Abstract
Mycoplasma penetrans, a potential human pathogen found mainly in HIV-infected individuals, uses a tip structure for both adherence and gliding motility. To improve our understanding of the molecular mechanism of M. penetrans gliding motility, we used chemical inhibitors of energy sources associated with motility of other organisms to determine which of these is used by M. penetrans, and also tested whether gliding speed responded to temperature and pH. M. penetrans gliding motility was not eliminated in the presence of a proton motive force inhibitor, a sodium motive force inhibitor, or an agent that depletes cellular ATP. At near-neutral pH, gliding speed increased as temperature increased. The absence of a clear chemical energy source for gliding motility and a positive correlation between speed and temperature suggest that energy derived from heat provides the major source of power for the gliding motor of M. penetrans.
Keywords: terminal organelle, arsenate, CCCP, amiloride, microcinematography
INTRODUCTION
Cellular motility is important for a variety of processes, including obtaining nutrients, evading threats, organizing cells for developmental processes, and cell division. Both thermal and chemical energy are employed as direct sources of energy for cellular motility. For example, many eukaryotic cells are driven forward by the formation of membrane protrusions through localized polymerization of actin, powered principally by thermal energy in the form of a Brownian ratchet (Peskin et al., 1993). Bacterial twitching motility is powered by ATP hydrolysis, which powers extension and retraction of type IV pili attached to a surface (Burrows, 2005). Rotation of bacterial flagella, which drive swimming and swarming movements, is powered by proton motive force (PMF) (Berg & Anderson, 1973), or rarely by sodium motive force (SMF) (McCarter, 2004). In both Flavobacterium johnsoniae and Myxococcus xanthus, gliding motility, the smooth movement of cells over a surface, is powered by PMF (Liu et al., 2007; Nan et al., 2010; Sun et al., 2011). Since gliding motility is carried out among diverse bacterial groups and uses diverse mechanisms (McBride, 2004), no single organism can be used to model a molecular mechanism for this process.
Several mycoplasmas exhibit gliding motility, enabling these bacteria to colonize and cause infection in their hosts (Jordan et al., 2007; Szczepanek et al., 2012). Among these species, only Mycoplasma mobile has been studied in depth to identify its motility energy source. Arsenate, a phosphate analogue that causes depletion of cellular ATP, rapidly and potently inhibits motility of M. mobile (Jaffe et al., 2004), and Triton X-100-permeabilized cells resume movement when ATP is added directly to the cells, demonstrating that the motor is directly dependent on ATP hydrolysis (Uenoyama et al., 2005). Little is known about the energy source necessary for gliding motility in other mycoplasmas. However, it is well-established that different mycoplasma species use compositionally dissimilar tip structures for gliding motility (Relich et al., 2009; Miyata, 2010; Jurkovic et al., 2012), making it impossible to generalize the motility mechanisms they use.
One mycoplasma species whose gliding mechanism is unknown is Mycoplasma penetrans, a putative human pathogen originally isolated from the urogenital tract of HIV-positive patients (Lo et al., 1991, 1992; Wang et al., 1992). Its lipoproteins are mitogenic toward B and T lymphocytes (Feng et al., 1994; Sasaki et al., 1995) and stimulate transcription of the HIV genome in vitro via toll-like receptors (Shimizu et al., 2004), implying a role for M. penetrans in the accelerated progression of AIDS. M. penetrans has a polar terminal organelle that leads during gliding motility and whose Triton X-100-insoluble cytoskeleton is distinct from those of most other species, including M. mobile (Jurkovic et al., 2012). Genomic analysis reveals the absence of clear homologs of terminal organelle-associated proteins of other species (Sasaki et al., 2002). The present study aims to identify potential sources of energy for gliding motility of M. penetrans, examining both chemical and thermal contributions to the movement of cells of this species.
MATERIALS AND METHODS
Bacterial culture conditions and passaging
M. penetrans strain HP88 was obtained through a series of passages of M. penetrans strain GTU-54-6A1 (Lo et al., 1992) in SP-4 motility media [SP-4 broth (Tully et al., 1979) supplemented with 3% gelatin]. A 100-µL aliquot of M. penetrans strain GTU-54-6A1 was added to 2 mL of SP-4 motility medium in a 24-well plate (TPP Techno Plastic Products AG). Upon a colour change in the medium from red to yellow, a 100-µL aliquot of the passaged M. penetrans was taken from the top of the well and transferred to a fresh 2 mL of SP-4 motility medium in the adjoining well. This process was repeated 75 times, generating strain HP88, which was subsequently cultured at 37°C in SP-4 broth or on SP-4 agar plates. As a control, M. mobile strain 163K (Kirchoff & Rosengarten, 1984) was cultured at room temperature in SP-4 broth or SP-4 motility medium.
