Abstract
Formation of an axon is the first morphological evidence of neuronal polarization, visible as a profound outgrowth of the axon compared to sibling neurites. One unsolved question on the mechanism of axon formation is the role of axon outgrowth in axon specification. This question was difficult to assess, because neurons freely extend their neurites in a conventional culture. Here, we leveraged surface nano/micro-modification techniques to fabricate a template substrate for constraining neurite lengths of cultured neurons. Using the template, we asked: (1) Do neurons polarize even if all neurites cannot grow sufficiently long? (2) Would the neurite be fated to become an axon if only one was allowed to grow long? A pattern with symmetrical short paths (20 μm) was used to address the former question, and an asymmetrical pattern with one path extended to 100 μm for the latter. Axon formation was evaluated by tau-1/MAP2 immunostaining and live-cell imaging of constitutively-active kinesin-1. We found that (1) neurons cannot polarize when extension of all neurites is restricted and that (2) when only a single neurite is permitted to grow long, neurons polarize and the longest neurite becomes the axon. These results provide clear evidence that axon outgrowth is required for its specification.
Keywords: neuronal polarization, hippocampal neuron, micropatterned surface, Kinesin, live-cell imaging
Most neurons in mammalian central nervous system are polarized, protruding a single axon and several dendrites. Axons and dendrites are molecularly and functionally distinct, and the mechanism of how the neuronal polarity emerges has been debated for over twenty years (Craig and Banker 1994; Andersen and Bi 2000; Arimura and Kaibuchi 2007; Barnes and Poulleux 2009). Establishment of the polarity occurs even in vitro when dissociated neurons are cultured in a biochemically homogeneous environment, and thus a cell-autonomous mechanism is sufficient to polarize a neuron (Barlett and Banker 1984; Caceres et al. 1984; Dotti et al. 1988). Cultured hippocampal neurons are the major model system for studying the cell-autonomous mechanism of the polarization, and their development is highly stereotyped (Dotti et al. 1988). Approximately half a day after adhesion, the neurons begin to sprout several undifferentiated neurites (developmental stage 2). After another day of culture, neurons break the symmetry, and one of the neurites abruptly elongates and differentiates into an axon (stage 3). Other sibling neurites later differentiate into dendrites (stage 4).
One important question concerning neuronal polarization in vitro is whether axon growth precedes its specification, or vice versa (Jiang and Rao 2005). These two factors have been difficult to distinguish in conventional culture, where neurons can freely grow their processes. Yet a number of previous observations, mostly made by cell-manipulation experiments, pointed to the former model. If an axon of a stage 3 hippocampal neuron was transected at a length nearly equal to other minor processes, the shortened axon stochastically became a dendrite while a new axon emerged from one of the minor processes (Dotti and Banker 1987; Goslin and Banker 1989). In another experiment, it was shown that forced elongation of an immature neurite of a stage 2 neuron with externally applied mechanical tension fated it to differentiate into an axon (Lamoureux et al. 2002). These suggest that asymmetric outgrowth of a future axon must precede its molecular specification, namely, that cultured hippocampal neurons are somehow capable of sensing neurite lengths for axon specification (Toriyama et al. 2010).
Here, in an attempt to directly confirm the role of neurite outgrowth in axon formation, we took advantage of surface nano/micro-modification techniques to fabricate μm-scaled patterns of cell-permissive and non-permissive monolayers on a cell-culture glass substrate. By culturing embryonic rat hippocampal neurons on these substrates, neurite extension lengths can be extrinsically controlled without further disturbance. We asked whether a neuron would polarize even if all neurites cannot grow sufficiently long and a neurite would become an axon if only one was allowed to grow long. Neurons were cultured on the surface for two to four days, and axon formation was subsequently evaluated using two non-morphological criteria; a tau-1/MAP2 immunostaining and a live-cell imaging for an early axonal marker, the truncated kinesin-1 (Kif5C560) (Jacobson et al. 2006).
