Abstract
Death of Central Nervous System (CNS) neurons following traumatic brain injury (TBI) is a complex process arising from a combination of factors, many of which are still unknown. It has been found that inhibition of transient receptor potential (TRP) channels constitutes an effective strategy for preventing death of CNS neurons following TBI. TRP channels are classified into seven related subfamilies, most of which are Ca2+ permeable and involved in many cellular functions, including neuronal cell death. We hypothesized that TRP channels of the TRPC subfamily may be involved in post-TBI pathophysiology and that the compound 5-isopropyl-2-methylphenol (carvacrol), by inhibition of TRP channels, may exert neuroprotective effect after TBI. To test these suppositions, carvacrol was given to mice after TBI and its effect on their functional recovery was followed for several weeks. Our results show that neurological recovery after TBI was significantly enhanced by application of carvacrol. To better define the type of the specific channel involved, the effect of carvacrol on the extent and speed of recovery after TBI was compared among mice lacking TRPC1, TRPC3, or TRPC5, relative to wild type controls. We found that neurological recovery after TBI was significantly enhanced by combining carvacrol with TRPC1 elimination, but not by the absence of TRPC3 or TRPC5, showing a synergistic effect between carvacrol application and TRPC1 elimination. We conclude that TRPC1-sensitive mechanisms are involved in TBI pathology, and that inhibition of this channel by carvacrol enhances recovery and should be considered for further studies in animal models and humans.
Key words: head trauma, neuronal cell death, TBI
Transient receptor potential (TRP) channels are essential components of biological sensors that detect environmental changes in response to a wide range of stimuli, such as temperature changes, mechanical force and mechanical injury, and changes in the chemical state of cells. They form an evolutionary conserved cation channel family consisting of seven subfamilies, which include nearly 30 human members.1 The founding member of this family was discovered in Drosophila and was designated TRP.2 TRP channels are classified into seven related subfamilies as described in detail in several reviews.1,3 TRP channels were shown to be involved in many cellular functions, including inflammation, cell adhesion, growth, differentiation, proliferation, death, and cell polarity.4–7
Most members of this superfamily are Ca2+ permeable non-selective ion channels, and as such may be crucially involved in the pathological events following traumatic and ischemic brain injury.
There is increasing evidence suggesting the involvement of TRP channels in neurotoxicity. In particular, TRPM2 and TRPM7 from the TRPM channel subfamily are involved in neurotoxicity. Both channels are permeable to Ca2+ and activated by reactive oxygen species or oxygen-glucose deprivation, two hallmarks of brain injury, and these channels have been associated with cell death caused by anoxia,8 oxidative stress,9 and stroke.10 TRPM7 was shown to mediate anoxic neuronal cell death,11 and it was reported that suppression of hippocampal TRPM7 prevented neuronal death following global cerebral ischemia.12
The mammalian canonical TRP channels (TRPC) are a subfamily of Ca2+ permeable nonselective cation channels, which are expressed in many tissues, including the brain. Members of this subfamily were found because of their sequence similarity to the Drosophila light-activated TRP channels. Overexpression of TRPC1 was shown to inhibit the activity of tumor necrosis factor (TNF)-induced transcription factor, NF-kB,13 and to be involved in cell proliferation. Such cellular effect was shown to be associated with improved functional recovery after TBI.14,15 In contrast, a high level of apoptosis was observed in response to ischemic-reperfusion injury in cells overexpressing TRPC3,16 and TRPC1 was shown to be involved in glutamate mediated neurotoxicity.17
Sensitivity to a wide range of small molecules produced by plants is a characteristic feature of TRP channels. A large variety of natural products are known to modulate TRP channels of the TRPV, TRPM, and TRPA subfamilies.18 The compound 5-isopropyl-2-methylphenol (carvacrol), a major constituent of the essential oil of the popular culinary herb Origanum vulgare (oregano) shows a complex pharmacological profile. It activates the thermosensitive TRP channel, TRPV3 and TRPA1,19 and inhibits non-thermo TRP channels, the Drosophila TRPL (a member of the TRPC family) and mammalian TRPM7.20 It is also anti-inflammatory by inhibiting cyclooxygenase (COX)-2 expression and activation of poly (ADP-ribose) polymerase (PARP).21 Even at high doses, carvacrol does not exert toxic effects,22,23 and, therefore, carvacrol is a natural compound with potential neuroprotective features.
