Abstract
OBJECTIVES
Angiotensin-converting enzyme (ACE) inhibitor and statin medications may preserve skeletal muscle. We examined associations between each medication class and baseline and mean annual change in physical performance measures and muscle strength in older women.
DESIGN
Prospective cohort study
PARTICIPANTS
Participants from the Women’s Health Initiative Clinical Trials who were aged 65–79 at baseline and had physical performance measures, self-report of health insurance and no prior history of stroke or congestive heart failure were included (n=5777). Women were recruited between 1993 and 1998.
MEASUREMENTS
Medication use was ascertained through a baseline inventory. Physical performance measures (timed 6-meter walk, repeated chair stands in 15 seconds) and grip strength were assessed at baseline and follow-up years 1, 3 and 6. Multivariable adjusted linear repeated- measures models adjusted for demographic and health characteristics.
RESULTS
ACE inhibitor use was negatively associated with mean grip strength at baseline (22.40 kg, 95% confidence interval [CI] 21.89, 22.91 versus 23.18 kg, 95% CI 23.02, 23.34; P = .005) and a greater mean annual change in number of chair stands (−.182, 95% CI −.217, −.147 versus −.145, 95% CI −.156, −.133; P = .05) compared to non-use. Statin use was not significantly associated with baseline or mean annual change for any outcome. A subgroup analysis suggested that statin use was associated with less mean annual change in chair stands (P = .006) in the oldest women.
CONCLUSION
These results do not support an association of statin or ACE inhibitor use with slower decline in physical performance or muscle strength, and thus do not support the use of these medications for preserving functional status in older adults.
Keywords: ACE inhibitors, statins, physical performance, grip strength
INTRODUCTION
Maintaining adequate physical function is important for older adults to continue independent living in the community. An objective of Healthy People 2020 is to “reduce the proportion of older adults who have moderate to severe functional limitations.”1 Performance based measures of functional status, such as timed walk, are useful in identifying individuals at risk for disability.2
Multiple factors appear to be involved in the decline in physical function and development of frailty that occurs with aging.3–5 Of special interest, a growing body of evidence suggests a relationship between chronic inflammation and age-related muscle loss, disability, frailty, low physical function, walking speed, and muscle strength.4–11 Two medication classes, ACE inhibitors and statins, have been identified as potential targets to reduce physical decline with aging.3–5 Although results from studies have been inconsistent, evidence exists to support a reduced risk of these outcomes with ACE inhibitors and statins, particularly in select samples.12–18
It is biologically plausible that these medications may prevent decline in physical function, beyond what might be expected by reducing vascular events. ACE inhibitors may have a direct effect on muscle or may reduce inflammation,3–5 whereas, statins may reduce systemic inflammation as indicated by specific markers (e.g., C-reactive protein [CRP]).19,20 However, it is possible that the benefits that statins may confer by reducing inflammation could be counteracted by the muscle-related adverse events (e.g., myalgia, muscle weakness) that may occur.21, 22
Since most studies to date have been conducted in select samples, it is important to examine this issue in large representative samples. Given this background, our objective was to examine the associations between each medication class and baseline and annual change in lower extremity physical performance measures and muscle strength in women ages 65 and older.
METHODS
Study Sample
This study uses data from the Women’s Health Initiative (WHI) clinical trials of 68,132 women ages 50 to 79 recruited from between 1993 and 1998 from 40 clinical centers in the United States. Women were eligible for study inclusion if they were postmenopausal and unlikely to relocate or die within 3 years. There were additional eligibility criteria specific to each clinical trial for reasons of safety, competing risk and adherence/retention. Further details regarding the design, recruitment strategy, and data collection methods have been published.23 The study was reviewed and approved by human subjects review committees at each participating institution.
The study population for this analysis includes the 25% random sample of clinical trial participants ages 65 and older who completed measures of physical performance (n=6025). Women were excluded from this analysis if they reported baseline congestive heart failure (n=57), history of stroke (n=98) or no health insurance (N =100), leaving an analytic sample of 5777 participants.
Outcomes: Physical Performance Measures and Muscle Strength
The three outcomes were assessed at baseline and at follow-up years 1, 3 and 6 by trained and certified staff using standard protocols. Timed walk and repeated chair stands were the measures of lower extremity physical performance assessed which represent two of three items of the Short Portable Performance Battery (SPPB).24 Slowed gait speed predicts disability and mortality in older adults.2, 25, 26 The 6 meter timed walk was performed at usual walking speed with use of ambulatory aids as needed. The test was repeated for a second trial, and the results were recorded as the mean number of seconds. The chair-stand test was conducted if the participant was able to stand at least once without using hands or arms from a straight-backed, nonpadded, flat-seated, armless chair. Two 15-second trials of repeated chair stands were performed with arms folded across the chest with a 1- to 2-minute rest in between trials and results were averaged.