Materials and Methods
Preparation of micropatterned substrates
Cell-permissive and non-permissive organosilane self-assembled monolayers (SAMs) were patterned on Pyrex glass substrates (15 mm × 15 mm × 0.5 mm-t) by electron-beam lithography (Fig. 1a). (3-trimethoxysilylpropyl) diethylenetriamine (DETA; Gelest) and n-octadecyltrimethoxysilane (ODS; Fluorochem) SAMs were employed as the permissive and non-permissive regions, respectively. Detailed fabrication method has been described previously (Yamamoto et al. 2011). Poly-D-lysine (PDL; Sigma P-0899) was used interchangeably with DETA SAM. For fabricating PDL/ODS patterns, the DETA SAM deposition step was replaced with an overnight immersion of the substrate in 50 μg/ml PDL/PBS solution. Prior to cell plating, paraffin wax was dotted at corners to provide spacing between glial feeder layers.
Fig. 1.
Schematics of micropatterned substrates. (a) Aminosilane and alkylsilane monolayers were patterned on a glass substrate, which served as cell permissive and non-permissive regions, respectively. (b,c) Geometry of micropatterns employed in this work. Pattern A consists of symmetrical 20-μm paths (denoted “x”) for supporting neurite outgrowths. Pattern B consists of three 20-μm paths (denoted “x”) and a 100-μm path (denoted “y”). Width of the paths was 1 μm, and the diameter of the circle for supporting soma adhesion was 15 μm.
Cell culture
All experiments were approved by institutional Animal Care & Use Committees. Timed-pregnant Sprague Dawley rats were obtained from Charles River Laboratories and CLEA Japan. Primary hippocampal neurons were obtained from embryonic day 18 rats and cultured as described previously (Kaech and Banker 2006) on the micropatterned substrates or on poly-L-lysine (PLL; Sigma P-2636)-coated coverslips. The dissociated neurons were plated at a concentration of 2.0–2.5 × 105 cells per 60-mm dish in plating media (minimal essential medium (MEM; Gibco 11095–080) + 5% FBS (Gibco) + 0.6% D-glucose (Sigma)). After 4 h of culture, the coverslips or substrates were transferred to dishes containing a monolayer of glial feeder layer, and were maintained in neuronal culture medium (MEM + N-2 supplement + 0.5 mg/ml ovalbumin (Sigma) + 10 mM HEPES (Gibco)).
The rat hippocampal culture system gives a very uniform population of pyramidal neurons (~90%) (Craig and Banker 1994), and on average, majority of cells (>70%) develop into stage 3 after 2 days in vitro (DIV) (Davare et al. 2009). This was confirmed in our culture by counting 148 randomly chosen cells (71%; Table 1).
Table 1.
Ratio of unpolarized and polarized neurons cultured on PLL-coated coverslips (Unpatterned) and on Patterns A-D.
| Unpatterned | Pattern A | Pattern B | Pattern C | Pattern D | |
|---|---|---|---|---|---|
| Longest path (μm) | 20 | 100 | 80 | 60 | |
| Number of cells | 148 | 111 | 145 | 114 | 166 |
| Unpolarized | 29% | 86% | 22% | 28% | 40% |
| Polarized | 71% | 14%* | 78% | 72% | 60% |
| Single axon | 64% | 14%* | 78% | 72% | 60% |
| Multiple axons | 7% | 0% | 0% | 0% | 0% |
Determined from tau-1/MAP2 immunostaining at 2 DIV. All of the polarized neurons on Pattern A (indicated with asterisks *) grew an axon longer than 20 μm that grew within the pattern.
