SUMMARY
Background and objective
Malaria is a disease of major public health importance in Kenya killing 26 000 children under 5 years of age annually. This paper seeks to assess the quality of sulphadoxine-pyrimethamine (SP) and amodiaquine (AQ) products available over-the-counter to communities in Kenya as most malaria fevers are self-medicated using drugs from the informal retail sector.
Methods
A retail audit of 880 retail outlets was carried in 2002 in four districts in Kenya, in which antimalarial drug stocks and their primary wholesale sources were noted. In addition, the expiry dates on audited products and the basic storage conditions were recorded on a proforma. The most commonly stocked SP and AQ products were then sampled from the top 10 wholesalers in each district and samples subjected to standard United States Pharmacopoeia (USP) tests of content and dissolution.
Results and discussion
SP and AQ were the most frequently stocked antimalarial drugs, accounting for approximately 75% of all the antimalarial drugs stocked in the four districts. Of 116 SP and AQ samples analysed, 47 (40·5%) did not meet the USP specifications for content and/or dissolution. Overall, approximately 45·3% of SP and 33·0% of AQ samples were found to be sub-standard. Of the sub-standard SP products, 55·2% were suspensions while 61·1% of the substandard AQ products were tablets. Most SP failures were because of the pyrimethamine component. Conclusion: There is a need to strengthen post-marketing surveillance systems to protect patients from being treated with sub-standard and counterfeit antimalarial drugs in Kenya.
Keywords: amodiaquine, antimalarial drugs, Kenya, malaria, quality, sulphadoxine-pyrimethamine
INTRODUCTION
Safety, efficacy and quality are the three most important criteria that have traditionally been used by regulatory authorities worldwide to ensure that populations derive the greatest benefit from pharmaceuticals (1). Although drug quality has been a public health concern from antiquity (2), it is currently receiving renewed international attention (3). In the era of economic liberalization and globalization, there is increasing cross-border trade in pharmaceuticals (4), and products containing the same active ingredient can be marketed by a myriad companies under different ‘brand’ names once the patent has expired, posing new challenges for drug regulation (5). It is, therefore, important to ensure products are manufactured to the highest quality regardless of the sources and regardless of the market for which such products are intended.
Of particular concern, is the quality of essential drugs in developing countries: there have been several reports of widespread availability of counterfeit and/or substandard essential drugs in Nigeria (6), Cameroun (7), Kenya (8-11), Tanzania (12-14), Uganda (15), Rwanda (12), India (16), Vietnam, Cambodia, Myanmar, Laos and Thailand(17-19). The quality of antimalarial drugs is especially crucial, given the widespread nature of the disease and its importance to global public health. For example, in 2002 alone, there were 515 million reported clinical episodes of Plasmodium falciparum malaria, with 70% of these cases concentrated in Africa (20). Further, in Africa, most people with malaria fever inappropriately self-medicate with antimalarial or antipyretic drugs obtained from retail shops, resulting in under dosing or over dosing with the same drug (21, 22). Poor regulation of the pharmaceutical market in sub-Saharan Africa means that consumers may not be adequately protected from access to counterfeit and sub-standard antimalarial drugs (23, 24).
In Kenya, the Ministry of Health (MoH) has developed the National Malaria Strategy (NMS) to guide malaria control programmes in the country by using evidence-based approaches. In this document, the MoH states that it will guarantee access to quality antimalarial drugs by working closely with the Pharmacy and Poisons’ Board (PPB, the drug regulatory authority), the National Quality Control Laboratory (NQCL) and other stakeholders in the pharmaceutical industry (25). However, such a partnership faces an uphill task as currently there is a dearth of knowledge on the quality of antimalarial drugs on the Kenyan market that could be used to support such a policy. To-date, the few studies that have been done on the quality of antimalarial drugs in Kenya, have been ad hoc, focused on products found in major urban centres or those submitted to the PPB for purposes of drug registration. There have been few independent studies on the quality of over-the-counter antimalarial drugs available at the peripheral (district) retail level where malaria therapies are routinely sought. In this paper, we describe the quality of sulphadoxine-pyrimethamine (SP) and amodiaquine (AQ) products available in the Kenyan retail sector in four study districts. SP and AQ were the first and second-line treatment for uncomplicated malaria in February to July 2002 when the study was conducted (26).
