Abstract
BACKGROUND
Secondary peritonitis continues to carry a high mortality rate despite aggressive use of imaging, drainage and antibiotics. Although host factors and microbial burden contribute to the outcome of peritonitis, here we propose a role bacterial virulence as a determinant of outcome from peritonitis. Bacterial virulence is an inducible trait that is activated in response to specific local “cues” that we have previously shown to be present in the mouse gut exposed to surgical stress and injury.
METHODS
Pseudomonas aeruginosa was harvested following its intestinal inoculation into the cecum of mice subjected to surgical injury (30% hepatectomy) or sham surgery (controls). Harvested strains were then injected into the peritoneum of non-injured (naïve) mice and mortality determined.
RESULTS
P. aeruginosa harvested from the intestines of surgically injured mice caused 100% mortality whereas strains harvested from control mice caused no mortality. Among recovered strains a distinct P. aeruginosa morphotype (wrinked shape) was demonstrated to cause lethal peritonitis compared to smooth shaped strains which were non-lethal. Wrinked strains were associated with a tendency to elicit a more pro inflammatory response in mice compared to smooth shaped strains.
CONCLUSIONS
Surgical injury transforms the morphotype of intestinal P. aeruginosa to express a hypervirulent response in the peritoneum of mice. Enhanced virulence of intestinal pathogens in response to surgical injury may play an important role in predicting the outcome of peritonitis.
INTRODUCTION
Recent large cohort studies comparing infection rates among surgical and non-surgical patients demonstrate that not only are infections more frequent among surgical patients, but they are also more lethal1,2. Even when controlling for co-morbid conditions, surgical patients experience more frequent and severe infections despite being healthier than their comparative non-surgical cohorts2. This conclusion remains statistically valid even when surgical site infections are eliminated from the comparison and suggests that surgical injury itself might shift the dynamics of the host pathogen interaction resulting in a greater degree of infectious related morbidity and mortality. Although current paradigms of the causes of surgical infection posit that infection develops merely as a result of the permissive effects of a dysfunctional immune system unable to contain or clear an invading pathogen, there is now increasing evidence that the ability of microbial organisms to dynamically sense host stress signals and respond with enhanced virulence governs the occurrence, course, and outcome of infection3-8. While this view does not dismiss the important role of the immune system, it raises the possibility that surgical patients may harbor more virulent strains of pathogenic bacteria because of the selective pressures imposed by the surgical injury itself and the ability of bacteria to sense host stress change their phenotype or genotype. Once bacteria are exposed to the key activating environmental “cues” released during host injury, they can process this information and respond in a context dependent manner. As most host injury is short lived (i.e elective surgery, rapid return of bowel function and normal oral intake), a return to homeostasis is the norm and usually ends in a type of molecular détente between the host and its newly acquired pathogenic flora. Yet for patients colonized by certain pathogens whose virulence is highly inducible, prolonged and severe surgical injury can lead to the release of host factors and environmental cues that trigger these otherwise dormant colonizers to become invasive and lethal pathogens. We have modeled such events with the human opportunistic pathogen Pseudomonas aeruginosa and determined that it is in vivo transformed to express enhanced virulence in response to host tissue factors released during surgical injury such as interferon gamma5, opioids9, and end-products of ischemia10 (i.e adenosine) via direct interactions with its quorum sensing system. Yet whether P. aeruginosa can express a phenotype to a degree sufficient to cause mortality in a non-injured and presumably immunocompetent host, has not yet been demonstrated. Therefore the aims of the present study were to determine if exposure of intestinal P. aeruginosa to surgical injury (30% hepatectomy) results in its transformation to a more pro-inflammatory and lethal phenotype when it is cross transferred into the peritoneal compartment of separate groups of untreated (naïve) mice.
MATERIAL AND METHODS
Bacterial Strains and Inoculum Preparation
P aeruginosa wild-type strain MPAO1 was obtained from the P aeruginosa mutant library11 and was routinely grown overnight before all experiments in tryptic soy broth (TSB), a phosphate-rich media containing 45 mmol/L phosphate. A single colony was selected and suspended in liquid TSB media overnight (12-14 hours). 200 μL of the bacterial suspension was centrifuged at 5,000 RPM for 5 minutes, the excess TSB removed, and the remaining pellet suspended in sterile 10% glycerol to a final optical density at 600 nm of 0.20, corresponding to 107 CFU/ml. This suspension was immediately placed on ice; bacteria were used within 2 hours of preparation.
