V-positive Neuro2A cells in a bell-shaped dose-dependent manner after exposure to CoCl2. At the most effective cPA-concentration (10 µM), the number of FITC-Annexin V-positive cells decreased to 30% of those in the vehicle control. LPA (10 µM) also exhibited neuroprotective effects on Neuro2A cells, as shown in Fig. 2B. However, the neuroprotective effects were not exhibited at lower concentrations (0.1 and 1 µM) of LPA (data not shown).
The cleavage of caspase-3 has been shown to be an important trigger for the execution of apoptosis [22], [23]. Treatment of Neuro2A cells with 300 µM CoCl2 significantly stimulated caspase-3 activity, which was attenuated by cPA in a bell-shaped dose-dependent manner, as shown in Fig. 2C. To confirm flow cytometric analysis, we performed western blot analysis to measure protein level of pro-caspase-3 using anti-caspase-3 antibody. It was revealed that level of pro-caspase-3 was significantly decreased by treatment of Neuro2A cells with 300 µM CoCl2 for 24 hours (data not shown), indicating pro-caspase-3 was activated. The decrease of pro-caspase-3 was attenuated by the addition of cPA 18∶1 or LPA 18∶1. These results are consistent with the results from flow cytometric analysis as shown in Fig. 2C.
In the case of measuring caspase-3 activity, 10 µM LPA but not lower concentration of LPA was observed to attenuate the caspase-3 activity. These results strongly indicate that cPA decreases the exposure of PS and caspase-3 activity and attenuates CoCl2-induced apoptosis.
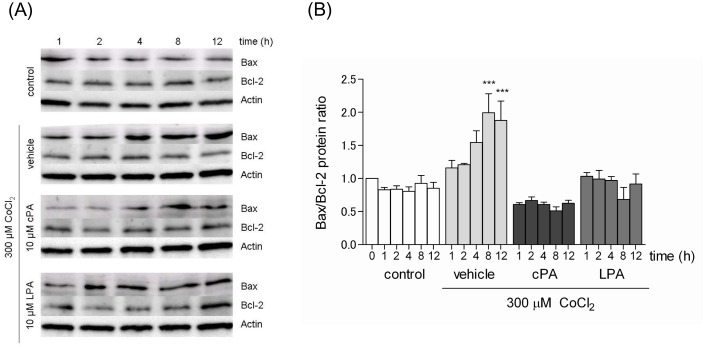
3. Effects of CoCl2 and cPA on the expression of the Bcl-2 protein family in Neuro2A cells
The Bcl-2 family is an important regulator of various apoptotic pathways [22]. Because hypoxia induces an increase in the expression of proapoptotic Bcl-2 family members, such as Bax, and decrease in the expression of antiapoptotic Bcl-2 family members, such as Bcl-2, we examined the effects of CoCl2-induced apoptotic signaling on Bax and Bcl-2. Bax and Bcl-2 protein levels were determined by western blot analysis, and a time-course analysis of Bax and Bcl-2 protein expression ratios was performed (Fig. 3). The Bax/Bcl-2 ratio increased over time with CoCl2 treatment. However, in the presence of either cPA or LPA (10 µM), the ratio did not increase and remained at the ratio seen in control cells for up to 12 hours. Thus, cPA and LPA suppressed the CoCl2-induced increase in the Bax/Bcl-2 ratio and prevented CoCl2-induced apoptosis.
Figure 3. Effects of cPA and LPA on the expression of the Bcl-2 protein family.
(A) Neuro2A cells were incubated with 300 µM CoCl2 in the presence of 10 µM cPA or LPA for the time indicated. Protein levels of Bax, Bcl-2, and β-actin were determined by western blot analysis. (B) The expression of Bax and Bcl-2 was determined using a densitometer, and the Bax/Bcl-2 ratio was calculated and normalized to cells without any treatment. The data represent the mean ± SE values from triplicate independent experiments (***P<0.001 vs. CoCl2-treated group).
