Abstract
The hepatitis B virus (HBV) encoded X protein (HBx) contributes centrally to the pathogenesis of hepatocellular carcinoma (HCC). Aberrant activation of the Hedgehog (Hh) pathway has been linked to many tumor types including HCC. Thus, experiments were designed to test the hypothesis that HBx promotes HCC via activation of Hh signaling. HBx expression correlated with an up-regulation of Hh markers in human liver cancer cell lines, in liver samples from HBV infected patients with HCC, and in the livers of HBx transgenic mice (HBxTg) that develop hepatitis, steatosis, and dysplasia, culminating in the appearance of HCC. The findings in human samples provide clinical validation for the in vitro results and those in the HBxTg. Blockade of Hh signaling inhibited HBx stimulation of cell migration, anchorage independent growth, tumor development in HBxTg and xenograft growth in nude mice. Results suggest that the ability of HBx to promote cancer is at least partially dependent upon the activation of the Hh pathway. This study provides biological evidence for the role of Hh signaling in the pathogenesis of HBV mediated HCC and suggests cause and effect for the first time. The observation that inhibition of Hh signaling partially blocked the ability of HBx to promote growth and migration in vitro and tumorigenesis in two animal models implies that Hh signaling may represent an “oncogene addiction” pathway for HBV associated HCC. This work could be central to designing specific treatments that target early development and progression of HBx mediated HCC.
Keywords: HBx transgenic mice, Hedgehog signaling, Gli2, GDC-0449
Introduction
The HBV “oncoprotein”, HBx, is a trans-activating protein that contributes to HCC by affecting cell cycle regulation, DNA repair, multiple signaling pathways as well as cellular genes that are important for cell proliferation, inflammation, angiogenesis, immune responses and epigenetics (1-4). Aberrant Hh pathway activation is seen in many tumor types where it accounts for about one-third of all cancer deaths (5). In the canonical pathway, Hh signaling is initiated by the binding of Hh ligands Sonic (Shh), Indian (Ihh), or Desert (Dhh) to the Patched (PTCH) receptor, which becomes internalized, leading to the activation of Smoothened (SMO) via release from PTCH dependent suppression. SMO activates the Gli transcription factors that regulate the expression of Hh target genes (6). Altered Hh signaling contributes to tumor progression and invasion (7, 8). HBx has been shown to stabilize Gli1 and Gli2 in vitro (9), but the biological implications of these findings are not clear. Thus, experiments were designed to test whether HBx promotes HCC, in part, through the activation of Hh signaling.
Recent work demonstrated that HBV and HCV increased hepatocyte production of ligands that activate Hh signaling, thereby expanding the pool of Hh-responsive cells that promote liver fibrosis and cancer (10). Hh activation occurs in response to liver injury (e.g., growth of hepatic progenitors, inflammation, vascular remodeling, and liver fibrosis) in chronic liver disease (CLD) (11, 12). Inhibition of Hh signaling in HCC cell lines decreased expression of Hh target genes and resulted in apoptosis (13). Gli2 and Gli1 were shown to be primary and secondary mediators of Hh signaling, respectively (14, 15). Specifically, Gli2 upregulates Gli1 by direct interaction with the Gli1 promoter (16). Gli2 also plays a predominant role in the proliferation of HCC cells (17). Thus, Gli2 was further investigated here in HBx mediated HCC.
Prior work has shown elevated Hh signaling markers in HCC (18), but their relationship to HBx, and whether they contributed to the cause or outcome of HCC, is not known. HBx correlated with the up-regulated expression of Hh markers in vitro (8), but the biological and pathological consequences of this up-regulation was not explored. In this work, these questions were addressed both in vitro and in vivo using two animal models. The first consisted of HBxTg that develop progressive pathology in the liver very similar to that observed among HBV carriers, culminating in the appearance of HCC (19, 20). In these mice, HBx expression is not seen until after birth, meaning that the mice are not tolerant to HBx. As HBx expression increases with age, so does the severity of CLD. This model permits evaluation of the relationships between HBx, up-regulation of Hh markers, and the pathogenesis of HCC. The second model consisted of HBx positive human HCC xenografts growing as subcutaneous tumor in nude mice. In this model, elevated Hh signaling was evaluated in tumor growth. The combined results support the hypothesis that HBx contributes to HCC by stimulating Hh signaling.
Materials and Methods
Cell lines
HepG2 cells were stably transfected with HBx (HepG2X) or the control bacterial chloramphenicol acetyltransferase (CAT; HepG2CAT) genes by recombinant retroviruses and cultured without the selection of individual clones as previously described (21). Huh7X and Huh7CAT cells were prepared and cultured in the same way. These cell lines have been used in numerous studies that have been published (21).
Patient Samples
Formalin fixed, paraffin embedded tumor (HCC)/nontumor (adjacent liver) tissues were obtained from Chinese patients who underwent surgery at the Third Military Medical University, Chongqing, China. All patients were hepatitis B surface antigen positive in blood; 21 were males, one was female, and the age range was from 35-60 (average: 47). Samples were used for diagnostic purposes and then for this study. Ten uninfected human liver tissue slides (Abcam) were used as controls. The use of these samples was approved by the Institutional Review Boards at all participating universities.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using the RNeasy kit (Qiagen). qRT-PCR was performed using SensiFAST SYBR kit (Bioline). Primer Sequences are shown in Table S1. Threshold cycles (Ct) were calculated by the StepOnePlus Detection System (Applied Biosystems). Target gene levels in the treated cells are presented as a ratio to levels detected in control cells according to the Ct method (22).
