Abstract
Background
Studies of the adverse neurobehavioural effects of maternal alcohol consumption on the fetus have been largely confined to the postnatal period, after exposure to alcohol has finished. This study explored the brain function of the fetus, at the time of exposure to alcohol, to examine its effect on information processing and stability of performance.
Methods
Five groups of fetuses, defined by maternal alcohol consumption patterns, were examined: control (no alcohol); moderate (5-10 units/week either drunk evenly across the week, or as a binge, in 2-3 days); heavy (20+units/week drunk evenly, or as a binge). Fetal habituation performance was examined on three occasions, separated by seven days, beginning at 35 weeks gestation. The number of trials required to habituate on each test session and the difference in performance across test sessions was recorded.
Results
Fetuses exposed to heavy binge drinking required significantly more trials to habituate and exhibited a greater variability in performance across all test sessions than the other groups. Maternal drinking, either heavily but evenly, or moderately as a binge, resulted in poorer habituation and moderate binge drinking resulted in greater variability compared to no, or even, drinking.
Conclusions
Decreased information processing, reflected by poorer habituation, and increased variability in performance may reflect the initial manifestations of structural damage caused by alcohol to the brain. These results will lead to a greater understanding of the effects of alcohol on the fetus's brain, enable the antenatal identification of FASD, and lead to the early implementation of better management strategies.
Keywords: Fetal alcohol spectrum disorders, binge drinking, habituation, brain function, neurobehavioural development
Introduction
Heavy maternal consumption of alcohol during pregnancy was found to give rise to a constellation of features in the child, comprising: a characteristic facial appearance; pre- and post-natal growth retardation; and, central nervous system (CNS) dysfunction (Lemoine et al., 1968), subsequently termed Fetal Alcohol Syndrome (Jones and Smith, 1973). Further studies revealed that antenatal exposure to alcohol resulted in a range of deficits, including physical, growth, organ, neuropsychological and neurobehavioural disorders, which may occur in isolation or combination and are encapsulated by the term, Fetal Alcohol Spectrum Disorders (FASD) (Goodlett, 2010; Riley et al., 2011). The incidence of FASD is largely unknown (BMA, 2007, Mattson and Riley, 2011) and a perhaps conservative figure of just less than 1 per 100 live births has been proposed (Sampson et al., 1997), but estimates vary widely and between specific populations.
The exact features displayed in response to antenatal alcohol exposure are influenced by the timing, type, amount and duration of exposure, as well as the individual characteristics of the mother and fetus (Goodlett et al., 2005). The most commonly occurring effect of antenatal alcohol exposure is CNS dysfunction (Mattson et al., 2011; Riley et al., 2011) which may be severe and debilitating (Riley et al., 2011). These effects include dysfunctions of learning, cognition, emotion, perception and motor performance, which may lead to behavioural difficulties and social problems (Kelly et al., 2000; Mattson et al., 2011; Riley and McGee, 2005). These outcomes may translate into secondary problems including difficulties at school, drug and alcohol problems, inappropriate sexual behaviour and “trouble with the law” (Streissguth, 1997).
One notable aspect of this evidence is that it has been gathered on individuals after birth. There is little information on the effects of alcohol on neurobehavioural functioning before birth (Hepper, 2007). This is perhaps surprising given that this is the time when the fetus is being exposed to alcohol and observations here could provide information on the acute and chronic effects of exposure as they emerge (Hepper, 2007). A few studies have examined the acute effects of alcohol exposure and found that it suppresses fetal breathing movements (McLeod et al., 1983) and disrupts fetal behavioural states (Mulder et al., 1998). Observations of the behaviour of the fetus when there is no alcohol in the mother's system suggest that alcohol induces a delay in neurobehavioural development (Hepper et al., 2005; Little et al., 2002). However, a comprehensive picture of the fetus's response to alcohol exposure remains elusive, but is vital to fully elucidate the underlying mediation of the subsequent neural, psychological and social deficits observed after birth (Hepper 2007).
One means of assessing antenatal neurobehavioural function is through the examination of the habituation response of the fetus (Dirix et al., 2009; Hepper and Leader, 1996; Krasnegor et al., 1998; Leader, 1995). Normal habituation is considered a learning process that requires an intact and integrated CNS, including the cortices (Jeffrey and Cohen, 1971; Thompson and Spencer, 1966), and thus the examination of habituation in the fetus provides a useful tool to examine its neural integrity and function (Hepper, 1995). Habituation has been used to examine fundamental psychological processes in the fetus, e.g. memory and its development (e.g. Dirix et al., 2009; van Heteren et al., 2000). Fetal habituation is considered to have great potential to assess fetal well-being and neonatal outcome as it reflects neural function and hence dysfunction (e.g. Hepper, 1997; Krasnegor et al., 1998; Leader, 1995). The habituation performance of the fetus has been demonstrated to be influenced by adverse maternal (Doherty and Hepper, 2000; Gonzalez-Gonzalez et al., 2009; Lynch et al. 2008), fetal (Hepper and Shahidullah, 1992; van Heteren et al., 2001; Leader at al., 1982), and environmental conditions (Leader, 1987; Leader and Baillie 1988).