4. The antiapoptotic function of cPA is mediated by LPA2
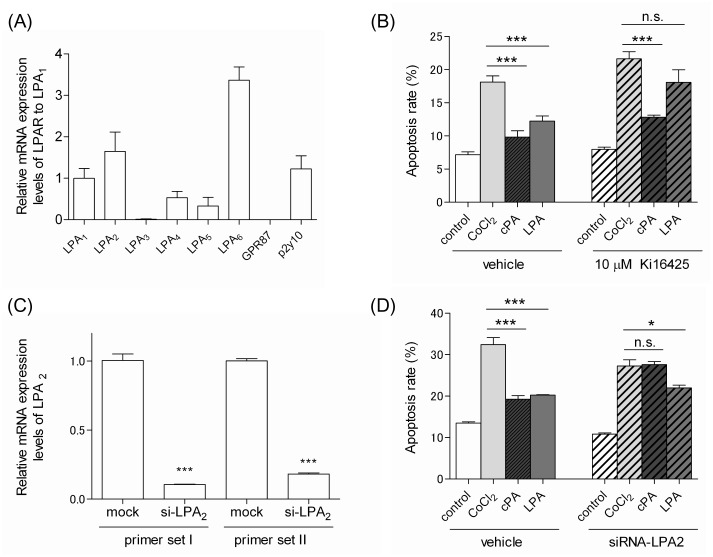
To gain insight into the molecular mechanism underlying the protective effects of cPA on CoCl2-induced apoptosis in Neuro2A cells, we focused on the signaling pathways initiated by LPA receptors. cPA has been shown to activate LPA1–5 [6], [17]. Recently, we examined whether cPA could activate LPA6 by measuring the LPA6-mediated neurite retraction of B103 cells as described previously [24], [25]. We observed that cPA could activate LPA6, although its efficacy and affinity were less potent than those of LPA (unpublished data). Quantitative PCR analysis showed that Neuro2A cells highly expressed LPA1, 2, 6 and p2y10 (Fig. 4A). Although LPA1, LPA2, and GPR87 have been speculated to have antiapoptotic activity [18], Neuro2A cells did not express GPR87. Therefore, we focused on the functions of the LPA1 and LPA2 in cPA-mediated antiapoptotic activity. In order to inhibit LPA1, we used Ki16425 (10 µM), which is an LPA receptor antagonist with selectivity for the LPA1 and LPA3 [26]. As shown in Fig. 4B, Ki16425 pretreatment attenuated LPA-mediated antiapoptotic activity for Neuro2A cells. However, the antiapoptotic activity of cPA was not affected by Ki16425. These results indicate that the antiapoptotic activity for Neuro2A of LPA may be mediated by LPA1, whereas that of cPA was not mediated by LPA1. We then used siRNA to knockdown LPA2 and assess the effects of reduced LPA2 expression on the antiapoptotic activity for Neuro2A cells of cPA and LPA. Compared to the mRNA expression level of LPA2 in non-targeting siRNA–transfected cells (control), the expression level in LPA2-targeted siRNA–transfected cells decreased by 14.4% (Fig. 4C). In addition, the antiapoptotic activity of cPA on CoCl2-induced Neuro2A cell apoptosis was largely abolished after knockdown of LPA2 (Fig. 4D). In the case of LPA, the effect of the suppression of its antiapoptotic activity on CoCl2-induced Neuro2A cell apoptosis was less apparent. Therefore, we propose that cPA-induced protection of Neuro2A cells from CoCl2-induced apoptosis might potentially be mediated by LPA2 and that LPA-induced protection of Neuro2A cells might be mediated mainly by LPA1 but not LPA2.
Figure 4. Effects of LPA receptors on the neuroprotective functions of cPA and LPA against CoCl2-induced apoptosis.
