Abstract
IL-27 modulates inflammatory responses by influencing cytokine secretion and CD4 T cell differentiation. Recently, IL-27 was demonstrated to inhibit HIV replication by inducing type I interferon (IFN) expression and subsequent IFN-dependent expression of apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like (APOBEC)-3 family members, a group of antiviral cytidine deaminases. To characterize other anti-viral genes modulated by IL-27, we examined another IFN-responsive gene: tetherin/bone marrow stromal cell antigen 2 (BST-2). Our study shows that IL-27 can directly induce BST-2 expression, independently of an intermediary type I IFN response. Quantitative RT-PCR analysis demonstrated IL-27-induced BST-2 mRNA expression as early as 2h after exposure of cells to IL-27. In the presence of the type I IFN-neutralizing protein, B18R, IL-27-induced BST-2 expression was maintained, demonstrating that IFN is not an intermediary in IL-27-induced BST-2. Taken together, our findings identify a novel function of IL-27 as a direct stimulator of BST-2 expression.
Interleukin-27 (IL-27) is an immunoregulatory cytokine that drives innate immune responses and adaptive immunity. IL-27 is a member of the IL-12 family of cytokines, comprised of molecules sharing subunits and receptor chain components. Produced by activated monocytes, macrophages and dendritic cells, IL-27 acts on a wide variety of cell types, with expression of the receptor subunits, IL-27Rα (WSX-1/TCCR) and gp130, reported in endothelial cells, mast cells, B cells, monocytes, Langerhan's cells, dendritic cells, and T cells1,2,3. Previous work demonstrated the ability of IL-27 to induce a similar profile of anti-viral genes to that of IFN-α4. Furthermore, the anti-viral gene profile induced by IL-27 inhibited the replication of HIV in both CD4 T cells and monocytes/macrophages4,5. This anti-HIV function of IL-27 was attributed to induction of the antiviral family of APOBEC cytidine deaminases, via an intermediate induction of type I IFN5. Our study identifies BST-2 (also known as CD317/tetherin) as an IL-27-inducible protein in HIV target cells: monocytes and T cells.
BST-2 is an interferon (IFN)-responsive host restriction factor expressed in various cell types6. Type I IFNs, IFN-α and IFN-β, play a key role in host antiviral defenses by upregulating expression of antiviral genes, like BST-2, which inhibits dissemination of virus7,8. BST-2 physically ‘tethers' or retains budding virions at the cell surface, restricting virus release and ongoing infection8. Indeed, two independent studies showed that BST-2 prevents the release of HIV, and that the viral accessory protein Vpu, could counteract this activity9,10. Additionally, BST-2 prevents the release of a broad spectrum of enveloped viruses, including other retroviruses, filoviruses, arenaviruses, paramyxovirus, gamma-herpesviruses, and rhabdoviruses11,12,13,14,15,16. The ability of BST-2 to tether this broad group of viruses is driven by common virus features, including lipid envelopes and budding through cholesterol-rich domains of the plasma membrane where BST-2 is concentrated17. Thus, expression of BST-2 can have an important influence on virus-host cell membrane interactions. Regulation of BST-2 expression is not well defined, and differences in expression levels on monocytes and T cells have been reported6,18,19,20. Furthermore, although BST-2 is widely recognized as an IFN-responsive gene, evidence exists to support the role for novel stimuli and signaling cascades leading to BST-2 expression6,21,22,23.
It has been shown that IL-27 can induce type I IFN-responsive genes in human macrophages, an effect that is dependent on intermediary IFN-α/β production5. Since viruses have mechanisms to block type I IFN expression and intracellular signaling pathways, the existence of other mechanisms regulating typical IFN-responsive genes is critical to anti-viral responses. Previous studies have challenged the notion that BST-2 is strictly a type I IFN-responsive gene. One study demonstrated that activated intracellular signalling proteins IRF-3 and IRF-7 can induce BST-2 expression in virus-infected cells, independently of IFN expression21. Analysis of the BST-2 promoter indicated binding sites for STAT3, in addition to IFN-responsive elements, pointing to a potential role for STAT3-activating cytokines in BST-2 regulation6,22. Our data provides the first evidence that a cytokine can induce expression of BST-2, independently of type I IFN intermediates. We show the immunoregulatory cytokine IL-27 can directly upregulate intracellular and cell surface expression of BST-2 on human monocytes and T cells.