Immunocytochemistry
For tau-1 and MAP2 immunostaining, cultured neurons were fixed at indicated times in PBS containing 4% paraformaldehyde (PFA)/4% sucrose for 10 min at 37°C. The neurons were then permeabilized with 0.1% Triton X-100 (Sigma)/PBS for 5 min and blocked with 0.5% fish skin gelatin (Sigma)/PBS for 1 h, both at room temperature (RT). The cells were then doubly stained for axonal (tau-1) and somatodendritic (MAP2) markers using: anti-tau-1 (Chemicon MAB3420, mouse monoclonal IgG2a, 3 μg/ml), anti-MAP2 (Sigma M4403, mouse monoclonal IgG1, 5 μg/ml), Cy-3 goat anti-mouse IgG2a (Jackson ImmunoResearch 115–165–206, 1.5μg/ml), and FITC goat anti-mouse IgG1 (Jackson ImmunoResearch 115–095–205, 0.75 μg/ml) antibodies. After secondary antibody incubation, the samples were washed with PBS, removed of paraffin dots, and were mounted on slide glasses.
The stained samples were imaged under an epifluorescence microscope (Zeiss Observer. Z1) equipped with 20x objective lens (Zeiss Plan-APOCHROMAT, NA=0.8). The images were obtained with Axiocam MRm CCD camera (Zeiss) and AxioVision software (Zeiss).
Live-imaging of Kif5C560
For live-imaging experiments, dissociated neurons were transfected with plasmids for constitutively-active kinesin-1 (Kif5C560) (Jacobson et al. 2006) fused with a red fluorescent protein tdTomato, which was driven by the β-actin promoter. Transfection was performed using the Nucleofection method (Amaxa) prior to plating. For imaging, the glass substrate was placed inside a sealed chamber (Warner Instruments) containing the culture medium and was maintained at 34°C. Fluorescence and phase images were obtained every 15 min using the same setup as mentioned above.
Results
To investigate the role of neurite extension in axon specification, embryonic rat hippocampal neurons were cultured on micropatterned substrates (Fig. 1a). Two geometric patterns were mainly employed in this study; both consisted of a 15μm-diameter circle for cell body adhesion and four 1μm-wide paths perpendicular to each other for supporting neurite outgrowth. The first pattern had four equally long 20μm paths (Pattern A; Fig. 1b). In the second pattern, one of the four paths was extended to 100 μm (Pattern B; Fig. 1c). These lengths were chosen to match average lengths of minor processes and axons in early stage 3 neurons; when evaluated at 1 DIV on unpatterned coverslips, the average lengths of tau-1 positive axons in stage 3 neurons were 96.2 ± 30.9 μm (n = 40 neurons; min = 51.9 μm, max = 197.5 μm). Neurons that adhered to the 15 μm-diameter circle and grew at least one neurite on the pathways were analyzed. Those that extended neurites outside the pattern (~20% of patterned neurons) were eliminated from analyses. For Pattern B, an additional criterion -a neurite to grow on the longest pathway- was imposed.
As a first experiment for determining polarization of the cultured neurons, we employed the well-established tau-1 and MAP2 immunostaining, which labels the axon and somatodendritic regions, respectively. Before polarization (stage 2), these markers label the cell body and all neurites equivalently (Mandell and Banker 1996). In stage 3 neurons, tau-1 labeling is enriched in the distal part of the longest process (axon), staining the shaft and the growth cone. Occasionally, tips of minor processes in stage 2 and 3 neurons are stained with tau-1. Since the tip localization of tau-1 is lost when stained after detergent extraction, these tau proteins are probably not bound to microtubules (Mandell and Banker 1996). Hence in this work, a process with tau-1 positive shaft was identified as an axon.
Figure 2a shows a typical tau-1/MAP2 staining of 2 DIV neurons that grew on Pattern A. Similar to stage 2 neurons on poly-lysine coated coverslips, tau-1 and MAP2 stained all neurites equivalently (86%; n = 96 cells). The remaining 14% of neurons established tau-positive axons, but these axons had extended more than 20 μm inside the pattern geometry by turning back at the distal end of the pathway. In the former unpolarized population, 6% (n = 7) had a single growth cone that was positive to tau-1, but no neuron had tau-1 positive shaft. Even after 4 DIV, no neurons became polarized when neurite lengths were restricted. At this age, 80% of neurons were unpolarized (n = 32). Remaining 20% formed axons but these had grown longer than 20 μm inside the pattern (n = 8). Hence we conclude that when neurite-lengths are constrained to 20μm, neurons cannot polarize and specify an axon even after sufficient days of culture.