(A) Expression of LPA receptors in Neuro2A cells. Total RNA was extracted from Neuro2A cells, and the expression level of each LPA receptor was determined by quantitative real-time PCR. The expression levels were normalized to those of LPA1 and expressed in terms of the mean ± SE values. (B) The effects of Ki16425 on the neuroprotective functions of cPA and LPA against CoCl2-induced apoptosis of Neuro2A cells. Neuro2A cells were pretreated with or without 10 µM Ki16425 for 20 min. Subsequently, the cells were incubated with 300 µM CoCl2 in the presence of 10 µM cPA or LPA for 24 hours. The cells were then stained with FITC-Annexin V and subjected to flow cytometric analysis. The data represent the mean ± SE values from triplicate independent experiments (***P<0.001 vs. the CoCl2-treated group). (C) Expression of LPA2 in Neuro2A cells. Neuro2A cells were transfected with siRNA against LPA2 or non-target siRNA. Total RNA was extracted from each transfected Neuro2A cell, and the expression level of each LPA receptor was determined by quantitative real-time PCR. The expression levels of LPA2 was normalized to those of Neuro2A cells transfected with non-target siRNA. The resulting data represent the mean ± SE values (***P<0.001 vs. the mock group). (D) The effects of LPA2 knockdown on the neuroprotective effects of cPA and LPA against CoCl2-induced apoptosis of Neuro2A cells. Neuro2A cells transfected with either siRNA against LPA2 or non-target siRNA were incubated with 300 µM CoCl2 in the presence of 10 µM cPA or LPA for 24 hours. Cells stained with FITC-Annexin V were subjected to flow cytometric analysis. The data represent the mean ± SE values from triplicate independent experiments (*P<0.05, ***P<0.001 vs. the CoCl2-treated group; n.s., not significant).
The LPA1 and LPA2 differ in their dose response to cPA and LPA. LPA1 can be activated by lower concentrations of cPA and LPA than LPA2 [6]. This difference in dose response has been speculated to have important biological implications [27]. These receptors can cooperate and respond over a wide range of LPA concentrations. For example, LPA1 is activated by LPA with 100% efficacy (EC50, 130 nM) and cPA with 66% efficacy (EC50, 1.7 µM). LPA2 is activated by LPA with 100% efficacy (EC50, 3 nM) and cPA with 140% efficacy (EC50, 180 nM) [6]. We speculate that the affinity for the ligand, efficacy of the ligand, and expression level of receptors in individual cells are important factors for LPA receptor signaling. Most cells express multiple LPA receptors and work cooperatively. Therefore, determining the signal response of individual LPA receptors is not easy. LPA1 and LPA2 are coexpressed in Neuro2A cells. The neuroprotective functions for Neuro2A of cPA could possibly be mediated by LPA2. Furthermore, the neuroprotective function of LPA might be mediated mainly by LPA1 but not LPA2 in Neuro2A cells. On the basis of these results, we suggest that 10 µM LPA could activate LPA1 but not LPA2. An LPA concentration of 10 µM is too high to activate LPA2. At high concentrations, LPA2 can undergo endocytic downregulation and degradation. On the other hand, 10 µM cPA activates only LPA2. The affinity of LPA2 for cPA is lower than that for LPA, which may prevent the receptors from undergoing endocytic downregulation on exposure to 10 µM of cPA. This may also be because the efficacy of cPA against LPA2 is higher than that of LPA. More remarkable effects were observed when the LPA2 was activated by cPA than when it was activated by LPA. Moreover, because LPA2 has a higher affinity for cPA than LPA1 does [6], cPA might prefer LPA2 over LPA1. Here, we have shown that cPA and LPA exhibit antiapoptotic activity for Neuro2A cells but their originating signaling pathways differ. We speculate that the receptors have differences in efficacy and affinity for cPA and LPA. Although many questions remain to be answered, the current study shows that lipid mediators that exhibit antiapoptotic activity and have overlapping receptors function via different receptors in apoptosis. We hypothesize that these differences in receptor preference and the efficacy of cPA and LPA can explain why cPA exhibits biological functions distinct from those of LPA.
We propose that cPA could be a promising therapeutic agent to treat ischemic brain injury. In order to develop novel compounds with medicinal properties, we have synthesized stable derivatives of cPA [6], [10], [28]. Moreover, we have also shown that each enantiomer of several cPA derivatives exhibits the same efficiency toward autotaxin inhibition [29]–[32]. On the basis of these findings, we are currently investigating effective therapeutic compounds to treat disorders such as cancer and neurodegeneration.
