Abstract
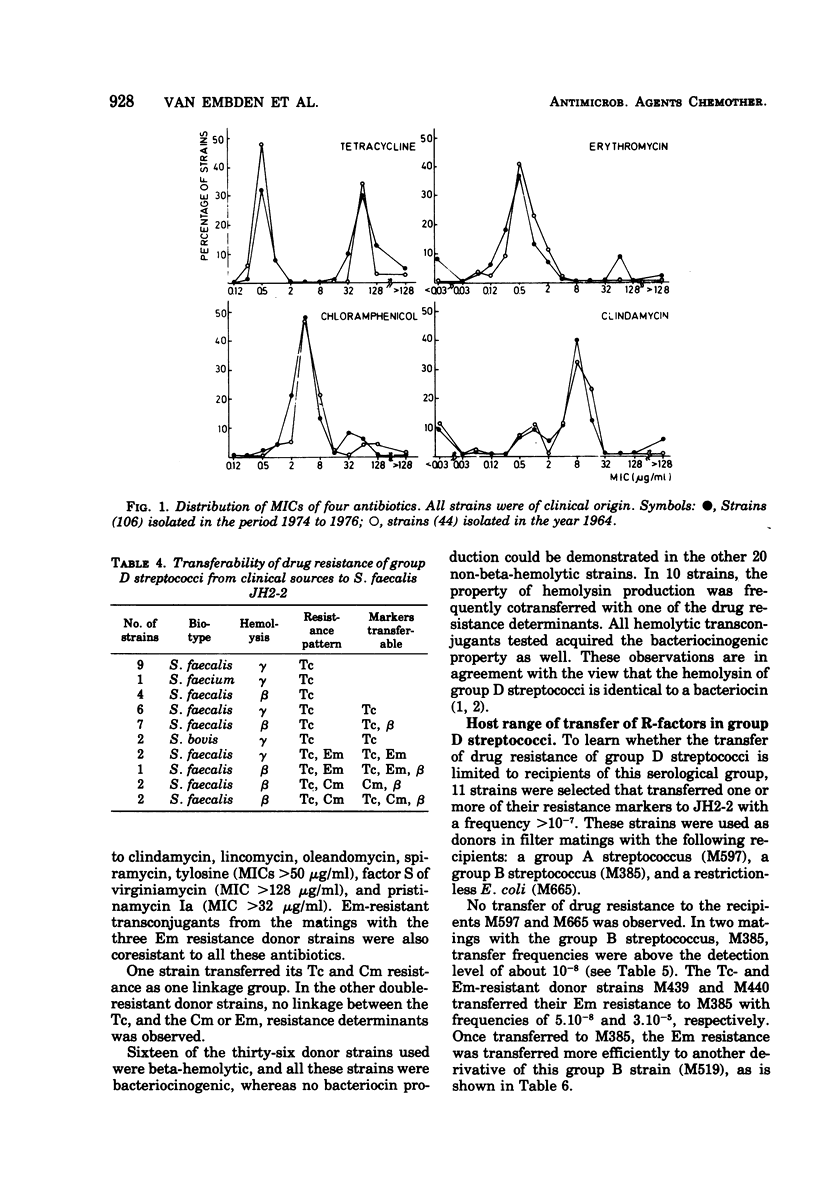
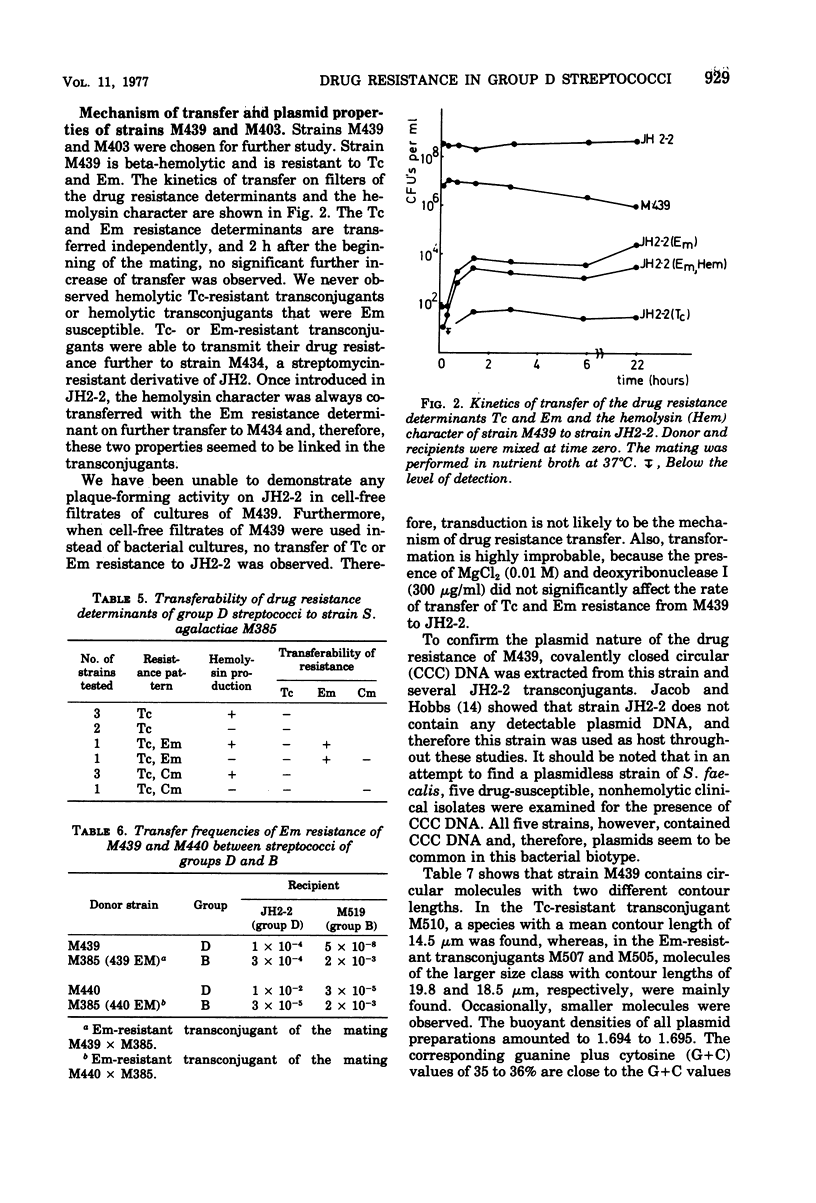
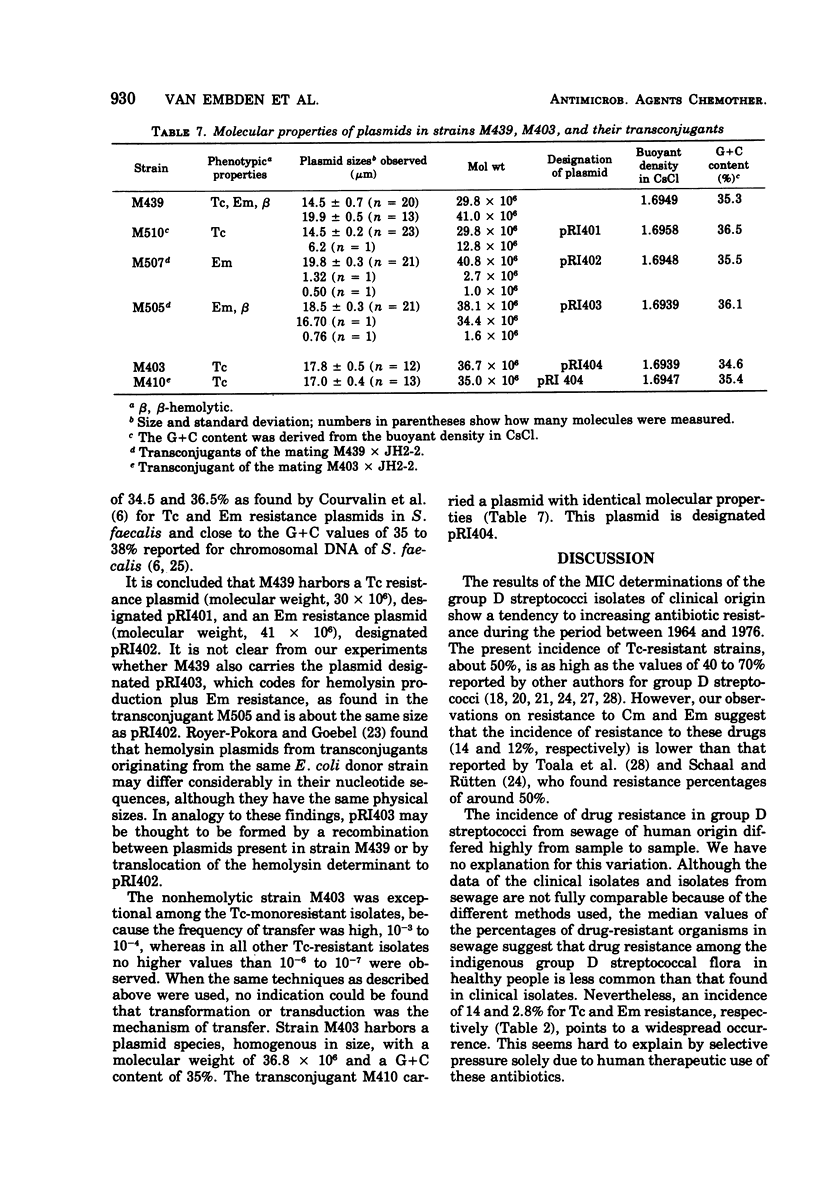
Group D streptococci isolated from clinical specimens and from sewage were investigated with regard to resistance to tetracycline (Tc), erythromycin (Em), and chloramphenicol (Cm). The median values of the percentages of resistant strains from sewage were: for Tc, 14%; for Em, 2.8%; and for Cm, 0.1%. For the recent isolates of clinical origin, resistance percentages found were 58% for Tc, 12% for Em, and 14% for Cm, and, in comparison to clinical isolates from 1964, the incidence of drug resistance slightly increased. In strains of both sources, the drug resistance was often found to be transferable to another group D streptococcus, probably by conjugation. Two strains were able to transfer their Em resistance to a streptococcus strain of group B. No transfer of drug resistance to a group A streptococcus and Escherichia coli was observed. All beta-hemolytic streptococci were also bacteriocinogenic, and frequently these properties were found to be transferable. The function, size, and base composition of the plasmids of two drug-resistant Streptococcus faecalis strains were investigated; strain M439 harbors at least two conjugative plasmids: pRI401, molecular weight 30 × 106, coding for Tc resistance, and pRI402, molecular weight 41 × 106, coding for Em resistance. Strain M403 carries one single conjugative plasmid species, coding