Fig. 2.
Tau-1/MAP2 immunostaining of micropatterned neurons. (a,b) Fluorescent micrographs of four representative neurons grown on Pattern A (a) and Pattern B (b). The cells were fixed at 2 DIV and stained for tau-1 (axonal marker; red) and MAP2 (somatodendritic marker; green). Tau-1 distributed equivalently in all neurites when neurons were grown on Pattern A (a). Contrarily, tau-1 positive axons grew on the 100 μm long paths when they were grown on Pattern B (b). (c) Distribution of lengths of neurites which grew on the 100 μm paths of Pattern B. Scale bars, 20 μm.
We next asked whether a neurite would be fated to become an axon if onlythat one neurite was permitted to grow longer than other processes. Pattern B was employed to address this question. Figure 2b shows the tau-1/MAP2 immunostaining of 2 DIV neurons that grew on Pattern B. Tau-1 positive axon was observed selectively in the longest pathway in 78% of the patterned neurons (n = 113), most of which grew toward the end of the 100 μm paths (Fig. 2c). Formation of multiple axons was not observed in the patterned neurons. Of the remaining 22% that were not polarized (n = 32), 12% had a short (<50 μm) neurite growing on the longest pathway (n = 18), and 6% had a single neurite extending from the cell body that grew on the longest pathway (n = 9), which is an irregular morphology for a multipolar pyramidal neuron. A high fraction of the former population will most probably polarize after another day of culture, which we did not quantify but did observe. When the length of the longest pathway was set to 80 μm (Pattern C), the percentage of polarized neurons was 72% (n = 114), comparable to that for neurons grown on unpatterned PLL-coated coverslips (71%). Interestingly, when the longest pathway was further shortened to 60 μm (Pattern D), the percentage decreased to 60% (n = 166). The number of neurons obeying the geometry of the cell-permissive pattern did not change significantly, but less percentage of cells showed localized tau-1 signal in the longest process. The results are summarized in Table 1.
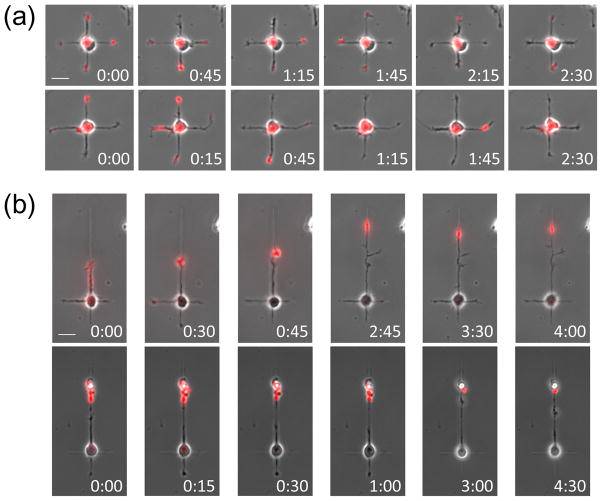
In order to confirm the immunostaining experiment, we performed live-cell time-lapse imaging of an axon-specific truncated kinesin in neurons grown on Patterns A and B. Kinesin-1 (Kif5) is a microtubule-dependent motor protein involved in active transport during axon specification (Nakata and Hirokawa 2003). Its truncated motor domain, Kif5C560, is constitutively-active and translocates randomly among neurite tips in stage 2 neurons and eventually accumulates stably in the tip of the axon after its specification (Jacobson et al. 2006).
