Summary
We conducted a study to determine whether clinical algorithms would be useful in malaria diagnosis among people living in an area of moderate malaria transmission within Kilifi District in Kenya. A total of 1602 people of all age groups participated. We took smears and recorded clinical signs and symptoms (prompted or spontaneous) of all those presenting to the study clinic with a history of fever. A malaria case was defined as a person presenting to the clinic with a history of fever and concurrent parasitaemia. A set of clinical signs and symptoms (algorithms) with the highest sensitivity and specificity for diagnosing a malaria case was selected for the age groups ≤5 years, 6–14 years and ≥15 years. These age-optimized derived algorithms were able to identify about 66% of the cases among those <15 years of age but only 23% of cases among adults. Were these algorithms to be used as a basis for a decision on treatment among those presenting to the clinic, 16% of children ≤5 years, 44% of those 6–14 years of age and 66% of the adults who had a history of fever and parasitaemia ≥5000 parasites/μl of blood would be sent home without treatment. Clinical algorithms therefore appear to have little utility in malaria diagnosis, performing even worse in the older age groups, where avoiding unnecessary use of antimalarials would make more drugs available to the really needy population of children under 5 years of age.
Keywords: malaria diagnosis, clinical algorithms, IMCI guidelines, Kenya
Introduction
There are estimated to be about 200 million episodes of clinical malaria every year in Africa (Snow et al. 1999). Most of these cases present to rural health centres that lack diagnostic facilities. Because malaria is one of the most common potentially fatal diseases, the Integrated Management of Childhood Illnesses (IMCI) guidelines attempt to ensure that all young children at risk receive appropriate treatment (Gove 1997). These guidelines recommend that all children living in areas of high malaria endemicity be treated for malaria if they present to a health facility with fever (which includes a history of fever, feels hot, or a temperature ≥37.5 °C), whereas children living in areas of low malaria endemicity be treated for malaria only if they present with fever (which includes a history of fever, feels hot, or a temperature ≥37.5 °C) in the absence of measles, running nose and any other identifiable cause of fever. These guidelines ensure that most children with parasitaemia and symptoms are treated, but many children without parasitaemia who fit the criteria are also treated in the process.
The IMCI guidelines apply to children ≤5 years of age, because this is the group with the highest risk of both general and malaria-specific morbidity and mortality. However, older children and adults, though generally at much lower risk of serious morbidity from malaria, account for a greater total use of antimalarial drugs in most situations (Snow et al. 2003). Among older children and adults, there are no generally accepted guidelines as to who should be treated in the absence of specific diagnostic facilities. Thus in all age groups, current practice leads to marked over-prescription of antimalarial drugs. When first-line drugs are cheap and safe, this is mostly of concern in relation to the generation of drug resistance, but with the introduction of much more expensive combination therapy, for which there are limited safety data, the need to target antimalarials at those who really need them has become even more pressing.
A number of studies have been conducted across Africa for improving the specificity of malaria case diagnosis using various clinical algorithms. Chandramohan et al. (2002) conducted a review of these studies and suggested that their use would lead to drug wastage in areas of low endemicity and an increased failure to treat in areas of high endemicity.
Few studies have examined the role of algorithms in improving clinical malaria diagnosis in older children and adults living in malaria-endemic areas of Africa. This paper describes the performance of algorithms for diagnosis of clinical malaria in an area of moderate malaria transmission among people of different age groups.
Materials and methods
Study area and sample selection
Two study areas were selected within Kilifi District on the coast of Kenya. The low-transmission area of Ngerenya where people are exposed to an average of 10 infective bites per person per year is located north of the Kilifi creek, whereas Chonyi, an area where people are exposed to 22–53 infective bites per person per year, is located south of the Kilifi creek (Mbogo et al. 1995, 2003). Both areas, which are approximately 20 km apart, have similar ethnic and socioeconomic characteristics and experience two rainy seasons, the ‘long’ rains in May–July and the ‘short’ rains in November.