Results
IL-27 induces BST-2 cell surface expression on human monocytes and T cells
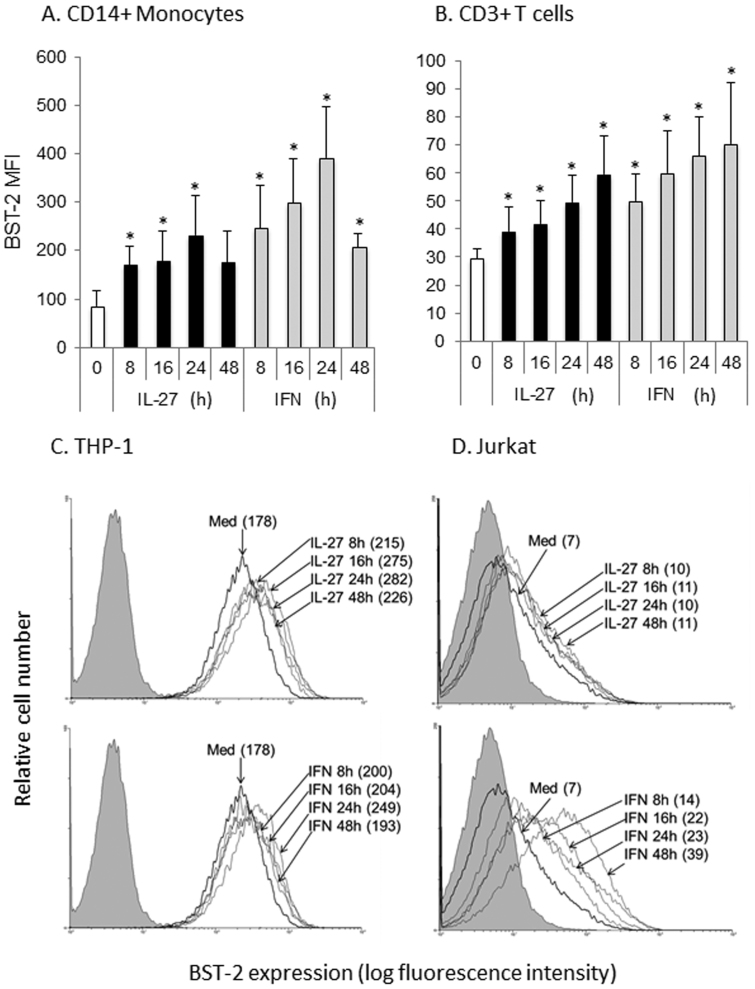
IL-27 was previously characterized to induce a similar profile of anti-viral gene expression to that of IFN-α in monocyte-derived macrophages and CD4 T cells4. Furthermore, Greenwell-Wild et al. showed IL-27 could induce expression of the type I IFN-responsive, anti-viral APOBEC family of cytidine deaminases5. Therefore, we reasoned that other anti-viral proteins may be modulated by IL-27 stimulation. Since BST-2 is a type I IFN-responsive protein, we investigated the impact of IL-27 on surface BST-2 expression. We previously found that recombinant IL-27 is biologically active on human monocytes at doses ranging from 50 to 200 ng/mL, with maximal responsiveness at ~100 ng/mL24. In this study we treated cells with 120 ng/mL of IL-27, a dose previously demonstrated to induce cytokine expression in human cells25. Considering that we previously observed serum IL-27 levels ranging from 0 to 50 ng/mL26, the dose of recombinant cytokine used herein is close to the physiological range expected in circulation. PBMC (CD14+ monocytes: Fig. 1A and CD3+ T cells: Fig. 1B), THP-1 cells (Fig. 1C) and Jurkat cells (Fig. 1D) were either left untreated or stimulated with IL-27 for times ranging from 8 to 24 h. As a positive control, cells were also treated with universal type I IFN, well-characterized to induce BST-2 expression6,10. BST-2 expression was measured on CD14+ monocytes and CD3+ T cells. We observed highest basal expression of BST-2 in cells of the monocytic lineage (CD14+ primary monocytes and THP-1 cells, Fig. 1A and C), while lower basal expression of BST-2 was observed in primary T cells (Fig. 1B), with the lowest expression of BST-2 on Jurkat cells (Fig. 1D). We observed statistically significant induction of BST-2 surface expression in as early as 8 h after IL-27 treatment. Additionally, IL-27 and IFN showed similar kinetics of BST-2 induction in both primary monocytes and T cells (Fig. 1A and B). A similar increase of IL-27-induced BST-2 in primary monocytes was observed in the THP-1 monocytic cell line (Fig. 1C). However, a relatively small increase in IL-27-induced BST-2 was observed in the Jurkat T cell line (Fig. 1D) compared to primary T cells (Fig. 1B). In contrast to previous studies20, we observed induction of BST-2 expression on Jurkat cells in response to IFN stimulation. Taken together, our results show that basal expression of BST-2 is higher on monocytes compared to T cells, and that IL-27 can significantly enhance surface BST-2 expression on both primary monocytes and T cells.
Figure 1. IL-27- and IFN-induced BST-2 cell surface expression on monocytes and T cells.
PBMC were cultured in the presence of IL-27 (120 ng/mL) or IFN (1000 U/mL) for time courses indicated, followed by surface staining for BST-2 and cell surface markers CD14 (monocytes, panel A) or CD3 (T cells, panel B). Acquired cells were first gated on live cells, followed by cell type specific gating on surface markers CD14 or CD3. Bars represent the average MFIs from 4 different subjects. Statistical significance of the IL-27/IFN treatment compared to untreated (0) is denoted with * when p<0.05. (C) THP-1 and (D) Jurkat cells were cultured with a time course of IL-27 (top panel) or IFN (bottom panel), and MFIs are denoted in brackets. Grey shaded histograms represent background autofluorescence of unstained cells, bold line histograms denoted ‘Med' represent cells cultured in medium alone (untreated).
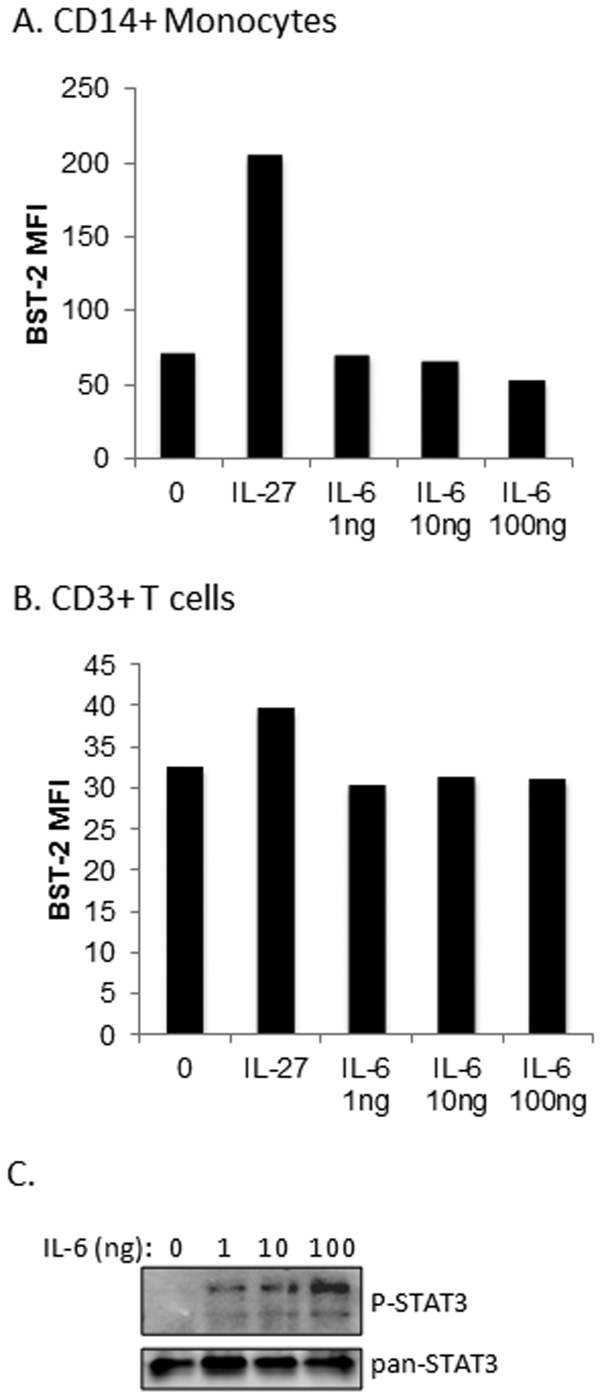
Despite similar signaling pathways to IL-27, IL-6 does not induce BST-2 on monocytes or T cells
Previous analyses indicated the presence of STAT-responsive elements in the BST-2 promoter21,22, implicating STAT transcription factors as potential drivers of BST-2 expression. IL-27 and the related cytokine family member, IL-6, signal through the common receptor signalling chain, gp130, to activate STAT327. Since a previous report identified the presence of a STAT3 binding sequence in the BST-2 promoter22, and IL-27 and IL-6 both induce STAT3 activation27, we reasoned that, like IL-27, IL-6 might enhance BST-2 expression. However, no increase in surface expression of BST-2 was observed in primary monocytes or T cells treated with IL-6 (Fig. 2A and B). The bioactivity of the recombinant IL-6 preparation was confirmed by IL-6-induced STAT3 phosphorylation in PBMC (Fig. 2C). Despite significant STAT3 phosphorylation in response to IL-6, our data indicated that BST-2 is not an IL-6-responsive gene.