MATERIALS AND METHODS
Sampling drug products
We conducted an audit of 880 retailers in 2002 in four districts in Kenya: Greater Kisii, Kwale, Bondo and Makueni. These districts are representative of the main malaria ecologies and broad demographics of the country and have been described elsewhere in detail (27, 28). The retail audit was structured in two rounds: in the first, we used a questionnaire to establish the types of antimalarial drugs in stock and the major wholesale suppliers of the products to the retail sector with a view to sampling the most commonly stocked products for chemical analysis. In the second, a more detailed questionnaire was administered on the types of drugs in stock, pharmacological types, their retail costs, storage conditions and shelf-life. Data on the range of brands, costs and detailed information on wholesale sources are presented elsewhere (29); data pertinent to the general quality of antimalarial drugs (storage and expiry) are reported here along with the results of the chemical analyses.
Data from the first round were assessed in terms of the stocking frequency of common products and major wholesale suppliers for AQ and SP products separately for each district. The top 10 wholesalers for antimalarial products in each district were visited to purchase AQ and SP branded products. To cover as many products as possible, if a given product had been purchased from the preceding district or wholesaler, preference was given to the one that had not been hitherto sampled. Between 60–100 tablets, or 7–10 bottles of suspensions/syrups/drops, of each product of interest were purchased. The samples were then evaluated by standard United States Pharmacopoeia (USP) tests (30) as described below.
Laboratory methods
Reagents
Reference standards for sulphadoxine (SDX, Lots F-1 and F-2), pyrimethamine (PMT, Lots G and H) and amodiaquine hydrochloride (Lot G-1) were purchased from the USP Commission, Rockville, MD. Phenacetin (Lot 13615–018) was purchased from the Sigma-Aldrich Chemicals Co. Dorset, UK. All other reagents and solvents were either of analytical or high-pressure liquid chromatographic (HPLC) grades and were purchased from BDH Chemicals Limited, Poole, UK.
Analytical methods
USP test for content
Content tests essentially determine the amount of active ingredient in a product, which is expressed as a percentage of the label claim. Sample preparation and assay for SP and AQ were performed according to USP specifications (30). AQ was assayed spectrophotometrically (UV/VIS PU8725 Philips scientific, Cambridge, UK), whereas SP was assayed by HPLC using a validated method developed in our laboratory. The USP monograph acceptance criteria states that the content of SDX or PMT in any sample should be 90–110% of label claim; the acceptance limits for content of AQ in a sample are given as 93–107% of label claim.
Dissolution test
Dissolution tests are done to determine the amount of active ingredient that is released from the dosage form and available for absorption, and are used as surrogate markers of in vivo bioavailability. Tests were performed on tablet formulations only. A six-station Erweka DT 600 dissolution apparatus (Erweka Gmbh, Frankfurt, Germany) was used. For SP, the dissolution medium was 1000 mL phosphate buffer, pH 6·8 maintained at 37 ± 0·5 °C, whereas for AQ 900 mL of distilled water maintained at 37 ± 0·5 °C was used as the dissolution medium. Paddle speed was set at 75 revolutions per minute (rpm) for SP and 50 rpm for AQ and amount of active ingredient in solution determined after 30 min, using the methods described above. The USP criteria for SP tablets passing the dissolution test require that the amount of SDX or PMT released in 30 min be at least 60% of label claim; whereas for AQ it is at least 75% of label claim (30).
RESULTS
SP and AQ products sampled
In all 852 (96·8%) of the retailers had antimalarial drugs in stock during the first round of the retail audit. Table 1 shows the top five SP and AQ products encountered in the retail sector. Results demonstrate that (i) the generic product Falcidin ® was the most frequent SP product while Malaratab ® was the most frequently encountered AQ, and that (ii) tablet formulations predominated compared to the suspensions. Both Falcidin ® and Malaratab ® are manufactured locally by Cosmos Limited.
Table 1.