We hypothesized that TRP channels of the TRPC subfamily may also be involved in pathophysiology after traumatic brain injury (TBI), and that carvacrol, by inhibition of TRP channels, may exert neuroprotective effects after TBI. To test these suppositions, carvacrol was given to mice after TBI. Mice were subjected to closed head injury (CHI) under isoflurane anesthesia, confirmed by loss of pupillary reflex, using a weight-drop device that falls over the left hemisphere, as previously described in detail.24 Carvacrol (Sigma-Aldrich, St Louis MO) was dissolved in anhydrous ethanol:emulphor:saline (1:1:18) and injected intraperitoneally 1 h after induction of injury at 100 μL per 10 g body weight.
One hour after CHI, the functional status of the mice was evaluated according to a set of 10 neurobehavioral tasks (neurological severity score [NSS]) that test reflexes, alertness, coordination, and motor abilities. One point was awarded for absence of reflex or failure to perform a particular task. Hence, a score of 10 reflected maximal neurological impairment. Immediately after evaluation of NSS at 1 h post-injury, mice were randomly assigned to vehicle or drug treatment. and NSS was evaluated again from 24 h up to 21 days. The effect of carvacrol treatment on functional recovery was followed for several weeks.
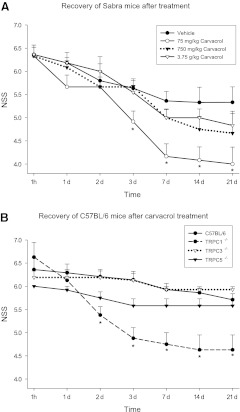
We first performed a dose-response study (0.5–25 mM, 75–3750 mg/kg) in Sabra mice (strain of the Hebrew University) and found that carvacrol at a dose of 75 mg/kg significantly improved the neurological outcome after CHI (see Fig. 1A) whereas higher doses were ineffective. Such a heterogeneous, bimodal dose-response curve is a typical behavior found for other natural products, either of plant or animal origin. Therefore, the cannabinoid (CB1) receptor agonist Δ9-tetrahydrocannabinol exerted a biphasic control of fear coping strategies,25 and when the endocannabinoid 2-arachidonoylglycerol was given to mice after CHI, only a dose of 5 but not 1 or 10 mg/kg significantly improved neurological outcome.26
FIG. 1.
(A) Effect of different doses of carvacrol and vehicle on Sabra mice. Mice were subjected to closed head injury (CHI), 1 h later, neurological severity score (NSS) was evaluated and mice were treated with a single dose of carvacrol or vehicle. NSS was followed up to 21 days. *p<0.01 when compared with vehicle-treated mice. (B) Effect of carvacrol on the recovery of TRPC−/− mice after CHI. NSS of mice treated with carvacrol. Mice were subjected to CHI, 1 h later, NSS was evaluated and mice were treated with a single dose of 75 mg/kg carvacrol. NSS was followed up to 21 days. *p<0.005 when compared with wild type (WT) mice. Error bars are standard error of the mean.
After establishing an effective dose, we tested the effect of carvacrol on C57BL/6 (n=7–9) mice lacking either TRPC1,27 TRPC3,28 or TRPC5, and compared the extent and speed of recovery after injury with that of their wild type (WT) C57BL/6 controls. Although TRPC5−/− mice showed a trend toward better recovery than did WT mice (Fig. 1B), the absence of TRPC3 seemed to impair the recovery process and showed no recovery in certain motor tests. Interestingly, genetic elimination of TRPC1 in combination with carvacrol treatment resulted in a robust significant functional recovery (Fig. 1B). From 2 days after brain injury until the end of the experiment, carvacrol-treated TRPC1−/− mice had highly significant (Mann–Whitney U test, p<0.005) better (i.e., lower) NSS scores than did WT mice (day 2: 5.4±0.2 vs. 6.2±0.2 and day 21: 4.6±0.3 vs. 5.7±0.1).