The Kenya Medical Research Institute (KEMRI) scientific steering committee and the national ethical review committee approved the study. Local leaders including divisional officers, chiefs, assistant chiefs, village elders and school headteachers were informed of the study and public meetings held to inform the people in the selected areas about the study.
Computer-generated random numbers were used to select households identified from an earlier, well-mapped census in Ngerenya. At the time of the study, there were no good census data for Chonyi. Homestead clustering is common in the area, and therefore five clusters with over 20 households were selected in accessible areas, a census conducted among them and participants selected.
Oral consent was sought from the head of each house-hold; one withheld consent and hence his household was excluded. This was followed by parental consent for children and individual consent for older children (≥15 years of age) and adults. The study was explained to the participants in the local dialect and a copy of the consent form left in the household for the family members. A total of 52 households were selected in Chonyi with 783 participants and 72 households in Ngerenya with 819 participants. All children under 10 years of age in these households were enrolled in the study, but not all adults as the study was weighted to children. Newborns into the selected households were enrolled continually during the 2 years of surveillance from May 1999 to May 2001.
Case detection
Each household was visited by a fieldworker at least once a week and the axillary temperature taken from every study participant using BD®thermometers (Becton-Dickinson UK Ltd, Oxford). Any temperature readings <36 °C were repeated twice to ensure that it was not because of poor placing of the thermometer. Any study participants with a fever (axillary temperature ≥37.5 °C) or a history of fever were given the fare to travel to the study clinic at the Kilifi District Hospital (KDH). At the study clinic, a smear was taken and a questionnaire with 27 clinical signs and 27 symptoms filled out by a study clinician. The symptoms and signs were selected on the basis of their association with malaria, in other studies conducted within Africa (Hendrickse et al. 1971; Okeahialam et al. 1972; Mkawagile & Kihamia 1986; Olivar et al. 1991; Rooth & Bjorkman 1992; Jonkman et al. 1995; Redd et al. 1996; Weber et al. 1997; Olaleye et al. 1998; Muhe et al. 1999). Symptoms were recorded as ‘spontaneous’ if they were mentioned in response to a general question as to the reason for presentation to the clinic or ‘prompted’ if the symptom was admitted to on subsequent systematic direct questioning by the clinician. Treatment with antimalarials was administered to all who presented to the study clinic with a history of fever and accompanying parasitaemia.
The data on people that reported to the clinic with a history of fever in the period May 1999 to May 2000 were used to derive clinical algorithms and data collected from May 2000 to May 2001 were used to validate these algorithms.
Data analysis
The term ‘history of fever’ will be used throughout the paper to describe people who presented to the clinic with febrile symptoms or measured fever (axillary temperature ≥37.5 °C). If history of fever occurred in two consecutive weeks in the same person, then only the first episode was used in the analysis (the second one being considered part of the same illness as the first episode). A clinical case of malaria for this analysis was defined as a person attending the clinic with a history of fever and accompanying parasitaemia of any level (all who received antimalarials). This would be the criterion for malaria treatment in a health centre with microscopy facilities. Data were analysed by age: children ≤5 years of age, children 6–14 years old and adults (≥15 years old). Two algorithms were derived using clinical signs and symptoms that were reported spontaneously, and using clinical signs and symptoms that were reported either spontaneously or prompted.
Deriving the algorithms
Using the data collected from May 1999 to May 2000, associations between various clinical symptoms or signs and clinical malaria were estimated using Mantel–Haenzel unadjusted odds ratios. Clinical symptoms or signs found to be associated with clinical malaria were identified (unadjusted odds ratios with P < 0.05) and termed ‘predictors’ of clinical malaria. Logistic regression models were used to estimate the association between each predictor and clinical malaria after adjusting for all the other predictors. Using both backward and forward logistic regression modeling, predictors independently associated with clinical malaria (adjusted odds ratios with P < 0.05) were identified. Sensitivities and specificities of these independent predictors were estimated. A malaria score was calculated for each individual using a simple count of these independent predictors, i.e. a malaria score ≥5 requires that the patient presents with at least 5 of the independent predictors. For each age group, sensitivity, specificity, positive and negative predictive values of each total score were estimated and the score with the highest sensitivity and specificity selected as the algorithm of choice.