Exposure to alcohol has been shown to alter habituation performance in fetal sheep (Leader et al., 1990). In humans, antenatal alcohol exposure through maternal drinking affects habituation performance in the new-born (Streissguth et al., 1983). Habituation may therefore be used to document changes in fetal brain function as a result of exposure to alcohol.
Observation of the effects of alcohol exposure in the fetus using habituation confers further advantages. Exploration of the behaviour of the fetus has enabled the antenatal ontogenetic origins of much postnatal behaviour to be observed (Hepper, 1992). Habituation is a paradigm that has been used after birth to examine cognitive and behavioural functions and its application before birth will enable these same psychological processes to be examined. Habituation performance before birth is correlated with information processing abilities at 6 months of age (Gaultney and Gingras, 2005). Moreover it is well established that after birth, following antenatal exposure to alcohol, psychological processes involved in habituation, e.g. attention and memory (e.g. Riley and McGee, 2005) are adversely affected. Therefore using habituation may provide the opportunity to explore the effects of alcohol on brain and behaviour from their first appearance antenatally, through various developmental stages, until their ‘final’ manifestation in adulthood. This will provide a much more detailed picture of the effects of antenatal alcohol exposure on the individual.
This research examined fetal brain function in response to exposure to alcohol during the period when the mother was consuming alcohol. The study explored neurobehavioural functioning in the fetus exposed to alcohol using habituation beginning at 35 weeks gestational age, a time when consistent and reliable habituation responses have been observed (Dirix et al., 2009; Morokuma et al., 2008). One aspect of normal brain operation that is fundamental to its performance is its stability of function. Acute alcohol exposure in adults disrupts the functioning of the brain and, even at small doses, adversely affects behaviour and cognitive processes (Sher et al., 2009); the brain no longer functions the same when exposed to alcohol. Given the fundamental importance of the consistency of brain functioning, this study also sought to assess whether this was affected in the fetus by antenatal exposure to alcohol. The same individuals were therefore tested on three separate occasions (35, 36 and 37 weeks of gestation) to examine the variability of the individual's brain function. No ‘safe’ level of alcohol has been identified and greater exposure leads to more significant problems, as does binge drinking (BMA, 2007). This study therefore examined habituation in the fetus in response to exposure to moderate and high levels of maternal alcohol consumption and compared the effects between mothers who drank as a binge, with those who drank in a more regular fashion.
Materials and Methods
Participants
Eighty-four non-smoking mothers with normal, apparently healthy, singleton pregnancies participated in this study. The mothers were recruited from the Royal Jubilee Maternity Service, Belfast. Mothers were given a comprehensive questionnaire on life-style habits, including smoking habits, current medications and use of drugs. This was followed by a semi-structured interview to confirm, or follow up, on the answers given in the questionnaire. None of the mothers recruited for the study smoked, were on medication, or were taking recreational drugs. All mothers were provided with information leaflets on alcohol and pregnancy available from the Health Promotion Agencies. Additional information was also provided as part of their normal hospital clinical management.
Alcohol consumption
Details of the mothers' alcohol consumption were obtained through a questionnaire completed at 12-14 and 18-20 weeks gestation and a semi-structured interview at 34 weeks gestation. The questionnaire asked mothers to detail the type of drink and its amount (no. of glasses) consumed each day over the previous week. A semi-structured interview was undertaken at 34 weeks of gestation, prior to the first scan of this study, to obtain information on drinking habits over the past two weeks and to clarify any issues from the questionnaires. For each time period, the amount of alcohol consumed per day was calculated in units (1 unit=10ml of ethanol, which is equivalent to one glass (50ml) of wine, ½ pint of ordinary strength beer, one 25ml short [spirit] measure) and averaged to provide a measure of alcohol consumed expressed as units/week. Further mothers were classified as either mothers who drank evenly, i.e. spread their drinking over 5-6 days per week, or mothers who binge drank, i.e. their drinking was concentrated in 2-3 consecutive days of the week.
Mothers were recruited into the study if they maintained a constant level of drinking across pregnancy and were able to attend the three test sessions at 7 day intervals. Mothers were included in the study if they maintained their level of drinking within three bands (zero units / week; 5-10 units/week; and 20+ units per week) and maintained their drinking pattern (drank evenly or drank in a binge) at all three time points.
Mothers were divided into 5 groups depending on their alcohol consumption patterns.
Group 1 – controls – mothers did not drink alcohol. Thirty-three mothers were initially recruited: three failed to complete the testing, two due to transport difficulties, and one for reason/s unknown. All were excluded from the data set. The final sample comprised 30 mothers.
Group 2 - Moderate even - mothers drank 5-10 units of alcohol evenly across the week. Seventeen mothers were initially recruited: two mothers failed to complete the testing, one was unable to travel for the final test and, one delivered prior to the final test. The final sample comprised 15 mothers.
Group 3 – Moderate binge - mothers drank 5-10 units of alcohol in 2-3 days/week. Thirteen mothers were recruited to this group and all completed testing.
Group 4 – Heavy even - mothers drank 20+ units of alcohol evenly across the week. Nine mothers were recruited to this group and all completed testing.
Group 5 – Heavy binge - mothers drank 20+ units of alcohol in 2-3 days/week. Twelve mothers were initially recruited to this group; one failed to complete the testing as she delivered before the final test, resulting in a final sample of 11 mothers.