Stocking frequencies of top five common sulphadoxine/sulphalene-pyrimethamine (SP) and amodiaquine (AQ) branded products across 856 retail outlets visited during the initial round of the retail audit survey in 2002. Data presented as proportion of audited retail outlets stocking a given product
| SP |
AQ |
||
|---|---|---|---|
| Brand | Stocking frequency* (%) |
Brand | Stocking frequency* (%) |
| Tablets | |||
| Falcidin | 159 (18·6) | Malaratab® | 735 (85·9) |
| Fansidar® | 81 (9·5) | Betaquine® | 115 (13·4) |
| Metakelfin® | 54 (6·3) | Alphaquine® | 37 (4·3) |
| Orodar® | 49 (5·7) | Emoquin® | 23 (2·7) |
| Malodar® | 16 (1·9) | Camoquin® | 17 (2·0) |
| Others | 115 (13·4) | Others | 42 (4·9) |
| Suspensions | |||
| Pyralfin® | 20 (2·3) | Amobin® | 30 (3·5) |
| Falcigo® | 14 (1·6) | Malaramed® | 24 (2·8) |
| Falcidin® | 13 (1·5) | Malaratab® | 17 (2·0) |
| Intadoxin® | 12 (1·4) | Falciquin® | 16 (1·9) |
| Medifan® | 11 (1·3) | Kamoc® | 13 (1·5) |
| Others | 41 (4·8) | Others | 34 (4·0) |
Proportion of audited outlets stocking a given product. Only 852 outlets were stocking antimalarial drugs in the initial round of the retail audit.
Twenty-three (23) brands of SP tablets were encountered during this first survey, of which 19 (82·6%) were sampled for laboratory analysis. Likewise, of 13 brands of AQ tablets encountered, 11 (84·6%) were sampled for laboratory analysis. For the liquid formulations, 13 SP suspensions/paediatric drops were encountered of which 12 (92·3%) were sampled; 12 AQ suspensions were encountered of which 11 (91·7%) were sampled for analysis. The sampling method used therefore yielded good brand or product coverage. Unbranded generic products (those using the International Non-proprietary Name) were not sampled for analysis. About half the sampled SP brands (48%) were imported from the Indian sub-continent, with most of the remaining being manufactured in Kenya. In contrast, 80% of AQ brands sampled were locally manufactured.
General quality of antimalarial drugs: storage and shelf-life
As stated earlier, storage and expiry dates of all antimalarial products were evaluated in the second round of the retail survey. The majority of products audited (97·2%) were found to satisfy the basic storage conditions set out in the study (namely stored off the floor, out of direct sunlight, in a dry area and away from food stuffs). In addition, over 90% of products were within their specified shelf-life. Figure 1 shows median remaining shelf-life (in months) of 2167 antimalarial products audited by their chemical class. Overall, AQ products had the longest remaining shelf-life [46 months, interquartile range (IQR) 30, 48], followed by SP (median 30 months, IQR 23, 35). Chloroquine (CQ), quinine (QN) and ‘other’ products (OTH in the figure refers to mefloquine, halofantrine, proguanil and mepacrine) had remaining shelf lives of 22 (IQR 15, 28), 17 (IQR 10, 25) and 19 (IQR 18, 27) months, respectively. Products containing artemisinin (ART) had remaining median shelf-life of 12 months (IQR 9, 23).
Fig. 1.
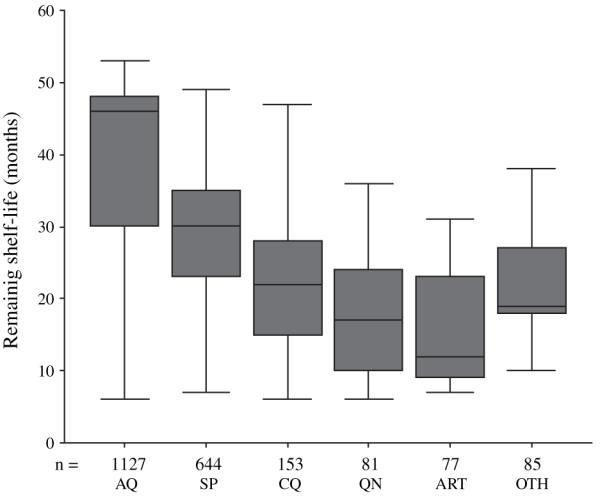
Shelf-life of 2167 audited, un-expired oral anti-malarial products in 876 retail outlets in four districts of Kenya by chemical group. AQ, amodiaquine; SP, sulphadoxine-pyrimethamine; CQ, chloroquine; QN, quinine; ART, artemisinin containing products; OTH, other antimalarial drugs.