To visualize kinesin dynamics in micropatterned neurons, dissociated neurons were transfected with tdTomato-tagged Kif5C560 prior to plating and cultured on the micropatterned substrates. In neurons grown on Pattern B, Kif5C560 was found to stably accumulate in the tip of the growing process that elongated on the 100 μm pathway at 1–2 DIV (n = 4; Fig. 3b), suggesting that the longest neurite has been fated to be the axon. No neuron was observed with stable Kif5C560 accumulation when its neurites were all confined to the 20 μm pathways using Pattern A. Instead, Kif5C560 translocated dynamically among different neurite tips, as expected for unpolarized neurons (n = 6; Fig. 3a). We were further able to confirm the persistence of this dynamic pattern of translocation as late as 3 and 4 DIV. These results are in agreement with the conclusions derived from the abovementioned results of immunostaining. Furthermore, they suggest that when a single neurite cannot grow sufficiently long, microtubule modifications required for specific direction of the kinesin (Konishi and Setou 2009; Hammond et al. 2010; Nakata et al. 2011) are not stabilized, and hence the molecular transport required for axon specification fails to consolidate.
Fig. 3.
Live-cell imaging of constitutively-active kinesin-1 (Kif5C560). Kif5C560 fluorescence (red) is overlaid on the corresponding phase-contrast images. (a,b) Time-lapse images of four representative neurons grown on Pattern A at 2 DIV (a) and on Pattern B at 1–2 DIV (b). Sustained translocation of Kif5C560 was confirmed for neurons grown on Pattern A, indicating that axonal process has not yet been specified (a). Contrarily, when one neurite was permitted to grow long, stable accumulation of Kif5C560 was confirmed on the longest neurite (b). Scale bars, 20μm.
Discussion
Distinction of axon specification from axon extension has been difficult to make with the conventional culture system (Jiang and Rao 2005). In the current study, we employed micropatterned substrates to control neurite length of cultured hippocampal neurons and provided experimental evidence that differential elongation of a single neurite is required for axon specification. The conclusion is consistent with previous observations made with axon transection experiments (Dotti and Banker 1987; Goslin and Banker 1989) or neurite-towing experiments (Lamoureux et al. 2002), which showed that a neuron somehow converts its morphological information that one neurite being longer than others to recruit positive signaling for axon specification.
The length-dependent enhancement of the positive feedback loop could be described by considering a balance of neurite length-independent anterograde transport of growth-promoting signals and length-dependent retrograde diffusion of the molecules (Goslin and Banker 1989). Among the several important signaling pathways involved in axon formation is the phosphatidylinositol 3-kinase (PI3K) pathway (Arimura and Kaibuchi 2007; Barnes and Poulleux 2009). In cultured hippocampal neurons, PI3K and the lipid product of PI3K, phosphatidylinositol-3,4,5-triphosphate (PIP3), accumulates at the tip of the prospective axon (Shi et al. 2003). PI3K is activated by extracellular signals mediated by cell-surface receptors and Ras GTPases (Yoshimura et al. 2006), but how the PI3K signaling becomes spatially biased in homogenous in vitro culture was not understood. Recently, Shootin1, an upstream regulator of PI3K, was shown to accumulate in neurite tips of stage 2 hippocampal neurons in a length-dependent manner (Toriyama et al. 2006; Toriyama et al. 2010). A simple mathematical model incorporating the experimentally measured parameters of anterograde transport via actin/myosin-dependent “waves” (Ruthel and Banker 1998) and the passive retrograde diffusion of Shootin1 was able to sufficiently reproduce the axon formation during stage 2/3 transition (Toriyama et al. 2010). The key idea is that the rate of the retrograde diffusion decreases with increasing neurite length, since the diffusion rate positively correlates with the concentration gradient between a neurite tip and the soma, and the gradient decreases when neurites become longer.