Hand grip strength was measured using a handheld dynamometer (Jamar hand dynamometer; Lafayette Instruments, Lafayette, IN). Low grip strength is a predictor of disability, mortality and other poor outcomes in older adults.27 Two measurements were made in the dominant hand with staff coaching for maximal performance and the mean of two trials was used.
ACE Inibitor and Statin Medication Ascertainment
WHI participants were asked to bring all medications taken on a regular basis in the past two weeks to their first screening interview. Trained clinic interviewers entered each medication name and strength from the containers directly into a database that assigned drug codes using Medi-Span software that was updated quarterly (First DataBank, Inc., San Bruno, CA). Women reported duration of use for each current medication. A woman was categorized as either a user or non-user of a statin (lovastatin, simvastatin, pravastatin, atorvastatin, fluvastatin) and/or ACE inhibitor (enalapril, benazapril, quinapril, ramipril, fosinopril, trandolapril, captopril) based on the medication inventory at screening. Duration of use was categorized as < 2 years, 2–5 years, or ≥ 5 years. Information was available on tablet strength but not on the prescribed dose.
Other Covariates
Data on demographic and health behavior characteristics (body mass index, smoking, alcohol use, leisure-time physical activity) were obtained at baseline. Body mass index (BMI) was calculated using measured height and weight as weight (kg) divided by height squared (m2). Alcohol consumption was estimated from a food-frequency questionnaire. Physical activity energy expenditure was calculated from self-reported recreational physical activity including walking, mild, moderate and strenuous physical activity (metabolic equivalent score [MET]-hours/wk).28 Medical conditions at baseline included self-reported physician diagnoses of treated diabetes (oral medication or insulin) and hypertension (on hypertensive medication and/or blood pressure > 140/90 mmHg). History of coronary heart disease (CHD) was based on a self-reported physician diagnosis of myocardial infarction, angina, coronary artery bypass graft or percutaneous transluminal coronary angioplasty. Depressive symptoms were assessed by a 6-item short form29, 30 of the Center for Epidemiologic Studies Depression Scale. Physical function was measured by the Rand-36 physical function scale (range 0–100), with higher scores indicating better physical function.31 Baseline medications used for hypertension other than ACE inhibitors (e.g. calcium channel blockers, beta-blockers, and diuretics), nonsteroidal anti-inflammatory drugs (NSAIDS), and menopausal hormone therapy were also ascertained.
Statistical Analysis
Baseline characteristics were compared for women according to use of statins or ACE inhibitors using chi-square tests for association for categorical variables and t-tests for continuous variables. Each exposure was examined in separate analyses. Multivariable adjusted linear repeated- measures models with an unstructured covariance matrix were used to examine the longitudinal association between each exposure and outcomes (physical performance measures and grip strength). To account for data that were likely not missing at random, values corresponding to the bottom 1% at each visit year for each measure were assigned to participants that attended their annual visit, but could not complete, refused, or did not attempt the task due to safety or health concerns. The percentage of data missing for these reasons was 1.3%, 2.7%, and 7.7% for the timed walk, grip strength, and chair stands, respectively.32 The models examine whether the mean scores on these outcome measures of exposure groups differ at baseline (P-intercept) or differ with respect to mean annual change over time (P-slope). The reasonableness of these linear fits was confirmed by comparing these estimates to results obtained by treating time as a categorical variable. To control for confounding, models were adjusted for age, ethnicity, education, BMI, alcohol consumption, systolic and diastolic blood pressure, self-reported health, number of antihypertensive medications, diabetes, depressive symptoms, history of CHD and hormone trial participation. Sensitivity analyses also included additional adjustment for baseline activity level by quartiles of MET-hrs/wk and baseline use of NSAIDs. Interactions with each exposure and age at baseline were examined. Additional analyses examined whether duration of medication use at baseline was associated with baseline and mean annual change in outcomes. Parameter estimates, 95% confidence intervals (CI), and two sided p-values were obtained using SAS PROC MIXED version 9.2 (SAS Institute, Cary, NC). Presentation of these summary statistics was graphed in R (version 2.11; R Development Core Team (2010) - http://www.R-project.org).
Several additional sensitivity analyses were conducted to examine the robustness of results and further examine confounding by indication. First we examined each exposure as a time varying covariate by updating exposure at year 3. We examined the interaction between exposure and Rand-36 physical function scale (tertiles: <75, 75 to 90, ≥90). For the ACE inhibitor analysis, we restricted the sample to those with hypertension. We also examined the interaction between current ACE inhibitor and statin use by testing the significance of cross-product terms.
RESULTS
Women were followed on average for 7.5 years (±SD 1.5) through the planned study closeout in Spring 2005. At that time 3.5% (N=202) of our sample had withdrawn or were lost to follow-up and 7.8% (N=450) of our sample had died. A description of the study sample at baseline is given in table 1. At baseline, 9.3% (N=539) of participants were current users of statins and of these women, 31% were users for a duration of between 2 to 5 years and 15.0% were users for more than 5 years. Likewise, 10.4 % (N=600) of participants were current users of ACE inhibitors and of these women 32.5% were users for a duration of between 2 to 5 years and 33.5% were users for more than 5 years. Concurrent use of both agents was reported by 83 (1.4%) women. Of those using an ACE inhibitor or statin at baseline, 72% and 82% were still using these respective medications at the year 3 visit. Physical performance measures were available at all four visits on 66.1% (N=3818) of participants, three visits on 20.6% (N=1187), two visits 8.9% (N=516), and available on a single visit for 4.4% (N=256) participants.