Western blots (WB)
Liver tissues were rinsed in ice-cold PBS and homogenized in lysis buffer (Cell Signaling) with protease inhibitor cocktail (Sigma). Cell debris was removed by double centrifugation at 14,000 x g for 15 minutes. Protein extracts from cells were prepared using same lysis buffer. For WB, 150 μg of protein extracts from liver tissues, 60 μg from Huh7CAT and Huh7X cells, and 100 μg from HepG2CAT and HepG2X cells were separated on SDS–polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell). Membranes were incubated with antibodies against Gli2, PTCH1 (Santa Cruz Biotechnology) or β-actin (Sigma). The blots were developed using the ECL plus kit (Amersham).
Immunohistochemistry
HBx transgenic and control mice, 3, 6, 9, 12 month old of age, were euthanized, their livers removed, fixed in formalin and embedded in paraffin (23). Liver morphology was evaluated by hematoxylin and eosin (H&E) staining. Mouse and human tissue sections were deparaffinized, dehydrated, treated with Uni-TRIEVE antigen retrieval (Innovex) and stained using the UltraVision Detection System (Thermo Scientific). For human tissues, antibodies used were anti-HBx (anti-99) (24), anti-Shh (Epitomics), anti-Gli2 (GenWay), anti-PTCH1 and anti-Ihh (Abcam). For mouse tissues, anti-HBx (anti-99), anti-Shh, anti-Ihh, anti-PTCH1 (Millipore) and anti-Gli2 (Abcam) were used. Normal mouse or rabbit IgG (Vector Labs) were used to rule out false-positive responses. Pre-absorption of primary antibodies with corresponding antigens was performed to insure specificity. Scoring was based upon colorimetric evaluation.
Hh signaling inhibition
For in vitro experiments, the Smo inhibitor GDC-0449 (Vismodegib; Selleck Chemicals) was reconstituted in DMSO (Sigma) and used at a final concentration of 1 μM for 24 h. The ligand inhibitor, Shh neutralizing antibody (5E1), was obtained from the Developmental Studies Hybridoma Bank at the University of Iowa (Des Moines, IA) and used at 10 μg/mL for 24 h. For in vivo experiments, GDC-0449 was reconstituted in 2-hydroxypropyl)-β-cyclodextrin (Sigma) in water 45% (w/v) and used at 25 mg/kg for HBxTg and at 30 mg/kg for nude mice.
Phenotypic assays
Cell migration, with or without GDC-0449, was evaluated using 24-well BD BioCoat™ Matrigel™ Invasion Chambers (BD). GDC-0449 was added for the duration of the assay. To assess anchorage independent growth (soft agar assay) cells were seeded (25) with or without GDC-0449. Colonies were counted after 22 days. Medium in all wells was changed twice a week.
Treatment of mice
HBxTg used herein have been previously described (19, 20). Twelve month old mice were treated daily with GDC-0449 or control vehicle by intraperitoneal (i.p.) injection (6 mice per group, total 19 injections). For xenograft experiments, male nude mice (Hsd:Athymic Nude-Foxn1nu, Harlan Labs) 3-8 weeks old, were injected subcutaneously in the flank with 0.2 ml containing 1 × 107 viable cells in PBS (5 mice for each cell line). Treatment with GDC-0449 or vehicle (19 injections) was started after tumor volumes reached about 0.6 cm3. Tumor volumes were estimated by caliper measurements as described (26). At the end of the experiment, tumors were removed. Volumes were also measured by water displacement, and their wet weights were determined.
Mice were housed in a pathogen-free room under controlled temperature and humidity. All animal protocols have been approved by the Temple University Institutional Animal Care and Use Committee.
Statistics
The relationship between HBx and Hh markers by immunohistochemistry was determined using 2 × 2 comparisons in the Chi square (χ2) test. Statistical significance was considered when p < 0.05. The Student’s t test was used to calculate the significance of mean difference in all other measurements. Significant relationships were identified when p < 0.05.
Results
HBx stimulates Hh signaling
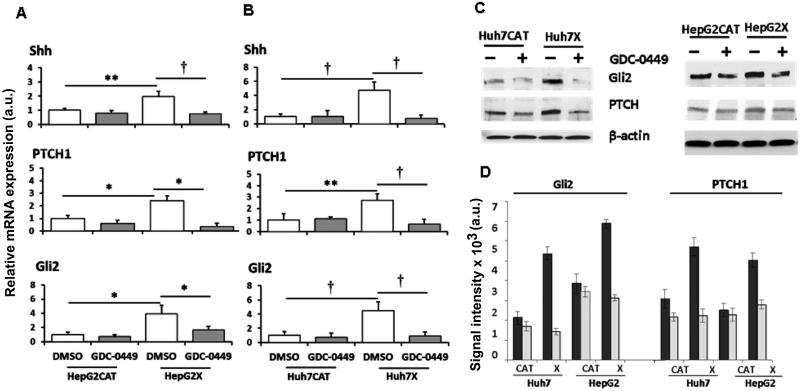
Lysates from HBx-expressing (HepG2X, Huh7X) and from HBx-negative (HepG2CAT, Huh7CAT) cells were analyzed for Hh components by qRT-PCR and WB. qRT-PCR showed increased levels of Shh (2-fold; P < 0.01), Gli2 (4-fold; P < 0.05), and PTCH1 (2.4-fold; P < 0.05) in HepG2X compared to HepG2CAT cells (Fig. 1). Up-regulation of these mRNAs in Huh7X compared to Huh7CAT cells was 4.7-fold for Shh (P < 0.005), 4.4-fold for Gli2 (P < 0.005) and 2.7-fold for PTCH1 (P < 0.01) (Fig. 1). The SMO antagonist, GDC-0449, now in Phase II trials for several cancers (27), was used to inhibit Hh signaling. GDC-0449 treatment of HepG2X cells decreased mRNA levels of Shh by 2.6-fold (61%, P < 0.005), PTCH1 by 6.8-fold (85%, P < 0.05), and Gli2 by 2.4-fold (58%, P < 0.05). In Huh7X cells, mRNA levels were reduced by 6.1-fold for Shh (84%, P < 0.005), by 4.1-fold for PTCH1 (76%, P < 0.005), and by 4.9-fold for Gli2 (80%, P < 0.005). In control cell cultures, there was no significant difference in these Hh markers with or without drug.