Time-lapse microcinematography
For motility assays of M. penetrans, a concentrated motility stock was made by growing 50 mL of culture to mid-log phase, indicated by a colour change in the medium from red to orange. Cells were harvested by centrifugation (17,400×g) at 4°C for 20 min, suspended in 2 mL fresh SP-4 broth, and passed through a 0.45-µm filter before aliquoting and storage. For motility assays at various temperatures and pH, HP88 motility stocks were thawed and inoculated into SP-4 motility medium with a pH of 5.8, 6.8, 7.8, or 8.8 and incubated at 30°C, 37°C, or 40°C for 3 h before analysis. To determine the average gliding speed of M. penetrans HP88, excluding rest periods, cells from frozen, mid-log phase stocks were passed through a 0.45-µm filter and incubated for 3 h at 37°C in glass chamber slides (Nunc) in SP-4 motility medium, and microcinematographic analysis was performed as previously described (Hatchel et al., 2006).
Energy source assays
To determine the effects of inhibitors of ATP metabolism and ion motive force on M. penetrans motility, cells were analyzed in buffers with or without the test reagent. M. penetrans motility stocks were incubated in SP-4 motility medium for 3 h at 37°C in a glass chamber slide. M. mobile cells from frozen mid-log phase growth were syringed 10 times before incubation in SP-4 motility media for 1 h at 25°C. For both species, the medium was then removed and each chamber was rinsed 5 times with the control or test buffer, incubated in the control or test buffer for 1 h, and analysed for motility as described above. The following buffers were used: phosphate-buffered saline supplemented with gelatin and glucose (PBS-G2; 150 mM NaCl, 32 mM NaH2PO4, 136 mM Na2HPO4, 10 mM glucose, 3% gelatin, pH 7.2); arsenate-buffered saline supplemented with gelatin and glucose (ArBS-G2K; 140 mM NaCl, 75 mM KCl, 10 mM glucose, 2.5 mM potassium arsenate, 4.75 mM sodium arsenate, 3% gelatin, pH 7.2); PBS-G2 supplemented with potassium (PBS-G2K; 140 mM NaCl, 10 mM KCl, 10 mM glucose, 50 mM sodium phosphate, pH 7.2); PBS-G2 supplemented with CCCP [C3PBS-G2; 150 mM NaCl, 3.2 mM NaH2PO4, 13.6 mM Na2HPO4, 10 mM glucose, 3% gelatin, 10 µM CCCP in dimethyl sulfoxide (DMSO), pH 7.2]; and PBS-G2 supplemented with amiloride (APBS-G2; 150 mM NaCl, 3.2 mM NaH2PO4, 13.6 mM Na2HPO4, 10 mM glucose, 3% gelatin, 10 µM amiloride, pH 7.2). All reagents were purchased from Sigma Aldrich.
Temperature/pH study
Motility stocks were incubated in SP-4 motility medium with the desired pH (5.8, 6.8, 7.8, 8.8) and temperature (30°C, 37°C, 40°C) in glass chamber slides. For motility analysis, 18 images were captured at 1000X magnification on a Leica DM IRB inverted phase-contrast/epifluorescence microscope at approximately 0.25-s intervals. Images were merged and analysed for 20–25 motile cells as previously described (Hatchel et al., 2006).
Statistical analysis
The temperature and pH data were analyzed using two-factor factorial analysis of variance (ANOVA) to examine the effects of both temperature and pH on motility speed. To determine the temperature and pH associated with maximal gliding speed, a statistical response surface model was fit to the data with an accompanying canonical analysis. The effects of energy source inhibitors on motility were analyzed by ANOVA. All statistical analyses were performed using SAS version 92 for Windows.