Table 1.
| Statin Use | P Value | ACE Inhibitor Use | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||
| Age, mean (±SD) | 70.0 | (3.6) | 69.8 | (3.7) | 0.21 | 70.1 | (3.7) | 69.8 | (3.7) | 0.04 |
| Education | 0.28 | 0.02 | ||||||||
| ≤ High school/GED or less | 138 | 25.8 | 1326 | 25.4 | 167 | 28.1 | 1297 | 25.2 | ||
| School after high school | 231 | 43.3 | 2105 | 40.4 | 256 | 43.0 | 2080 | 40.4 | ||
| College degree or higher | 165 | 30.9 | 1782 | 34.2 | 172 | 28.9 | 1775 | 34.5 | ||
| Race/ethnicity | 0.04 | 0.25 | ||||||||
| White | 452 | 83.9 | 4535 | 86.6 | 506 | 84.3 | 4481 | 86.6 | ||
| Black | 46 | 8.5 | 387 | 7.4 | 60 | 10.0 | 373 | 7.2 | ||
| Hispanic | 9 | 1.7 | 133 | 2.5 | 14 | 2.3 | 128 | 2.5 | ||
| American Indian | 1 | 0.2 | 11 | 0.2 | 1 | 0.2 | 11 | 0.2 | ||
| Asian/Pacific Islander | 21 | 3.9 | 111 | 2.1 | 11 | 1.8 | 121 | 2.3 | ||
| Unknown | 10 | 1.9 | 61 | 1.2 | 8 | 1.3 | 63 | 1.2 | ||
| Living alone | 175 | 32.6 | 1579 | 30.4 | 0.28 | 177 | 29.8 | 1577 | 30.7 | 0.66 |
| BMI, mean (±SD) | 28.9 | (5.6) | 28.5 | (5.6) | 0.09 | 30.6 | (6.3) | 28.3 | (5.5) | <0.001 |
| Smoking status | 0.12 | 0.40 | ||||||||
| Never | 268 | 50.9 | 2855 | 55.3 | 322 | 54.2 | 2801 | 55.0 | ||
| Past | 231 | 43.8 | 2029 | 39.3 | 246 | 41.4 | 2014 | 39.5 | ||
| Current | 28 | 5.3 | 279 | 5.4 | 26 | 4.4 | 281 | 5.5 | ||
| Alcohol consumption | 0.71 | <0.001 | ||||||||
| Non drinker | 245 | 45.6 | 2302 | 44.1 | 313 | 52.2 | 2234 | 43.3 | ||
| ≤ 1 drink/day | 233 | 43.4 | 2365 | 45.3 | 228 | 38.0 | 2370 | 45.9 | ||
| > 1 drink/day | 59 | 11.0 | 557 | 10.7 | 59 | 9.8 | 557 | 10.8 | ||
| Physical Activity (MET-hours per week), mean (±SD) | 11.3 | (12.4) | 11.4 | (12.8) | 0.90 | 9.1 | (10.5) | 11.6 | (13.0) | <0.001 |
| Self-reported health | <0.001 | <0.001 | ||||||||
| Excellent | 45 | 8.4 | 779 | 15.0 | 23 | 3.8 | 801 | 15.6 | ||
| Very good | 197 | 36.7 | 2178 | 41.8 | 212 | 35.5 | 2163 | 42.0 | ||
| Good | 238 | 44.3 | 1828 | 35.1 | 284 | 47.5 | 1782 | 34.6 | ||
| Fair/poor | 57 | 10.6 | 424 | 8.1 | 79 | 13.2 | 402 | 7.8 | ||
| Treated diabetes (pills or shots) | 50 | 9.3 | 258 | 4.9 | <0.001 | 70 | 11.7 | 238 | 4.6 | <0.001 |
| Hypertension | 334 | 62.3 | 2730 | 52.5 | <0.001 | 578 | 97.6 | 2486 | 48.3 | <0.001 |
| History of coronary heart diseasec | 106 | 20.0 | 377 | 7.3 | <0.001 | 95 | 16.4 | 388 | 7.6 | <0.001 |
| No. of Depressive symptoms | 0.73 | 0.02 | ||||||||
| 0 | 145 | 27.3 | 1307 | 25.4 | 135 | 22.8 | 1317 | 25.9 | ||
| 1–2 | 197 | 37.0 | 2021 | 39.3 | 252 | 42.6 | 1966 | 38.7 | ||
| 3–4 | 121 | 22.7 | 1162 | 22.6 | 115 | 19.5 | 1168 | 23.0 | ||
| 5+ | 69 | 13.0 | 655 | 12.7 | 89 | 15.1 | 635 | 12.5 | ||
| Blood pressure | ||||||||||
| Systolic (mm Hg), mean (±SD) | 133.4 | (18.1) | 132.0 | (17.3) | 0.07 | 139.7 | (18.1) | 131.3 | (17.1) | <0.001 |
| Diastolic (mm Hg), mean (±SD) | 75.1 | (9.6) | 74.8 | (9.1) | 0.49 | 77.1 | (10.1) | 74.6 | (9.0) | <0.001 |