Fig. 1.
Changes in markers of Hh signaling were determined in HBx positive (X) and negative (CAT) HepG2 and Huh7 cells treated with DMSO or GDC-0449. (A and B) qRT-PCR results are shown as the mean ± SEM of triplicate experiments; *p<0.05, **p<0.01, †p<0.005. (C) Representative western blots of total extracts from the cells above. (D) Quantification of protein levels (mean expression ± S.D. of 3 assays for each marker). DMSO controls are the black bars and cells treated with 1 μM GDC-0449 are the white bars.
Hh signaling was also inhibited by the neutralizing antibody to Shh (5E1), which prevents Shh from binding to PTCH1. In HepG2X cells, this resulted in decreased Shh mRNA (5.9-fold; 83%, P < 0.005), PTCH1 mRNA (2.8-fold; 64%, P < 0.05), and Gli2 mRNA (2.3-fold; 57%, P < 0.05). In Huh7X cells, reduction was 9.1-fold for Shh (89%, P < 0.05), 2.7-fold for PTCH1 (63%, P < 0.01), and 7.7-fold for Gli2 (87%, P < 0.01) (Fig. S1). In control cells, 5E1 had no effect, implying that HBx trans-activates Hh signaling.
When Gli2 and PTCH1 levels were evaluated by WB, both were elevated in HepG2X compared to HepG2CAT cells (1.8-fold; P < 0.02 for Gli2 and 2-fold; P < 0.02 for PTCH1) (Fig. 1). They were also elevated in Huh7X compared to Huh7CAT cells (2.5-fold; P < 0.01 for Gli2 and 1.9-fold; P < 0.03 for PTCH1) (Fig. 1). It was not possible to perform accurate WB for Shh and Ihh, since they are mostly extracellular. GDC-0449 reduced Gli2 in HepG2X cells (2.2-fold; 55%, P < 0.02) and in Huh7X cells (3.8-fold; 74%, P < 0.02), but not in treated compared to untreated control cells (Fig. 1). GDC-0449 also reduced PTCH1 in HepG2X (1.8-fold; 44%, P < 0.02) and Huh7X cells (2.6-fold; 61%, P < 0.01), but not in the HBx negative cultures. Hence, HBx stimulates expression of Hh components in human liver cancer cells.
HBx promotion of cell migration and growth in soft agar is largely Hh dependent
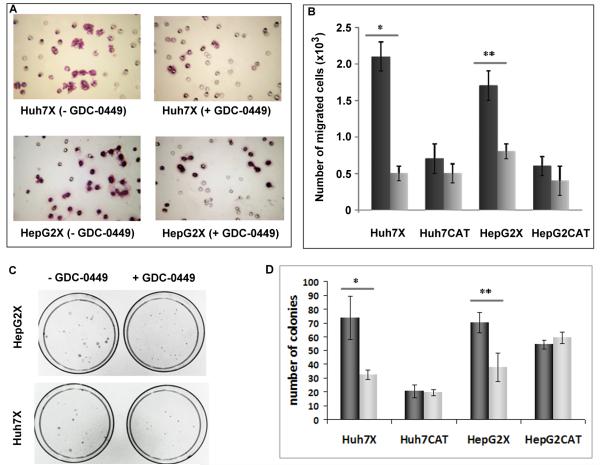
Both Hh signaling (28) and HBx (29) promote cell migration. To determine whether HBx-stimulated migration was Hh dependent, cells were treated with GDC-0449. Migration of Huh7X cells was blocked an average of 4.4-fold (P < 0.01) and HepG2X cells by 2.2-fold (P < 0.02), but there was no effect of treatment upon HBx negative cells (Fig. 2). Hence, HBx stimulation of cell migration correlated with up-regulation of Hh markers, while the migration of HBx negative cells was largely independent of Hh pathway activity.
Fig. 2.
Phenotypic changes associated with Hh signaling in HBx positive and negative cells with or without GDC-0449. (A) Representative images of HBx expressing cells that migrated through Matrigel basement membrane (200x). (B) Quantification of the results in panel A (mean expression ± S.D. of 3 assays). Cells were treated with DMSO (dark bars) or with GDC-0449 (light bars): *p<0.01; **p<0.02. (C) Anchorage independent growth of Huh7X and HepG2X with or without GDC-0449. (D) Quantification of the results in panel C (mean expression ± S.D. of 3 assays). Cells were treated with DMSO (dark bars) or with GDC-0449 (light bars): *p<0.01; **p<0.03.