Testing the algorithms
The performance of these selected algorithms was then tested on data collected from May 2000 to May 2001. For each age group, estimates of sensitivity, specificity, positive and negative predictive values of these algorithms for clinical malaria diagnosis were made. These age-optimised derived algorithms were compared to the generic IMCI guidelines for an area of low malaria endemicity (the IMCI guidelines for higher endemicity areas would result in all patients with a history of fever being treated).
The total number of cases defined as ‘clinical malaria’ by the algorithms was compared to the actual observed number of clinical malaria cases (i.e. illness with a history of fever and peripheral parasitaemia) during this period. The proportions of clinical malaria cases with a parasitaemia ≥5000 parasites/μl of blood detected by these algorithms was also estimated.
Results
Table 1 shows the algorithms that would best predict the people that ought to be given antimalarials in the three age groups. For children ≤5 years of age, an algorithm that involved the use of clinical signs and both spontaneous and prompted symptoms had the best predictive value for clinical malaria diagnosis, whereas for children 6–14 years of age as well as adults, the use of clinical signs and spontaneous symptoms only had the best predictive value for clinical malaria diagnosis.
Table 1.
The best algorithm to predict clinical malaria in people of different age groups (data collected May 1999 to May 2000)
| Spontaneous symptoms |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spontaneous and prompted symptoms (0–5 years) |
Children (6–14 years) |
Adults (≥15 years) |
|||||||
| Clinical signs and symptoms | Odds ratio | Sen (%) | Spec (%) | Odds ratio | Sen (%) | Spec (%) | Odds ratio | Sen (%) | Spec (%) |
| Fever | |||||||||
| Hot on palpation* | 2.1 | 39.7 | 79 | – | – | – | 2.9 | 9.2 | 96.9 |
| Axillary temperature* (≥37.5 °C) | 1.4 | 44 | 73.2 | 1.9 | 26 | 83.7 | – | – | – |
| Shivering | 3.7 | 26.6 | 93.7 | 1.6 | 17.2 | 89.6 | – | – | – |
| Digestive problems | |||||||||
| Vomiting | 2.2 | 30.9 | 85.3 | 2 | 16.9 | 92.3 | – | – | – |
| Absence of diarrhoea | 3.1 | 92.5 | 14.3 | – | – | – | – | – | |
| Respiratory problems | |||||||||
| Normal chest sounds* | 4.5 | 97.5 | 11.5 | 3.4 | 99 | 3 | – | – | – |
| Absence of rhinitis* | 3.0 | 97.9 | 7.2 | 3.4 | 99 | 3.4 | – | – | – |
| Cough not heard* | 1.4 | 89.4 | 19 | 1.8 | 72 | 43 | – | – | – |
| Normal respiratory rate* | 1.8 | 95.8 | 8.1 | – | – | – | – | – | – |
| Absence of cough | 1.5 | 53.8 | 61.7 | – | – | – | – | – | – |
| Absence of running nose | 1.5 | 87.2 | 22 | – | – | – | – | – | – |
| Absence of chest pains | – | – | – | – | – | 2.4 | 91.4 | 18.6 | |
| Skin problems | |||||||||
| Absence of wounds* | 3.0 | 98.6 | 4.6 | 6.6 | 99.5 | 3.4 | – | – | – |
| Absence of rashes | 4.0 | 98.6 | 4.6 | 7 | 99.7 | 2.7 | – | – | – |
| Other | |||||||||
| Pallor* | 3.4 | 16.6 | 95.8 | – | – | – | – | – | – |
| Palpable spleen* | 4.2 | 18.2 | 96.5 | 3.5 | 16.7 | 95 | – | – | – |
| Absence of red discharging eyes |
4.7 | 99.7 | 1.4 | – | – | – | – | – | – |
| Sleepiness | 2.6 | 7.9 | 97.9 | – | – | – | – | – | – |
| Joint pains | – | – | – | – | – | – | 1.8 | 25.8 | 84 |
| Best score | ≥10 | ≥5 | ≥2 | ||||||
| Sensitivity and specificity | Sen = 82%, Spec = 59% | Sen = 86%, Spec = 72% | Sen = 29%, Spec = 85% | ||||||
| NPV and PPV | NPV = 83%, PPV = 57% | NPV = 36%, PPV = 86% | NPV = 83%, PPV = 31% | ||||||
Sen, sensitivity; spec, specificity; NPV, negative predictive value; PPV, positive predictive value.