RESULTS
Gliding speed and HA
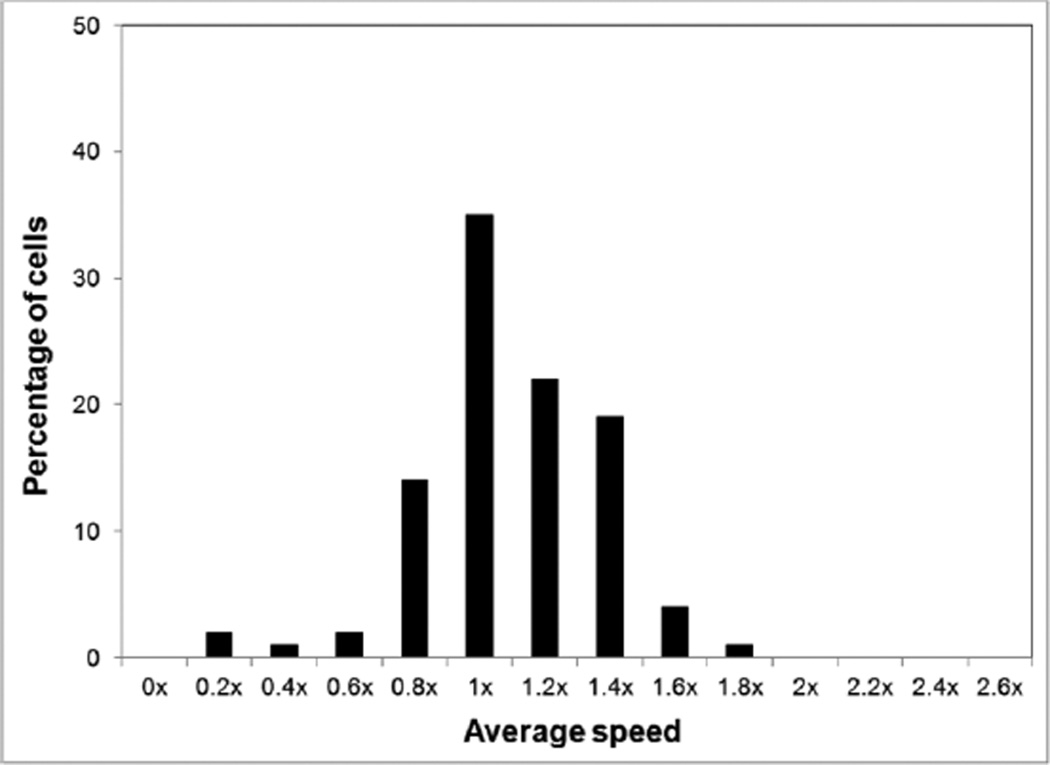
The advantage of using a fast-gliding strain for analysis of motility-associated phenomena is that increased gliding speed allows clearer resolution of changes in speed under different conditions. High passage M. penetrans strain HP88 glided in one direction with an average speed of 1201 ± 326 nm s−1 (n=103), twice as fast as strain GTU-54-6A1 and >20 times faster than strain HF-2 (Jurkovic et al., 2012). The gliding speed of this strain, which was used for all experiments, spanned a range of 158–2115 nm s−1, corresponding to 0.2–1.8 times the average gliding speed (Figure 1). For subsequent experiments, values were normalized to the gliding speed observed at 37°C and pH 7.8 in the appropriate control buffers.
Figure 1.
Distribution of M. penetrans HP88 gliding speeds about the mean. Gliding speeds of the individual motile cells were grouped into bins of 0.2x of the mean, designated by x.
Effect of arsenate on motility and growth
Arsenate enters prokaryotic and eukaryotic cells via phosphate transporters (Rosen, 2002) and inhibits many reactions involving phosphate. These reactions include substrate-level phosphorylation events leading to ATP synthesis via the glycolysis (Warburg & Christian, 1939) and arginine dihydrolase (Knivett, 1953) pathways, the only two means of ATP synthesis available to M. penetrans (Lo et al., 1992; Sasaki et al., 2002), as mycoplasma membrane ATP synthase actually hydrolyses ATP to create a proton gradient (Linker & Wilson, 1985). To confirm toxicity of arsenate to M. penetrans, cells were cultured in the presence of 10 mM sodium arsenate or sodium phosphate, pH 7.2. After 2 d of incubation at 37°C, growth of M. penetrans was observed with added sodium phosphate, but not arsenate, (not shown), confirming that M. penetrans takes up arsenate and its growth is inhibited at relatively low arsenate concentrations. As mycoplasmas lack electron transport chain-associated respiration, arsenate toxicity can be attributed to ATP depletion.