Ethical approval for the study was obtained from the Medical Research Ethical Committee, Queen's University of Belfast and each participant gave full informed consent to participate.
The following demographic information was obtained from all mothers: parity; maternal age; problems encountered during pregnancy; delivery method; gestational age at delivery; infant sex; infant birth weight; Apgar score at five minutes after birth; time since last drink; and, alcohol consumption per week. All babies were born healthy and none had signs of FAS.
Apparatus
The fetus was observed using either an ATL Ultramark 4plus, or Dornier AI3200 ultrasound machine with a 3.5MHz curvilinear scan head. A Fetal Acoustic Stimulator, Model 146 (Corometrics Medical Systems Inc.) provided the sound stimulus. This emits sound at a frequency of 75Hz± 10% with harmonics ranging from 20 to 90 Hz, at 82dB measured one meter from the stimulator face. The stimulator was computer controlled and a visual indication of stimulus presentation was superimposed over the ultrasound picture. This visual cue provided an easy reference to define the period during which the fetus was considered to have responded to the stimulus.
A Lion Alcometer (SD-400, Lion Laboratories plc, UK) was used to assess maternal breath alcohol level.
Procedure
Testing began at 35-36 weeks gestation. Mothers were asked to refrain from drinking on the day before they attended for their scan, and were scanned approximately two hours after their last meal. Mothers lay in a semi-recumbent position and a longitudinal view of the fetus was obtained so that the fetal head, upper body and arms could be visualised; this view was maintained throughout the study. At this point, fetal measurements (biparietal diameter, abdominal circumference, femur length) were taken to confirm gestational age. Mothers also blew into the breathalyser to assess their current alcohol level. No mother had alcohol in her body at the time of study.
The stimulator was placed on the maternal abdomen directly above the fetal head. A series of stimuli, produced by the stimulator of 2 sec duration and 5 sec inter-stimulus-interval, were presented to the fetus. This continued until no fetal response was observed on five consecutive stimulus presentations. The fetus was considered to have responded if it made an observable movement of the head, arms or upper body, within 4.5 sec of the onset of the stimulus. One of the experimenters (PH) was present at all scans and determined for each participant if the fetus responded within the 4.5 sec response period and when the criteria for habituation had been met. Fetuses were observed on three occasions separated by exactly seven days.
The number of trials (i.e the number of stimulus presentations) to habituate (excluding the five no response trials) was recorded for each fetus for each of the three test sessions. To assess the stability of habituation, and hence consistency of brain performance, a further measure, RANGE, was calculated. This was determined for each fetus by considering the number of trials required to habituate for each of the three test sessions and then subtracting the smallest number of trials from the largest number of trials. In ideal conditions, performance would be the same on each test session and hence the score for RANGE would be 0. With increasing variability in performance across the three sessions, the score for RANGE would increase. The larger the RANGE score, the poorer the consistency in habituation performance as a result of less stable brain functioning.
Intra- and Inter-observer reliability
To ensure the accuracy of documenting the fetus's responses, both inter- and intra-reliability measures were undertaken. One researcher (PH) and an independent observer separately assessed the number of trials required for habituation in a random selection of tests from a videotape recording of the original scan. Both observers had undertaken at least 100 hours of observation of the human fetus. Six control mothers (group 1) and three mothers from each of the other groups (12 in total) were re-scored for all three tests. Both observers were blind to the fetus's group at the time of this analysis. Agreement between the two scorers was assessed by Kappa co-efficient.
Inter-rater reliability was very high, with agreement between scorers at 0.969 when all three test sessions were combined and for session 1 - 0.954, session 2 - 1.000 and session 3 - 0.951. Agreement by kappa for intra-rater reliability (comparing the original score and same participant's score when scored blind a second time) was also very high at 1.0 for all test sessions and each session (1, 2, 3) individually. Although the experimenter was not ‘blinded’ to the mother's group at the time of scan, when documenting the habituation response, the high inter- and intra-rater reliability confirm the accuracy of the data recording.
Analysis
A one-way-analysis of variance was performed to determine if groups differed in: parity; maternal age; gestational age at delivery; infant birth weight; Apgar score at five minutes after birth; and, for groups 2-5, the time since last drink. Possible differences between groups in: problems encountered during pregnancy; delivery method; and, infant sex, were explored by chi-squared analysis.
To assess whether the number of trials required to habituate differed between groups, a mixed model analysis of variance was performed for the between subjects factor of group (1-5) and within subject factor of test session (wk 35,36,37). To assess whether maternal alcohol consumption exerted an effect on the stability of brain functioning, a one-way-between-subject analysis of variance for group (1-5) was performed using the score of RANGE. Post-hoc LSD comparisons were used to explore any significant results.
Results
Demographic details
There were no significant differences between the groups in parity; maternal age; problems encountered during pregnancy; delivery method; gestational age at delivery; infant sex; infant birth weight; Apgar score at five minutes after birth and for groups 2-5, the time since last drink (see Table 1).
Table 1.