Prior work has shown that HBx promotes anchorage independent growth (21). To determine whether this depends upon Hh signaling, HBx positive and negative cells were seeded into soft agar with or without GDC-0449. GDC-0449 decreased the clonability of Huh7X cells by 2.2-fold compared to untreated cells (P < 0.01), and of HepG2X cells by 1.8-fold compared to untreated cells (P < 0.03) (Fig. 2). Growth of HBx negative cells was not significantly different under identical conditions, suggesting that Hh signaling contributes to HBx associated anchorage independent growth.
Hh markers in human liver and HCC samples
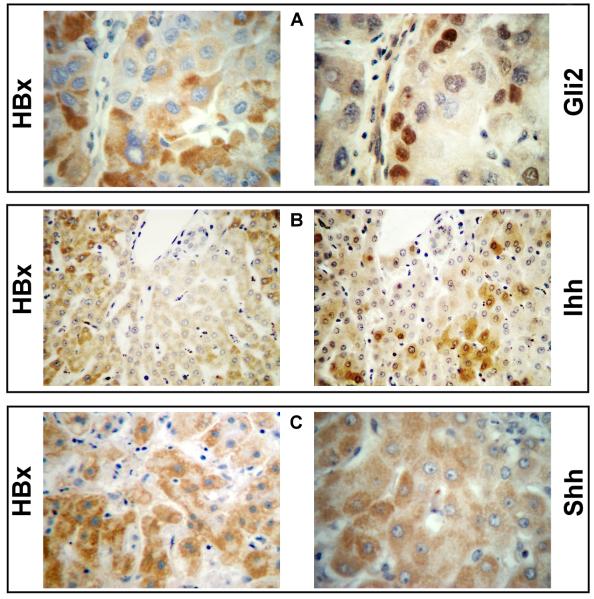
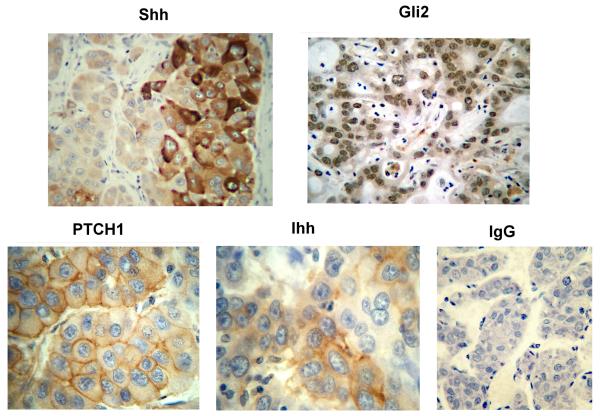
Paraffin embedded tissues from 22 de-identified patients were used to evaluate HBx and Hh markers by immunohistochemistry. Among these, 17 patients had tumor and adjacent non-tumor liver, 3 only had tumor, and 2 had non-tumor liver. HBx staining, mostly cytoplasmic (Fig. 3), was seen in 15 of 20 tumors (75%) and in all 19 non-tumor livers (100%) (Table S3). Cytoplasmic Shh staining (Fig. 3) was observed in 12 of 19 non-tumor samples (63%) and in 12 of 20 tumors (60%). Five patients had Shh staining in tumor and non-tumor (Table S3). In HCC, strong Shh staining was seen at the growing margin of tumors (Fig. 4). Cytoplasmic Ihh was seen in 14 of 19 non-tumor cases (74%), in 6 of 14 cases of HCC (43%), and in both compartments of 4 patients (Table S3, Figs. 3 and 4). Nuclear Gli2 was observed in 11 of 19 non-tumor samples (58%), in 11 of 20 tumors (55%), and in 6 patients in both compartments (Figs. 3 and 4). Membranous PTCH1 was observed in 10 of 19 non-tumor samples (53%), in 9 of 20 tumors (45%), and in both compartments in 4 patients (Fig. 4, Tables S2 and S3). Ten commercially available liver sections from uninfected individuals were negative for HBx, Gli2, Shh, PTCH1, and Ihh (data not shown). Staining with normal IgG proved specificity of staining (Fig. 4). Thus, Hh signaling is activated in non-tumor and tumor of HBV patients with HCC.
Fig. 3.
Co-staining of HBx with (A) Gli2 (x400), with (B) Ihh (x200), and with (C) Shh (x400) in nontumor sections of clinical samples from HBV infected patients.
Fig. 4.
Staining for Shh and Gli2 (x200) as well as PTCH1 and Ihh (x400) in HCC samples from HBV carriers. The control panel is HCC stained with normal IgG (x200).
When these relationships were evaluated by Chi-square analysis (Table S2), there was a strong correlation between HBx and all Hh markers in non-tumor liver, where HBx staining was stronger and widespread, as previously reported (30), but not in adjacent tumor, where HBx expression was often among scattered cells. This suggests a tight correlation between HBx expression and activated Hh signaling in non-tumor liver. It appears that once triggered by HBx, Hh signaling remains activated in HCC even in cells without detectable HBx expression.