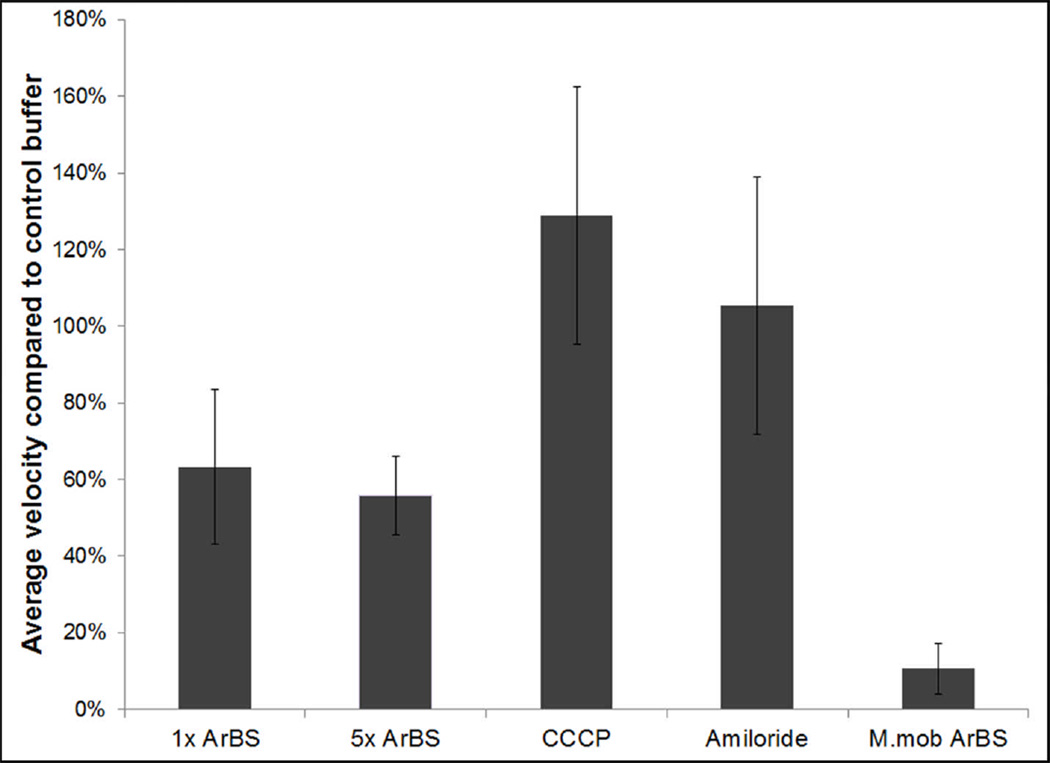
Arsenate was added to M. penetrans cells to determine whether ATP hydrolysis by a motor-associated component directly provides energy for gliding, as proposed for M. mobile, upon whose gliding motility arsenate has an immediate negative impact (Jaffe et al., 2004). M. penetrans continued to glide in the presence of 50 mM arsenate, five times the amount in which growth was prevented (see above), at incubation times ranging from 1 to 8 h. In 50 mM arsenate, the gliding speeds of both M. mobile [F(1, 144)=13331, p<0.0003] and M. penetrans [F(1, 144)=7670, p<0.0003] were significantly reduced. However, the 37% decrease for M. penetrans was much smaller than that of M. mobile, which exhibited an 89% decrease in speed (Figure 2), essentially in agreement with the observations of an absence of M. mobile cells moving faster than 10% of normal gliding speed after 10 min under similar conditions (Jaffe et al., 2004). Although the change in speed of M. penetrans was statistically significant, the moderate value of the decrease and the continued movement of the cells after 8 h (not shown) suggest that direct inhibition of the motor by ATP depletion was unlikely. Increasing the arsenate concentration fivefold further, to 250 mM, had a negligible effect on M. penetrans motility (Figure 2). Thus, ATP hydrolysis is an unlikely energy source for gliding by M. penetrans.
Figure 2.
M. penetrans relative gliding speed in the presence and absence of arsenate, CCCP, and amiloride compared to the average gliding speed of M. mobile in the presence of 50 mM arsenate. Values were normalized to respective control buffers. 1x ArBS, arsenate-buffered saline with 50 mM arsenate; 5x ArBS, arsenate-buffered saline with 250 mM arsenate.
Effect of CCCP and amiloride on motility
The presence of membrane potential has been reported in a variety of mycoplasma species (Benyoucef et al., 1981; Schiefer & Schummer, 1982). To determine whether PMF supplies the energy needed for M. penetrans gliding motility, we observed motility in the presence of the ionophore CCCP, which collapses the proton gradient. Cells were incubated for 1 h in the presence of 10 mM CCCP in DMSO and in PBS-G2K containing the same volume of DMSO used in the test buffer. After 1 h, gliding speed actually increased by 29% compared to the control buffer (p<0.0001) (Figure 2), ruling out PMF as an energy source for gliding motility of M. penetrans. To test SMF as a potential energy source for M. penetrans gliding, cells were observed in the presence of amiloride, an inhibitor of Na+/H+ antiporters and sodium channels which competes with Na+ in the medium (Benos, 1982). M. penetrans gliding speed was not significantly affected by 1 h of incubation in amiloride (p=0.6) (Figure 2), ruling out SMF as an energy source.