Mouse model of surgical injury and cross transfer of intestinal Pseudomonas aeruginosa into the peritoneum of sham operated mice
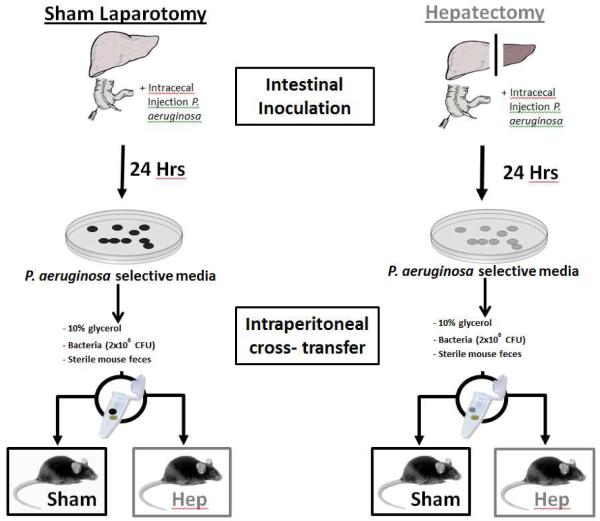
All experiments were approved by the Animal Care and use Committee at the University of Chicago (IACUC Protocol 72090). Specific pathogen free C57B/L6 mice weighing 18 to 22g and 6-8 weeks old were used for all experiments. Mice were routinely fed tap water and Harland Teklad feed under 12 hour light/dark cycles and were allowed to acclimate for at least 48 hours prior to surgery. To test in-vivo virulence transformation of P. aeruginosa, mice were sterilely draped and underwent either sham laparotomy or 30% hepatectomy immediately followed by inoculation of 2 × 106 CFU of P. aeruginosa into the cecum via injection into the terminal ileum (Intestinal inoculation). This model was developed in our lab and has been repeatedly reported to result in sepsis and high mortality (60-100%) in mice following hepatectomy and no mortality and healthy appearing mice in sham operated mice at 48 hours. Partial (30%) hepatectomy is associated with in vivo virulence activation of P. aeruginosa in the cecum of mice but not in the cecum of sham operated mice. After 24 hours, cecal contents were collected from both groups of mice and plated on Pseudomonas isolation agar (PIA) to isolate P. aeruginosa from the stool of each group. After overnight culture, 2 × 106 CFU of pure P. aeruginosa were suspended in 10% glycerol and admixed with sterile control (untreated) mouse feces and a small fixed weight pellet cross transferred and implanted in the right lower quadrant of separate groups of mice following either sham laparotomy or 30% hepatectomy. This lead to cross transfer of intestinal P. aeruginosa from sham operated (Sham) and hepatectomy (Hep) mice into the peritoneum of sham operated and hepatectomy mice generating four treatment groups (Figure 1 and Table I).
Figure 1.
The experimental protocol consisted of two phases, beginning with intestinal inoculation of P. aeruginosa following either a 30% hepatectomy or sham laparotomy. After 24 hours, cecal contents from both groups were harvested and P. aeruginosa was isolated on selective media. Bacterial colonies from both groups were then admixed with sterile stool pellets and cross-transferred into the peritoneum of mice undergoing either sham laparotomy or 30% hepatectomy.
| GROUP | INTESTINAL INOCULATION |
INTRAPERITONEAL CROSS-TRANSFER |
|
|---|---|---|---|
| I | Shamintestine | → | Shamperitoneum |
| II | Shamintestine | → | Hepperitoneum |
| III | Hepintestine | → | Shamperitoneum |
| IV | Hepintestine | → | Hepperitoneum |
Morphotype Identification
P. aeruginosa was retrieved from cecal stool contents from mice in the intestinal inoculation phase 24 hours after the initial procedure. Mice were sacrificed and cecal stool contents were collected under sterile conditions. Samples were diluted with sterile normal saline and plated onto PIA. After overnight culture, plates were observed under light microscopy to identify the morphotype patterns of individual colonies. Two predominant morphotypes were identified and defined as either “smooth” or “round.” These morphotypes were then sub-cultured overnight and subsequently suspended in 10% glycerol to an OD of 0.20 for mixture with sterile stool and used in intraperitoneal cross transfer studies.