| HRT use status | 0.73 | 0.18 | ||||||||
| Never used | 297 | 55.2 | 2819 | 53.8 | 303 | 50.5 | 2813 | 54.4 | ||
| Past user | 109 | 20.3 | 1052 | 20.1 | 126 | 21.0 | 1035 | 20.0 | ||
| Current user | 132 | 24.5 | 1365 | 26.1 | 171 | 28.5 | 1326 | 25.6 | ||
| No. of antihypertensive medications | <0.001 | <0.001 | ||||||||
| 0 | 238 | 44.2 | 3437 | 65.6 | 0 | 0.0 | 3675 | 71.0 | ||
| 1 | 180 | 33.4 | 1176 | 22.5 | 296 | 49.3 | 1060 | 20.5 | ||
| 2 | 97 | 18.0 | 522 | 10.0 | 229 | 38.2 | 390 | 7.5 | ||
| 3+ | 24 | 4.5 | 103 | 2.0 | 75 | 12.5 | 52 | 1.0 | ||
ACE= Angiotensin-Converting Enzyme
Results expressed as number (percent) unless indicated otherwise
P value based on a chi-squared test of association for categorical variables and t-test for continuous variables.
Myocardial infarction, angina, coronary artery bypass graft, percutaneous transluminal coronary angioplasty
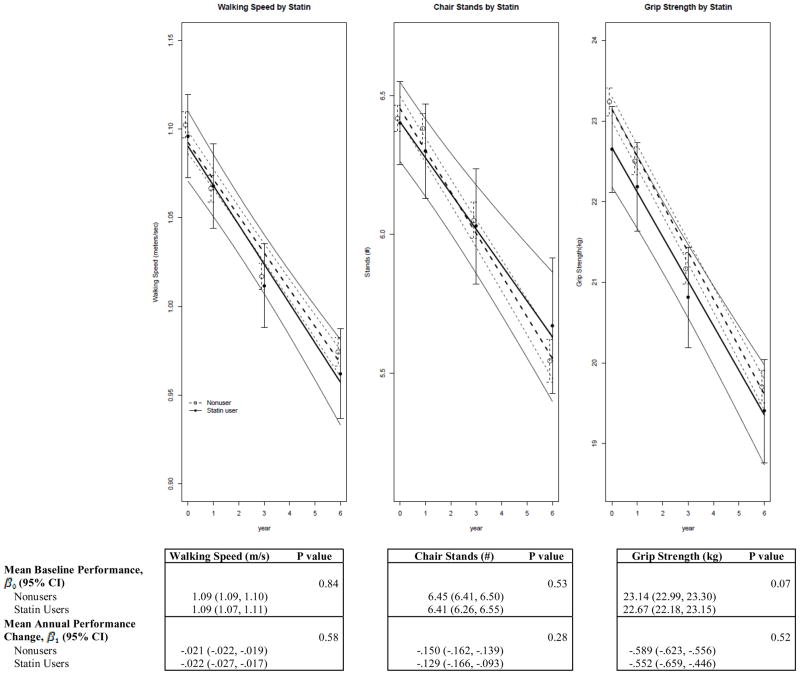
Figure 1 shows the trajectory of each outcome according to baseline statin use adjusted for covariates. There were no differences in baseline walking speed, chair stands or grip strength (P-intercept .84, .53, .07 respectively) or mean annual change (P-slope .58, .28, .52 respectively) between statin users and nonusers. The relationship between the duration of statin use and each outcome were not statistically significant. We next examined the interaction between and age and statin use for physical performance measures and grip strength. At baseline, walking speed was the only outcome in which a significant interaction was found between age and statin use (P-trend-intercept = .01). Baseline walking speed was similar among statin users, regardless of age; mean (95% confidence interval [CI]) = 1.09 (1.06, 1.13), 1.09 (1.06, 1.13), and 1.08 (1.04, 1.11) meters/second by increasing age groups (65–67 years, 68–71 years, and 72–79 years respectively). However, baseline walking speed was negatively associated with age in statin nonusers; mean (95% CI) = 1.13 (1.12, 1.14), 1.10 (1.09, 1.11) and 1.05 (1.03, 1.06) meters/second for increasing age groups. When examining mean annual change, chair stands was the only outcome in which an interaction between age and statin use was found (P-trend- slope .006). The mean annual change (decline) in the number of chairs stands performed was relatively constant across increasing age groups for statin users, with mean (95% CI) values of −0.157 (−0.221, −0.093), −0.124 (−0.182, −0.066), −0.105 (−0.173, −0.037) by increasing age groups. However, the mean annual change in performance among the oldest statin non-users was nearly twice that of youngest non-users; mean (95% CI) = −0.117 (−0.137, −0.098), −0.139 (−0.158, −0.120), and −0.204 (−0.226, −0.183). Age did not modify the association of statin use on baseline or mean annual change in grip strength.