Alternatively, localization of cytoskeletal modulators which promote neurite extension could also be responsible. For example, plasma membrane ganglioside sialidase (PMGS), which promotes TrkA signaling that destabilizes actin by inactivating RhoA GTPase, accumulates to a prospective axon in early stage 2 neurons (Da Silva et al. 2005). Actin destabilization leads to enhanced polymerization and stabilization of microtubules, which play a critical role in neurite extension (Bradke and Dotti 1999; Andersen and Bi 2000; Witte et al. 2008). Therefore PMGS accumulation can trigger neurite extension and subsequent axon specification (Jiang and Rao 2005). How PMGS localizes in a single process before polarization is not yet understood.
Tension-induced signaling (Shibasaki et al. 2010) may also play a role, since lengthening of a neurite positively correlates with the tension exerted on the neurite (Lamoureux et al. 1989). Roth et al. recently showed that by using micropatterns of curved paths to compensate linear vectorial tension in neurites, a neurite that grew on a straight path and subjected to the largest tension was most likely to be fated as the axon (Roth et al. 2012).
In any case, our experimental paradigm using micropatterned substrates offer a valuable, new tool for studying the role of signaling molecules in neuronal polarization. Proper cargo transport by Kif5 is also required for neuronal polarization, since dominant-negative inhibition of Kif5 prevents axon specification (Kimura et al. 2005), and in this study, we were able to show that constitutively-active Kif5 (Kif5C560) fails accumulate stably in a single neurite when extension of all neurites is restricted. This showed that active transport required for axon specification cannot be sustained without axon outgrowth. Post-translational modifications (Konishi and Setou 2009; Hammond et al. 2010) or GTP-binding (Nakata et al. 2011) of tubulins in axonal microtubules are proposed as mechanisms underlying the specific transportation of Kif5, and it is of interest to investigate how these depend on neurite length.
Our result that the percentage of polarized neurons decreased in Pattern D (Table 1) was somewhat surprising, because axon transection experiments have shown that a hippocampal neuron is capable of sensing 10 μm lengthening of a neurite and fates the longest one as its axon (Goslin and Banker 1989). Our interpretation is that Δx = 10 μm is sufficient to induce a shift of the equilibrium to a polarized state with the longest neurite being its axon, whereas Δx > 40 μm is required to stabilize the polarized state; here Δx is the difference in length of a prospective axon from average length of sibling minor processes. Whether this threshold length differs among different neuronal subtypes is unknown, but we speculate that they would share a common threshold since signaling pathways for axon specification is well-conserved (Barnes and Poulleux 2009).
To our knowledge, observation that neurons cannot form an axon when extension of neurites is restricted has not been reported in any other literature. It is not clear at this point whether these neurons are confined in developmental stage 2, or in some other state that is not observed in ordinary culture. Further experiment using in vitro-surface modification (Yamamoto et al. 2011) to convert an originally non-permissive region to a permissive one after several days of culture and allow neurites to re-grow would provide an answer to this question.
From the viewpoint of surface chemistry, one challenge has been to develop a method to control neuronal polarity using surface cues. Two lines of approaches are currently available; one is to immobilize multiple molecular cues on a substrate so that neurons would biochemically recognize the cues for growing axons and dendrites (Oliva et al. 2003; Shi et al. 2007; Nevill et al. 2011), and the other is to prepare a differential geometrical pattern (Stenger et al. 1998; Vogt et al. 2004; Fricke et al. 2011; Greene et al. 2011). For the latter approach, Stenger et al. proposed that axons and dendrites can be differentially guided by creating “speed-bump”-like structures for dendrite growth (Stenger et al. 1998). Yet this was contradicted by a later work, insisting that the “speed-bumps” were not effective in directing polarization (Vogt et al. 2004). Taking our current data into account, it is now possible to interpret the critical difference in the above two experiments. Each of the patterns used by Stenger et al. was isolated as ones we used in this study. On the other hand, ones used by Vogt et al. were continuously sided with neighboring patterns. Our findings suggest that the discrepancy was likely to be the effect of insufficient constraint of minor processes (future dendrites) in the latter paper.