Figure 2. Despite similar signaling pathways to IL-27, IL-6 does not induce BST-2 on monocytes or T cells.
PBMC were cultured with IL-27 for 16 h or with increasing doses (as indicated) of IL-6 for 16 h, followed by surface staining for BST-2 and surface markers CD14 or CD3. Live cells were gated, followed by (A) CD14-positive (CD14+) cell gating or (B) CD3-positive (CD3+) cell gating. (C) Western blot analysis for phospho-STAT3 (P-STAT3) and total STAT3 (pan-STAT3) expression in PBMC lysates.
IL-27 enhances intracellular expression of BST-2
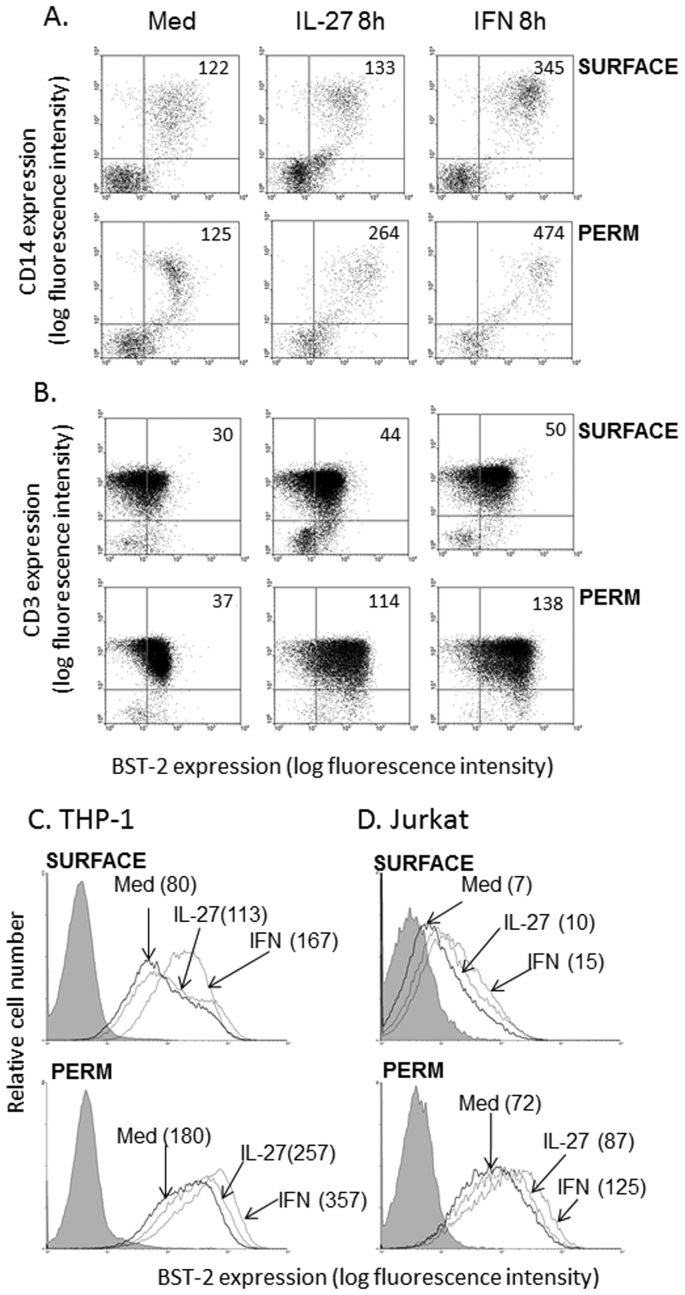
Since we observed early upregulation of surface BST-2 in response to IL-27 (8 h treatment), and a similar pattern of IL-27-induced BST-2 kinetics to that of IFN-induced BST-2, we decided to measure intracellular BST-2 expression to further confirm that IL-27 mediates induction of BST-2 expression. Accordingly, we stimulated PBMC with IL-27 or IFN for 8 h, followed by staining for either cell surface BST-2 or permeabilization and staining for quantification of total cellular BST-2 expression. Subsequent gating on CD14+ monocytes (Fig. 3A) and CD3+ T cells (Fig. 3B) permitted a comparison of BST-2 induction only on the cell surface (non-permeabilized BST-2 staining, Fig. 3A and B, top panels) versus total cellular levels of BST-2 (permeabilized BST-2 staining, Fig. 3A and B, bottom panels). In CD14+ monocytes (Fig. 3A) and CD3+ T cells (Fig. 3B), we observed a striking increase in IL-27-mediated and IFN-mediated BST-2 upregulation, with greater than 2-fold increases over basal levels in IL-27-stimulated permeabilized cells. Furthermore, we observed a stronger IL-27-mediated increase of BST-2 in permeabilized cells compared to surface stained cells. When we performed a similar staining comparison in THP-1 and Jurkat cell lines, we observed relatively smaller increases in BST-2 expression upon permeabilization of the cells (Fig. 3C and D). The relatively fast kinetics of IL-27-induced BST-2 expression, suggests that IL-27 directly induces BST-2 expression.
Figure 3. IL-27 enhances BST-2 expression in permeabilized cells.