Pyocyanin Assay
P aeruginosa was retrieved from cecal stool contents from mice in the intestinal inoculation at 24 hours after the initial procedure. Mice were sacrificed and cecal stool contents were collected under sterile conditions. Samples were diluted with sterile normal saline and plated onto PIA for pyocyanin formation as previously described6. Briefly, after overnight culture on PIA, bacterial cultures were spun down by centrifugation and 1 mL of supernatant was extracted using 500 μL of chloroform, then re-extracted with 150 μL of 0.2 mol/L HCl. Absorbance was measured at OD 520 nm. Appropriate controls were analyzed including bacterial suspensions containing high phosphate (15 mmol/L), low phosphate (0.1 mmol/L), as well as phosphate solutions containing no bacteria. All experiments were performed in triplicate.
Cytokine Assay
Assessments of cytokine profiles were performed using a commercially available assay by Millipore (Milliplex MAP Mouse Cytokine/Chemokine–Premixed 12 Plex). Simultaneous measurement of 12 cytokines/chemokines (IL-1 beta, IL-6, IL-12(p40), IL-12(p70), IL-15, TNF-α, IL-10, IL-13, IL-17, IFN-γ, IL-9, IP-10) was performed. All assays were performed according to the manufacturer’s protocols. Samples were collected from the blood and peritoneal washings of mice following intraperitoneal cross transfer at 24 hours post-procedure, and diluted 1:1 in the assay buffer provided with the kit, and at least two replicate wells were plated per sample. Cytokine concentrations were determined using BeadView software (Millipore).
Photon camera imaging of P. aeruginosa in the peritoneum of mice
In order to demonstrate that the P. aeruginosa fecal pellet admixture distributed within the peritoneum to cause peritonitis as others have described12, we performed photon camera imaging of bioluminescent bacteria following 6 and 12 hours following intraperitoneal injection. P. aeruginosa distributed from the fecal pellet to the generalized peritoneal cavity within 12 hours.
Statistical Analysis
For survival curves standard Kaplan Meir statistical analysis was applied. Virulence factor analysis was compared using student’s t test. Non-parametric Kruskall-Wallis sum rank ANOVA was used to compare the results of the cytokine data. Standard statistical software was used for all analyses (STSS Chicago, Illinois).
RESULTS
P. aeruginosa isolated from the intestine (cecum) of mice subjected to 30% hepatectomy produces significantly increased pyocyanin production compared to isolates from sham laparotomy mice
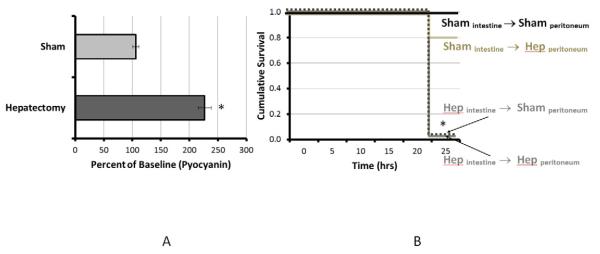
When stool from mice subjected to 30% hepatectomy was cultured on selective media for P. aeruginosa, it appeared grossly green compared to stool cultures from sham operated mice. Pyocyanin assay reveled that P. aeruginosa cultured from mice subjected to 30% hepatectomy demonstrated a statistically significant increase in pyocyanin production compared to that from sham operated mice (p<0.01) (Figure 2A).
Figure 2.