Hh markers in HBx transgenic mice
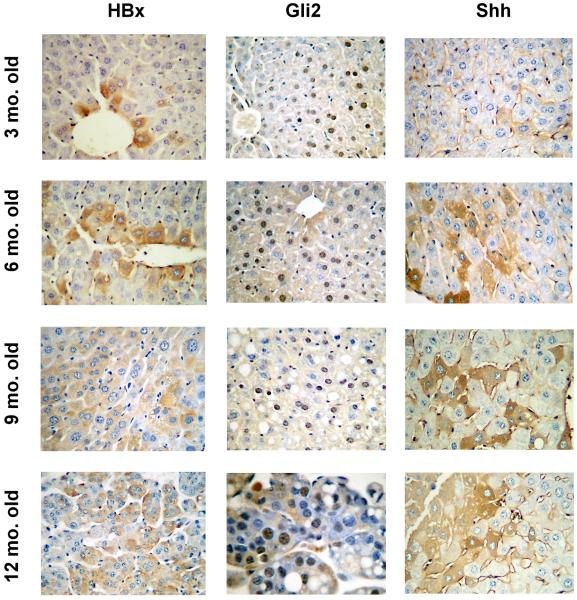
The centrality of HBx to the development of HCC is recapitulated in HBxTg that develop progressive lesions in the liver as in human carriers (19, 20). These mice develop hepatitis and steatosis by 5-6 months of age, dysplastic nodules by 8-9 months, and visible HCC by 12 months in 100% of mice. This is accompanied by increased HBx expression with age in the liver. Non-transgenic littermates had no lesions in their livers at any age (Fig. S2). Staining for HBx, Gli2 and Shh in livers from 3, 6, 9 and 12 month old transgenic mice showed an increase in all these markers with age (Fig. 5). As in human livers, HBx and Shh staining was mostly cytoplasmic, although membranous Shh was also seen. Gli2 staining was nuclear, with some cytoplasmic localization (Fig. 5). PTCH1 was membranous, while Ihh was cytoplasmic, membranous, and within some liver sinusoids (Fig. S3 [A]). Livers from non-transgenic littermates were negative for all Hh markers (Fig. S3 [B]). Thus, Hh signaling is increasingly activated in HBxTg with age and the severity of CLD.
Fig. 5.
Staining for HBx, Shh, and Gli2 in the livers from 3, 6, 9 and in HCC from 12 month old HBxTg. Magnification is x200 except for the 12 month old Gli2 image which is x400.
When Chi-square analysis was performed on these markers in mice of different ages, there were statistically significant relationships between HBx and each of the Hh markers in the liver. The most striking relationship in the livers of 3 month old mice was between HBx and Gli2 (Table S4). In tumors from 12 month old mice, the correlation between HBx and Hh markers no longer existed.
Hh signaling in the pathogenesis of HBx associated HCC
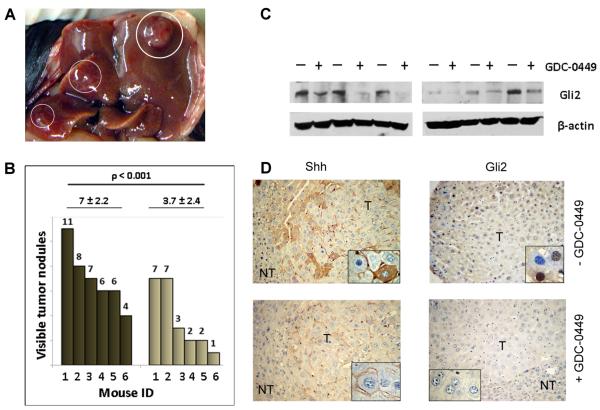
To determine whether HBx promotes hepatocarcinogenesis via Hh signaling, the effect of GDC-0449 on tumor development in HBxTg and tumor growth in xenograft bearing nude mice was evaluated. Untreated 12 month old HBxTg had multiple tumors on the surface of their livers (Fig. 6A) while most GDC-0449 treated mice had fewer tumors. These differences were statistically significant (Fig. 6B). Excised tumors showed lower levels of Gli2 in GDC-0449 treated mice compared to controls (Fig. 6C). The latter was confirmed by staining, where no Gli2 was observed in treated compared to untreated mice (Fig. 6D). Shh staining was much weaker and dispersed compared to untreated mice (Fig. 6D). Thus, inhibition of Hh signaling resulted in decreased number of tumors.
Fig. 6.
Relationship between Hh signaling and HCC in HBxTg. (A) HCC nodules (circled) on the surface of the liver. (B) The number of visible nodules observed on livers (n = 6 HBxTg per group) after injections of vehicle (dark bars) or GDC-0449 (light bars). Tumor numbers for individual mice are shown above each bar. The average tumor number is shown above each group. (C) WB for Gli2 in livers from transgenic mice treated with vehicle (−) or GDC-0449 (+). (D) Staining for Gli2 and Shh on serial sections of tumors (T) and nontumor (NT) livers from HBxTg treated with vehicle (upper panels) or GDC-0449 (lower panels). Magnification is x100 for each panel and x400 for each insert.