Figure 1.
Multivariable-Adjusted Linear Repeated Measures Analyses of Physical Performance Measures and Grip Strength by Baseline Statin Use
Linear estimate and 95%CI (solid and dashed lines) from a multivariable adjusted linear repeated measures model. Models were adjusted for age, ethnicity, education, BMI, alcohol consumption, systolic blood pressure, diastolic blood pressure, self-reported health, number of antihypertensive medications, diabetes, depressive symptoms, history of CHD, hormone trial randomization, and ACE use. The minimum sample size (baseline, year 1, year 3, year 6) for three outcome measures was n= (496, 436, 419,377) for statin users and n= (4852, 4243, 4189, 3768) for non-users.
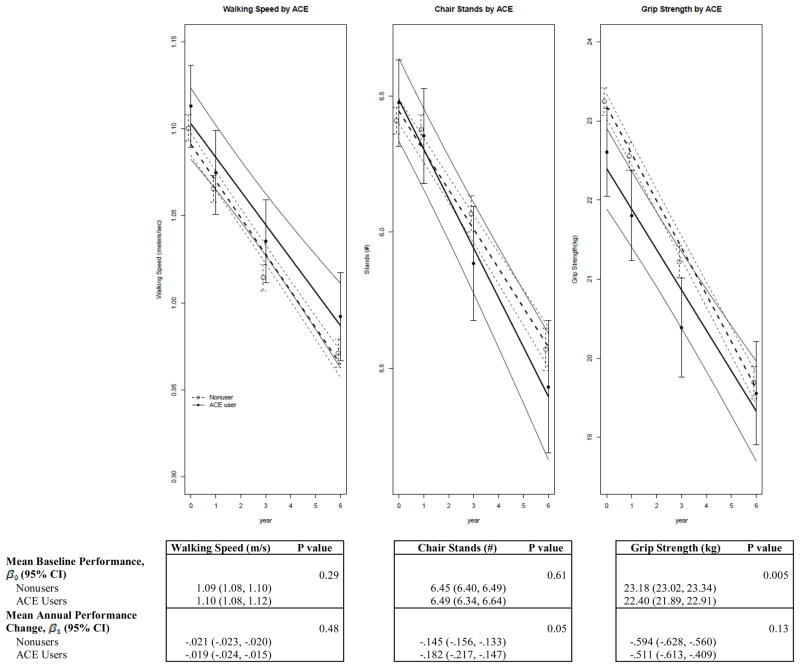
Figure 2 shows the trajectory of each outcome according to baseline ACE inhibitor use adjusted for covariates. There were no differences in baseline walking speed or mean annual change in performance between users and nonusers of ACE inhibitors. For chair stands, there was not a difference in baseline performance among users and nonusers (P-intercept= .61); however, there was suggestion of a greater annual decline in chair stand performance among users (P-slope =.05). ACE inhibitor use was associated with a reduced grip strength at baseline (P-intercept =.005). Similar results were obtained when linearity was not assumed and year was modeled as a categorical variable (P=.03). There was no difference in mean annual change in grip strength over time (P-slope= .13). When examining mean annual change according to duration of use, longer duration of ACE inhibitor use was not associated with better performance for any outcome. The interactions between age and ACE inhibitor use were not significant for any outcome.
Figure 2.
Multivariable-Adjusted Linear Repeated Measures Analyses of Physical Performance Measures and Grip Strength by Baseline Angiotensin-Converting Enzyme (ACE) Inhibitor Use
Linear estimate and 95%CI (solid and dashed lines) from a multivariable adjusted linear repeated measures model. Models were adjusted for age, ethnicity, education, BMI, alcohol consumption, systolic blood pressure, diastolic blood pressure, self-reported health, number of antihypertensive medications, diabetes, depressive symptoms, history of CHD, hormone trial randomization, and statin use. The minimum sample size (baseline, year 1, year 3, year 6) for the three outcome measures was n= (551, 477, 460, 410) for ACE users and n= (4797, 4201, 4148, 3734) for non-users.