Pyocyanin production (2A) was significantly increased in P. aeruginosa harvested from the ceca of mice following 30% hepatectomy versus sham laparotomty (controls). P. aeruginosa harvested from the cecum of mice subjected to 30% hepatectomy caused 100% mortality at 24 hours (2B) after peritoneal cross-transfer. P. aeruginosa harvested from the cecum of mice subjected to sham laparotomy (control) appeared healthy throughout the experimental period and did not demonstrate sepsis. * P_< 0.01
Intestinal P. aeruginosa isolates from mice following intestinal inoculation and 30% hepatectomy, in contrast to isolates from sham operated mice, cause high mortality rates following intraperitoneal cross transfer
Cross transfer of intestinal P. aeruginosa from sham operated mice to the peritoneum of sham operated mice (Shamintestine→Shamperitoneum) group I resulted in 100% survival (6/6) and healthy appearing mice at 24 hours. Cross transfer of intestinal P. aeruginosa from sham operated mice to the peritoneum of 30% hepatectomy (Hep) mice (Shamintestine →Hepperitoneum, group II), demonstrated a lower survival rate (83%; 5/6) at 24 hours that was not statistically significant (P=NS) with all surviving mice appearing healthy. However cross transfer of P. aeruginosa from the cecum of 30% hepatectomy mice to the peritoneum of sham operated mice (Hepintestine→Shamperitoneum), group III, resulted in rapid appearing sepsis and 100% mortality (8/8 p<0.05). Similarly cross transfer of intestinal P. aeruginosa from 30% hepatectomy mice to the peritoneum of 30% hepatectomy mice (Hepintestine →Hepperitoneum, group IV) also resulted in rapid appearing sepsis and 100% mortality (N=6, p<0.05). All mice were sacrificed at 24 hours and autopsied for the presence of peritonitis (Figure 2B).
Intraperitoneal P. aeruginosa within a sterile fecal pellet distributes within the general peritoneum within 12 hours
In mice that developed severe peritonitis and death, photon camera imaging of the abdominal cavity of bioluminescent strains confirmed that the fecal pellet containing P. aeruginosa diffusely distributed in the general peritoneal cavity within 12 hours following their intraperitoneal injection as has been reported by others12 (Figure 3).
Figure 3.
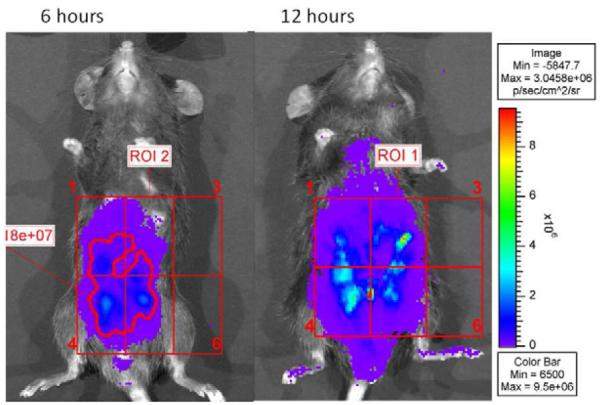
Photon camera imaging of intraperitoneal bioluminescent strains at 6 and 12 hours post injection. Images demonstrate a typical spatiotemporal progression and distribution of the localized bioluminescent P. aeruginosa inoculum from 6 to 12 hours post exposure.
Intestinal P. aeruginosa isolates from mice subjected to 30% hepatectomy are transformed to distinct wrinkled surface morphotypes
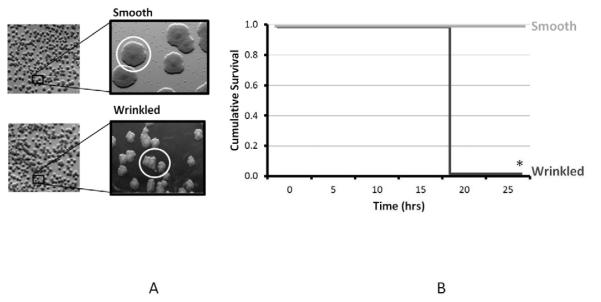
When stock wild type strains of P. aeruginosa PAO1 are observed under light microscopy they demonstrate a round/smooth surface morphotype on PIA. Similarly strains harvested from the intestine of mice undergoing sham laparotomy following the intestinal inoculation phase displayed a similar round appearance. In contrast, isolates taken from mice undergoing 30% hepatectomy demonstrated a high proportion of the wrinkled morphotype (as depicted in Figure 4A), while proportionally demonstrating a smaller number of the smooth surface morphotype.
Figure 4.