In this work, we showed that the polarization axis of neurons can be exogenously determined by constraining their growth on asymmetric patterns (Pattern B). This simple strategy is highly efficient. About 78% of cells extended their axons in the desired direction. With the recent advance of surface modification techniques, we are now capable of manipulating cell-affinity of their scaffolds in cell-culture environment on a μm-scale (Kaji et al. 2004; Nakanishi et al. 2004; Sugio et al. 2004; Robertus et al. 2010; Okano et al. 2011; Rolli et al. 2012). These are also applicable to neuronal cells to guide their neurites (Sugio et al. 2004; Yamamoto et al. 2011). Combining the in vitro-surface modification methods with directed-polarization from surface cues would enable a reconstruction of well-defined neuronal circuits in vitro, which would provide us a novel simplified system for investigating the network dynamics of the circuits and how different molecules are involved in their function.
Acknowledgments
We thank Ms. Barbara Smoody for assistance with the cell culture, Dr. Stefanie Kaech for advice on imaging, Dr. Anthony P. Barnes for discussion on neuronal polarization, and the members of the Banker lab for many fruitful discussions. This work was supported by the Research Fellowships for Young Scientists (HY) and the Institutional Program for Young Researcher Overseas Visits (HY) from the Japan Society for the Promotion of Science and the National Institutes of Health Grant MH066179 (GB).
Footnotes
The authors declare no conflict of interest.
References
- Andersen SSL, Bi GQ. Axon formation: a molecular model for the generation of neuronal polarity. Bioessays. 2000;22:172–179. doi: 10.1002/(SICI)1521-1878(200002)22:2<172::AID-BIES8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I Cells which develop without intercellular contacts. J Neurosci. 1984;4:1944–1953. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Da Silva JS, Hasegawa T, Miyagi T, Dotti CG, Abad-Rodriguez J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat Neurosci. 2005;8:606–615. doi: 10.1038/nn1442. [DOI] [PubMed] [Google Scholar]
- Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, Soderling TR, Wayman GA. Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Iγ to promote axon formation in hippocampal neurons. J Neurosci. 2009;29:9794–9808. doi: 10.1523/JNEUROSCI.1544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke R, Zentis PD, Rajappa LT, Hofmann B, Banzet M, Offenhäusser A, Meffert SH. Axon guidance of rat cortical neurons by microcontact printed gradients. Biomaterials. 2011;32:2070–2076. doi: 10.1016/j.biomaterials.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Greene AC, Washburn CM, Bachand GD, James CD. Combined chemical and topographical guidance cues for directing cytoarchitectural polarization in primary neurons. Biomaterials. 2011;32:8860–8869. doi: 10.1016/j.biomaterials.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rao Y. Axon formation: fate versus growth. Nat Neurosci. 2005;8:544–546. doi: 10.1038/nn0505-544. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Prot. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kaji H, Tsukidate K, Matsue T, Nishizawa M. In situ control of cellular growth and migration on substrates using microelectrodes. J Am Chem Soc. 2004;126:15026–15027. doi: 10.1021/ja045702t. [DOI] [PubMed] [Google Scholar]
- Kimura T, Watanabe H, Iwamatsu A, Kaibuchi K. Tubulin and CRMP-2 complex is transported via Kinesin-1. J Neurochem. 2005;93:1371–1382. doi: 10.1111/j.1471-4159.2005.03063.x. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Lamoureux P, Buxbaum RE, Heidemann SR. Direct evidence that growth cones pull. Nature. 1989;340:159–162. doi: 10.1038/340159a0. [DOI] [PubMed] [Google Scholar]