Sensitivity Analyses
Models adjusting for baseline activity level by quartiles of MET-hrs/wk or baseline use of NSAIDs produced similar estimates to those derived from the primary analyses. Results similar to the primary analyses were obtained when statin and ACE inhibitor use were modeled as time-varying exposures by updating exposure at year 3. No significant associations were observed between statin use and each outcome. While the strength of the association between ACE inhibitor use and baseline grip strength was attenuated, the result was still statistically significant (P value intercept changed from .005 to .04). The association between ACE inhibitor use and mean annual change in chair stand performance was strengthened with non-users experiencing less decline compared with users (P value intercept changed from .05 to .006). We examined the interaction between each exposure and physical functioning subgroups as measured by the Rand-36 physical function scale (tertiles: <75, 75 90, >=90). Neither statin nor ACE inhibitor use interacted with baseline physical functioning. For statins, tests of trend for both regression parameters yielded P values > .20. For ACE inhibitors, tests of trend for both regression parameters yielded P values > .14. There was not a significant interaction between current statin and ACE inhibitor use with any outcome (all P values > .18). Lastly, similar results were obtained with ACE inhibitor use and each outcome when restricting the sample to those with hypertension; an attempt to examine confounding by indication.
DISCUSSION
In this large prospective study in older women with an average of 7.5 years of follow-up, we did not find a consistent association between statin or ACE inhibitor use and two measures of lower extremity physical performance or grip strength. A major contribution of this study is the examination of a clinically relevant performance based measure of physical function (i.e. gait speed) in a large representative sample of older women. An advantage of performance based measures over self-reported functional status (e.g. mobility disability33) is the ability to examine relationships between medication use and physical function earlier on the disablement continuum. Thus, our results provide additional information to a growing body of literature suggesting that these medications may not be beneficial for slowing age-related decline in physical performance.
Statins
Statin use was not associated with baseline or mean annual change in physical performance measures or grip strength. Of interest, statin use was associated with less decline in performance on chair stands in the oldest women, suggesting that some aspect of health status or exposure in this group is overshadowing the influence of age. However, this finding should be viewed as preliminary and requires confirmation. Statin users had a slightly better performance on timed chair stands compared to nonusers in a one-year longitudinal study in older men (0.5 seconds, P=.04).18 Additional data supporting statin medications and positive function-related outcomes have come from small randomized trials15, 34 and a longitudinal study13 in patients with peripheral arterial disease. In fact, Giri et al. did not find an association between statin use and functional decline in those without peripheral artery disease.13 Our overall results are consistent with studies conducted in more representative sample.33, 35–37 Large observational studies found that statin use was not related to lower incidence of frailty in post-menopausal women,36 self-reported mobility disability,33 or a decline in lower extremity muscle strength.37
Several potential explanations may explain these discrepant findings. First, the positive associations between statins and physical functioning in those with peripheral arterial disease may be due to improved endothelial function resulting in enhanced lower extremity blood flow13 rather than a reduction in inflammation-mediated sarcopenia. Second, use of statin medications is associated with dose-related muscle complaints; these adverse events could negate any positive association with physical performance due to reduction in inflammation. Muscle adverse events may occur in up to 10% of those receiving high-dose treatment,38 however precise estimates may not be known for older frail adults. When examining the association of statins with physical performance measures in a population study, such as ours, average population estimates are obtained and potential beneficial associations in subgroups could be masked. It is encouraging that there is no evidence from this study that statin use is associated with deteriorating performance. However, it is possible that those who experience statin-related muscle adverse events discontinue therapy before the long-term consequence of functional limitations develop, which would not be captured in our study. Information from on an on-going trial examining the effect of high dose atorvastatin on muscle parameters in adults older than 20 may help clarify some of these unanswered questions.39
ACE inhibitors
To our knowledge, this is the first study to report a negative association between ACE inhibitor use and physical performance (e.g. chair stand performance) or muscle strength (e.g. baseline grip strength). Prior studies have reported positive or neutral associations of ACE inhibitor use with physical function measures. The studies most relevant for comparison are those that used performance measures similar to those in the present study, which include two randomized controlled trials and one longitudinal study. A randomized controlled trial in older adults with self-reported functional impairment without heart failure reported that ACE inhibitors increased 6-minute walking distance, a measure of exercise capacity, but had no effect on secondary measures of physical performance that are comparable to the outcomes of our study (sit to stand test, get up and go).40 Likewise, a six month randomized controlled trial also did not find that ACE inhibitor treatment improved a well-established measure of physical performance (i.e., the SPPB) and hand grip strength in older adults.41 In contrast to these, ACE inhibitor use was related to less decline in muscle strength and walking speed in older disabled women with hypertension in a longitudinal study.12 Studies conducted in small select samples found that ACE inhibitor use improved walking distance in those with heart failure and peripheral arterial disease.16, 17 improvements speculated to be related to improvements in cardiovascular function. In contrast, results from longitudinal studies in more representative samples have not found associations between ACE inhibitor use with mobility disability, frailty or grip strength.33, 42–44 Given the mixed findings among available studies on the association between ACE inhibitors and physical functioning, and because of the greater decline observed on one performance measure in the present study, we believe that additional research is needed to further clarify these relationships.