Morphotypes identification of P. aeruginosa (4A) isolates demonstrating a shift toward a “wrinkled” surface morphotypes when isolates were harvested from mice subjected to 30% hepatectomy compared to the round/smooth appearing isolates harvested from the cecum of mice subjected to sham laparotomy (control). Distinct morphotypes were then subcultured and cross-transferred into the peritoneum (4B); wrinkled morphotyes produced high mortality rates at 24 hours compared to round/smooth morphotypes which cause no sepsis or mortality. * P <0.01.
Wrinkled surface P. aeruginosa isolates cause high mortality rates in mice following intraperitoneal inoculation compared to no mortality with smooth surface isolates
To evaluate the relationship between morphotype and virulence, wrinkled or round/smooth isolates of P. aeruginosa were directly harvested from the culture plates generated during the intestinal inoculation experiments. Selected wrinkled or round/smooth isolates were then re-introduced into naïve mice via intraperitoneal inoculation without additional surgical stress. Interestingly, mice who received inoculations of smooth morphotype isolates taken from both sham and hepatectomy mice appeared healthy and had 100% survival at 24 hours. However, wrinkled morphotype isolates taken from hepatectomy mice cause 100% mortality by 24 hours, suggesting that a wrinkled surface morphotype confers a virulent and lethal phenotype (Figure 4B) (n=8/group, p<0.01).
Differential effect of P. aeruginosa isolates on serum and local peritoneal cytokine production
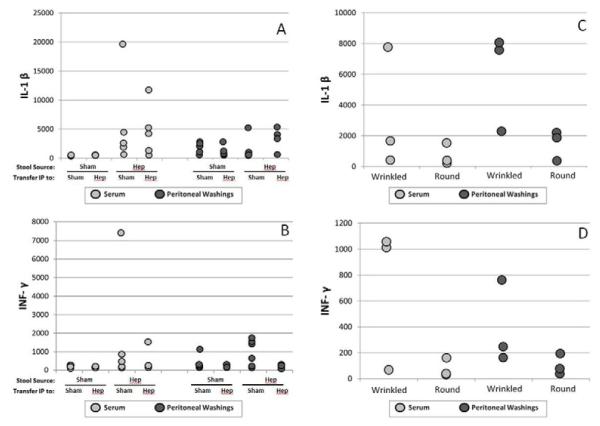
Cytokines expression across groups (I-IV) showed a trend toward activation of the serum cytokines IL-1β and INF-λ when isolates from hepatectomy mice were intraperitoneally transferred into separate groups of mice although this was not statistically significant. Wrinkled isolates compared to round isolates also demonstrated a trend toward a pro-inflammatory phenotype, although again, this was not statistically significant (see Figure 5).
Figure 5.
Cytokines were measured in serum (A-B) and peritoneal washings (C-D) of mice 24 hours after peritoneal cross-transfer of P. aeruginosa. A trend toward a more pro-inflammatory response was observed in mice inoculated with the wrinkled versus smooth/round morphotypes of P. aeruginosa.
DISCUSSION
Data from the present study underscore the importance of phenotype transformation or phase variation that develops as microbes adapt and respond to novel environments. Here we show for the first time, that the local intestinal microenvironment during surgical injury can shape the phenotype of colonizing pathogens like P. aeruginosa to play a causal role in mortality. That this effect is due to a particular property of the pathogen and not to impaired immune clearance per se is suggested by the use of cross transfer experiments of intestinal P. aeruginosa. These experiments allow for the independent assessment of microbial phenotype while maintaining the host immune response constant using mice not previously exposed (i.e naïve) to P. aeruginosa inoculation. We have previously observed and reported this round/smooth versus wrinkled or rough surface phenotype in P. aeruginosa13. However the ability of these morphotypes to differentially express a lethal potential was not assessed. Further work to determine the precise mutation, epigenetic, or phenotype switching mechanisms that are responsible for the expression of this lethal phenotype wil be necessary. Yet this will be methologically challenging given that the smooth and round phenotypes are not stable upon subculture and revert back and forth (data not shown). This finding makes pure DNA and RNA extraction from a single phenotype challenging. Phase variation or phenotype switching is a well described phenomenon among heterogeneous bacterial populations14. Phase variation can be defined as the random switching of phenotype that appears to occur at frequencies much higher than reported spontaneous mutation rates. Phase variation is a major mechanism by which bacteria express virulence and population heterogeneity15,16. Phase variation might be accomplished by riboswitches, small molecules or mRNA that can bind to target molecules and affect _ENREF_15gene expression without necessarily affection mutation or gene structure. This could be yet another mechanism by which bacteria sense unique host environments and respond in a contextually appropriate manner17-19_ENREF_15_ENREF_15_ENREF_15. That riboswitches turn on and off particular phenotypes has been proposed by others and could also explain the variation in mortality among the isolates observed in the present study20. The round/smooth versus wrinkled phenotype switch has been observed with other strains of Pseudomonas and appears to be highly dependent on adaptation to a fluctuating environment21 . Sequence analysis of the various isolates in present model is being pursued and will clarify the various mechanisms that shift P. aeruginosa from an indolent colonizer to lethal pathogen when present in the gut of a surgically stressed host.