Mean (+ 95% ci) or number for demographic variables assessed between groups. GA = gestational age.
| Control | Moderate even | Moderate binge | Heavy even | Heavy binge | |
|---|---|---|---|---|---|
| Number of participants | 30 | 15 | 13 | 9 | 11 |
| Maternal age (yrs) | 25.53 (23.6-27.5) |
24.46 (21.7-27.2) |
25.38 (22.5-28.2) |
25.44 (22.3-28.6) |
25.24 (23.1-27.3) |
| GA at delivery | 39.6 (39.2-40.0) |
39.2 (38.8-39.6) |
39.9 (39.2-40.5) |
39.3 (38.7-40.0) |
39.4 (9.3-39.7) |
| Birthweight (gms) | 3374 (3255-3491) |
3287 (3115-3457) |
3444 (3275-3612) |
3179 (3008-3350) |
3237 (3071-3403) |
| Start date (GA) of 1st trial | 35.17 (34.9-35.4) |
35.5 (35.2-35.8) |
35.2 (35.0-35.5) |
35.1 (34.9-35.4) |
35.05 (35.1-35.8) |
| Apgar score at 5 min | 8.9 (8.79-9.01) |
8.7 (8.40-8.94) |
8.9 (8.76-9.09) |
8.7 (8.28-9.05) |
8.6 (8.30-8.98) |
| Delivery | |||||
| Vaginal | 22 | 8 | 10 | 4 | 9 |
| Caesarean | 8 | 7 | 3 | 5 | 2 |
| No. obstetric complications | 0 | 0 | 0 | 0 | 0 |
| Sex | |||||
| Male | 15 | 9 | 5 | 5 | 4 |
| Female | 15 | 6 | 8 | 4 | 7 |
| Parity | 1.0 (0.63-1.37) |
0.8 (0.28-1.32) |
1.38 (0.51-2.26) |
0.33 (-0.21-0.88) |
0.96 (0.33-1.85) |
| Units of alcohol/week | |||||
| 12-14 wks | 0 | 7.4 (6.82-7.98 |
7.5 (6.84-8.08) |
22.0 (20.67-23.32) |
22.64 (21.44-23.84 |
| 18-20 wks | 0 | 7.3 (6.47-8.06 |
7.5 (6.69-8.39) |
22.3 (20.95-23.72) |
22.46 (21.20-23.71) |
| 34 wks | 0 | 7.3 (6.44-8.09) |
7.5 (6.56-8.51) |
22.4 (20.44-24.45) |
22.7 (21.35-24.11) |
| Time since last alcohol (hrs) | |||||
| Mean 3 trials | 0 | 42·0 (37.5-46.5) |
42.4 (37.6-47.2) |
41.6 (35.7-47.3) |
43.3 (38.1-48.6) |
| Trial 1 | 0 | 42.5 (38.1-47.0) |
42.3 (35.8-47.3) |
41.6 (35.8-47.3) |
43.4 (38.2-48.6) |
| Trial 2 | 0 | 41.3 (36.8-45.7 |
42.2 (37.5-47.0) |
41.6 (35.8-47.3) |
43.3 (38.1-48.5) |
| Trial 3 | 0 | 42.1 (37.5-46.8) |
42.5 (37.5-47.6) |
42.5 (37.5-47.5) |
43.4 (37.9-48.8) |
There were obvious significant differences between groups in the amount of alcohol consumed by mothers, but not between the two groups (even/binge) in the moderate or heavy conditions (see Table 1).
The number of trials to habituate
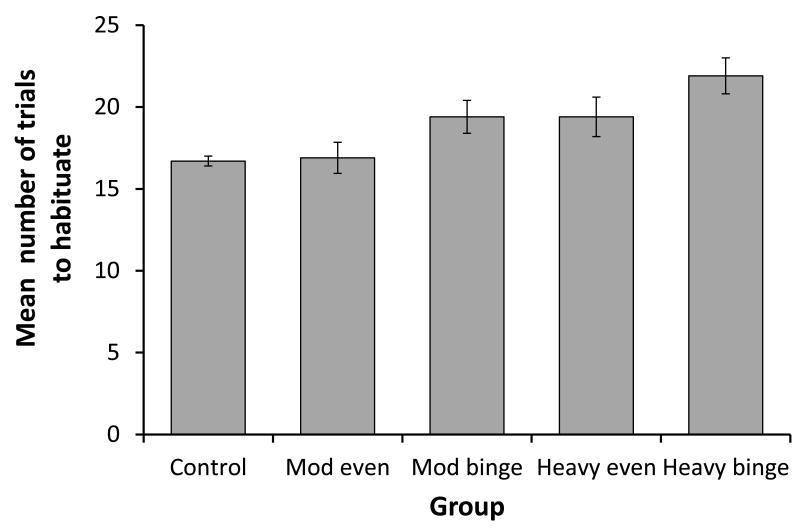
There was no significant effect of test session (F[2,146]=1.807, p=0.168), nor an interaction between session and group (F[8,146]=0.421, p=0.907), on the number of trials required to habituate. There was, however, a significant effect of group on this variable (F[4,73]=20.450, p<0.001, see Figure 1). Post-hoc tests revealed that the ‘heavy binge’ group required significantly (p<0.005) more trials to habituate than all other groups. The ‘heavy even’ group did not differ from the ‘moderate binge’ group, but both groups required significantly more trials to habituate (p<0.001) than the ‘moderate even’ and control groups. There was no significant difference between the control and ‘moderate even’ groups in the number of trials required to habituate.