Clinical signs.
Pallor – includes both conjuctival and palm pallor.
A dash indicates that the odds ratio for the association between predictors and clinical malaria was not statistically significant (P > 0.05).
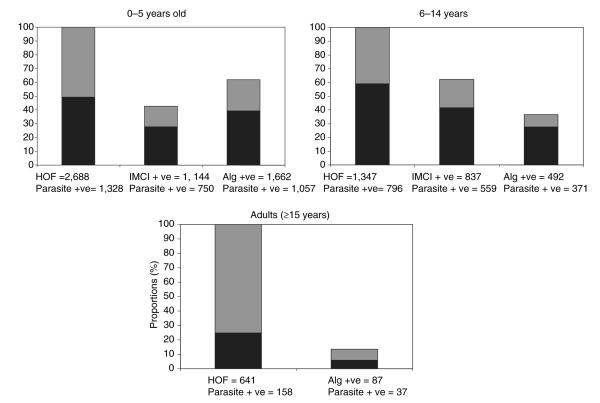
Table 2 shows the sensitivity, specificity, positive and negative predictive values of the age-optimized derived algorithms as they performed on data collected in the following year (May 2000 to May 2001). Estimate trends followed the pattern of the original estimates except for the sensitivity and negative predictive values of the algorithms for children 6–14 years of age (sensitivity 86% compared with 46.6% and NPV of 50.3% compared with 36%). Among the children ≤5 years of age, the IMCI guidelines had a higher specificity but lower sensitivity compared with the use of the age-optimized derived algorithms, whereas among children 6–14 years of age, the IMCI guidelines had a higher sensitivity but lower specificity compared with the derived algorithms. This is illustrated in Figure 1, which shows the number and proportion of study clinic attendants presenting with a history of fever and accompanying parasitaemia (i.e. required an antimalarial) and those who would have been treated for clinical malaria using either the age-optimized derived algorithms or the IMCI guidelines.
Table 2.
Estimates of sensitivity (Sen), specificity (Spec), positive and negative predictive values (PPV and NPV) for the algorithms selected for each age group (data collected May 2000 to May 2001)
| Age group |
Algorithm | Sen (%) |
Spec (%) |
NPV (%) |
PPV (%) |
|---|---|---|---|---|---|
| 0–5 years | Study | 79.6 | 55.5 | 73.6 | 63.6 |
| IMCI | 58.4 | 67.6 | 62.5 | 63.8 | |
| 6–14 years | Study | 46.6 | 78 | 50.3 | 75.4 |
| IMCI | 72.2 | 45.4 | 53.1 | 65.6 | |
| Adults (≥15 years) |
Study | 23.4 | 89.6 | 78.2 | 42.5 |
The ‘study’ algorithm refers to the age-optimized algorithms derived in this study.
Figure 1.
The number and proportion of all who attended the study clinic with a history of fever from Chonyi and Ngerenya in the period May 2000 to May 2001 that would have been diagnosed with malaria using IMCI guidelines or the age-optimized derived algorithm. Black bars represent those that were parasite positive, whereas grey represents those without parasitaemia. HOF – total with a history of fever, represents 100% on the proportions scale; parasite positive – those with any parasitaemia in each category; IMCI positive – those that would have been diagnosed with clinical malaria using the IMCI guidelines for an area of low transmission (history of fever in the absence of measles, running nose and other causes of fever, in our case the absence of respiratory symptoms and diarrhoea); Alg positive – those that would have been diagnosed with clinical malaria using the age-optimized derived algorithm.