The variation in immune response between intestinal P. aeruginosa isolated from mice following hepatectomy versus sham laparotomy is intriguing but will require further study. Although many cytokines were measured, the data were very noisy and not statistically significant. Accurate cytokine measurements in these types of studies are heavily dependent on timing, degree of mouse illness, hydration status, normalization to a background protein etc. For these reasons we did not pursue additional studies in this regard as we felt they would only show that when mice appeared healthy, were feeding well, and were well hydrated, their cytokines profiles would remain at baseline and when they appeared frankly septic, they would be altered. Similarly results from experiments in which wrinkled isolates were compared to round/smooth isolates for their serum and peritoneal cytokine response following intraperitoneal injection demonstrated a trend toward a pro-inflammatory phenotype. However again this was not statistically significant. We also did not pursue these studies further because depending on the strain and character of quorum sensing molecule expression, P. aeruginosa can either suppress or stimulate cytokine production4_ENREF_4. In addition depending on the particular environmental context (i.e in vivo cell growth dynamics, local physico-chemical cues (i.e pH, redox, presence of immune cells etc)), a uniform cytokine pattern may not emerge between groups under the conditions of the present study. Nonetheless, studies are in progress to examine the effect of wrinkled versus round/smooth variants on the immune response using in vitro assays where P. aeruginosa phenotypes can be stabilized (i.e they do not revert back and forth) and the local environment controlled. This will require that strains be fully sequenced and media conditions rigorously controlled.
Finally, results from the present study could explain, in part, the poor prognosis of patients who develop secondary or tertiary peritonitis22. It is generally recognized that when hospitalized patients develop intestinal perforations or require multiple laparotomies to treat peritonitis, mortality rates are high23. While this is often attributed to exhaustion of the immune system, malnutrition, etc, enhanced virulence expression among the offending organisms could also play an important role. Emerging technologies that will allow for in depth analysis of virulence phenotype among recovered organisms will help clarify this issue. One these mechanisms are uncovered, application of anti-virulence compounds might offer promise as an adjunctive measure to current treatment approaches in addition to antibiotics.