Figure 1.
The mean (+95% ci) number of trials required to habituate for each of the study groups.
The RANGE of trials to habituate
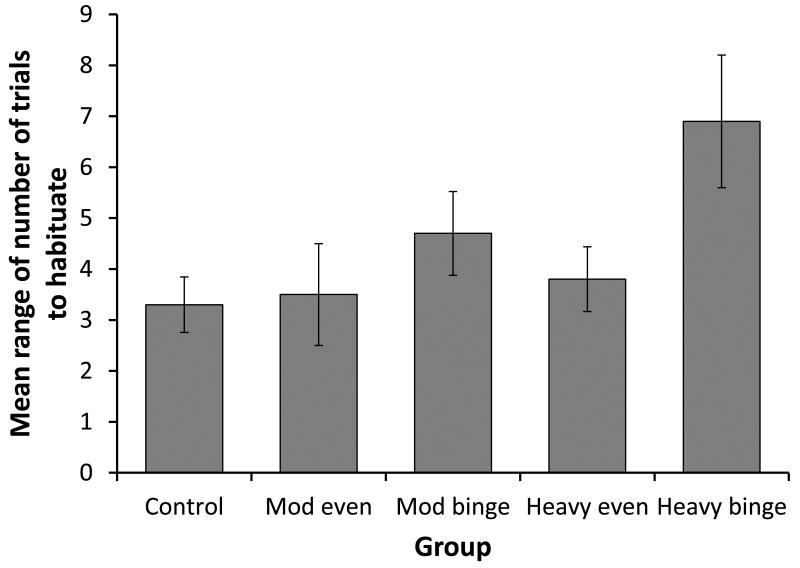
There was a significant effect of group (F[4,73]=29.045, p<0.001, see Figure 2) on the RANGE of trials fetuses required to habituate across the three sessions. Post-hoc tests revealed that the ‘heavy binge’ group exhibited a significantly (p<0.001) greater RANGE in habituation scores than all other groups. The ‘moderate binge’ group exhibited a significantly (p<0.01) greater RANGE than the control (p<0.01), and an almost significant (p=0.051) difference than the ‘moderate even’ group. The control group differed in RANGE from both binge drinking groups (heavy, p<0.001; moderate, p<0.01), but not the even groups.
Figure 2.
The mean (+95%ci) RANGE of trials required to habituate for each of the study groups.
Discussion
The results demonstrate that maternal alcohol consumption influences the functioning of the fetus's brain. Binge drinking increased the number of trials required to habituate compared to drinking evenly across the week, and heavy drinking (22.5 units/week) had a greater effect on performance than moderate drinking (7.5 units/week). Moderate drinking evenly across the week did not produce performance any different from non-alcohol exposed fetuses. With regard to the consistency of brain function, heavy binge drinking, in particular, increased variability in performance, i.e. decreased the functional stability of the brain.
The results reflect findings from studies conducted after birth in showing that maternal binge drinking and greater consumption of alcohol exert a larger adverse long-term effect on the fetus (BMA, 2007). Moderate drinking evenly across the week, approximately 1 unit per day, had no influence on the measures of fetal brain function assessed here. Some caution must be exercised in concluding that moderate exposure had no effect. Habituation in fetal sheep progressed faster following small amounts of alcohol exposure (Leader et al., 1990), an effect thought to be due to catecholamine stimulation of the CNS. Higher levels of alcohol depressed CNS function. It may be that a residual small amount of alcohol remained in the fetus's system in the ‘moderate even’ group that led to faster responding, equivalent to the control group. The effects of low dose alcohol exposure require more investigation.
Habituation is a measure of basic information processing that requires integration between various brain parts, including cortical structures to operate (Hepper and Leader, 1996; Jeffrey and Cohen, 1971; Morokuma et al., 2004; Sokolov, 1963; Thompson and Spencer, 1966). Faster habituation (although not too fast) is indicative of more efficient processing. The slower habituation performance reported here indicates that both heavy drinking and binge drinking (even at moderate levels) reduce the efficiency of the fetal brain to process information. Whilst it is accepted that the fetus's habituation response is a reflection of its neural integrity (Dirix et al., 2009; Hepper and Leader, 1996; Krasnegor et al., 1998; Leader, 1995; Morokuma et al., 2004), the underlying brain areas involved in habituation, in the fetus or adult, are unknown. However, it is believed that the hippocampus and pre-frontal cortex, which are implicated in the detection of novelty (Knight and Scabini, 1998), attention and orienting and the control of these processes (Lindsley, 1982), are involved. Alcohol exposure in the third trimester results in damage to the hippocampus and pre-frontal cortex (Guerri et al., 2009). The observation of deficits in habituation observed here may represent the initial effects of structural damage to these areas as a result of alcohol exposure. Indeed, they are consistent with the deficits in attention, memory, perceptual and learning processes observed after birth (Riley and McGee, 2005). Exactly how any alcohol-induced structural damage results in poorer habituation is presently unknown. Alternatively, exposure to alcohol may result in a developmental delay, leading to less well developed (slower) habituation performance. Low dose alcohol exposure has been observed to lead to a delay in the inhibition of spontaneous startles by the fetus in early pregnancy, but there is a ‘catch-up’ in the last trimester (Hepper et al., 2005; Little et al., 2002). There is considerable debate as to whether there is any developmental progression in the fetus's habituation response with advancing gestation. Some have reported the number of trials does decrease across gestation (e.g. Groome et al., 1993; McCorry and Hepper, 2007), whilst others have not found any change (e.g. Madison et al., 1986). If the fetus's habituation response ‘develops’ (fewer trials required to habituate) with advancing gestational age, the results observed here may be due to a delay induced by exposure to alcohol.