PBMC were stimulated with IL-27 or IFN for 8 h, then in parallel, surface stained or permeabilized and intracellularly stained for BST-2 expression. (A) Co-staining for BST-2 and CD14 on non-permeabilized PBMC (top panel, ‘surface') and permeabilized PBMC (bottom panel, ‘perm'). Mean fluorescence intensities (MFIs) for CD14+ monocytes are denoted in upper right quadrants of dot plots. (B) Co-staining for BST-2 and CD3 on non-permeabilized PBMC (top panel) and permeabilized PBMC (bottom panel). MFIs for CD3+ T cells are denoted in upper right quadrants of dot plots. Histograms represent similar stimulation and staining (non-permeabilized versus permeabilized) procedures performed in THP-1 (C) and Jurkat (D) cell lines. Grey shaded histograms represent background autofluorescence of unstained cells, bold line histograms denoted ‘Med' represent cells cultured in medium alone (untreated).
IL-27 induces BST-2 mRNA expression
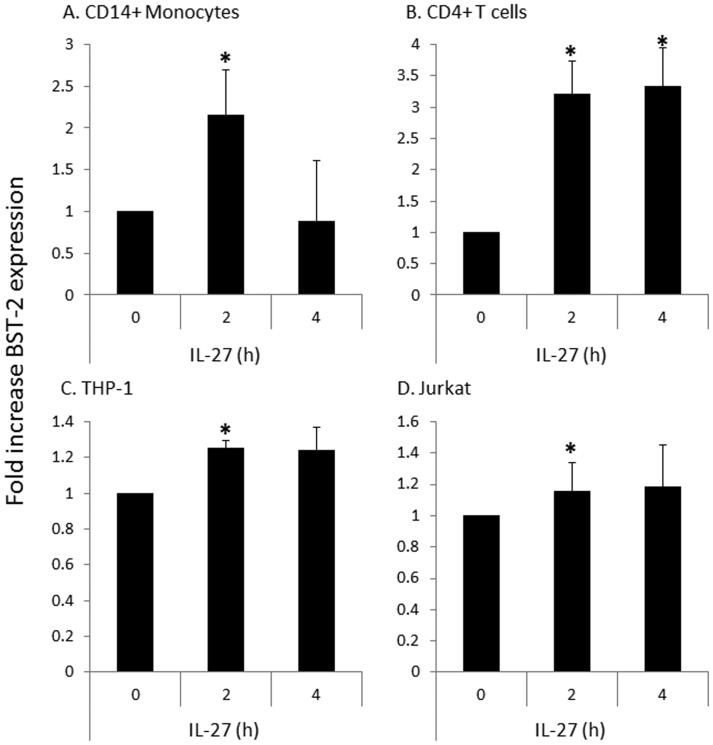
To further confirm that IL-27 induces expression of BST-2, we isolated monocytes and CD4+ T cells from PBMC fractions and performed a short time course of IL-27 treatment (2–4 h). Subsequent quantitative RT-PCR analysis indicated IL-27-mediated induction of BST-2 mRNA expression in primary monocytes and T cells, observed as a significant increase in BST-2 transcript as early as 2 h post exposure to IL-27 (Fig. 4A and B). Similar IL-27 treatments were also performed using THP-1 and Jurkat cell lines, and revealed significant BST-2 induction at 2 h, although to a lesser magnitude than that of primary cells (Fig. 4C and D).
Figure 4. IL-27 enhances mRNA expression of BST-2.
Quantitative RT-PCR analysis was performed on purified monocytes and T cells from PBMC fractions, as well as THP-1 and Jurkat cell lines. Cells were treated with a short time course of IL-27 treatment (2–4 h), followed by amplification of BST-2 transcript expression. 18S rRNA served as the housekeeping gene to which each sample was normalized. Data is presented as fold increase in BST-2 expression relative to untreated (0) cells. Statistical significance of the IL-27 treatment compared to untreated is denoted with * when p<0.05. Data shown are representative averages of two primary cell (CD14 monocytes and CD4 T cells) donors and four cell line biological replicates.
IL-27 induces BST-2 expression independently of a type I IFN intermediate
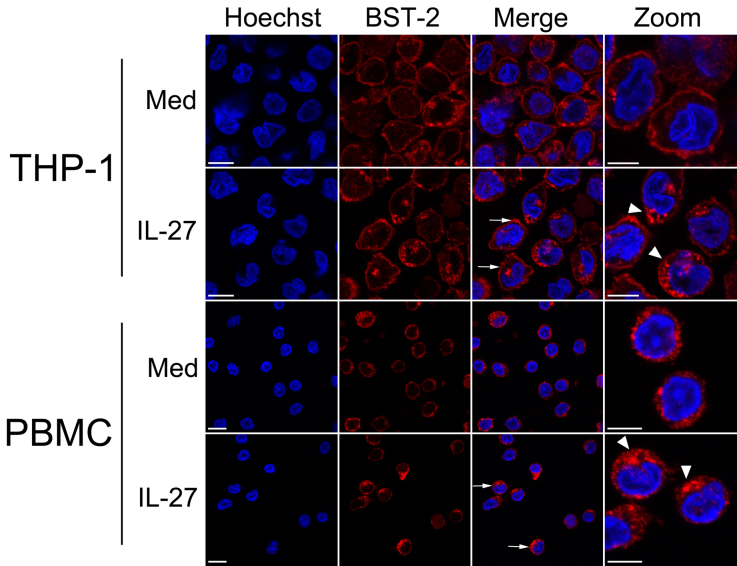
IL-27 was previously shown to induce antiviral gene expression by two mechanisms: one, an indirect effect via inducing type I IFN production5, and the other, a direct mechanism whereby IL-27 induces an IFN-like gene expression profile, in the presence of IFN-neutralizing antibodies4. To address whether the effect of IL-27 on BST-2 expression is direct, we first examined the intracellular expression of BST-2 in response to IL-27. We examined BST-2 localization by confocal immunofluorescence microscopy in THP-1 cells (Fig. 5, top 2 rows) and PBMC (Fig. 5, bottom 2 rows). Since IL-27 had relatively small effects on BST-2 expression in Jurkat cells, this cell line was omitted from these analyses. These experiments demonstrated IL-27 enhanced BST-2 expression in all cells and that the BST-2 was localized predominantly to the plasma membrane and cytoplasm (Fig. 5, rows 2 and 4, arrows). As observed in the zoomed in images (right-most panel, arrowheads), IL-27 stimulation for 8 h resulted in increased punctate BST-2 staining in both THP-1 cells and PBMC. This supports our above findings, in particular those detected by flow cytometry in permeabilized cells (Fig. 3), that IL-27 enhances BST-2 expression.