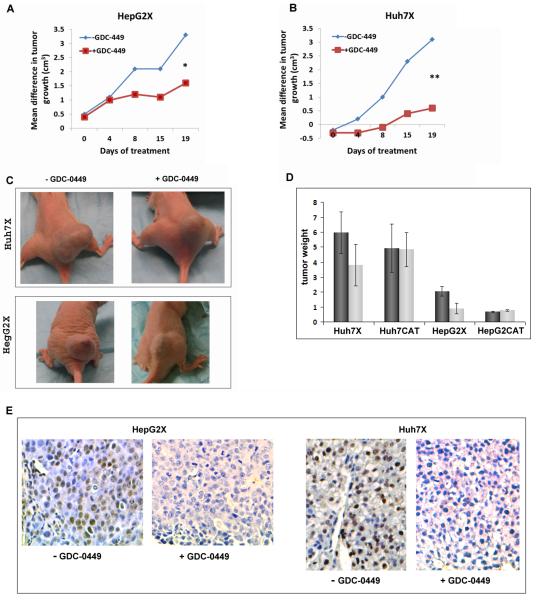
In the xenograft experiments, nude mice bearing HBx positive or negative xenografts were treated with GDC-0449 or vehicle (Fig. 7 and Figs. S4). The contribution of HBx only to cell growth via Hh signaling was assessed as described in the legend to Fig. 7. GDC-0449 inhibited the growth of HepG2X compared to HepG2CAT tumors by ~2-fold (P < 0.01) (Fig. 7A). Identical experiments with Huh7X cells showed differences of 5.6-fold (P < 0.005) (Fig. 7B). There was little difference in the tumor size of HepG2CAT and Huh7CAT cells whether or not they were treated (data not shown). Tumor volumes determined by water displacement showed similar to those determined by caliper measurements (data not shown). The average weight tumors was also smaller in drug treated compared to control mice for Huh7X (1.9-fold, P < 0.05) and HepG2X (2.2-fold, P < 0.01) (Fig. 7D). Tumor cell growth was verified by positive Ki67 staining (data not shown). These results suggest that HBx promoted tumor growth by stimulating Hh signaling, while in the absence of HBx, tumor growth was not dependent upon Hh signaling activity.
Fig. 7.
HBx expressing xenografts in nude mice treated with vehicle or GDC-0449. The mean difference in tumor size for (A) HepG2X and (B) Huh7X tumors (to evaluate the contribution of HBx only) was calculated as follows: the average size of HepG2CAT tumors without drug was subtracted from the average size of HepG2X tumors without drug and plotted for each time point. Likewise, the average size of HepG2CAT cells with drug was subtracted from the average size of the HepG2X tumors with drug. Parallel calculations were performed for Huh7 cells; *p < 0.01; **p < 0.005 at day 19. (C) Nude mice with Huh7X and HepG2X xenografts following treatment with GDC-0449 or vehicle. (D) Mean wet weights (in grams) of tumors from HBx positive and negative HepG2 and Huh7 xenografts in animals treated with drug or vehicle; *p < 0.01; **p < 0.05. (E) IHC staining for Gli2 in Huh7X and HepG2X xenografts after treatment with GDC-0449 or vehicle.
Discussion
This is the first report showing that activated Hh signaling is linked to the expression of HBx in the pathogenesis of HCC. Elevated Hh markers in HBx positive HepG2 and Huh7 cells correlated with the ability of HBx to promote cell migration and growth in soft agar. GDC-0449 and Shh neutralizing antibody reduced the expression of Hh markers in these cells, cell migration and growth (Figs. 1, 2 and S1). Importantly, elevated Hh marker expression was seen in HBxTg (but not in non-transgenic littermates) (Fig. 5). These results were validated in tissue samples from patients with HBV associated HCC, where co-staining between HBx and Hh markers was seen in the livers but not in HCC nodules (Fig.3, Table S2), suggesting that once Hh markers were up-regulated by HBx, they remained elevated even when HBx was no longer detectable. Treatment of HBxTg with GDC-0449 yielded significantly fewer tumors as well as suppressed Gli2 expression (Fig. 6), suggesting that Hh signaling contributed to tumor development. GDC-0449 also inhibited the growth of HBx expressing xenografts in nude mice (Fig. 7). Thus, the ability of HBx to promote HCC appears to depend upon the activation of Hh signaling, suggesting that HCC may be “addicted” to an activated Hh pathway in chronic HBV infection.
The results from Fig. 1 show upregulated expression of Shh, PTCH1 and Gli2 in the presence of HBx, although the underlying mechanism(s) remain to be defined. While these results suggest that these Hh components are transcriptionally activated by HBx, other work (9) has shown that HBx does not up-regulate Gli transcription factors, but post-translationally stabilizes them. Although both studies used HepG2 and Huh7 cells, the experimental designs were different. In particular, prior work used transient transfection, while the work here was carried out with stably transfected cell lines. The latter is more representative of the host-virus relationship in the chronically infected liver and also reflects the relationship between HBx and Hh markers in cell culture and in HBxTg. Gli2 can be post-translationally stabilized by deacetylation (31), which may occur by the recruitment of mSin3A-HDAC1 deacetylase complex by HBx (4, 32). Gli2 transcriptional activation by HBx is also possible. The Gli2 promoter has SMAD and TCF/LEF binding sites, making it responsive to TGF-β and β-catenin (25, 33), both of which are trans-activated by HBx (34, 35). Since Gli2 is expressed in the absence of Hh signaling (36), it may be activated by HBx through TGF-β1.
Although the HBx activation of Hh signaling may upregulate Hh target genes (such as PTCH1), the elevated expression of Shh, which is not an Hh target gene, must occur by other mechanisms. The up-regulation of Shh in HBx expressing cells (Fig. 1) could be mediated through HBx activation of NF-κB (37) which binds to the Shh promoter and induces Shh expression (38). HBx activation of canonical Hh signaling is also suggested by the correlation between HBx and Hh markers in chronically infected human liver (Fig. 3) and in HBxTg livers with age (Fig. 5, Table S4). TGF-β1 may also promote canonical Hh signaling, since TGF-β1 up-regulates Shh mRNA and protein (39). The finding that treatment of HBx expressing cells with Shh neutralizing antibody 5E1 resulted in decreased levels of Gli2 and PTCH1 mRNAs (Fig. S1) also supports a role for canonical signaling in HBx mediated Hh activation. Thus, HBx may promote Shh expression by multiple pathways, and may underlie differences in the presence, frequency and distribution of some of the Hh markers evaluated by staining.