Content and dissolution rates
The results for the analysis of SP and AQ for active ingredient content and tablet dissolution rates are summarized in Tables 2 and 3, respectively. Overall, 116 samples of SP and AQ were analysed of which 47 (40·5%) did not meet the USP specifications for content and/or dissolution. Details are given in the following sections for each drug class separately.
Table 2.
Results of 37 sulphadoxine-pyrimethamine (SP) tablets and 27 SP suspensions analysed in 2002
| Content (%) |
Dissolution (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low fail |
Pass |
High fail |
Low fail |
Pass |
||||||
| SDX | PMT | SDX | PMT | SDX | PMT | SDX | PMT | SDX | PMT | |
| SP tablets | 0 | 3 (8·1) | 37 (100·0) | 34 (91·9) | 0 | 0 | 1 (2·7) | 11 (29·7) | 36 (97·3) | 26 (70·3) |
| SP suspensions | 2 (7·4) | 12 (44·4) | 21 (77·8) | 12 (44·4) | 4 (14·8) | 3 (11·1) | na | na | na | na |
na, Not applicable.
Amount sulphadoxine (SDX) and pyrimethamine (PMT) is expressed as per cent label claim. USP limits for content of SDX and PMT range from 90% to 110% and amount SDX/PMT released into dissolution medium within 30 min should be ≥60%. Pass means within limit, and low and high fail means lower or higher than limit, respectively.
Table 3.
Results of 29 amodiaquine (AQ) tablets and 23 AQ suspensions analysed in 2002
| Content (%) |
Dissolution (%) |
||||
|---|---|---|---|---|---|
| Low fail |
Pass | High fail |
Low fail |
Pass | |
| AQ tablets | 10 (34·5) |
19 (65·5) |
0 | 4 (13·8) |
25 (86·2) |
| AQ suspensions |
6 (26·1) |
16 (69·6) |
1 (4·3) |
na | na |
na, Not applicable.
Amount AQ is expressed as per cent label claim. USP limits for content of AQ range from 93% to 107% and amount AQ released into dissolution medium within 30 min should be ≥75%. Pass means within limit, and low and high fail means lower or higher than limit, respectively.
SP samples
Sixty-four SP samples were analysed for content (37 tablet batches and 27 suspensions). Overall, almost half (45·3%) of the SP samples failed to meet these criteria. For the tablet forms 13 (35·1%) samples failed to meet the official requirements: 10 samples (76·9% of failures) failed dissolution alone, two (15·4%) samples failed content alone and one (7·7%) failed both tests. A disproportionate number of SP products failing dissolution and content tests (90% and 100%, respectively) had problems with the PMT component. Further, the only sample which failed both dissolution and content did so with respect to PMT alone. Twenty-seven (27) SP suspensions were analysed for content. Results in Table 2 show that sixteen samples (59·3%) failed to meet official requirements, most of them (81·3%) with respect to PMT. One sample had no detectable PMT and can only be said to be substandard (not counterfeit) according to the WHO, which defines fake or counterfeit drugs as those ‘...deliberately and fraudulently mislabelled with respect to identity and/or source...’ (3).
AQ samples
Fifty-two AQ samples (29 tablet forms and 23 suspensions) were analysed for content of active ingredient. The results presented in Table 3 show that 11 (37·9%) tablet samples failed the requisite tests: one (9·1% of AQ tablet failures) for dissolution alone, seven (63·6%) for AQ content alone and three (27·3%) failed both tests. Results indicate that seven (30·4%) suspensions failed the content test with six (85·7% of failures) being below the lower limit and one sample above the higher limit (Table 3).