The brains of fetuses exposed to alcohol, especially high levels through binge drinking, display increased variability in performance when tested repeatedly, as evidenced through the RANGE score. Given that the testing procedure was identical (and all mothers consumed there last drink at the same time before testing [Table 1]) it is unlikely that a methodological or procedural difference between groups explains this result. This study suggests that exposure to alcohol, especially through binge drinking, affects this fundamental property of brain function, i.e. its ability to function the same from moment to moment. Whether the inconsistency in the performance of the brain is transient or permanent is unknown. Some studies have reported increased variability in reaction times in infants exposed to alcohol, but not in older children (Jacobsen et al., 1994; Olsen et al., 1998). Variability in reaction times is observed in older children with FAS but not those exposed to alcohol and not exhibiting FAS (Simmons et al., 2010). If this variability in performance were permanent, it may contribute to subsequent psychological, cognitive and behavioural problems. Early development, even antenatally, is a time of learning through active and passive processes that set the foundation for subsequent psychological, social and behavioural performance and achievements (Hepper, 1992). Variability in brain performance may significantly influence the ability to acquire information during this time and to use existing skills and tasks to negotiate the world. Further studies, from our Centre, are exploring this.
The effects observed here were exacerbated by both heavy and binge drinking. The placenta presents little barrier to alcohol and the fetus will experience the alcohol consumed by the mother with few physiological resources to break it down. Alcohol clearance by the fetus, due to the immaturity of its liver function, is greatly reduced (Brien et al., 1983). Although there was no alcohol in the mother's system at the time of testing it may have been present in fetal system, especially when high levels of alcohol were consumed (Nava-Ocampo, et al., 2004; Pikkarainen and Raiha, 1967). Alcohol may persist in the fetus' blood stream and pool in the amniotic fluid. As the fetus swallows amniotic fluid from 15 weeks gestation (Hepper, 1992), exposure may further continue via this route. Thus the fetus may be affected by the alcohol consumed by their mother for a considerable time after its effects in the mother have ended. Heavy binge drinking would result in the greatest concentration of alcohol in the fetus and this may be the reason for the largest effects being observed in this group. This greater concentration will also have taken longer to be broken down by the fetus and the possibility exists, that for this group in particular, alcohol remained active within the fetus's system and this contributed to the observed effects.
With regard to possible limitations to the study a note of caution should be expressed with respect to sample size. Many women decrease alcohol consumption across pregnancy however this study focussed on those women who maintained a constant level of drinking and drinking style. This reduced the pool of women available for study. However it provided a greater uniformity within each group, reducing intra-group variability with respect to the key variable under examination, alcohol consumption. Thus whilst the numbers in some groups were small, the variability within groups was small and, the levels of difference between the groups as reflected by the significance tests were very high (p < 0.001) which provides reassurance that the results are most likely due to differing levels of alcohol consumption and not the small sample size. Additionally although the inter-rater reliability was high, an improvement in the study could be made by having observers blind to the mother's group. Finally, breathalyser tests at the time of study revealed no alcohol in the mother's system at this point in time. All mothers reported that they refrained from alcohol prior to each scan and exploration of this through additional questioning at the scan supported the mother's response. Thus, whilst we cannot be absolutely sure that mothers refrained from alcohol consumption, we are very confident they did report accurately their drinking behaviour over the 24 hours leading up to each scan.
The incidence of FASD is unknown due to difficulties in establishing diagnostic criteria (BMA, 2007; Mattson and Riley, 2011). Observation of the fetus's neurobehavioural response at the time of exposure to alcohol may be a useful early indicator of FASD. The study indicates that the behaviour of the fetus is affected by maternal consumption of alcohol, and the actual effects of alcohol on the individual's brain may be observed through observation of its behaviour. To realise the benefits from this approach as a diagnostic tool large scale studies are needed with individuals followed up after birth, at least to childhood, to evaluate whether patterns of behaviour (group and individual) observed before birth are predictive of outcomes. Given the individual differences in outcome that arise from pregnant mothers seemingly consuming equivalent amounts of alcohol, the technique reported here may be able to identify those fetuses who have been affected by alcohol through comparison of the individual's behaviour against a ‘gold standard’ of normal fetal behaviour (Hepper, 1995).
In summary, the functioning of the fetus's brain is affected by maternal alcohol consumption. Both heavy, and binge, drinking exert an effect on indices of information processing, and, in combination, exert a significant effect on the stability of brain functioning, which may be a result of both structural damage and acute exposure. Both effects may have long-term consequences for the individual, as this altered brain function may influence development after birth. Previous reports (BMA, 2007) have identified the need for research to examine the exact mechanisms of alcohol teratogenesis and how these relate to the dysfunctions apparent after birth. The exploration of the neurobehavioural ontogenesis of FASD at the time of alcohol exposure will identify the earliest behavioural manifestations of alcohol teratogenesis and enable these to be followed from their initial appearance before birth through to their manifestation in the infant, child and adult. This will enable better diagnosis, a deeper understanding of the effects of alcohol on the fetus, and the earlier implementation of management strategies to better manage the neurobehavioural, psychological and social manifestations of FASD.