In summary, the gut of a surgically stress host represent a complex ecology that has the potential to shape the virulence of opportunistic pathogens resulting in the expression of a lethal phenotype. Further elucidation of this new dimension in the host pathogen interaction may allow for a more discriminative analysis to explain the mechanisms by which surgical patients have higher infectious related morbidity and mortality beyond the operative site.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Craven DE, Kunches LM, Lichtenberg DA, Kollisch NR, Barry MA, Heeren TC, et al. Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med. 1988;148(5):1161–8. [PubMed] [Google Scholar]
- 2.Sax H, Uckay I, Balmelli C, Bernasconi E, Boubaker K, Muhlemann K, et al. Overall burden of healthcare-associated infections among surgical patients. Results of a national study. Ann Surg. 2011;253(2):365–70. doi: 10.1097/SLA.0b013e318202fda9. [DOI] [PubMed] [Google Scholar]
- 3.Hegde M, Wood TK, Jayaraman A. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl Microbiol Biotechnol. 2009;84(4):763–76. doi: 10.1007/s00253-009-2045-1. [DOI] [PubMed] [Google Scholar]
- 4.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, et al. Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science. 2008;321(5886):259–63. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–7. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 6.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007;3(3):e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yung SC, Parenti D, Murphy PM. Host chemokines bind to Staphylococcus aureus and stimulate protein A release. J Biol Chem. 2011;286(7):5069–77. doi: 10.1074/jbc.M110.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratt JT, Ismail AM, Camilli A. PhoB regulates both environmental and virulence gene expression in Vibrio cholerae. Mol Microbiol. 2010;77(6):1595–605. doi: 10.1111/j.1365-2958.2010.07310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, et al. Pseudomonas aeruginosa Virulence Expression Is Directly Activated by Morphine and Is Capable of Causing Lethal Gut-Derived Sepsis in Mice During Chronic Morphine Administration. Ann Surg. 2011 doi: 10.1097/SLA.0b013e3182331870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink D, Romanowski K, Valuckaite V, Babrowski T, Kim M, Matthews JB, et al. Pseudomonas aeruginosa Potentiates the Lethal Effect of Intestinal Ischemia-Reperfusion Injury: The Role of In Vivo Virulence Activation. J Trauma. 2011 doi: 10.1097/TA.0b013e31821cb7e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100(24):14339–44. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma PK, Engels E, Van Oeveren W, Ploeg RJ, van Henny der Mei C, Busscher HJ, et al. Spatiotemporal progression of localized bacterial peritonitis before and after open abdomen lavage monitored by in vivo bioluminescent imaging. Surgery. 2010;147(1):89–97. doi: 10.1016/j.surg.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Zaborina O, Holbrook C, Chen Y, Long J, Zaborin A, Morozova I, et al. Structure-function aspects of PstS in multi-drug-resistant Pseudomonas aeruginosa. PLoS Pathog. 2008;4(2):e43. doi: 10.1371/journal.ppat.0040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayliss CD. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol Rev. 2009;33(3):504–20. doi: 10.1111/j.1574-6976.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 15.Struve C, Bojer M, Krogfelt KA. Characterization of Klebsiella pneumoniae type 1 fimbriae by detection of phase variation during colonization and infection and impact on virulence. Infect Immun. 2008;76(9):4055–65. doi: 10.1128/IAI.00494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broadbent SE, Davies MR, van der Woude MW. Phase variation controls expression of Salmonella lipopolysaccharide modification genes by a DNA methylation-dependent mechanism. Mol Microbiol. 2010;77(2):337–53. doi: 10.1111/j.1365-2958.2010.07203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainey PB, Beaumont HJ, Ferguson GC, Gallie J, Kost C, Libby E, et al. The evolutionary emergence of stochastic phenotype switching in bacteria. Microb Cell Fact. 2011;10(Suppl 1):S14. doi: 10.1186/1475-2859-10-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gripenland J, Netterling S, Loh E, Tiensuu T, Toledo-Arana A, Johansson J. RNAs: regulators of bacterial virulence. Nat Rev Microbiol. 2010;8(12):857–66. doi: 10.1038/nrmicro2457. [DOI] [PubMed] [Google Scholar]
- 19.Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, Mandin P, et al. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009;139(4):770–9. doi: 10.1016/j.cell.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 20.Smith KD, Lipchock SV, Ames TD, Wang J, Breaker RR, Strobel SA. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat Struct Mol Biol. 2009;16(12):1218–23. doi: 10.1038/nsmb.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaumont HJ, Gallie J, Kost C, Ferguson GC, Rainey PB. Experimental evolution of bet hedging. Nature. 2009;462(7269):90–3. doi: 10.1038/nature08504. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Lopez A, Castano-Avila S, Maynar-Moliner FJ, Urturi-Matos JA, Manzano-Ramirez A, Martin-Lopez HP. Tertiary peritonitis: as difficult to define as it is to treat. Cir Esp. 2011 [Google Scholar]
- 23.Chromik AM, Meiser A, Holling J, Sulberg D, Daigeler A, Meurer K, et al. Identification of patients at risk for development of tertiary peritonitis on a surgical intensive care unit. J Gastrointest Surg. 2009;13(7):1358–67. doi: 10.1007/s11605-009-0882-y. [DOI] [PubMed] [Google Scholar]