DISCUSSION
Although storage and product shelf-life were deemed adequate for over 90% of products in the survey, the content and dissolution tests revealed a large number of substandard SP and AQ products in the market (40·5%), which is consistent with reports of substandard essential drugs across the developing world (6). These results also suggest that for SP products, pyrimethamine accounts for a disproportionate number of dissolution and content failures, an observation in accord with previous reports (11, 31). Of particular concern is the poor quality of SP suspensions in the market; 59·3% failed to meet the official requirements, especially with regard to the pyrimethamine component. This is despite the fact that the burden of malaria is greatest in children under five who are expected to use these preparations. There is therefore, a need for greater controls on the quality of antimalarial suspensions in the market.
Although a substantial proportion of samples did not comply with the USP specifications, most products which failed did so marginally. These failures can be attributed, in part, to poor quality control during manufacture (6, 11), and may reflect the fact that most developing countries including Kenya have weak regulatory mechanisms for enforcing Good Manufacturing Practices (GMP). In Kenya, for example, with over 40 pharmaceutical manufacturing plants, the Pharmaceutical Inspectorate department of the MoH is understaffed and can only manage to inspect a given plant at most once a year. There is, therefore, a need for more personnel for the drug regulatory authority to ensure sub-standard products are not released into the market by local manufacturers of pharmaceuticals. However, this only addresses part of the problem as most products are imported into the country and there is an equal need to tighten controls at ports of entry. One practical and cheap way of detecting poor quality drugs at ports of entry is through the use of semi-quantitative tests such as the Mini-Lab™ which relies on simple colour reactions to quickly identify substandard products (32) at the ports of entry into the country, or in the market, while results of a more thorough and confirmatory laboratory tests are awaited. This may be one way of enhancing the effectiveness of the Pharmaceutical Inspectorate department of the MoH even with the current problem of understaffing.
Although the products analysed in the current study were sampled from the private retail sector, the results arguably represent the situation in Kenya generally. In a recent WHO study of the quality of antimalarial drugs in nine African countries, no differences were observed in the quality of antimalarial drugs between the public and private sectors, public sector outlets (central medical stores, regional stores, hospitals, health centres, dispensaries, etc.) were just as likely to have poor quality antimalarial drugs as their private sector counterparts (pharmacies, retail shops, street vendors, etc.) (31).
In April 2004, Kenya recommended artemether-lumefantrine (AL) combination as the new first-line therapy for uncomplicated malaria (33). Currently, AL is a single-source, high value product, manufactured by a reputable international company (Novartis Pharma AG) to GMP. Therefore, sub-standard AL is unlikely to be a problem in the near future. However, as is common with such high-value products, unscrupulous businessmen eager for quick profits are likely to counterfeit it. There is, therefore, a need to strengthen post-marketing surveillance of products in the market to identify sub-standard and counterfeit antimalarial drugs and to deal appropriately with such practices. The observation that the median remaining shelf-life of ART products in the private sector (12 months) was lower than for other drugs means that drug distribution mechanisms in the Kenyan public sector (historically less efficient than the private sector) need to be strengthened to avoid expiry and drug wastages (AL has short shelf-life of 24 months and because of the hydrophilic nature of ARTs, this applies to all ART-based combination therapies). In addition, those in the private sector need to be inspected regularly and informed clearly about shelve lives of antimalarial drugs and be encouraged to remove expired products from their shelves.
In the case of malaria, a poor quality drug increases the risks of therapeutic failure even when the parasites are fully sensitive to the ingested compounds. This is because most drug failures are because of lower contents or dissolution scores, which is comparable to taking low doses of the drug. Sub-therapeutic drug levels could in turn lead to selection of drug resistant strains of P. falciparum with resistance soon spreading to the rest of the parasite population (34). The widespread resistance to pyrimethamine, and the fact that most SP samples which fail, do so with respect to the pyrimethamine component, is probably not a coincidence. Given the fact that the range of affordable antimalarial drugs is limited, the impact of poor quality on the ‘Useful Therapeutic Life’ (UTL) of antimalarial drugs needs urgent attention. The high prevalence of sub-standard drugs found in this study contrasts markedly with the more positive results reported by Hebron et al. (35).