Figure 5. Subcellular localization of IL-27-induced BST-2.
THP-1 cells (top 2 rows) and PBMC (bottom 2 rows) were cultured in the presence/absence (Med, medium control) of IL-27 for 8 h prior to fixation and permeabilization. Cells were stained for BST-2 (red fluorescence) and with Hoechst 33342 to identify nuclei (blue fluorescence). In the merged panels, arrows indicate representative cells having increased plasma membrane-localized BST-2. In the panels containing zoomed merged images, arrowheads indicate perinuclear cytoplasmic puncta where BST-2 is concentrated. Scale bars are 10 μm. Data shown are representative of two primary monocyte donors and four cell line biological replicates.
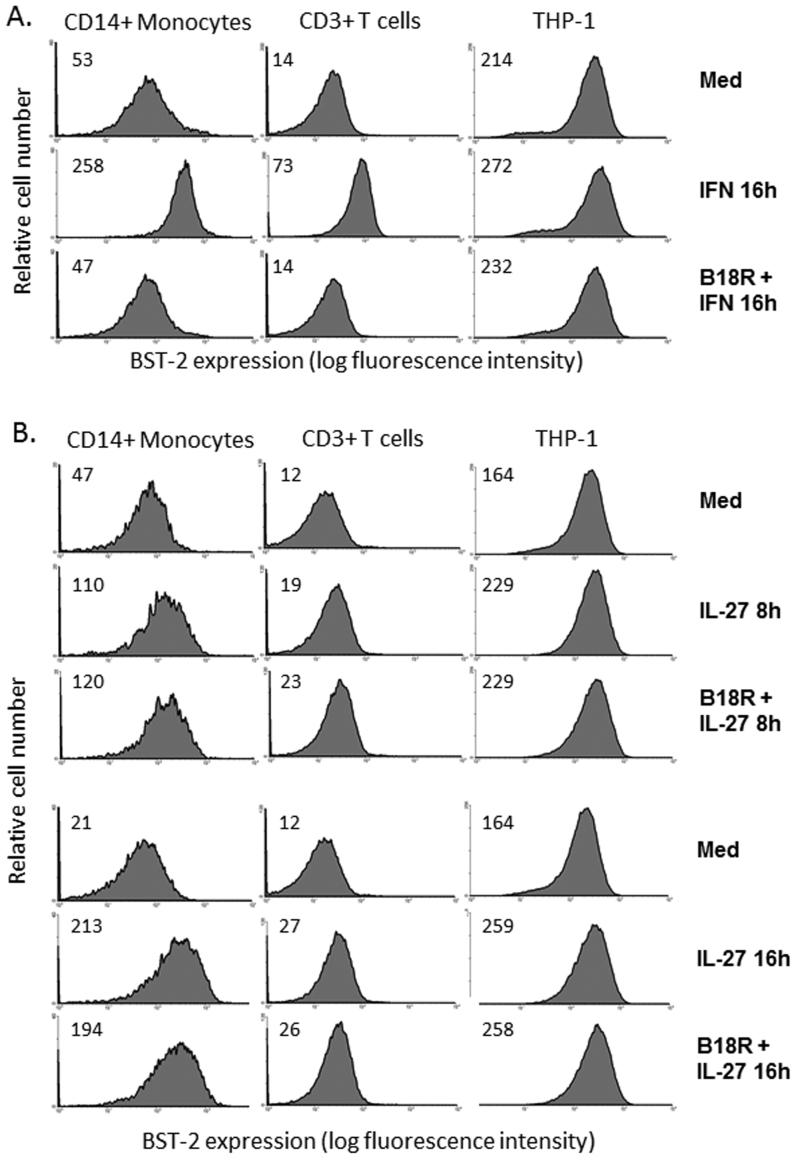
To investigate whether type I IFN was responsible for IL-27-induced BST-2 expression, we employed a soluble vaccinia virus-encoded type I-IFN receptor (B18R), previously shown to have potent IFN neutralizing effects21,28. PBMC and THP-1 cells were treated with B18R and universal type I IFN followed by analysis of surface expression of BST-2 by flow cytometry. In the presence of B18R (0.1 μg/mL), cells treated with IFN for 16 h showed complete abrogation of BST-2 induction (Fig. 6A). As an additional control, cells treated with IFN for 8 h showed a similar potent blockade of the IFN response in the presence of B18R (data not shown). By contrast, IL-27-mediated induction of BST-2 expression was not inhibited in the presence of B18R. As seen in Fig. 6B (top 3 rows), primary monocytes, T cells and THP-1 cells maintained IL-27-induced BST-2 expression after 8 h IL-27 stimulation in the presence of B18R. Similarly, in cells treated for 16 h with IL-27, in the presence of B18R, we did not observe significant decreases in IL-27-induced BST-2 expression (Fig. 6B, bottom 3 rows). Taken together, the data demonstrate that IL-27 induces BST-2 expression independently of a type I IFN intermediate.
Figure 6. IL-27 induces BST-2 expression independently of a type I IFN intermediate.
(A) To confirm potency of B18R for IFN inhibition, cells were cultured in medium alone (Med), in the presence of 1000 U/mL of IFN for 16 h (IFN 16 h), or the presence of 1 μg/mL of B18R + IFN (B18R + IFN 16 h). CD14+ cells gated from PBMC are shown in column 1, CD3+ cells gated from PBMC in column 2, and THP-1 cells in column 3. Histograms represent live gated cells denoted with mean fluorescence intensity of BST-2 staining in top left corners. (B) To observe the role of IFN in IL-27-induced BST-2 expression, cells were cultured in medium alone, in the presence of IL-27 for 8 h, or in the presence of B18R + IL-27 8 h (top 3 rows). To observe a later time point of induction, cells were also assayed 16 h, in the presence of IL-27 for 16 h, or in the presence of B18R + IL-27 16 h (bottom 3 rows). Histograms represent live gated cells denoted with mean fluorescence intensity of BST-2 staining in top left corners. Data are representative of 5 separate PBMC donors and 3 biological replicates for THP-1 cells.