Although the IMCI guidelines were developed for children ≤5 years of age, in this analysis, the guidelines were tested on all children <15 years of age. Using the IMCI guidelines would result in a higher proportion of children 6–14 years being treated for clinical malaria than children ≤5 years of age (62.4% vs. 42.6%, χ2 = 137.63, P < 0.001); however, the proportion with parasitaemia among those that would be diagnosed using the selected algorithms was the same in the two age groups (66.8% vs. 65.6%, χ2 = 0.32, P = 0.6). On the contrary, most of the presenting to the clinic with a history of fever did not have parasitaemia and the derived algorithm performed poorly, selecting only 23% of those with parasitaemia.
We examined whether clinical algorithms might be better at identifying patients with a high parasitaemia. This was tested by estimating the proportion of patients with parasitaemia ≥5000 parasites/μl of blood that would be selected by the algorithm. Among children ≤5 years of age, the algorithm was able to select 84% (803/960) of those with a parasitaemia ≥5000 parasites/μl of blood, whereas for those 6–14 years of age, that algorithm selected 56% (265/472) compared with 34% (16/47) among adults. The derived algorithm for younger children was therefore better at selecting those with a high parasitaemia. However, were this algorithm to be used for treating clinical malaria in these areas, 16% of children ≤5 years of age and 44% of those 6–14 years of age would be sent home without treatment despite having high parasitaemia.
Discussion
Among young children in sub-Saharan Africa, clinical malaria is one of the commonest illnesses and a leading cause of death. In endemic areas, among older children and adolescents, it is a common cause of fever though rarely causing life-threatening disease, while in adults malaria still causes episodes of self-limiting fever, although incidence rates are typically <5% of those occurring in younger children. The picture is, however, complicated by the fact that in most areas both patients and health care workers label practically any acute episode of illness ‘malaria’. Ideally, the decision to use an antimalarial drug should be based on a definitive demonstration of parasites in the peripheral blood film. In endemic areas, asymptomatic parasitisation is common and a positive blood film does not necessarily establish that the parasites are the cause of the illness, but from a pragmatic point of view anyone with an acute febrile illness and a positive film should certainly receive antimalarials.
The vast majority of people with acute febrile illness in Africa are treated on the basis of their presenting symptoms without access to any specific diagnostic techniques. There is a clear balance between the need to ensure adequate coverage of a potentially fatal disease and the need to avoid the expense and risk of unnecessary treatment. In the case of children ≤5 years of age living in areas of stable malaria endemicity, the IMCI guidelines lean heavily towards over-treatment by recommending antimalarials for all febrile illnesses. There are no generally accepted guidelines for older children and adults (although they form a significant load on the health services) but, in practice, it is likely that the same sort of approach is applied at all ages.
Clearly, the proportion of febrile cases who are parasite-positive will vary from place to place but the populations we describe, drawn from areas of low to moderate transmission, are probably representative of many populations in Africa. Overall 57% of the 4676 people presenting to the study clinic with a history of fever were children ≤5 years of age and in this age group, 49% had a positive blood film. Twenty-nine per cent of those presenting to the clinic with a history of fever were older children and adolescents, but interestingly a higher proportion in this group (59%) had a positive film. Only 14% of those presenting to the clinic with a history of fever were adults and 25% had a positive blood film. This figure in adults is broadly comparable to those reported elsewhere in Africa ranging from 14% in a district hospital in Tanzania (Oster et al. 2000) to 30% in an adult outpatient hospital in Blantyre (Jonkman et al. 1995). In studies conducted in endemic areas outside Africa, this figure was 12% in India (Chandramohan et al. 2001) and 51% in Papua New Guinea (Genton et al. 1994). Using a history of fever to treat adults for clinical malaria in malaria-endemic areas would therefore result in considerable waste of antimalarials among a population that is not at a high risk of death from clinical malaria.