Effects of pH and temperature on gliding motility
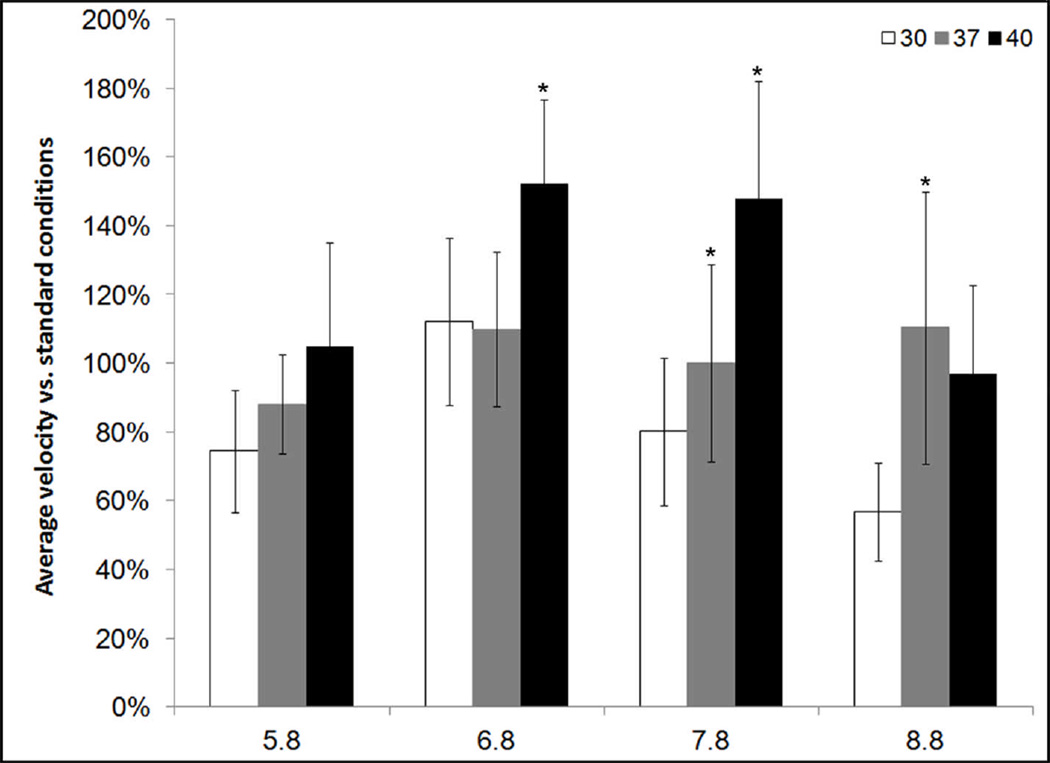
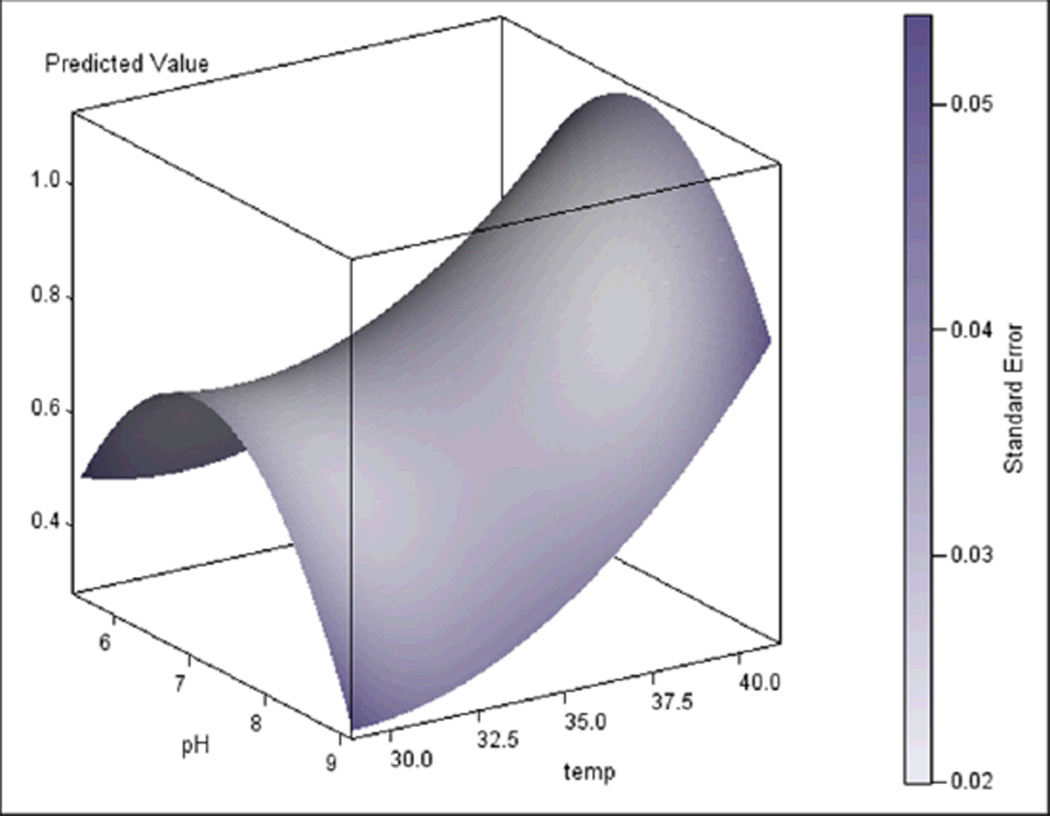
To determine the role of thermal energy in the motility mechanism of M. penetrans, we analysed its gliding speed under conditions of differing temperature. If radiant energy from ambient heat is a significant power source, then we would predict increased speed even at temperatures in excess of those normally encountered physiologically. We analysed gliding speed at temperatures ranging from 30°C to 40°C and pH levels ranging from 5.8 to 8.8 (Figure 3). Speed increased with temperature, but at acidic or alkaline pH, the trend was less distinct. Additionally, speed was greater closer to neutral pH. The interaction between temperature and pH was significant [F(6,283)=989, p<0.0001], suggesting that the effects of temperature depend on the pH. To determine the temperature and pH parameters for maximal speed, a statistical response surface model was fitted to the data obtained from the temperature and pH assays, along with accompanying canonical analysis (Figure 4). There were highly significant linear and curvilinear effects, as well as a marginally significant interaction effect of both temperature and pH, and both were found to be significant contributors to gliding speed. The surface model revealed a rising ridge along the temperature gradient, suggesting that maximal speed occurs at a temperature higher than 40°C. Ridge analysis suggested that maximal speed was well maintained near neutral pH levels, and was found on a strongly linear trajectory in increasing temperature. At 45°C almost no cells adhered, marking 40°C as an upper limit to the experiment. These data suggest that thermal energy is limiting for gliding speed as long as the adherence and motility machinery is capable of functioning.