Conclusion
CoCl2 treatment induced apoptosis in Neuro2A cells, involving an increase in ROS generation and the Bax/Bcl-2 ratio. cPA increased the cell survival rate, while decreasing the number of FITC-Annexin V- and FITC-DEVD-FMK-positive cells among CoCl2-treated Neuro2A cells in a bell-shaped dose-dependent manner. The protective effect of cPA on CoCl2-induced apoptosis in Neuro2A cells was not inhibited by the LPA1 and LPA3 antagonist Ki16425. However, the effect of LPA was inhibited by Ki16425. Knockdown of LPA2 in Neuro2A cells largely affected the neuroprotective response of cPA but not that of LPA. Therefore, we propose that the molecular mechanism underlying the protective effect of cPA might differ from that for LPA. In addition, we suggest that cPA-induced protection of Neuro2A cells from CoCl2-induced hypoxia damage might be mediated via LPA2.
Funding Statement
This work was supported in part by the Princess Takamatsu Cancer Research Fund, the Rational Evolutionary Design of Advanced Biomolecules (REDS3) Project, Central Saitama Area in the Program for Fostering Regional Innovation (City Area Type), a Grant-in-Aid for Scientific Research (KAKENHI, No. 22790203) from the Ministry for Education, Culture, Sports, Science and Technology of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Murakami-Murofushi K, Shioda M, Kaji K, Yoshida S, Murofushi H (1992) Inhibition of eukaryotic DNA polymerase α with a novel lysophosphatidic acid (PHYLPA) isolated from myxoamoebae of Physarum polycephalum . J Biol Chem 267: 21512–21517. [PubMed] [Google Scholar]
- 2. Kobayashi T, Tanaka-Ishii R, Taguchi R, Ikezawa H, Murakami-Murofushi K (1999) Existence of a bioactive lipid, cyclic phosphatidic acid, bound to human serum albumin. Life Sci 65: 2185–2191. [DOI] [PubMed] [Google Scholar]
- 3. Shan L, Li S, Jaffe K, Davis L (2008) Quantitative determination of cyclic phosphatidic acid in human serum by LC/ESI/MS/MS. J Chromatogr B 862: 161–167. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi Y, Shimada T, Shioda M, Yoshida S, Murofushi H, et al. (1993) Isolation of a new species of Physarum lysophosphatidic acid (PHYLPA) having an inhibitory activity on DNA polymerase α. Cell Struct Funct 18: 135–138. [DOI] [PubMed] [Google Scholar]
- 5. Murakami-Murofushi K, Uchiyama A, Fujiwara Y, Kobayashi T, Kobayashi S, et al. (2002) Biological functions of a novel lipid mediator, cyclic phosphatidic acid. Biochim Biophys Acta 1582: 1–7. [DOI] [PubMed] [Google Scholar]
- 6. Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, et al. (2006) Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem 281: 22786–22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujiwara Y (2008) Cyclic phosphatidic acid-A unique bioactive phospholipid. Biochim Biophys Acta 1781: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakiuchi Y, Nagai J, Gotoh M, Hotta H, Murofushi H, et al. (2011) Antinociceptive effect of cyclic phosphatidic acid and its derivative on animal models of acute and chronic pain. Mol Pain 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gotoh M, Fujiwara Y, Yue J, Liu J, Lee S, et al. (2012) Controlling cancer through the autotaxin-lysophosphatidic acid receptor axis. Biochem Soc Trans 40: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uchiyama A, Mukai M, Fujiwara Y, Kobayashi S, Kawai N, et al. (2007) Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim Biophys Acta 1771: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujiwara Y, Sebök A, Meakin S, Kobayashi T, Murakami-Murofushi K, et al. (2003) Cyclic phosphatidic acid elicits neurotrophin-like actions in embryonic hippocampal neurons. J Neurochem 87: 1272–1283. [DOI] [PubMed] [Google Scholar]
- 12. Gotoh M, Hotta H, Murakami-Murofushi K (2010) Effects of cyclic phosphatidic acid on delayed neuronal death following transient ischemia in rat hippocampal CA1. Eur J Pharmacol 649: 206–209. [DOI] [PubMed] [Google Scholar]
- 13. Zou W, Yan M, Xu W, Huo H, Sun L, et al. (2001) Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J Neurosci Res 64: 646–653. [DOI] [PubMed] [Google Scholar]