ACKNOWLEDGEMENTS
Funds were provided by the Department for International Development-Kenya (#031555054), The Wellcome Trust, UK (#058992), The Ministry of Health, Government of Kenya, and the Kenya Medical Research Institute. None of these institutions was involved in study conception, data collection, analysis, and interpretation of results. The authors are grateful to the National Quality Control Laboratory, Mission for Essential Drugs and Supplies for their support in the QC work, Medical Officers of Health for Makueni, Kisii, Gucha, Bondo and Kwale districts for availing some of their staff during sample collection. RWS is a Senior Wellcome Trust Fellow. This paper is published with the permission of the director KEMRI.
Footnotes
CONFLICT OF INTEREST STATEMENT
None to declare.
REFERENCES
- 1.Waller P. Pharmacoepidemiology – a tool for public health. Pharmacoepidemiology and Drug Safety. 2001;10:165–172. doi: 10.1002/pds.579. [DOI] [PubMed] [Google Scholar]
- 2.Shah RR. Thalidomide, drug safety and early drug regulation in the UK. Adverse Drug Reaction and Toxicology Review. 2001;20:199–255. [PubMed] [Google Scholar]
- 3.WHO . Counterfeit drugs: guidelines for the development of measures to combat counterfeit drugs. WHO; Geneva: 1999. pp. 1–60. [Google Scholar]
- 4.Krosnar K. Cross-border trade in medicines causes concern in the EU. Lancet. 2005;365:1297–1298. doi: 10.1016/S0140-6736(05)61011-2. [DOI] [PubMed] [Google Scholar]
- 5.WHO . Use of the WHO certification scheme on the quality of pharmaceutical products moving in international commerce. WHO; Geneva: 1995. pp. 1–146. [PubMed] [Google Scholar]
- 6.Taylor RB, Shakoor O, Behrens RH, et al. Pharmacopoeial quality of drugs supplied by Nigerian pharmacies. Lancet. 2001;357:1933–1936. doi: 10.1016/s0140-6736(00)05065-0. [DOI] [PubMed] [Google Scholar]
- 7.Basco LK. Molecular epidemiology of malaria in Cameroon. XIX. Quality of antimalarial drugs used for self-medication. American Journal of Tropical Medicine and Hygiene. 2004;70:245–250. [PubMed] [Google Scholar]
- 8.Kibwage IO, Ondari CO, Mureithi IG, Thuranira JK, Hoogmartens J. Analysis of co-trimoxazole products on the Kenyan market. East and Central African Journal of Pharmaceutical Sciences. 1998;1:34–38. [Google Scholar]
- 9.Kibwage IO, Ondari CO, Ndemo FA. Inequivalence of some pharmaceuticals on the Kenyan market. Pharmaceutical Journal of Kenya. 1988;1:8–9. [Google Scholar]
- 10.Kibwage IO, Thuranira J, Migosi D. Quality performance of metronidazole tablet products on the Kenyan market. East African Medical Journal. 1991;68:365–371. [PubMed] [Google Scholar]
- 11.Kibwage IO, Ngugi JK. Sulphadoxine/pyrimethamine tablet products on the Kenyan market: quality concerns. East and Central African Journal of Pharmaceutical Sciences. 2000;3:14–19. [Google Scholar]
- 12.Kayumba PC, Risha PG, Shewiyo D, et al. The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: influence of tropical storage conditions on in vitro dissolution. Journal of Clinical Pharmacy and Therapeutics. 2004;29:331–338. doi: 10.1111/j.1365-2710.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- 13.Abdi YA, Rimoy G, Ericsson O, Alm C, Massele AY. Quality of chloroquine preparations marketed in Dar es Salaam, Tanzania. Lancet. 1995;346:1161. doi: 10.1016/s0140-6736(95)91834-5. [DOI] [PubMed] [Google Scholar]
- 14.Risha PG, Shewiyo D, Msami A, Masuki G, Vergote G, Vervaet C. In vitro evaluation of the quality of essential drugs on the Tanzanian market. Tropical Medicine and International Health. 2002;7:701–707. doi: 10.1046/j.1365-3156.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogwal-Okeng JW, Okello DO, Odyek O. Quality of oral and parenteral chloroquine in Kampala. East African Medical Journal. 1998;75:692–694. [PubMed] [Google Scholar]