Figure 3.
M. penetrans relative gliding speed under conditions of varying temperature and pH. Values were normalized to mean gliding speed at 37°C and pH 7.8. Asterisks indicate significant differences from the next lower temperature at the same pH (p<0.0001) as determined by two-factor factorial analysis of variance.
Figure 4.
Fitted response surface of M. penetrans motility generated by the temperature and pH motility experiment.
DISCUSSION
Energy source for M. penetrans gliding motility
The molecular mechanism of M. penetrans gliding motility is unknown and no homologues of known motility proteins in the better-characterized species, M. pneumoniae and M. mobile, are present. In an effort to identify the energy source used to power gliding, the motility behavior of M. penetrans was observed in the presence of chemical inhibitors previously used to characterized motility energetics in other species of mycoplasmas and bacteria. Arsenate did not have the same degree of impact on M. penetrans gliding as it did on M. mobile, with a much smaller reduction in speed. Furthermore, M. penetrans cells were still able to glide well after 8 h in the presence of arsenate, and at concentrations fivefold greater than those tested for M. mobile, both of which are conditions under which ATP is nearly completely depleted through inhibition of the reactions catalysed by glyceraldehyde 3-phosphate dehydrogenase (Warburg & Christian, 1939) and ornithine carbamoyltransferase (Knivett, 1954). As mycoplasma membrane ATP synthase actually operates in reverse to maintain a proton gradient functioning in sodium extrusion and cell volume maintenance (Linker & Wilson, 1985), and is therefore not involved in ATP synthesis, it is overwhelmingly likely that ATP is depleted under our experimental conditions, which include incubation in 25 times the concentration of arsenate that prevents growth. These data suggest that ATP hydrolysis is at best an indirect source of energy for motility in M. penetrans, perhaps only providing the energy necessary to replenish less stable molecular components of the motor and/or to maintain these components, such as by phosphorylation, which is essential for normal function of motility-associated proteins in M. pneumoniae (Schmidl et al., 2010). Furthermore, neither a PMF inhibitor nor an SMF inhibitor decreased gliding speed. We therefore could not identify a convincing source of chemically derived energy for gliding.
Temperature and pH effects on gliding motility
To examine the possibility of a thermal component to the energy source for gliding, motility was observed under different temperature and pH conditions. We found that at any tested temperature, the pH optimum was between 6.8 and 7.8, although even at pH of both 5.8 and 8.8 the gliding speed was still substantially greater than the previously reported speed for strain HF-2 (Jurkovic et al., 2012). At near-neutral pH, there was a clear increase in gliding speed with increasing temperature, even though normal physiological temperature was exceeded at 40°C. Therefore, near neutral pH, there is a linear relationship between temperature and motility speed. These data suggest that thermal energy is a substantive energy source for M. penetrans gliding motility, whereas a chemical energy source typically observed for bacterial motility was not identified. Given the role of gliding in M. penetrans cell division (Jurkovic et al., 2012), it is conceivable that the difference in gliding speed between strains GTU-54-6A1, isolated from the urine, and HF-2, isolated from the respiratory tract, is attributable to selection for sufficient speed at the lower pH of the urogenital tract environment.