Discussion
We define a novel role for IL-27 as a regulator of BST-2 expression, as evidenced by significant upregulation of BST-2 expression in response to IL-27 on human monocytes and T cells. The data support the notion that IL-27-induced BST-2 expression is a direct effect, as we observed upregulated mRNA expression of BST-2 as early as 2 h after IL-27 stimulation and increased intracellular and surface BST-2 staining after 8 h of IL-27 stimulation. We confirmed that IL-27-induced BST-2 expression occurs independently of type I IFN, as in the presence of an IFN-neutralizing protein (B18R), IL-27-induced BST-2 expression was maintained. This study is the first to identify IL-27 as an inducer of BST-2 expression, which represents a new anti-viral function for IL-27.
Consistent with the findings of others, we report greater steady-state levels of cell-surface BST-2 on monocytes versus T cells19,21,29. However, these results contrast previous studies which reported undetectable basal expression of BST-2 in primary CD3+ T cells18 and undetectable basal and IFN-inducible BST-2 in Jurkat cells20. Our work shows that type I IFN enhances BST-2 expression on primary T cells and the Jurkat T cell line, contrasting previous reports of weak IFN-induced BST-2 on lymphocytes from tonsil tissues18. These differences in BST-2 basal and inducible expression may be attributed to culture conditions and varying sensitivities of detection. Accordingly, we examined both surface and intracellular BST-2 induction by flow cytometry together with mRNA expression levels and, collectively, our data show that IL-27 induces lower BST-2 expression on T cells compared to that induced by type I IFN. Furthermore, we report that IL-27 can induce BST-2 expression on both primary human monocytes and THP-1 cells.
Upon identifying the ability of IL-27 to induce BST-2 expression, we decided to investigate whether IL-6 could induce BST-2 in the same way as IL-27, since both cytokines signal via gp130 to activate STAT327. We observed no IL-6-mediated induction of BST-2 on monocytes or T cells. Our finding was in accordance with previous results which observed no changes in induction of cell-surface BST-2 on PBMC in response to IL-619. The BST-2 promoter region encodes putative binding sites for STAT3 transcription factors, in addition to the IFN-response elements IRF-1/2, ISGF3, and GAS22. Furthermore, sequence alignment of human, mouse, and rhesus BST-2 promoters showed STAT binding sites to be conserved, indicating a likely dependency on STAT3 for effective BST-2 gene expression22. Since our data indicates that IL-27, but not IL-6, induces BST-2, it is likely that gp130-mediated STAT3 induction alone may not be sufficient for BST-2 upregulation. Indeed, virus-mediated induction of IRF-3 and IRF-7 were demonstrated to be sufficient for BST-2 upregulation in the absence of type I IFN21. IL-27 has also been shown to induce the activation of NF-κB24,25,30, for which a putative binding site has been identified in the BST-2 promoter21. However, stimulation of PBMC with TNF-α, an inducer of NF-κB activity, did not enhance BST-2 expression, while a dependency on STAT1 for IFN-induced BST-2 induction has been demonstrated21. Since we and others have shown that IL-27 induces activation of STAT1 in both monocytes and T cells, it is possible that this transcription factor may be involved in BST-2 induction25,27. Therefore a combination IL-27-mediated activation of STAT1/3 in addition to that of NF-κB may cooperate with other factors to influence BST-2 expression.
To extend our findings of upregulated surface BST-2 in response to IL-27, we performed analysis on total cellular content of BST-2 in permeabilized cells. Interestingly, we observed greater increases in IL-27-mediated upregulation of BST-2 at 8 h in permeabilized versus non-permeabilized cells showing that total cellular levels of BST-2 are increased in IL-27-stimulated compared to basal levels. The increased magnitude of IL-27-induced BST-2 expression was observed in both permeabilized primary monocytes and T cells; however, this trend was not as significant in the cell lines, THP-1 and Jurkat. It is possible that mechanisms of gene regulation are different in cell lines compared to primary cells, as a consequence of the immortal characteristics of these cells and unique differentiation lineages. BST-2 expression levels in Jurkat cells may not change significantly in response to IL-27; while instead, IL-27 may impact the BST-2 membrane recycling in these cells. Indeed, inducible BST-2 expression on T cells (both primary and cell line) has been previously reported to be inconclusive in the literature, and despite dedicated study to more precise mechanisms governing BST-2 expression specific to T cells, the events still remain unclear18,19.
To investigate the ability of IL-27 to directly induce BST-2 expression, we initially performed quantitative RT-PCR analysis on cells treated with IL-27. In these experiments we observed increased BST-2 gene expression in as early as 2 h after IL-27 stimulation. We performed confocal microscopy to assess expression levels as well as the subcellular localization of BST-2 following 8 h of IL-27 stimulation. Increased cell surface expression of BST-2 was readily seen in IL-27 stimulated cells. Additionally, we observed IL-27-stimulated cells to have enhanced intracellular expression of BST-2 that localized predominantly to a perinuclear vesicular compartment reminiscent of the Golgi (Fig. 5, arrows). Furthermore, the increase of BST-2 staining in punctate cytoplasmic structures, may represent the presence of BST-2 in secretory vesicles targeted for the plasma membrane. Indeed, BST-2 has been previously shown to localize intracellularly within elements of the secretory pathway and to the plasma membrane via lipid raft association6,17.
Two previous studies have investigated the impact of HIV infection on IL-27 expression. He et al. demonstrated a modest, albeit statistically significant, increase in IL-27 expression in HIV positive, treatment-naive patients31, while our own work indicated a trend in decreased in serum IL-27 expression associated with a relatively high viral load in HIV positive patients26. However, both of these reports were small-scale correlation studies, and whether or not HIV infection directly affects IL-27 expression has yet to be determined in in vivo or in vitro infection models. IL-27 is known to inhibit HIV replication via induction of IFN-dependent APOBEC proteins5. Furthermore, the anti-viral gene profile induced by IL-27 inhibited the replication of HIV in both CD4 T cells and monocytes/macrophages4,5. Interestingly, both unstimulated and IFN-α-treated PBMC isolated from HIV positive individuals showed enhanced expression of surface BST-2 compared to HIV negative or untreated cells respectively19. Although previous work detected trace levels of IFN-α secretion (~50 pg/mL) in human macrophage supernatants following 7 h of IL-27 stimulation5, we found that IL-27-induced type I IFN was not responsible for mediating the upregulation of BST-2 expression through the use of the type I IFN neutralizing protein, B18R.