Acknowledgments
This work was partially funded by a grant (RRG3.7) from the R&D Office, Dept. of Health and Social Services and Public Safety, NI, and a grant (5 U24 AA014828) from the National Institute on Alcohol and Alcohol Abuse (NIAAA) and done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Additional information about CIFASD can be found at www.cifasd.org. We thank D Wells for her contribution to inter-rater reliability.
Support: R&D Office, Dept. of Health and Social Services and Public Safety, NI, and National Institute on Alcohol and Alcohol Abuse (NIAAA).
References
- BMA. A guide for healthcare professionals. BMA; London: 2007. Fetal alcohol spectrum disorders. [Google Scholar]
- Brien JF, Loomis CW, Tranmer J, McGrath M. Disposition of ethanol in human maternal venous blood and amniotic fluid. Am J Obstet Gynecol. 1983;146:181–186. doi: 10.1016/0002-9378(83)91050-5. [DOI] [PubMed] [Google Scholar]
- Dirix CEH, Nijhuis JG, Jongsma HW, Hornstra G. Aspects of fetal learning and memory. Child Dev. 2009;80:1251–1258. doi: 10.1111/j.1467-8624.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- Doherty NN, Hepper PG. Habituation in fetuses of diabetic mothers. Ear Hum Dev. 2000;59:85–93. doi: 10.1016/s0378-3782(00)00089-x. [DOI] [PubMed] [Google Scholar]
- Gaultney JF, Gingras JL. Fetal rate of behavioural inhibition and preference for novelty during infancy. Ear Hum Dev. 2005;81:379–386. doi: 10.1016/j.earlhumdev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez NL, Medina V, Padron E, Domenech E, Gomez NMD, Armas H, Bartha JL. Fetal and neonatal habituation in infants of diabetic mothers. J Pediat. 2009;154:492–497. doi: 10.1016/j.jpeds.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Goodlett CR. Fetal alcohol spectrum disorders; new perspectives on diagnosis and intervention. Alcohol. 2010;44:579–582. doi: 10.1016/j.alcohol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Med Biol. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Gotlieb SJ, Neely CL, Waters MD. Developmental-trends in fetal habituation to vibroacoustic stimulation. Am J Perinatol. 1993;10:46–49. doi: 10.1055/s-2007-994700. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcoholism. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepper PG. In: Fetal psychology: an embryonic science, in Fetal behaviour: Developmental and perinatal aspects. Nijhuis JG, editor. Oxford University Press; Oxford: 1992. pp. 129–156. [Google Scholar]
- Hepper PG. The behaviour of the foetus as an indicator of neural functioning, in Fetal development. In: Lecanuet JP, Fifer W, Krasnegor N, Smotherman W, editors. A psychobiological perspective. Lawrence Erlbaum; Hillsdale, NJ: 1995. pp. 405–417. [Google Scholar]
- Hepper PG. Fetal habituation. Another Pandora's Box? Dev Med Child Neurol. 1997;39:274–278. doi: 10.1111/j.1469-8749.1997.tb07426.x. [DOI] [PubMed] [Google Scholar]
- Hepper PG. The effect of maternal consumption of alcohol on the behaviour of the human fetus: A review. Int J Dis Hum Dev. 2007;6:153–159. [Google Scholar]
- Hepper PG, Dornan JC, Little JF. Maternal alcohol consumption during pregnancy may delay the development of spontaneous fetal startle behavior. Physiol Behav. 2005;83:711–714. doi: 10.1016/j.physbeh.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Leader LR. Fetal habituation. Fet Mat Med Rev. 1996;8:109–123. [Google Scholar]
- Hepper PG, Shahidullah S. Habituation in normal and Down Syndrome fetuses. Quart J Exp Psych. 1992;44B:305–317. doi: 10.1080/02724999208250617. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. 1994;18:1125–1132. doi: 10.1111/j.1530-0277.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Jeffrey WE, Cohen LB. In: Habituation in the human infant, in Advances in child development and behaviour. Reese H, editor. Academic Press; New York: 1971. pp. 63–97. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behaviour in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of evoked related potentials and their relationship to novelty detection in humans. J Clin Neuropsychol. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Krasnegor NA, Fifer W, Maulik D, McNellis D, Romero R, Smotherman W. Fetal behavioural development: Measurement of habituation, state transitions, and movement to assess fetal well being and to predict outcome. J Mat Fetal Invest. 1998;8:51–57. [PubMed] [Google Scholar]
- Leader LR. In: The effects of cigarette smoking and maternal hypoxia on fetal habituation, in The fetus as a patient. Maeda K, editor. Elsevier; Amsterdam: 1987. pp. 83–88. [Google Scholar]
- Leader LR. The potential value of habituation in the prenate, in Fetal development. In: Lecanuet JP, Fifer W, Krasnegor N, Smotherman W, editors. A psychobiological perspective. Lawrence Erlbaum; Hillsdale, NJ: 1995. pp. 383–404. [Google Scholar]