Mechanism of M. penetrans gliding motility
Two models have been proposed for gliding motility in M. mobile and M. pneumoniae, the centipede and inchworm model, respectively (Miyata, 2010). In the better elucidated centipede model, adhesins reversibly bind substrate in a manner dependent upon ATP hydrolysis. There is no direct evidence in support of a particular motility model in M. pneumoniae, but the inchworm model has been proposed based on electron cryotomography data. In this model, flexing of the cytoskeleton within the attachment organelle causes the displacement and association of adhesins to the cell surface, moving the cell forward (Henderson & Jensen, 2006). Although it remains unclear whether either of these occurs in M. penetrans, our data indicate that the mechanism of motility has an important thermal component. M. mobile speed also correlates positively with temperature (Miyata & Uenoyama, 2002), but in that organism ATP hydrolysis is absolutely required for movement (Jaffe et al., 2004; Uenoyama et al., 2005), unlike in M. penetrans. If M. penetrans gliding motility is in fact driven by a Brownian ratchet mechanism that converts thermal energy into forward movement, then this is unique among prokaryotes, and suggests the existence of a yet uncharacterized cytoskeletal component capable of polarized polymerization and depolymerization. Further investigation of the structure and composition of the M. penetrans motor is warranted.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (Public Health Service grant R15 AI073994). We gratefully acknowledge the assistance of G. Huang with the statistical analysis, and thank D.C. Krause for helpful comments.
REFERENCES
- Benos DJ. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982;242:131–145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Benyoucef M, Rigaud JL, Leblanc G. The electrochecmial proton gradient in mycoplasma cells. Eur J Biochem. 1981;113:491–498. doi: 10.1111/j.1432-1033.1981.tb05090.x. [DOI] [PubMed] [Google Scholar]
- Berg HC, Anderson RA. Bacteria swim by rotating their flagellar filaments. Nature. 1973;19:380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Burrows LL. Weapons of mass retraction. Mol Microbiol. 2005;57:878–888. doi: 10.1111/j.1365-2958.2005.04703.x. [DOI] [PubMed] [Google Scholar]
- Feng SH, Lo SC. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect Immun. 1994;62:3916–3921. doi: 10.1128/iai.62.9.3916-3921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchel JM, Balish RS, Duley ML, Balish MF. Ultrastructure and gliding motility of Mycoplasma amphoriforme, a possible human respiratory pathogen. Microbiology. 2006;152:2181–2189. doi: 10.1099/mic.0.28905-0. [DOI] [PubMed] [Google Scholar]
- Henderson GP, Jensen GJ. Three-dimensional structure of Mycoplasma pneumoniae’s attachment organelle and a model for its role in gliding motility. Mol Microbiol. 2006;60:376–385. doi: 10.1111/j.1365-2958.2006.05113.x. [DOI] [PubMed] [Google Scholar]
- Jaffe JD, Miyata M, Berg HC. Energetics of gliding motility in Mycoplasma mobile. J Bacteriol. 2004;186:4254–4261. doi: 10.1128/JB.186.13.4254-4261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan JL, Chang HY, Balish MF, Holt LS, Bose SR, Hasselbring BM, Waldo RH, III, Krunkosky TM, Krause DC. Protein P200 is dispensable for Mycoplasma pneumoniae hemadsorption but not gliding motility or colonization of differentiated bronchial epithelium. Infect Immun. 2007;75:518–522. doi: 10.1128/IAI.01344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkovic DA, Newman JT, Balish MF. Conserved terminal organelle morphology and function in Mycoplasma penetrans and Mycoplasma iowae. J Bacteriol. 2012;194:2877–2883. doi: 10.1128/JB.00060-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H, Rosengarten R. Isolation of motile mycoplasma from fish. J Gen Microbiol. 1984;130:2439–2445. doi: 10.1099/00221287-130-9-2439. [DOI] [PubMed] [Google Scholar]
- Knivett VA. The effect of arsenate on bacterial citrulline breakdown. Biochem J. 1954;56:606–610. doi: 10.1042/bj0560606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Yamamoto K, Kawagishi I, Homma M. The polar flagellar motor of Vibrio cholerae is driven by a Na+ motive force. J Bacteriol. 1999;181:1927–1930. doi: 10.1128/jb.181.6.1927-1930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C, Wilson TH. Sodium and proton transport in Mycoplasma gallisepticum. J Bacteriol. 1985;163:1250–1257. doi: 10.1128/jb.163.3.1250-1257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McBride M, Subramaniam S. Cell surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J Bacteriol. 2007;189:7503–7506. doi: 10.1128/JB.00957-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SC, Hayes NM, Tully JG, Wang RY, Kotani H, Pierce PF, Rose DL, Shih JW. Mycoplasma penetrans sp. nov., from the urogenital tract of patients with AIDS. Int J Syst Bacteriol. 1992;42:357–364. doi: 10.1099/00207713-42-3-357. [DOI] [PubMed] [Google Scholar]