for Tc resistance. The molecular weight of this plasmid, pRI404, was 37 × 106. The guanine plus cytosine content of these plasmids was 35 to 36%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D., DAVIE J. M. PROBABLE IDENTITY OF A GROUP D HEMOLYSIN WITH A BACTERIOCINE. J Bacteriol. 1963 Oct;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKWALL M. K., HARTMAN P. A. COMPARISON OF DIRECT PLATING MEDIA FOR THE ISOLATION AND ENUMERATION OF ENTEROCOCCI IN CERTAIN FROZEN FOODS. Appl Microbiol. 1964 Jan;12:18–23. doi: 10.1128/am.12.1.18-23.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinger S. F., Jackson R. W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968 Dec;96(6):1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN H. E., HJORT G. H. Spleen-shielding in x-irradiation-acclerated experimental amyloidosis in mice. Acta Pathol Microbiol Scand. 1960;48:1–12. doi: 10.1111/j.1699-0463.1960.tb04729.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES W. F., Jr, FINLAND M. Susceptibility of enterococcus to eleven antibiotics in vitro; with special reference to species differences. Am J Clin Pathol. 1957 Apr;27(4):467–481. doi: 10.1093/ajcp/27.4_ts.467. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Marder H. P., Kayser F. H. Epidemiologische und genetische Untersuchungen der Antibiotikaresistenz bei Enterokokken. Pathol Microbiol (Basel) 1974;41(3-4):131–132. [PubMed] [Google Scholar]