The application of clinical algorithms, either the generic IMCI algorithms for low endemicity areas or the age-optimized derived algorithms, would have resulted in substantially fewer treatments with antimalarials being given compared to treating all people that present to the clinic with a history of fever for malaria. Thus the proportion receiving antimalarials after application of the age-specific derived algorithms would have fallen to 62%, 37% and 14% for the three age groups (Figure 1). However, this would have resulted in failure to treat 20%, 53% and 77%, respectively, of those requiring it (i.e. those with a history of fever with accompanying parasitaemia). In the group at highest risk of death (children ≤5 years of age), a potential failure of 20% to treat is unacceptable. While it could be argued that failing to treat immediately in older children and adults may be acceptable in some circumstances, the proportions missed using the algorithmic approach are so high that in practice it is hard to imagine such an approach having utility.
It has sometimes been argued that algorithms may be better at detecting cases with higher parasitaemia (more likely to be ‘true’ cases) and that failure to treat low-density parasitaemia may not represent such a large risk though so far as we know, there is no evidence to support this in symptomatic individuals. As the algorithms in this study were derived using a malaria definition aimed at treating all with malaria (but in the process may treat some presenting with a history of fever due to other causes but have concurrent parasitaemia, as is common in endemic areas) we sought to find out whether the algorithms would detect cases with higher parasitaemia. However, in this study, the ability of the algorithmic approach to detect cases with parasitaemia ≥5000 parasites/μl of blood was not substantially better than that for detecting cases with any level of parasitaemia.
The poor performance of clinical algorithms in identifying children in need of antimalarial treatment is in line with the review by Chandramohan et al. (2002), who suggested that their use would lead to drug wastage in areas of low endemicity and an increased failure to treat in those of high endemicity. Another weakness of this approach is that algorithms tend to be site-specific but perhaps more disappointing is the failure of careful age-specific approaches to produce useful algorithms in older age groups where there is more latitude for delaying treatment and enormous scope for saving on unnecessary treatments.
Malaria control programmes across Africa are currently in the process of adopting combination chemotherapy as the optimum first-line treatment for clinical malaria. Whilst there are expected to be major advantages in terms of efficacy and avoiding drug resistance, combination therapy is considerably more expensive, and data safety is limited. There is thus an urgent need to target treatment more effectively to those who actually need it. Unfortunately, despite their attractiveness, it appears that clinical algorithms have little if any utility in achieving this. An alternative solution appears to be age-specific (older children and adults) rationing of specific diagnostic facilities. In this situation, all children ≤5 years of age in a malaria-endemic area presenting to a health facility with a history of fever ought to be treated with an effective anti-malarial; however, older children and adults should only be treated for malaria if they are found to be parasite positive by either microscopy or rapid tests. This, in itself, represents a cost but it may be one that we cannot afford not to pay.
Acknowledgements
This investigation received financial support from The Wellcome Trust and the Kenya Medical Research Institute. We wish to thank all the clinical staff of the KEMRI Unit at Kilifi who made this study possible, especially Norbert Peshu. We are grateful to the fieldworkers who carried out all the surveys and the interviews for their dedication. The authors are especially grateful to Johnson Masha for supervising most of the fieldwork and Monica for data entry. KM and RWS are supported by The Wellcome Trust as Senior Research Fellows (nos 631342 and 058992). This paper is published with the permission of the director of KEMRI.