One of the individual tests that comprise the NSS is walking on beams of different widths. Following carvacrol treatment, only TRPC1−/− mice were able to regain the ability to walk on the 3 cm beam (only 12.5% failed this task from day 3 on), whereas 58% of the TRPC5−/− mice and 93% of the TRPC3−/− mice still failed to perform this task at the end of the 3rd week post-injury. In the WT group, the failure rate was 93% on day 3 and dropped to 71% by day 21. Interestingly, when testing the more difficult task, walking on a 1 cm wide beam, all WT, as well as all TRPC3−/− and TRPC5−/− mice, failed, whereas from 48 h post-injury, 40% of the TRPC1−/− mice were able to perform the task.
In order to examine the possibility that the absence of TRPC1 is responsible for the improved recovery, we repeated the experiments with TRPC1−/− and WT mice (n=11) in the absence of treatment, to evaluate spontaneous recovery. There was a non-significant trend toward better recovery in TRPC1−/− mice (Fig. 2). Based on the experimental results, we conclude that carvacrol treatment and TRPC1 elimination have synergistic neuroprotective effects.
FIG. 2.
Spontaneous recovery of wild type (WT) and TRPC1−/− mice (no treatment with carvacrol), same experimental paradigm as in Figure 1, but without treatment.
Our experiments identified additional ion channels involved in neurological recovery after head trauma, namely TRPC1 and TRPC3, which have opposing effects. TRPC3 seems to be important in the recovery of motor functions, whereas the absence of TRPC1 is not beneficial by itself, but becomes enhanced after carvacrol treatment.
The TRPC1 channel was shown to be involved in glutamate-mediated toxicity in hippocampal cultures.17 Previous studies have shown that TRP channels are involved in neurotoxicity after TBI. An additional study revealed that carvacrol is a potent antagonist of TRPM7, a channel that is activated under conditions of glucose-oxygen deprivation, probably by released reactive oxygen species. Moreover, it was shown that carvacrol is also a potent antagonist of the Drosophila TRPL channel, which belongs to the TRPC subfamily, whereas TRPC1, a member of this subfamily, was shown to participate in glutamate-mediated toxicity of hippocampal neurons. Together, the above mentioned findings suggest that treatment with carvacrol in addition to TRPC1 elimination might be neuroprotective after TBI.
In the present study, we first, as a proof of concept and dose-repsonse study, found that carvacrol significantly improved functional recovery of Sabra mice at the lowest dose tested, whereas TRPC1 elimination had only a marginal effect on neurological recovery after CHI, as compared with WT controls. Strikingly, the combination of carvacrol application combined with TRPC1 elimination largely improved recovery after CHI, and this effect was specific to TRPC1, and was not obtained by TRPC5 or TRPC3 elimination.
TRPC1 expressed in tissue culture cells does not produce a significant current in isolation. However, when expressed together with other signaling proteins/channels, a novel current appears (e.g., when co-expressed with TRPC5), suggesting that TRPC1 forms a heteromultimeric channel. Based on the results from the knockout animals, it is unlikely that carvacrol targets any of the tested TRPC channels. But surprisingly, the combination of carvacrol application and genetic elimination of TRPC1 exerts a synergistic neuroprotective effect. It is unlikely that carvacrol's effects are mediated by a single protein, but rather by multiple effectors, such as hypothermia induced by TRPV3 activation and protection from calcium overload by TRPM7 inhibition. As up-to-date interventions with a single pharmacological agent failed to show neuroprotective effects in human trials, our results point toward a strategy involving multiple targets. The TRPC channel usually forms heteromultimers; therefore, the absence of one TRPC protein can be compensated for by other TRPCs. Furthermore the pharmacological properties and biophysical properties are different depending upon the exact subunit composition. It is possible that by eliminating TRPC1 from a TRPC complex, the complex becomes more sensitive to carvacrol. Because of the complex biophysics of TRPC1, no specific pharmacological inhibitors were available to test this hypothesis.