- Lamoureux P, Ruthel G, Buxbaum RE, Heidemann SR. Mechanical tension can specify axonal fate in hippocampal neurons. J Cell Biol. 2002;159:499–508. doi: 10.1083/jcb.200207174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi J, Kikuchi Y, Takarada T, Nakayama H, Yamaguchi K, Maeda M. Photoactivation of a substrate for cell adhesion under standard fluorescence microscopes. J Am Chem Soc. 2004;126:16314–16315. doi: 10.1021/ja044684c. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. J Cell Biol. 2011;194:245–255. doi: 10.1083/jcb.201104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill JT, Mo A, Cord BJ, Palmer TD, Poo MM, Lee LP, Heilshorn SC. Vacuum soft lithography to direct neuronal polarization. Soft Matter. 2011;7:343–347. [Google Scholar]
- Oliva AA, James CD, Kingman CE, Craighead HG, Banker GA. Patterning axonal guidance molecules using a novel strategy for microcontact printing. Neurochem Res. 2003;28:1639–1648. doi: 10.1023/a:1026052820129. [DOI] [PubMed] [Google Scholar]
- Okano K, Yu D, Matsui A, Maezawa Y, Hosokawa Y, Kira A, Matsubara M, Liau I, Tsubokawa H, Masuhara H. Induction of cell-cell connections by using in situ laser lithography on a perfluoroalkyl-coated cultivation platform. ChemBioChem. 2011;12:795–801. doi: 10.1002/cbic.201000497. [DOI] [PubMed] [Google Scholar]
- Robertus J, Browne WR, Feringa BL. Dynamic control over cell adhesive properties using molecular-based surface engineering strategies. Chem Soc Rev. 2010;39:354–378. doi: 10.1039/b906608j. [DOI] [PubMed] [Google Scholar]
- Rolli CG, Nakayama H, Yamaguchi K, Spatz JP, Kemkemer R, Nakanishi J. Switchable adhesive substrates: Revealing geometry dependence in collective cell behavior. Biomaterials. 2012;33:2409–2418. doi: 10.1016/j.biomaterials.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Roth S, Bisbal M, Brocard J, Bugnicourt G, Saoudi Y, Andrieux A, Gory-Fauré S, Villard C. How Morphological Constraints Affect Axonal Polarity in Mouse Neurons. PLoS one. 2012;7(3):e33623. doi: 10.1371/journal.pone.0033623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: A novel form of axonal transport? Cell Motil Cytoskeleton. 1998;40(2):160–173. doi: 10.1002/(SICI)1097-0169(1998)40:2<160::AID-CM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 Enhances Axon Outgrowth through Activation by Membrane Stretch in Developing Sensory and Motor Neurons. J Neurosci. 2010;30:4601–4612. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- Shi P, Shen K, Kam LC. Local presentation of L1 and N-cadherin in multicomponent, microscale patterns differentially direct neuron function in vitro. Develop Neurobiol. 2007;Nov; 67 (13):1765–1776. doi: 10.1002/dneu.20553. [DOI] [PubMed] [Google Scholar]
- Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, Shaffer K, Schaffner K, Cribbs DH, Cotman CW. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. J Neurosci Methods. 1998;82:167–173. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
- Sugio Y, Kojima K, Moriguchi H, Takahashi K, Kaneko T, Yasuda K. An agar-based on-chip neural-cell-cultivation system for stepwise control of network pattern generation during cultivation. Sens Actuators B. 2004;99:156–162. [Google Scholar]
- Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, Katsuta K, Sakumura Y, Roepstorff P, Inagaki N. Shootin1: A protein involved in the organization of an asymmetric signal for neuronal polarization. J Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama M, Sakumura Y, Shimada T, Ishii S, Inagaki N. A diffusion-based neurite length-sensing mechanism involved in neuronal symmetry breaking. Mol Syst Biol. 2010;6:394. doi: 10.1038/msb.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt AK, Stefani FD, Best A, Nelles G, Yasuda A, Knoll W, Offenhäusser A. Impact of micropatterned surfaces on neuronal polarity. J Neurosci Methods. 2004;134:191–198. doi: 10.1016/j.jneumeth.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Okano K, Demura T, Hosokawa Y, Masuhara H, Tanii T, Nakamura S. In-situ guidance of individual neuronal processes by wet femtosecond-laser processing of self-assembled monolayers. Appl Phys Lett. 2011;99:163701. doi: 10.1063/1.3651291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3β/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340:62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]