How IL-27 stimulation modulates BST-2 expression in PBMC isolated from HIV positive individuals has yet to be reported, and results from such investigations will be critical to furthering our understanding of the role played by the IL-27-BST-2 axis in HIV infection. Whether BST-2 antiviral activity is accomplished by inhibiting virus spread via tethering of newly formed HIV virions has not been specifically determined; however, several studies have demonstrated that BST-2 can tether HIV-1 to the cell surface and, thus, inhibit its release9,32,33. This may result in a restriction of virus spread, limiting virus infection. Liberatore and Bieniasz showed that when BST-2 deficient mice were challenged with a strain of Moloney murine leukemia virus that is known to induce immune activation and type I IFN expression, viremia and exacerbated pathology were observed in comparison to wild type mice34. Furthermore, Jones et al. demonstrated that siRNA silencing of BST-2 enhanced mouse mammary tumor virus replication in vivo, indicating that BST-2 functions to restrict virus replication32. Although the HIV-1 protein Vpu is known to counteract BST-29,32,33, Homann et al. demonstrated that IFN-α-mediated induction of BST-2 expression was able to overcome the effects of Vpu and inhibit HIV release in vitro19. It is possible that IL-27-induced BST-2 may function in a similar manner in the setting of HIV infection. Since we demonstrated that IL-27-mediated induction of cell surface BST-2 expression is observed up to 24–48 h post-stimulation, it is possible that these enhanced levels of BST-2 may function to inhibit virus release. Moreover, the effects of IL-27 on Vpu expression and activity have not been defined and represent an important area for future investigation. In light of the previously established ability of IL-27 to inhibit HIV replication4,5, the impact of IL-27 on BST-2 expression may represent an additional mechanism by which IL-27 can accomplish this function; this is of particular importance if recombinant IL-27 was to be considered as a potential adjunct to anti-retroviral therapy.
In this study we confirm expression levels of BST-2 on monocytes and T cells, and for the first time, report inducible BST-2 expression on monocytes and T cells in response to IL-27 stimulation. Expression of BST-2, a viral restriction factor, on these specific cell types is particularly interesting, as these cell types are targets for infection by HIV. Since BST-2 can inhibit HIV infection, and IL-27 can inhibit HIV replication, it is likely that the influence of IL-27 on BST-2 expression represents a newly defined anti-viral function for IL-27.
Methods
Cell culture and reagents
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood donations obtained in agreement with the Queen's University Research Ethics Board approval. Briefly, whole blood was diluted with an equal volume of PBS-EDTA (1 mM)/2% FBS and layered over Lympholyte (Cedarlane Laboratories, Burlington, ON, Canada) and subjected to density centrifugation. The PBMC fraction was extracted, washed twice in PBS-EDTA, and re-suspended in media for stimulations or further processed by magnetic cell separation to isolate CD4+ T cells or monocytes (StemCell Technologies, Vancouver, BC, Canada). Both cell lines, THP-1 (pro-monocytic leukemic cells) and Jurkat (T cells), were obtained from ATCC. All cells were cultured in Iscove's Modified Dulbecco's Medium (Invitrogen, Burlington, ON, Canada) supplemented with 10% fetal bovine serum (FBS) (ThermoScientific, Ottawa, ON, Canada). Recombinant cytokines (IL-6 and IL-27) were purchased from R&D Systems (Minneapolis, MN, USA). All IFN stimulations were performed at 1000 U/mL with the Universal Type I Interferon, an alpha IFN hybrid (PBL Interferon Source, Piscataway, NJ, USA). The vaccinia virus-encoded type I IFN receptor, B18R, was used to neutralize type I IFN intermediates, as previously described (eBioscience, San Diego, CA, USA)21.
Flow cytometry
Cells were washed in PBS-azide-FBS (PBS-0.1% azide,1% FBS) and stained with fluorochrome-conjugated antibodies for surface markers as follows: CD14-PE (Beckman Coulter, Mississauga, ON, Canada) and CD3-ECD (Beckman Coulter). BST-2 staining was performed for 30 min at room temperature using rabbit anti-human BST-2 (1:2000) obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Anti-Bst-2 (cat# 11722) from Drs. Klaus Strebel and Amy Andrew23. Subsequent staining with a fluorochrome labelled goat-anti-rabbit IgG-Alexa Fluor 488 (Molecular Probes Inc., Eugene, OR, USA), permitted detection of BST-2 expression. As a control, cells were assessed for background staining by treatment with pre-immune rabbit serum followed by staining with the goat-anti-rabbit IgG-Alexa Fluor 488. The mean fluorescence intensity was less than 5 for all cell types. For intracellular staining, cells were first fixed in 4% paraformaldehyde and then surface stained with CD14 and CD3. This was followed by permeabilization with 0.1% saponin and intracellular staining with anti-BST-2, and subsequently stained with secondary antibody (anti-rabbit-Alexa Fluor 488). Data were acquired with the Epics XL-MCL flow cytometer. Analysis was performed using the WinMDI version 2.9 software package (J. Trotter, Scripps Institute, San Diego). PBMC were gated on CD14-positive cells for monocyte analysis, or CD3-positive cells for T cell analysis.
Western blot
PBMC were lysed and protein concentrations measured by the Bradford Assay. Samples (50 μg protein) were subjected to 10% polyacrylamide SDS-PAGE followed by transfer onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Membranes were probed with anti-phospho-STAT3 and anti-pan-STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:500 dilution in 2.5% bovine serum albumin. Immunoblots were visualized by Enhanced Chemiluminescence (Amersham Biosciences, Baie d'Urfe, QC, Canada) on the AlphaInnotech HD2 Imager; the brightness and contrast of the final image was adjusted to all parts of the image equally.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from cell pellets using TRI Reagent RNA Isolation Reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's protocol. Reverse transcription of 0.5 μg (CD4+ T cells and CD14+ monocytes), 1 μg (Jurkat cells) or 5 μg RNA (THP-1 cells) was performed with reverse transcriptase from Moloney Murine Leukemia Virus (Invitrogen, Burlington, ON, Canada). To quantify the level of BST-2 mRNA, qRT-PCR was performed using SsoFast EvaGreen Supermix (Biorad, Mississauga, Ontario, Canada) and the following primer sets. BST-2 forward: 5′-TTCTCAGTCGCTCCACCT-3′, BST-2 reverse: 5′-CACCTGCAACCACACTGT-3′, 18S rRNA forward: 5′-TTCGGAACTGAGGCCATGAT-3′, 18S rRNA reverse: 5′-CGAACCTCCGACTTTCGTTT-3′. PCR cycling was performed with CFX96 Real-time PCR Detection System (Biorad, Mississauga, Ontario, Canada), at an annealing temperature of 60.9°C. Relative amount of BST-2 mRNA was calculated by the ΔCt method using 18S rRNA: relative ratio = 2∧(18S rRNA Ct – Target Gene Ct). Relative fold increase was calculated by normalizing relative ratios against the 0 hour (h) time point.