- Lo SC, Hayes MM, Wang RY, Pierce PF, Kotani H, Shih JW. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415–1418. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- Luciano J, Agrebi R, LeGall AV, Wartel M, Fiegna F Ducret A, Brochier-Armanet C, Mignot T. Emergence of modular evolution of a novel motility machinery in bacteria. PLoS Genet. 2001;7:e1002268. doi: 10.1371/journal.pgen.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MJ. Cytophaga-Flavobacterium gliding motility. J Mol Microbiol Biotechnol. 2004;7:63–71. doi: 10.1159/000077870. [DOI] [PubMed] [Google Scholar]
- McCarter LL. Dual flagellar systems enable motility under different circumstances. J Mol Microbiol Biotechnol. 2004;7:18–29. doi: 10.1159/000077866. [DOI] [PubMed] [Google Scholar]
- Miyata M, Uenoyama A. Movement on the cell surface of the gliding bacterium, Mycoplasma mobile, is limited to its head-like structure. FEMS Microbiol Lett. 2002;215:285–289. doi: 10.1111/j.1574-6968.2002.tb11404.x. [DOI] [PubMed] [Google Scholar]
- Miyata M. Unique centipede mechanism of Mycoplasma gliding. Annu Rev Microbiol. 2010;64:519–537. doi: 10.1146/annurev.micro.112408.134116. [DOI] [PubMed] [Google Scholar]
- Nan B, Mauriello EMF, Sun I, Wong A, Zusman DR. A multi-protein complex from Mycococcus xanthus required for bacterial gliding motility. Mol Microbiol. 2010;76:1539–1554. doi: 10.1111/j.1365-2958.2010.07184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S, Yogev D, Naot A. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relich RF, Friedberg AJ, Balish MF. Novel cellular organization in a gliding mycoplasma, Mycoplasma insons. J Bacteriol. 2009;191:5312–5314. doi: 10.1128/JB.00474-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BP. Biochemistry of arsenic detoxification. FEBS Lett. 2002;529:86–92. doi: 10.1016/s0014-5793(02)03186-1. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Blanchard A, Watson HL, Garcia S, Dulioust A, Montagnier L, Gougeon ML. In vitro influence of Mycoplasma penetrans on activation of peripheral T lymphocytes from health donors or human immunodeficiency virus-infected individuals. Infect Immun. 1995;63:4277–4283. doi: 10.1128/iai.63.11.4277-4283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Ishikawa J, Yamashita A, Oshima K, Kenri T, Furuya K, Yoshino C, Horino A, Shiba T, Sasaki T, Hattori M. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 2002;23:5293–5230. doi: 10.1093/nar/gkf667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer HG, Schummer U. The electrochemical potential across mycoplasmal membranes. Rev Infect Dis. 1982;4:S65–S70. doi: 10.1093/clinids/4.supplement_1.s65. [DOI] [PubMed] [Google Scholar]
- Schmidl SR, Gronau K, Hames C, Busse J, Becher D, Hecker M, Stülke J. The stability of cytadherence proteins in Mycoplasma pneumoniae requires activity of the protein kinase PrkC. Infect Immun. 2010;78:184–192. doi: 10.1128/IAI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kida Y, Kuwano K. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology. 2004;113:121–129. doi: 10.1111/j.1365-2567.2004.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Wartel M, Cascales E, Shaevitz JW, Mignot T. Motor-driven intracellular transport powers bacterial gliding motility. Proc Natl Acad Sci USA. 2011;18:7559–7564. doi: 10.1073/pnas.1101101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanek SM, Majumder S, Sheppard ES, Liao X, Rood D, Tulman ER, Wyand S, Krause DC, Silbart LK, Geary SJ. Vaccination of BALB/c mice with an avirulent Mycoplasma pneumoniae P30 mutant results in disease exacerbation upon challenge with a virulent strain. Infect Immun. 2012;80:1007–1014. doi: 10.1128/IAI.06078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully JG, Rose DL, Whitcomb RF, Wenzel RP. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified cultured medium. J Infect Dis. 1979;139:478–482. doi: 10.1093/infdis/139.4.478. [DOI] [PubMed] [Google Scholar]
- Uenoyama A, Kusumoto A, Miyata M. Identification of a 349-kilodalton protein (Gli349) responsible for cytadherence and glass binding during gliding of Mycoplasma mobile. J Bacteriol. 2004;186:1537–1545. doi: 10.1128/JB.186.5.1537-1545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Shih JW, Grandinetti T, Pierce PF, Hayes MM, Wear DJ, Alter HJ, Lo SC. High frequency of antibodies to Mycoplasma penetrans in HIV-infected patients. Lancet. 1992;340:1312–1316. doi: 10.1016/0140-6736(92)92493-y. [DOI] [PubMed] [Google Scholar]
- Warburg O, Christian W. Isolierung und Krystallisation des Proteins des oxydierenden Garungsferments. Biochem Z. 1939;303:40–68. [Google Scholar]