The importance of HBx promoting tumorigenesis through the activation of Hh signaling is underscored by experiments using GDC-0449 which blocked the ability of HBx to stimulate cell migration and anchorage independent growth (Fig. 2). These findings correlated with suppressed levels of Gli2, PTCH1 and Shh (Fig. 1). Stimulation of migration is a part of epithelial-to-mesenchymal transition that results in the remodeling of liver during CLD and promotion of metastasis during cancer progression. The role of Hh signaling in HBx mediated tumor progression was confirmed in xenograft experiments and in HBxTg, where GDC-0449 inhibited tumor growth (Figs. 6 and 7). Among transgenic mice, GDC-0449 treatment also correlated with decreased expression of Gli2 and Shh (Fig. 6). While it is not clear how Shh is suppressed after GDC-0449 treatment (Fig. 1), this has been shown elsewhere (40, 41), implying an unidentified feedback loop in Hh signaling. Thus, Hh signaling may be important in the early stages of hepatocarcinogesis. This is further indicated by the strong correlation between HBx staining and the appearance of Hh components prior to the detection of HCC in HBxTg (Fig. 5) and in infected human liver and HCC (Fig. 3). If HBx mediated activation of Hh signaling results in the altered expression of Hh target genes, it may contribute to the pleiotropic properties of HBx. Thus, HBx may constitutively activate Hh signaling in the pathogenesis of HCC, suggesting that Hh signaling may be a therapeutic target in this tumor type.
Although aberrant Hh activation associated with mutations has been documented in several tumor types, such mutations are rare in HCC (18). Aberrant Hh activation also occurs in nontumor liver (Figs. 3 and 5), suggesting that HBx may trigger Hh signaling prior to tumor development. The oxidative environment in CLD appears to trigger Hh signaling and promotes HBx expression which contributes to tumor development (42). The fact that normal human hepatocytes are resistant to Hh ligand mediated signaling, that Hh responsive cells often consist of immature hepatocytes and/or tissue progenitors (17), and that HBx promotes the development of “stemness” in the liver (25), also suggests that HBx activates Hh signaling prior to the development of tumor.
HBx mediated activation of Hh signaling might also be involved in the “oncogene addiction” of HCC. Two pathways, Raf/MEK/MAPK and PI3K/Akt, are known to be oncogene “addicted” in HCC (43). These pathways potentiate Hh signaling through non-canonical pathways (7, 44, 45) that are activated by HBx (46, 47). If so, this would provide strong rationale for the development of combination therapies that focus upon Gli2. Since there are some 50 drug candidates being tested in roughly 200 clinical trials (48), a major problem contributing to the development of resistance and failure of so many trials may be the lack of combination therapies targeting pathways associated with “oncogene addiction.” Perhaps the linkage of HBx to activated Hh signaling in the pathogenesis of HCC will result in therapies that are better targeted to prevent tumor appearance and/or block the growth and relapse of established tumors.
Supplementary Material
Acknowledgements
The authors thank Drs. Yongwen Chen and Cheng-ying Yang from the Third Military Medical University for the tissue samples.
Grant Support: This work was supported by grants AI076535 awarded to Dr. Feitelson and 5K08DK080980-03 awarded to Dr. Choi.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Feitelson MA. Hepatitis B virus in hepatocarcinogenesis. J Cell Physiol. 1999;181:188–202. doi: 10.1002/(SICI)1097-4652(199911)181:2<188::AID-JCP2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J. Gastroenterol. 2011;46:974–990. doi: 10.1007/s00535-011-0415-9. [DOI] [PubMed] [Google Scholar]
- 3.Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, et al. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 4.Zheng DL, Zhang L, Cheng N, Xu X, Deng Q, Teng XM, et al. Epigenetic modification induced by hepatitis B virus X protein viainteraction with de novo DNA methyltransferase DNMT3A. J Hepatol. 2009;50:377–387. doi: 10.1016/j.jhep.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 6.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 7.Mimeault M, Batra S. Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies. Pharmacol Rev. 2010;62:497–524. doi: 10.1124/pr.109.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Cho HK, Hong SP, Cheong J. Hepatitis B virus X protein stimulates the Hedgehog-Gli activation through protein stabilization and nuclear localization of GLI1 in liver cancer cells. Cancer Lett. 2011;309:176–184. doi: 10.1016/j.canlet.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Pereira TA, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690–1703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 12.Omenetti A, Choi S, Michelotti G, Dehl AM. Hedgehog signaling in the liver. J of Hepatology. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27:1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of CLI2 and CLI3 activities by an amino-terminal repression domain: implication of CLI2 and CLI3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 15.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 16.Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, et al. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol. 2004;22:1503–1509. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, Marcusson EG. Selective down-regulation of CLI2 inhibits the proliferation of hepatocellular carcinoma cells. Cancer Res. 2007;67:3583–3593. doi: 10.1158/0008-5472.CAN-06-3040. [DOI] [PubMed] [Google Scholar]
- 18.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–757. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 19.Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, et al. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31:123–132. doi: 10.1016/s0168-8278(99)80172-x. [DOI] [PubMed] [Google Scholar]