References
- Chandramohan D, Carneiro I, Kavishwar A, Brugha R, Desai V, Greenwood BM. A clinical algorithm for the diagnosis of malaria: results of an evaluation in an area of low endemicity. Tropical Medicine and International Health. 2001;6:505–510. doi: 10.1046/j.1365-3156.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- Chandramohan D, Jaffar S, Greenwood BM. Use of clinical algorithms for diagnosing malaria. Tropical Medicine and International Health. 2002;7:45–52. doi: 10.1046/j.1365-3156.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- Genton B, Smith T, Baea K, et al. Malaria: how useful are clinical criteria for improving the diagnosis in a highly endemic area? Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88:537–541. doi: 10.1016/0035-9203(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. Bulletin of the World Health Organization. 1997;75(Suppl. 1):7–24. [PMC free article] [PubMed] [Google Scholar]
- Hendrickse RG, Hasan AH, Olumide LO, Akinkunmi A. Malaria in early childhood. Annals of Tropical Medicine and Parasitology. 1971;65:1–20. [PubMed] [Google Scholar]
- Jonkman A, Chibwe RA, Khoromana CO, et al. Cost-saving through microscopy-based versus presumptive diagnosis of malaria in adult outpatients in Malawi. Bulletin of the World Health Organization. 1995;73:223–227. [PMC free article] [PubMed] [Google Scholar]
- Mbogo CNM, W SR, Khamala CPM, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. American Journal of Tropical Medicine and Hygiene. 1995;52:201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- Mbogo CNM, Mwangangi JM, Nzovu J, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. American Journal of Tropical Medicine and Hygiene. 2003;68:734–742. [PubMed] [Google Scholar]
- Mkawagile DSM, Kihamia CM. Relationship between clinical diagnosis of malaria and parasitaemia in adult patients attending Mwananyamala Dispensary Dar es Salaam. The Central African Journal of Medicine. 1986;32:2–5. [PubMed] [Google Scholar]
- Muhe L, Oljira B, Degefu H, Enquesellassie F, Weber MW. Clinical algorithm for malaria during low and high transmission seasons. Archives of Disease in Childhood. 1999;81:216–220. doi: 10.1136/adc.81.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeahialam TC, Kilama WL, Ramji BD. The clinical significance of malaria parasitaemia in children. East African Medical Journal. 1972;49:962–969. [PubMed] [Google Scholar]
- Olaleye BO, Williams LA, Dalessandro U, et al. Clinical predictors of malaria in Gambian children with fever or a history of fever. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92:300–304. doi: 10.1016/s0035-9203(98)91021-5. [DOI] [PubMed] [Google Scholar]
- Olivar M, Develoux M, Chegou Abari A, Loutan L. Presumptive diagnosis of malaria results in a significant risk of mistreatment of children in urban Sahel. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85:729–730. doi: 10.1016/0035-9203(91)90432-x. [DOI] [PubMed] [Google Scholar]
- Oster N, Krause E, Hatz CH. Towards a rational malaria management at district hospital level: exploratory case series of febrile adult patients in a holo-endemic area of Tanzania. Tropical Doctor. 2000;30:203–207. doi: 10.1177/004947550003000407. [DOI] [PubMed] [Google Scholar]
- Redd SC, Kazembe PN, Luby SP, et al. Clinical algorithm for treatment of Plasmodium falciparum malaria in children. Lancet. 1996;347:223–227. doi: 10.1016/s0140-6736(96)90404-3. [DOI] [PubMed] [Google Scholar]
- Rooth I, Bjorkman A. Fever episodes in a holoendemic malaria area of Tanzania: parasitological and clinical findings and diagnostic aspects related to malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:479–482. doi: 10.1016/0035-9203(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bulletin of the World Health Organization. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Eckert E, Teklehaimanot A. Estimating the needs for artesunate-based combination therapy for malaria case-management in Africa. Trends in Parasitology. 2003;19:363–369. doi: 10.1016/s1471-4922(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Weber MW, Mulholland K, Jaffar S, Troedsson H, Gove S, Greenwood BM. Evaluation of an algorithm for the intergrated management of childhood illness in an area with seasonal malaria in the Gambia. Bulletin of the World Health Organisation. 1997;75(Suppl. 1):25–32. [PMC free article] [PubMed] [Google Scholar]