Indirect immunofluorescence microscopy
Cells were fixed in 4% paraformaldehyde for 10 minutes at room temperature, washed three times in PBS-1% FBS, and permeabilized in 0.1% saponin. Cells were subsequently stained with rabbit-anti-human BST-2 (1:500) (see ‘Flow Cytometry' methods), washed and stained with secondary fluorochrome-conjugated antibody, goat-anti-rabbit-Alexa Flour 568 (1:500) (Molecular Probes Inc., Eugene, OR, USA). Cells were washed once and then stained with Hoechst 33342 to visualize nuclei as previously described27. Cells were washed three times and plated on poly-lysine-coated glass bottom dishes (MatTek, Ashland, MA, USA) 1 hour prior to microscopy to allow settling of cells on dishes for optimal imaging. Images were captured using an Olympus FV1000 laser scanning confocal microscope and Fluoview 1.7.3.0 software, using a 60X (1.42 NA) oil immersion objective. Composites of representative images were prepared using Adobe Photoshop CS3 software and the brightness and contrast of the final image was adjusted to all parts of the image equally.
Statistical analysis
To assess significance of IL-27-induced BST-2 expression the two-tailed paired Student's t-test was used, with p<0.05 considered significant.
Author Contributions
CG performed experiments and wrote the manuscript, MJ performed qRT PCR, AG assisted with PCR, and BWB assisted with microscopy staining, imaging, and analysis. CG and KG designed experiments, and all authors edited the manuscript. KG oversaw all aspects of the study. CG was supported by a CIHR Vanier graduate scholarship. This research was supported by grants from Queen's University and the National Sciences and Engineering Council of Canada (NSERC) to KG and Canada Foundation for Innovation award and Canadian Institutes of Health Research (CIHR) grant to BWB.
References
- Larousserie F. et al. Differential effects of IL-27 on human B cell subsets. J. Immunol. 176(10), 5890–5897 (2006). [DOI] [PubMed] [Google Scholar]
- Lucas S. et al. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. U. S. A. 100(25), 15047–15052 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 16(6), 779–790 (2002). [DOI] [PubMed] [Google Scholar]
- Imamichi T. et al. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS 22(1), 39–45 (2008). [DOI] [PubMed] [Google Scholar]
- Greenwell-Wild T. et al. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood. 114(9), 1864–1874 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius A. L. et al. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177(5), 3260–3265 (2006). [DOI] [PubMed] [Google Scholar]
- Stetson D. B. & Medzhitov R. Type I interferons in host defense. Immunity. 25(3), 373–381 (2006). [DOI] [PubMed] [Google Scholar]
- Tokarev A. et al. Antiviral activity of the interferon-induced cellular protein BST-2/tetherin. AIDS Res. Hum. Retroviruses. 25(12), 1197–1210 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil S. J., Zang T. & Bieniasz P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 451(7177), 425–430 (2008). [DOI] [PubMed] [Google Scholar]
- Van, Damme N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host. Microbe. 3(4), 245–252 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N. et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83(4), 1837–1844 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R. L. et al. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106(8), 2886–2891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T. et al. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83(5), 2382–2385 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshitzky S. R. et al. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J. Virol. 84(20), 10569–10580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M. et al. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83(19), 9672–9681 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner J. M. et al. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 84(24), 12646–12657 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S. et al. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 4(10), 694–709 (2003). [DOI] [PubMed] [Google Scholar]
- Erikson E. et al. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc. Natl. Acad. Sci. U. S. A. 108(33), 13688–13693 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann S. et al. Upregulation of BST-2/Tetherin by HIV infection in vivo. J. Virol. 85(20), 10659–10668 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi E. et al. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106(8), 2868–2873 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bego M. G., Mercier J. & Cohen E. A. Virus-activated interferon regulatory factor 7 upregulates expression of the interferon-regulated BST2 gene independently of interferon signaling. J. Virol. 86(7), 3513–3527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtomo T. et al. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 258(3), 583–591 (1999). [DOI] [PubMed] [Google Scholar]
- Matsuda A. et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 22(21), 3307–3318 (2003). [DOI] [PubMed] [Google Scholar]
- Guzzo C. et al. IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J. Immunol. 188(2), 864–873 (2012). [DOI] [PubMed] [Google Scholar]
- Guzzo C., Che Mat N. F. & Gee K. Interleukin-27 induces a STAT1/3 and NF-kappaB dependent proinflammatory cytokine profile in human monocytes. J. Biol. Chem. 285(32), 24404–24411 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo C. et al. Impact of HIV infection, highly active antiretroviral therapy, and hepatitis C coinfection on serum interleukin-27. AIDS. 24(9), 1371–1374 (2010). [DOI] [PubMed] [Google Scholar]
- Pflanz S. et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172(4), 2225–2231 (2004). [DOI] [PubMed] [Google Scholar]
- Symons J. A. Alcami A. & Smith G. L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 81(4), 551–560 (1995). [DOI] [PubMed] [Google Scholar]
- Kawa S. et al. Construction of a conventional non-radioisotope method to quantify HM1.24 antigens: correlation of HM1.24 levels and ADCC activity of the humanized antibody against HM1.24. Leuk. Res. 30(8), 949–956 (2006). [DOI] [PubMed] [Google Scholar]
- Kanda N. & Watanabe S. IL-12, IL-23, and IL-27 enhance human beta-defensin-2 production in human keratinocytes. Eur. J. Immunol. 38(5), 1287–1296 (2008). [DOI] [PubMed] [Google Scholar]
- He L. et al. Upregulation of interleukin-27 expression is correlated with higher CD4+ T cell counts in treatment of naive human immunodeficiency virus-infected Chinese. 3(1), 6–10 (2011).
- Jones P. H. et al. Bone marrow stromal cell antigen 2 (BST-2) restricts mouse mammary tumor virus (MMTV) replication in vivo. Retrovirology. 9: 10, 10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M. et al. Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83(9), 4574–4590 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore R. A. & Bieniasz P. D. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 108(44), 18097–18101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]