- 16.Singh J, Dutta AK, Khare S, et al. Diethylene glycol poisoning in Gurgaon, India, 1998. Bulletin of the World Health Organization. 2001;79:88–95. [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . Counterfeit and sub-standard drugs in Myanmar and Vietnam. WHO; Geneva: 1999. pp. 1–55. [Google Scholar]
- 18.Newton P, Proux S, Green M, et al. Fake artesunate in southeast Asia. Lancet. 2001;357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- 19.Dondorp AM, Newton PN, Mayxay M, et al. Fake antimalarials in Southeast Asia are a major impediment to malaria control: multinational cross-sectional survey on the prevalence of fake antimalarials. Tropical Medicine and International Health. 2004;9:1241–1246. doi: 10.1111/j.1365-3156.2004.01342.x. [DOI] [PubMed] [Google Scholar]
- 20.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCombie SC. Self-treatment for malaria: the evidence and methodological issues. Health Policy and Planning. 2002;17:333–344. doi: 10.1093/heapol/17.4.333. [DOI] [PubMed] [Google Scholar]
- 22.Marsh VM, Mutemi WM, Muturi J, Haaland A, Watkins WM, Otieno G, Marsh K. Changing home treatment of childhood fevers by training shopkeepers in rural Kenya. Tropical Medicine and International Health. 1999;4:383–389. doi: 10.1046/j.1365-3156.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 23.Siringi S. Over-the-counter sale of antimalaria drugs stalls Kenyan disease strategy. Lancet. 2001;357:1862. doi: 10.1016/S0140-6736(00)05025-X. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Effective drug regulation: what can countries do? WHO; Geneva: 1999. pp. 1–53. [Google Scholar]
- 25.DOMC . National Malaria Strategy: 2001–2010. Division of Malaria Control (DOMC), Ministry of Health; Nairobi: 2001. pp. 1–50. [Google Scholar]
- 26.DOMC . National guidelines for diagnosis, treatment and prevention of malaria for health workers. Division of Malaria Control (DOMC), Ministry of Health; Nairobi: 1998. pp. 1–49. [Google Scholar]
- 27.Amin AA, Marsh VM, Noor AM, Ochola SA, Snow RW. The use of formal and informal curative services in the management of paediatric fevers in four districts in Kenya. Tropical Medicine and International Health. 2003;8:1143–1152. doi: 10.1046/j.1360-2276.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 28.Noor AM, Gikandi P, Hay SI, Muga RO, Snow RW. Creating spatially defined databases for equitable health service planning in low-income countries: the example of Kenya. Acta Tropica. 2004;91:239–251. doi: 10.1016/j.actatropica.2004.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin AA, Snow RW. Brands, costs and registration status of antimalarial drugs in the Kenyan retail sector. Malaria Journal. 2005;4:36. doi: 10.1186/1475-2875-4-36. Available at: http://www.malariajournal.com/content/pdf/1475-2875-4-36.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.USP . United States Pharmacopoeia, USP 24 NF, 19th edn. Pharmacopoeial Convention INC; Rockville: 2000. [Google Scholar]
- 31.WHO . The quality of antimalarials. A study in selected African countries. World Health Organization; Geneva: 2003. pp. 1–67. [Google Scholar]
- 32.Jahnke RWO, Kusters G. Low-cost quality assurance of medicines using the GPHF-Minilab. Drug Information Journal. 2001;35:941–945. [Google Scholar]
- 33.MoH National symposium on next anti-malaria treatment policy in Kenya; Naivasha: Ministry of Health, Republic of Kenya. 5th–6th April 2004.2004. pp. 1–29. [Google Scholar]
- 34.Taylor RB, Shakoor O, Behrens RH. Drug quality, a contributor to drug resistance? Lancet. 1995;346:122. doi: 10.1016/s0140-6736(95)92145-1. [DOI] [PubMed] [Google Scholar]
- 35.Hebron Y, Tettey JNA, Poumamdari M, Watson DG. The chemical and pharmaceutical equivalence of sulfadoxine/pyrimethamine tablets sold in the Tanzanian market. Journal of Clinical Pharmacy and Therapeutics. 2005;30:563–569. doi: 10.1111/j.1365-2710.2005.00687.x. [DOI] [PubMed] [Google Scholar]