Strengths of this study include the prospective design, the range of age in this older well-characterized sample of postmenopausal women, availability of serially obtained standardized physical performance measures, and ability to adjust for a large number of covariates that may be confounders. However this study has certain limitations. Dose of medication was not available and medication adherence was unknown. Lack of dose information is particularly relevant when examining the association between statins and physical performance, where one might expect that the benefit would be limited to lower doses. Furthermore, these healthy women had small average annual declines in gait speed (adjusted average annual decline ranged from −.019 to −.022 m/s), perhaps making it difficult to observe differences according to medication use. To put these findings in perspective, a change in gait speed of 0.05 m/s has been proposed as a small clinically meaningful change.45 Finally, despite the measures we took to control for confounding such as stratification and adjustment, all observational studies of pharmacologic exposures are subject to issues related to confounding by indication. This issue may be particularly relevant for the negative association found for some outcomes and ACE inhibitor use.
CONCLUSION
In summary, in this prospective study of well-functioning older women ACE inhibitor or statin medication use was not related to less decline in physical performance or grip strength. Given the multi-factorial nature of age and disease-related functional decline, modification of one potential factor may not be sufficient to delay decline. Taken together with the existing conflicting results from other investigators, there is paucity of evidence to support using these medications for preserving functional status. Randomized controlled trials in older adults would provide much needed information regarding the potential differential effect of statin dose on measures of muscle strength or physical performance.
Appendix A.
A Comparison of Minimally1 and Fully2 Adjusted Linear Repeated Measures Analyses of Physical Performance Measures and Grip Strength by Baseline Statin Use and Baseline Angiotensin-Converting Enzyme (ACE) Inhibitor Use.
| Walking Speed (m/s) | Chair Stands (#) | Grip Strength (kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimally Adjusted | Fully Adjusted | Minimally Adjusted | Fully Adjusted | Minimally Adjusted | Fully Adjusted | ||||||||
|
| |||||||||||||
| Estimate(95%CI) | P value | Estimate(95%CI) | P value | Estimate(95%CI) | P value | Estimate(95%CI) | P value | Estimate(95%CI) | P value | Estimate(95%CI) | P value | ||
| Statin | Mean Baseline Performance, β0 (95% CI) | 0.16 | 0.84 | 0.08 | 0.53 | 0.01 | 0.07 | ||||||
| Nonusers | 1.09 (1.08, 1.10) | 1.09 (1.09, 1.10) | 6.45 (6.40, 6.49) | 6.45 (6.41, 6.50) | 23.14 (22.99, 23.30) | 23.14 (22.99, 23.30) | |||||||
| Statin Users | 1.08 (1.06, 1.09) | 1.09 (1.07, 1.11) | 6.32 (6.17, 6.46) | 6.41 (6.26, 6.55) | 22.51 (22.04, 22.99) | 22.67 (22.18, 23.15) | |||||||
| Mean Annual Performance Change, β1 (95% CI) | 0.75 | 0.58 | 0.40 | 0.28 | 0.46 | 0.52 | |||||||
| Nonusers | −.020 (−.022, −.019) | −.021 (−.022, −.019) | −.151 (−.162, −.140) | −.150 (−.162, −.139) | −.591 (−.625, −.558) | −.589 (−.623, −.556) | |||||||
| Statin Users | −.021 (−.026, −.016) | −.022 (−.027, −.017) | −.135 (−.171, −.098) | −.129 (−.166, −.093) | −.549 (−.655, −.443) | −.552 (−.659, −.446) | |||||||
|
| |||||||||||||
| ACE | Mean Baseline Performance, β0 (95% CI) | 0.18 | 0.29 | 0.15 | 0.61 | <0.001 | 0.005 | ||||||
| Nonusers | 1.09 (1.08, 1.10) | 1.09 (1.08, 1.10) | 6.45 (6.40, 6.49) | 6.45 (6.40, 6.49) | 23.17 (23.02, 23.33) | 23.18 (23.02, 23.34) | |||||||
| ACE Users | 1.08 (1.06, 1.10) | 1.10 (1.08, 1.12) | 6.34 (6.21, 6.48) | 6.49 (6.34, 6.64) | 22.32 (21.87, 22.78) | 22.40 (21.89, 22.91) | |||||||
| Mean Annual Performance Change, β1 (95% CI) | 0.77 | 0.48 | 0.01 | 0.05 | 0.15 | 0.13 | |||||||
| Nonusers | −.020 (−.022, −.019) | −.021 (−.023, −.020) | −.145 (−.156, −.133) | −.145 (−.156, −.133) | −.595 (−.629, −.562) | −.594 (−.628, −.560) | |||||||
| ACE Users | −.020 (−.024, −.015) | −.019 (−.024, −.015) | −.190 (−.225, −.156) | −.182 (−.217, −.147) | −.518 (−.619, −.416) | −.511 (−.613, −.409) | |||||||
Acknowledgments
This study was supported by grant R01 AG025441 from the National Institute of Aging.
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf.
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Jennifer G. Robinson, MD, MPH has received grants (to Institution) from the following companies that have products included in the current study: Abbott and Merck. Other grants received from Daiichi-Sankyo, Esperion, and Glaxo-Smith Kline.