- Leader LR, Baillie P. The changes in fetal habituation patterns due to a decrease in inspired maternal oxygen. Brit J Obstet Gynecol. 1988;95:664–668. doi: 10.1111/j.1471-0528.1988.tb06527.x. [DOI] [PubMed] [Google Scholar]
- Leader LR, Baillie P, Martin B, Vermeulen E. Fetal habituation in high risk pregnancies. Brit J Obstet Gynecol. 1982;89:441–446. doi: 10.1111/j.1471-0528.1982.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Leader LR, Smith FG, Lumbers ER. The effect of ethanol on habituation and the cardiovascular response to stimulation in fetal sheep. Eur J Obstet Gynecol and Rep Biol. 1990;36:87–95. doi: 10.1016/0028-2243(90)90054-5. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Les enfants de parents alcooliques. Anomalies observes; A propos de 127 cas. Quest Med. 1968;21:476–482. [Google Scholar]
- Lindsley DB. In: Neural mechanisms of arousal, attention and information processing, in Neuropsychology after Lashley: fifty years since the publication of brain mechanisms and intelligence. Orbach J, editor. Erlbaum; Hillsdale, NJ: 1982. pp. 315–407. [Google Scholar]
- Little JF, Hepper PG, Dornan JC. Maternal alcohol consumption during pregnancy and fetal startle behaviour. Physiol Behav. 2002;76:691–694. doi: 10.1016/s0031-9384(02)00804-1. [DOI] [PubMed] [Google Scholar]
- Lynch C, Hepper P, Morrow J. Habituation in fetuses exposed to antiepileptic drugs. Epilepsia. 2008;50:202. Abs. [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioural features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. The quest for a neurobehavioural profile of heavy prenatal alcohol exposure. Alch Res Health. 2011;34:51–55. [PMC free article] [PubMed] [Google Scholar]
- McCorry NK, Hepper PG. Fetal habituation performance: gestational age and sex effects. Brit J Dev Psych. 2007;25:277–292. [Google Scholar]
- McLeod W, Brien JF, Loomis C, Carmichael L, Probert C, Patrick J. Effects of maternal ethanol ingestion on fetal breathing movements gross body movements and heart rate at 37 to 40 weeks gestational age. Am J Obstet Gynecol. 1983;145:251–257. doi: 10.1016/0002-9378(83)90501-x. [DOI] [PubMed] [Google Scholar]
- Morokuma S, Doria V, Ierullo A, Kinukawa N, Fukushima K, Nakano H, Arulkumaran S, Papageorghiou AT. Developmental change in fetal response to repeated low-intensity sound. Dev Sci. 2008;11:47–52. doi: 10.1111/j.1467-7687.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- Morokuma S, Fukushima K, Kawai N, Tomonaga M, Satoh S, Nakano H. Fetal habituation correlates with functional brain development. Behav Brain Res. 2004;153:459–463. doi: 10.1016/j.bbr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Mulder EJH, Morssink LP, van der Schee T, Visser GHA. Acute maternal alcohol consumption disrupts behavioral state organization in the near-term fetus. Ped Res. 1998;44:774–779. doi: 10.1203/00006450-199811000-00022. [DOI] [PubMed] [Google Scholar]
- Nava-Ocampo AA, Velázquez-Armenta Y, Brien JF, Koren G. Elimination kinetics of ethanol in pregnant women. Rep Toxicol. 2004;18:613–617. doi: 10.1016/j.reprotox.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Olsen HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Pikkarainen PH, Raiha NC. Development of alcohol dehydrogenase activity in the human liver. Ped Res. 1967;1:165–168. doi: 10.1203/00006450-196705000-00001. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal Alcohol Spectrum Disorders: An overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behaviour. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Litte RE, Clarren SK, Dehaene P, Hanson JW, Graham JM. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratol. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sher L, Kandel I, Merrick J. Research and clinical perspectives. Nova Science; New York: 2009. Alcohol-related cognitive disorders. [Google Scholar]
- Simmons RW, Thomas JD, Levy SS, Riley EP. Motor response programming and movement time in children with heavy prenatal alcohol exposure. Alcohol. 2010;44:371–378. doi: 10.1016/j.alcohol.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Macmillan; New York: 1963. [Google Scholar]
- Streissguth AP. Fetal Alcohol Syndrome; A guide for families and communities. Paul H Brooks; Baltimore: 1997. [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton Scale. Child Dev. 1983;54:1109–1118. [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behaviour. Psych Rev. 1966;173:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- van Heteren CF, Boekkooi PF, Jongsma HW, Nijhuis JG. Fetal learning and memory. Lancet. 2000;356:1169–1170. doi: 10.1016/S0140-6736(00)02766-5. [DOI] [PubMed] [Google Scholar]
- van Heteren CF, Boekkooi PF, Jongsma HW, Nijhuis JG. The responses to repeated vibroacoustic stimulation in a fetus with trisomy 18. Eur J Obstet Gynecol Reprod Biol. 2001;96:123–125. doi: 10.1016/s0301-2115(00)00396-1. [DOI] [PubMed] [Google Scholar]