Its polypharmacological profile makes carvacrol a promising compound for cytoprotective interventions. During the course of our study, an independent study was published. Carvacrol also showed protective effects in a middle cerebral artery occlusion mouse model. A dose similar (50 mg/kg) to the one used in our study reduced infarct volume and increased the level of phosphorylated Akt, an anti-apoptotic kinase.29 Akt and TRPC1 regulate each other bidirectionally, whereas TRPC1 can be directly activated by Akt,30 Akt activity is lower in TRPC1−/− mice.31 Another possible mechanism for the polymodal action of carvacrol is not by directly modulating TRP channels, but by modulating associated proteins, such as Homer or stromal interaction molecule (STIM)1.32
Our study demonstrates that both TRPC1 and TRPC3 are involved by opposing mechanisms in recovery after head trauma, such that whereas absence of TRPC3 prevents functional recovery, regardless of carvacrol treatment, elimination of TRPC1 is beneficial after treatment with carvacrol. The absence of TRPC5 does not seem to affect the intensity of or recovery from head trauma. In addition, we could show that carvacrol is a novel neuoprotective agent, the effects of which are potentiated by the absence of TRPC1.
Our results show that TRPC1 elimination in combination with carvacrol treatment is beneficial after CHI. Whereas direct pharmacological inhibition of TRPC1 is not a promising strategy, disrupting TRPC1 containing complexes with high-affinity peptides should be similar to genetic elimination of TRPC1. This strategy was successfully applied to prevent the neurotoxic effects of binding of NMDA-receptors to PDZ domains.33
Carvacrol treatment in combination with disruption of TRPC1 complexes should be further explored in an animal model, with possible relatively rapid translation to the clinic, because of the safety profile of carvacrol.
Acknowledgments
This study was approved by the Institutional Animal Care Committee of the Hebrew University. We thank Ben Katz for critical reading of the manuscript. This research was supported by grants from the Rosetrees Trust (to Dr. Minke) and by the Intramural Research Program of the National Institutes of Health (NIH) (project Z01-ES-101684 to Dr. Lutz Birnbaumer).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Parnas M. Peters M. Minke B. 6.4 Biophysics of TRP channels. In: Edward H.E., editor. Comprehensive Biophysics. Elsevier: Amsterdam; 2012. pp. 68–107. [Google Scholar]
- 2.Minke B. Wu C. Pak W.L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 3.Nilius B. Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramowitz J. Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dadon D. Minke B. Cellular functions of transient receptor potential channels. Int. J. Biochem. Cell Biol. 2010;42:1430–1445. doi: 10.1016/j.biocel.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller B.A. The role of TRP channels in oxidative stress-induced cell death. J. Membr. Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- 7.Nishida M. Hara Y. Yoshida T. Inoue R. Mori Y. TRP channels: molecular diversity and physiological function. Microcirculation. 2006;13:535–550. doi: 10.1080/10739680600885111. [DOI] [PubMed] [Google Scholar]
- 8.Schuhmann M.U. Mokhtarzadeh M. Stichtenoth D.O. Skardelly M. Klinge P.M. Gutzki F.M. Samii M. Brinker T. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol Res. 2003;25:481–491. doi: 10.1179/016164103101201896. [DOI] [PubMed] [Google Scholar]
- 9.Hara Y. Wakamori M. Ishii M. Maeno E. Nishida M. Yoshida T. Yamada H. Shimizu S. Mori E. Kudoh J. Shimizu N. Kurose H. Okada Y. Imoto K. Mori Y. LTRPC2 Ca 2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 10.Tseveleki V. Rubio R. Vamvakas S.S. White J. Taoufik E. Petit E. Quackenbush J. Probert L. Comparative gene expression analysis in mouse models for multiple sclerosis, Alzheimer's disease and stroke for identifying commonly regulated and disease-specific gene changes. Genomics. 2010;96:82–91. doi: 10.1016/j.ygeno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aarts M. Iihara K. Wei W.L. Xiong Z.G. Arundine M. Cerwinski W. MacDonald J.F. Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 12.Sun H.S. Jackson M.F. Martin L.J. Jansen K. Teves L. Cui H. Kiyonaka S. Mori Y. Jones M. Forder J.P. Golde T.E. Orser B.A. Macdonald J.F. Tymianski M. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- 13.Marasa B.S. Rao J.N. Zou T. Liu L. Keledjian K.M. Zhang A.H. Xiao L. Chen J. Turner D.J. Wang J.Y. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF- k B activation through Ca 2+ influx. Biochem. J. 2006;397:77–87. doi: 10.1042/BJ20060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beni S.M. Tsenter J. Alexandrovich A.G. Galron–Krool N. Barzilai A. Kohen R. Grigoriadis N. Simeonidou C. Shohami E. CuZn-SOD deficiency, rather than overexpression, is associated with enhanced recovery and attenuated activation of NF-kappaB after brain trauma in mice. J. Cereb. Blood Flow Metab. 2006;26:478–490. doi: 10.1038/sj.jcbfm.9600209. [DOI] [PubMed] [Google Scholar]
- 15.Panikashvili D. Mechoulam R. Beni S.M. Alexandrovich A. Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J. Cereb. Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- 16.Shan D. Marchase R.B. Chatham J.C. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am. J. Physiol. Cell Physiol. 2008;294:C833–C841. doi: 10.1152/ajpcell.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanan K.L. Irmady K. Subramaniam S. Unsicker K. von Bohlen und Halbach O. Evidence that TRPC1 is involved in hippocampal glutamate-induced cell death. Neurosci. Lett. 2008;446:117–122. doi: 10.1016/j.neulet.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Vriens J. Appendino G. Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 19.Xu H. Delling M. Jun J.C. Clapham D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 20.Parnas M. Peters M. Dadon D. Lev S. Vertkin I. Slutsky I. Minke B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009;45:300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotta M. Nakata R. Katsukawa M. Hori K. Takahashi S. Inoue H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J. Lipid Res. 2010;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen A. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol. Int. J. Toxicol. 2006;25(Suppl. 1):29–127. doi: 10.1080/10915810600716653. [DOI] [PubMed] [Google Scholar]
- 23.De Vincenzi M. Stammati A. De Vincenzi A. Silano M. Constituents of aromatic plants: carvacrol. Fitoterapia. 2004;75:801–804. doi: 10.1016/j.fitote.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Flierl M.A. Stahel P.F. Beauchamp K.M. Morgan S.J. Smith W.R. Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009;4:1328–1337. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- 25.Metna–Laurent M. Soria–Gómez E. Verrier D. Conforzi M. Jégo P. Lafenêtre P. Marsicano G. Bimodal control of fear-coping strategies by CB1 cannabinoid receptors. J. Neurosci. 2012;32:7109–7118. doi: 10.1523/JNEUROSCI.1054-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panikashvili D. Simeonidou C. Ben–Shabat S. Hanus L. Breuer A. Mechoulam R. Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 27.Liu X. Cheng K.T. Bandyopadhyay B.C. Pani B. Dietrich A. Paria B.C. Swaim W.D. Beech D. Yildrim E. Singh B.B. Birnbaumer L. Ambudkar I.S. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17,542–17,547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann J. Dragicevic E. Adelsberger H. Henning H.A. Sumser M. Abramowitz J. Blum R. Dietrich A. Freichel M. Flockerzi V. Birnbaumer L. Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H. Zhang Z.L. Chen J. Pei A. Hua F. Qian X. He J. Liu C.F. Xu X. Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PLoS One. 2012;7:e33584. doi: 10.1371/journal.pone.0033584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao H. Peng F. Dhillon N. Callen S. Bokhari S. Stehno–Bittel L. Ahmad S.O. Wang J.Q. Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J. Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanou N. Schakman O. Louis P. Ruegg U.T. Dietrich A. Birnbaumer L. Gailly P. Trpc1 ion channel modulates phosphatidylinositol 3-kinase/Akt pathway during myoblast differentiation and muscle regeneration. J. Biol. Chem. 2012;287:14,524–14,534. doi: 10.1074/jbc.M112.341784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan J.P. Lee K.P. Hong J.H. Muallem S. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiol (Oxf) 2012;204:238–247. doi: 10.1111/j.1748-1716.2011.02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook D.J. Teves L. Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]