Sponsor’s Role: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Footnotes
Minimally adjusted models included age, race/ethnicity, education, and BMI.
Results from fully adjusted models (shaded portion of the table) were presented earlier in Figures 1 & 2, and presented again for ease of comparison. Full covariates adjustment included age, ethnicity, education, BMI, alcohol consumption, systolic and diastolic blood pressure, self-reported health, number of antihypertensive medications, diabetes, depressive symptoms, history of CHD and hormone trial participation.
Author Contributions: All authors contributed to study concept and design, interpretation of data, and preparation of manuscript. Drs. Gray and LaCroix, and Mr. Aragaki contributed to data analysis. Drs. LaCroix, Cochrane and Woods contributed to acquisition of subjects and/or data.
References
- 1. [Accessed October 20, 2011];Healthy People 2020 Objectives. http://www.healthypeople.gov/2020/topicsobjectives2020/default.aspx.
- 2.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Corsonello A, Garasto S, Abbatecola A, et al. Targeting inflammation to slow or delay functional decline: Where are we? Biogerontology. 2010;11:603–614. doi: 10.1007/s10522-010-9289-0. [DOI] [PubMed] [Google Scholar]
- 5.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217–228. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 9.Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-Reactive Protein with physical performance in elderly persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 11.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 12.Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 13.Giri J, McDermott MM, Greenland P, et al. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47:998–1004. doi: 10.1016/j.jacc.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Guralnik JM, Greenland P, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107:757–761. doi: 10.1161/01.cir.0000050380.64025.07. [DOI] [PubMed] [Google Scholar]
- 15.Mondillo S, Ballo P, Barbati R, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–364. doi: 10.1016/s0002-9343(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 16.Hutcheon SD, Gillespie ND, Crombie IK, et al. Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: A randomised double blind placebo controlled trial. Heart. 2002;88:373–377. doi: 10.1136/heart.88.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahimastos AA, Lawler A, Reid CM, et al. Brief communication: Ramipril markedly improves walking ability in patients with peripheral arterial disease. Ann Intern Med. 2006;144:660–664. doi: 10.7326/0003-4819-144-9-200605020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Agostini JV, Tinetti ME, Han L, et al. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55:420–425. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 20.Albert MA, Danielson E, Rifai N, et al. for the PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Silva MA, Swanson AC, Gandhi PJ, et al. Statin-related adverse events: A meta-analysis. Clin Ther. 2006;28:26–35. doi: 10.1016/j.clinthera.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Golomb BA, Evans MA. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31:3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 28.Meyer AM, Evenson KR, Morimoto L, et al. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnam MA, Wells KB, Leake B, et al. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Tuunainen A, Langer RD, Klauber MR, et al. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103:261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 32.Onder G, Penninx BW, Lapuerta P, et al. Change in physical performance over time in older women: The Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57:M289–293. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 33.Gray SL, Boudreau RM, Newman AB, et al. Angiotensin-converting enzyme inhibitor and statin medication use and incident mobility limitation in community older adults. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2011;59:2226–2232. doi: 10.1111/j.1532-5415.2011.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohler ER, III, Hiatt WR, Creager MA for the Study Investigators. Cholesterol reduction with Atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–1486. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 36.LaCroix AZ, Gray SL, Aragaki A, et al. Statin use and incident frailty in women ages 65 and older: Prospective findings from the Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci. 2008;63:369–375. doi: 10.1093/gerona/63.4.369. [DOI] [PubMed] [Google Scholar]
- 37.Scott D, Blizzard L, Fell J, et al. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM. 2009;102:625–633. doi: 10.1093/qjmed/hcp093. [DOI] [PubMed] [Google Scholar]
- 38.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 39.Thompson PD, Parker BA, Clarkson PM, et al. A randomized clinical trial to assess the effect of statins on skeletal muscle function and performance: Rationale and study design. Prev Cardiol. 2010;13:104–111. doi: 10.1111/j.1751-7141.2009.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumukadas D, Witham MD, Struthers AD, et al. Effect of perindopril on physical function in elderly people with functional impairment: A randomized controlled trial. CMAJ. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cesari M, Pedone C, Incalzi RA, et al. ACE-inhibition and physical function: Results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11:26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schellenbaum GD, Smith NL, Heckbert SR, et al. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertension. J Am Geriatr Soc. 2005;53:1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x. [DOI] [PubMed] [Google Scholar]
- 43.Cao YJ, Mager DE, Simonsick EM, et al. Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther. 2008;83:422–429. doi: 10.1038/sj.clpt.6100303. [DOI] [PubMed] [Google Scholar]
- 44.Gray SL, LaCroix AZ, Aragaki AK, et al. Angiotensin-converting enzyme inhibitor use and incident frailty in women aged 65 and older: Prospective findings from the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2009;57:297–303. doi: 10.1111/j.1532-5415.2008.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]