- Nord C. E., Wadström T. Susceptibility of haemolytic oral enterococci to eight antibiotics in vitro. Acta Odontol Scand. 1973 Dec;31(6):395–399. doi: 10.3109/00016357309002527. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Goebel W. Plasmids controlling synthesis of hemolysin in Escherichia coli. II. Polynucleotide sequence relationship among hemolytic plasmids. Mol Gen Genet. 1976 Mar 22;144(2):177–183. doi: 10.1007/BF02428106. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schaal K. P., Rütten D. Zur Resistenz der Enterokokken gegen Penizilline, Cephalosporine und klassische Breitbandantibiotika. Med Welt. 1973 Mar 16;24(11):402–405. [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Facklam R. R. Antibiotic susceptibility of Streptococcus bovis and other group D streptococci causing endocarditis. Antimicrob Agents Chemother. 1974 Mar;5(3):228–233. doi: 10.1128/aac.5.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Tomura T., Hirano T., Ito T., Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group D streptococci. Jpn J Microbiol. 1973 Nov;17(6):445–452. doi: 10.1111/j.1348-0421.1973.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Yag Y., Franke A. E., Clewell D. B. Plasmid-determined resistance to erythromycin: comparison of strains of streptococcus faecalis and streptococcus pyogenes with regard to plasmid hmology and resistance inducibility. Antimicrob Agents Chemother. 1975 Jun;7(6):871–873. doi: 10.1128/aac.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