- 20.Koo JS, Seong JK, Park C, Yu DY, Oh BK, Oh SH, et al. Large liver cell dysplasia in hepatitis B virus x transgenic mouse liver and human chronic hepatitis B virus-infected liver. Intervirology. 2005;48:16–22. doi: 10.1159/000082090. [DOI] [PubMed] [Google Scholar]
- 21.Lian Z, Pan J, Liu J, Zhang S, Zhu M, Arbuthnot P, et al. The translation initiation factor, hu-Sui1 may be a target of hepatitis B X antigen in hepatocarcinogenesis. Oncogene. 1999;18:1677–1587. doi: 10.1038/sj.onc.1202470. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(delta-delta) C(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Fischer AH, Jacobson KA, Rose J, Zeller R. Paraffin embedding tissue samples for sectioning. CSH Protoc. 2008 May 1; doi: 10.1101/pdb.prot4989. pdb.prot4989. [DOI] [PubMed] [Google Scholar]
- 24.Feitelson MA, Millman I, Duncan GD, Blumberg BS. Presence of antibodies to the polymerase gene product(s) of hepatitis B and woodchuck hepatitis virus in natural and experimental infections. J Med Virol. 1988;24:121–136. doi: 10.1002/jmv.1890240202. [DOI] [PubMed] [Google Scholar]
- 25.Arzumanyan A, Friedman T, Ng IO, Clayton M, Lian Z, Feitelson MA. Does the hepatitis B antigen HBx promote the appearance of liver cancer stem cells? Cancer Res. 2011;71:3701–3708. doi: 10.1158/0008-5472.CAN-10-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SA, Lee SY, Cho IH, Oh MA, Kang ES, Kim YB, et al. Tetraspanin TM4SF5 mediates loss of contact inhibition through epithelial-mesenchymal transition in human hepatocarcinoma. J Clin Invest. 2008;118:1354–1366. doi: 10.1172/JCI33768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Smaele E, Ferretti E, Gulino A. Vismodegib, a small-molecule inhibitor of the hedgehog pathway for the treatment of advanced cancers. Curr Opin Investig Drugs. 2010;11:707–718. [PubMed] [Google Scholar]
- 28.Liao X, Siu M, Au C, Wong E, Chan HY, Ip P, et al. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–140. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lara-Pezzi E, Gómez-Gaviro MV, Gálvez BG, Mira E, Iñiguez MA, Fresno M, et al. The hepatitis B virus X protein promotes tumor cell invasion by inducing MMP-1 and COX-2 expression. J Clin Invest. 2002;110:1831–1838. doi: 10.1172/JCI200215887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, London WT, Feitelson MA. HBxAg in HBV carrier patients with liver cancer. Cancer Res. 1991;51:4971–4977. [PubMed] [Google Scholar]
- 31.Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, et al. Histone deacetylase and Cullin3-REN (KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12:132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 32.Arzumanyan A, Friedman T, Kotei E, Ng IO, Lian Z, Feitelson MA. Epigenetic repression of E-cadherin expression by hepatitis B virus x antigen in liver cancer. Oncogene. 2012;31:563–572. doi: 10.1038/onc.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennler S, André J, Verrecchia F, Mauviel A. Cloning of the human CLI2 promoter: transcriptional activation by TGF-beta via SMAD3/beta-catenin cooperation. J Bio Chem. 2009;284:31523–31531. doi: 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo YD, Ueda H, Park K, Flanders KC, Lee YI, Jay G, et al. Regulation of TGF-beta 1 expression by the hepatitis B virus (HBV) X transactivator. Role in HBV pathogenesis. J Clin Invest. 1996;97:388–395. doi: 10.1172/JCI118427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DK, Park SH, Yi Y, Choi SG, Lee C, Parks WT, et al. The hepatitis B virus encoded oncoprotein pX amplifies TGF-beta family signaling through direct interaction with Smad4: potential mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev. 2001;15:455–466. doi: 10.1101/gad.856201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su F, Schneider RJ. Hepatitis B virus HBx protein activates transcription factor NF-kappa B by acting on multiple cytoplasmic inhibitors of rel-related proteins. J Virol. 1996;70:4558–4566. doi: 10.1128/jvi.70.7.4558-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-κB target gene that promotes NF-κB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21–33. doi: 10.1096/fj.08-111096. [DOI] [PubMed] [Google Scholar]
- 39.Maitah MY, Ali S, Ahmad A, Gadgeel S, Sarkar FH. Up-regulation of sonic hedgehog contributes to TGF-β1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One. 2011;6:e16068. doi: 10.1371/journal.pone.0016068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Phillips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dormoy V, Danilin S, Lindner V, Thomas L, Rothhut S, Coquard C, et al. The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth. Mol Cancer. 2009;8:123. doi: 10.1186/1476-4598-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha HL, Shin HJ, Feitelson M, Yu DY. Oxidative stress and antioxidants in hepatitis B virus infected hepatic pathogenesis. World J Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka S, Arii S. Molecular targeted therapy for hepatocellular carcinoma in the current and potential next strategies. J Gastroenterol. 2011;46:289–296. doi: 10.1007/s00535-011-0387-9. [DOI] [PubMed] [Google Scholar]
- 44.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riobó NA, Lu K, Ai X, Haines GM, Emerson CP. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung TW, Lee YC, Kim CH. Hepatitis B viral HBx induces MMP-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J. 2004;18:1123–1125. doi: 10.1096/fj.03-1429fje. [DOI] [PubMed] [Google Scholar]
- 47.Tarn C, Lee S, Hu Y, Ashendel C, Andrisani OM. Hepatitis B virus X protein differentially activates RAS-RAF-MAPK and JNK pathways in X-transforming versus non-transforming AML12 hepatocytes. J Biol Chem. 2001;276:34671–34680. doi: 10.1074/jbc.M104105200. [DOI] [PubMed] [Google Scholar]
- 48.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.