- 14. Zou W, Zeng J, Zhuo M, Xu W, Sun L, et al. (2002) Involvement of caspase-3 and p38 mitogen-activated protein kinase in cobalt chloride-induced apoptosis in PC12 cells. J Neurosci Res 67: 837–843. [DOI] [PubMed] [Google Scholar]
- 15. Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG, et al. (2007) Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12. Life Sci 80: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 16. Zhang S, Chen X, Yang Y, Zhou X, Liu J, et al. (2011) Neuroprotection against cobalt chloride-induced cell apoptosis of primary cultured cortical neurons by salidroside. Mol Cell Biochem 354: 161–170. [DOI] [PubMed] [Google Scholar]
- 17. Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, et al. (2009) Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem 284: 17304–17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aoki J, Inoue A, Okudaira S (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781: 513–518. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi S, Tokunoh R, Shibasaki M, Shinagawa R, Murakami-Murofushi K (1993) Synthesis of 1-O-acylgrycerol 2,3-cyclic phosphate: Determination of the absolute structure of PHYLPA, A specific inhibitor of DNA polymerase α. Tetrahedron Lett 34: 4047–4050. [Google Scholar]
- 20. Valentine WJ, Kiss GN, Liu J, Shuyh E, Gotoh M, et al. (2010) (S)-FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a pan-antagonist of sphingosine 1-phosphate GPCR signaling and inhibits autotaxin activity. Cell Signal 22: 1543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu N, Zhou H, Lin YH, Chen ZQ, Pan Y, et al. (2007) Oxidative stress mediates CoCl2-induced prostate tumour cell adhesion: Role of protein kinase C and p38 mitogen-activated protein kinase. Basic Clin Pharmacol Toxicol 101: 41–46. [DOI] [PubMed] [Google Scholar]
- 22. Sarada SKS, Himadri P, Ruma D, Sharma SK, Pauline T, et al. (2008) Selenium protects the hypoxia induced apoptosis in neuroblastoma cells through upregulation of Bcl-2. Brain Res 1209: 29–39. [DOI] [PubMed] [Google Scholar]
- 23. Elmore S (2007) Apoptosis: A review of programmed cell death,. Toxicol Pathol 35: 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, et al. (2009) Identification and characterization of novel lysophosphatidic acid receptor, p2y5/LPA6 . J Biol Chem 284: 17731–17741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CW, Rivera R, Dubin AE, Chun J (2007) LPA4/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing Gs-, Gq/Gi-mediated calcium signaling and G12/13-mediated Rho activation. J Biol Chem 282: 4310–4317. [DOI] [PubMed] [Google Scholar]
- 26. Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, et al. (2006) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharm 64: 994–1005. [DOI] [PubMed] [Google Scholar]
- 27. Chen M, Towers LN, Oconnor KL (2007) LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am J Physiol Cell Physiol 292: C1927–C1933. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka R, Kato M, Suzuki T, Nakazaki A, Nozaki E, et al. (2011) Efficient synthesis of 3-O-thia-cPA and preliminary analysis of its biological activity toward autotaxin. Bioorg Med Chem Lett 21: 4180–4182. [DOI] [PubMed] [Google Scholar]
- 29. Gupte R, Siddam A, Lu Y, Li W, Fujiwara Y, et al. (2010) Synthesis and pharmacological evaluation of the stereoisomers of 3-carba cyclic-phosphatidic acid. Bioorg Med Chem Lett 20: 7525–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nozaki E, Gotoh M, Hotta H, Hanazawa S, Kobayashi S, et al. (2011) Synthesis of enantiopure 2-carba-cyclic phosphatidic acid and effects of its chirality on biological functions. Biochim Biophys Acta 1811: 271–277. [DOI] [PubMed] [Google Scholar]
- 31. Nozaki E, Gotoh M, Hanazawa S, Mori H, Kobayashi S, et al. (2011) Comparison of inhibitory activities of stereo-isomers of cyclic phosphatidic acid (cPA) on autotaxin. Cytologia 76: 73–80. [Google Scholar]
- 32. Nozaki E, Gotoh M, Tanaka R, Kato M, Suzuki T, et al. (2012) Pharmacological evaluation of a novel cyclic phosphatidic acid derivative 3-S-cyclic phosphatidic acid (3-S-cPA). Bioorg Med Chem Lett 20: 3196–3201. [DOI] [PubMed] [Google Scholar]